Submitted:
27 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolation of Streptomyces
2.2. Screening of Streptomyces Strains with Anti-Phytophthora Activity and Antifungal Spectrum Assays
2.3. Genome Assembly and Annotation
2.4. Comparative Genomic Analysis
2.5. Preparation of Ethyl Acetate Extract
2.6. Inhibition Rate of Mycelial Growth by ASG80 Extract
2.7. Pot Experiment for Disease Control with ASG80 Extract
3. Results
3.1. Screening of Streptomyces
3.2. Antifungal Spectrum of Strain ASG80
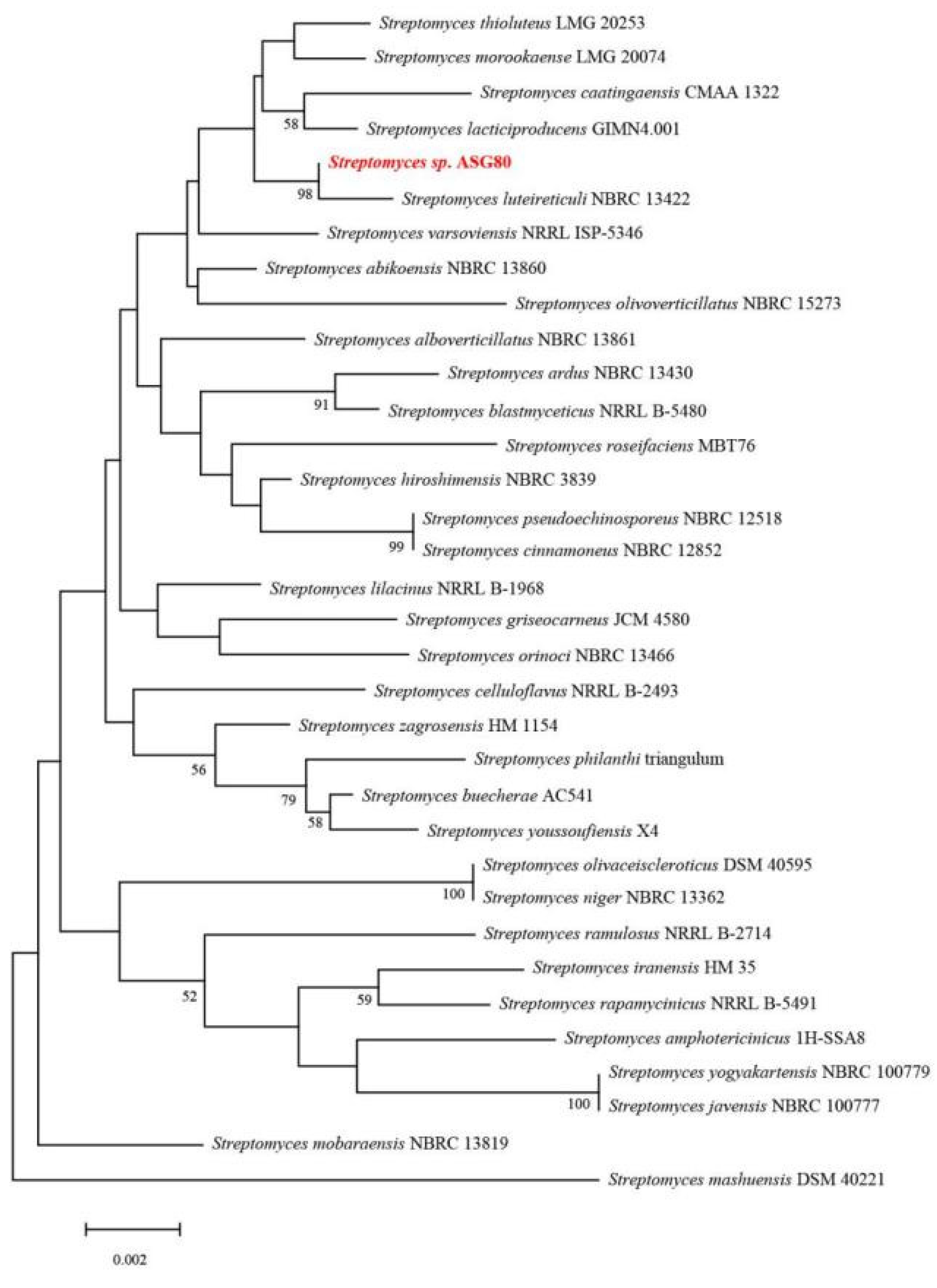
3.3. Complete Genome Sequence and Biosynthesis-Related Gene Clusters of Strain ASG80
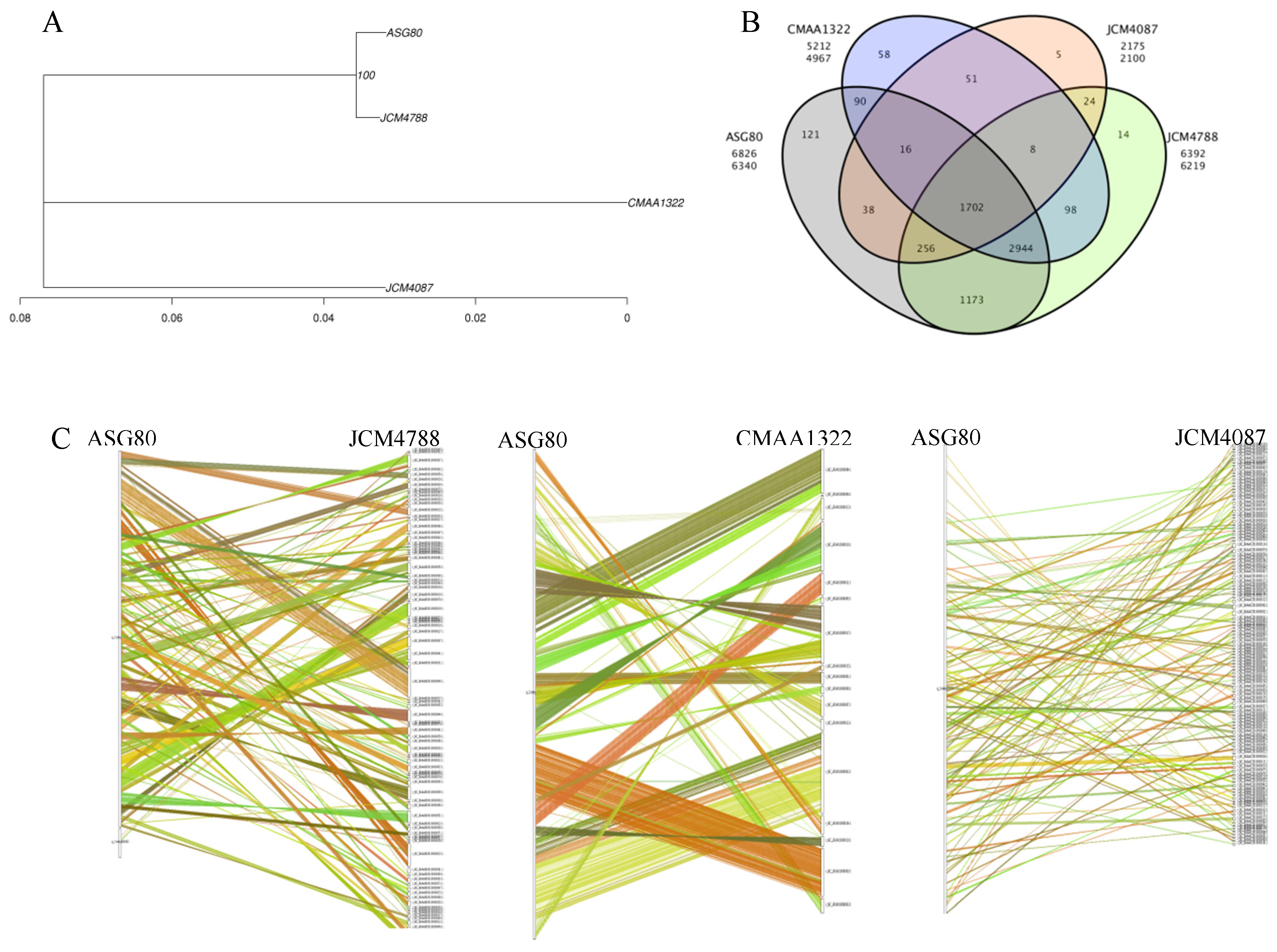
3.4. Comparative Genomic Analysis
3.5. Broad-Spectrum Antifungal Activity Assay
3.6. Effect of Strain ASG80 Extract on Sisal Zebra Disease under Greenhouse Conditions
4. Discussion
5. Conclusion
Supplementary Materials
Acknowledgments
References
- Judelson, H.S.; Blanco, F.A. The spores of Phytophthora: weapons of the plant destroyer. Nature reviews. Microbiology 2005, 3, 47-58, doi:10.1038/nrmicro1064. [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nature Reviews Microbiology 2023, 21, 640-656, doi:10.1038/s41579-023-00900-7. [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. NATURE ECOLOGY & EVOLUTION 2019, 3, 430-+, doi:10.1038/s41559-018-0793-y. [CrossRef]
- Grunwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular plant pathology 2008, 9, 729-740, doi:10.1111/j.1364-3703.2008.00500.x. [CrossRef]
- Farooq, Q.U.A.; McComb, J.; Hardy, G.E.S.J.; Burgess, T.I. Soil amendments for management of Phytophthora root rot in avocado and their impact on the soil microbiome. JOURNAL OF PLANT PATHOLOGY 2024, 106, 439-455, doi:10.1007/s42161-024-01604-4. [CrossRef]
- Lu, X.H.; Zhu, S.S.; Bi, Y.; Liu, X.L.; Hao, J.J. Baseline Sensitivity and Resistance-Risk Assessment of Phytophthora capsici to Iprovalicarb. PHYTOPATHOLOGY 2010, 100, 1162-1168, doi:10.1094/PHYTO-12-09-0351. [CrossRef]
- Fang, Y.; Wang, Z.; Zhang, S.; Peng, Q.; Liu, X. Characterization and proteome analysis of the extracellular vesicles of Phytophthora capsici. JOURNAL OF PROTEOMICS 2021, 238, doi:10.1016/j.jprot.2021.104137. [CrossRef]
- Cheng, W.; Lin, M.; Qiu, M.; Kong, L.; Xu, Y.; Li, Y.; Wang, Y.; Ye, W.; Dong, S.; He, S.; et al. Chitin synthase is involved in vegetative growth, asexual reproduction and pathogenesis of Phytophthora capsici and Phytophthora sojae. ENVIRONMENTAL MICROBIOLOGY 2019, 21, 4537-4547, doi:10.1111/1462-2920.14744. [CrossRef]
- Gallup, C.A.; McCorkle, K.L.; Ivors, K.L.; Shew, D. Characterization of the Black Shank Pathogen, Phytophthora nicotianae, Across North Carolina Tobacco Production Areas. PLANT DISEASE 2018, 102, 1108-1114, doi:10.1094/PDIS-02-17-0295-RE. [CrossRef]
- Garcia-Gaona, M.; Botero-Rozo, D.; Araque, L.; Romero, H.M. The Dynamic Interaction between Oil Palm and Phytophthora palmivora in Bud Rot Disease: Insights from Transcriptomic Analysis and Network Modelling. JOURNAL OF FUNGI 2024, 10, doi:10.3390/jof10030164. [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a potential natural compound to manage plant diseases. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2022, 220, 998-1009, doi:10.1016/j.ijbiomac.2022.08.109. [CrossRef]
- Liu, K.; McInroy, J.A.; Hu, C.-H.; Kloepper, J.W. Mixtures of Plant-Growth-Promoting Rhizobacteria Enhance Biological Control of Multiple Plant Diseases and Plant-Growth Promotion in the Presence of Pathogens. PLANT DISEASE 2018, 102, 67-72, doi:10.1094/PDIS-04-17-0478-RE. [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. MICROORGANISMS 2022, 10, doi:10.3390/microorganisms10030596. [CrossRef]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.M.; Cai, L. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. NATURE COMMUNICATIONS 2022, 13, doi:10.1038/s41467-022-35452-6. [CrossRef]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Pratap Singh, S.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as Bio-factories for Antifungal Secondary Metabolites: Innovation Beyond Whole Organism Formulations. MICROBIAL ECOLOGY 2023, 86, 1-24, doi:10.1007/s00248-022-02044-2. [CrossRef]
- Kulkova, I.; Dobrzynski, J.; Kowalczyk, P.; Belzecki, G.; Kramkowski, K. Plant Growth Promotion Using Bacillus cereus. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2023, 24, doi:10.3390/ijms24119759. [CrossRef]
- Hou, Q.; Kolodkin-Gal, I. Harvesting the complex pathways of antibiotic production and resistance of soil bacilli for optimizing plant microbiome. FEMS MICROBIOLOGY ECOLOGY 2020, 96, doi:10.1093/femsec/fiaa142. [CrossRef]
- Liu, L.; Zhao, K.; Cai, L.; Zhang, Y.; Fu, Q.; Huang, S. Combination effects of tebuconazole with Bacillus subtilis to control rice false smut and the related synergistic mechanism. PEST MANAGEMENT SCIENCE 2023, 79, 234-243, doi:10.1002/ps.7193. [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Li, R.; Kowalchuk, G.A.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. SOIL BIOLOGY & BIOCHEMISTRY 2017, 114, 238-247, doi:10.1016/j.soilbio.2017.07.016. [CrossRef]
- Santos, M.; Dianez, F.; Sanchez-Montesinos, B.; Huertas, V.; Moreno-Gavira, A.; Esteban Garcia, B.; Garrido-Cardenas, J.A.; Gea, F.J.J. Biocontrol of Diseases Caused by Phytophthora capsici and P. parasitica in Pepper Plants. JOURNAL OF FUNGI 2023, 9, doi:10.3390/jof9030360. [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. PATHOGENS 2022, 11, doi:10.3390/pathogens11020273. [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. MICROORGANISMS 2020, 8, doi:10.3390/microorganisms8030401. [CrossRef]
- Zhan, X.; Khan, R.A.A.; Zhang, J.; Chen, J.; Yin, Y.; Tang, Z.; Wang, R.; Lu, B.; Liu, T. Control of postharvest stem-end rot on mango by antifungal metabolites of Trichoderma pinnatum LS029-3. SCIENTIA HORTICULTURAE 2023, 310, doi:10.1016/j.scienta.2022.111696. [CrossRef]
- Duan, Y.; Pang, Z.; Yin, S.; Xiao, W.; Hu, H.; Xie, J.; Moussa, E.J. Screening and Analysis of Antifungal Strains Bacillus subtilis JF-4 and B. amylum JF-5 for the Biological Control of Fusarium Wilt of Banana. JOURNAL OF FUNGI 2023, 9, doi:10.3390/jof9090886. [CrossRef]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. JOURNAL OF FUNGI 2023, 9, doi:10.3390/jof9111047. [CrossRef]
- Chaudhary, A.K.; Dhakal, D.; Sohng, J.K. An Insight into the "-Omics" Based Engineering of Streptomycetes for Secondary Metabolite Overproduction. BIOMED RESEARCH INTERNATIONAL 2013, 2013, doi:10.1155/2013/968518. [CrossRef]
- Nguyen, C.T.; Dhakal, D.; Pham, V.T.T.; Nguyen, H.T.; Sohng, J.-K. Recent Advances in Strategies for Activation and Discovery/Characterization of Cryptic Biosynthetic Gene Clusters in Streptomyces. MICROORGANISMS 2020, 8, doi:10.3390/microorganisms8040616. [CrossRef]
- Rey, T.; Dumas, B. Plenty Is No Plague: Streptomyces Symbiosis with Crops. TRENDS IN PLANT SCIENCE 2017, 22, 30-37, doi:10.1016/j.tplants.2016.10.008. [CrossRef]
- Park, H.J.; Lee, J.Y.; Hwang, I.S.; Yun, B.S.; Kim, B.S.; Hwang, B.K. Isolation and Antifungal and Antioomycete Activities of Staurosporine from Streptomyces roseoflavus Strain LS-A24. Journal of Agricultural and Food Chemistry 2006, 54, 3041-3046, doi:10.1021/jf0532617. [CrossRef]
- Sun, Y.; Wu, H.; Xu, S.; Tang, S.; Hao, J.; Liu, X.; Zhang, H.; Han, L. Roles of the EPS66A polysaccharide from Streptomyces sp. in inducing tobacco resistance to tobacco mosaic virus. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2022, 209, 885-894, doi:10.1016/j.ijbiomac.2022.04.081. [CrossRef]
- Wang, X.-J.; Wang, M.; Wang, J.-D.; Jiang, L.; Wang, J.-J.; Xiang, W.-S. Isolation and Identification of Novel Macrocyclic Lactones from Streptomyces avermitilis NEAU1069 with Acaricidal and Nematocidal Activity. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 2010, 58, 2710-2714, doi:10.1021/jf902496d. [CrossRef]
- Arasu, M.V.; Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp AP-123 and its cytotoxic effect. CHEMOSPHERE 2013, 90, 479-487, doi:10.1016/j.chemosphere.2012.08.006. [CrossRef]
- Li, X.; Jing, T.; Zhou, D.; Zhang, M.; Qi, D.; Zang, X.; Zhao, Y.; Li, K.; Tang, W.; Chen, Y.; et al. Biocontrol efficacy and possible mechanism of Streptomyces sp. H4 against postharvest anthracnose caused by Colletotrichum fragariae on strawberry fruit. POSTHARVEST BIOLOGY AND TECHNOLOGY 2021, 175, doi:10.1016/j.postharvbio.2020.111401. [CrossRef]
- Wang, Y.; Zhao, Q.; Sun, Z.; Li, Y.; He, H.; Zhang, Y.; Yang, X.; Wang, D.; Dong, B.; Zhou, H.; et al. Whole-genome analysis revealed the growth-promoting mechanism of endophytic bacterial strain Q2H1 in potato plants. FRONTIERS IN MICROBIOLOGY 2022, 13, doi:10.3389/fmicb.2022.1035901. [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC BIOINFORMATICS 2010, 11, doi:10.1186/1471-2105-11-119. [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. BIOINFORMATICS 2013, 29, 2933-2935, doi:10.1093/bioinformatics/btt509. [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods in molecular biology (Clifton, N.J.) 2019, 1962, 1-14, doi:10.1007/978-1-4939-9173-0_1. [CrossRef]
- Naughton, L.M.; Romano, S.; O'Gara, F.; Dobson, A.D.W. Identification of Secondary Metabolite Gene Clusters in the Pseudovibrio Genus Reveals Encouraging Biosynthetic Potential toward the Production of Novel Bioactive Compounds. FRONTIERS IN MICROBIOLOGY 2017, 8, doi:10.3389/fmicb.2017.01494. [CrossRef]
- Li, L.; Stoeckert, C.J., Jr.; Roos, D.S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome research 2003, 13, 2178-2189, doi:10.1101/gr.1224503. [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. NUCLEIC ACIDS RESEARCH 2012, 40, doi:10.1093/nar/gkr1293. [CrossRef]
- Van Ewijk, P.H.; Hoekstra, J.A. Calculation of the EC50 and its confidence interval when subtoxic stimulus is present. Ecotoxicology and environmental safety 1993, 25, 25-32, doi:10.1006/eesa.1993.1003. [CrossRef]
- Zhang, X.; Wang, Y.; Han, X.; Gou, J.; Li, W.; Zhang, C. A Novel Bio-Fertilizer Produced by Prickly Ash Seeds with Biochar Addition Induces Soil Suppressiveness against Black Shank Disease on Tobacco. APPLIED SCIENCES-BASEL 2021, 11, doi:10.3390/app11167261. [CrossRef]
- Zhong, J.; Sui, W.W.; Bai, X.Y.; Qiu, Z.L.; Li, X.G.; Zhu, J.Z. Characterization and biocontrol mechanism of Streptomyces olivoreticuli as a potential biocontrol agent against Rhizoctonia solani. Pesticide Biochemistry and Physiology 2023, 197, 105681, doi:doi.org/10.1016/j.pestbp.2023.105681. [CrossRef]
- Cao, M.; Cheng, Q.; Cai, B.; Chen, Y.; Wei, Y.; Qi, D.; Li, Y.; Yan, L.; Li, X.; Long, W.; et al. Antifungal Mechanism of Metabolites from Newly Isolated Streptomyces sp. Y1-14 against Banana Fusarium Wilt Disease Using Metabolomics. JOURNAL OF FUNGI 2022, 8, doi:10.3390/jof8121291. [CrossRef]
- Dong, W.; Wu, W.-J.; Song, C.-Y.; Li, T.; Zhang, J.-Z. Jinggangmycin stimulates reproduction and increases CHCs-dependent desiccation tolerance in Drosophila melanogaster. PESTICIDE BIOCHEMISTRY AND PHYSIOLOGY 2023, 194, doi:10.1016/j.pestbp.2023.105484. [CrossRef]
- Li, M.; Chen, Z.; Zhang, X.; Song, Y.; Wen, Y.; Li, J. Enhancement of avermectin and ivermectin production by overexpression of the maltose ATP-binding cassette transporter in Streptomyces avermitilis. BIORESOURCE TECHNOLOGY 2010, 101, 9228-9235, doi:10.1016/j.biortech.2010.06.132. [CrossRef]
- Sharma, V.; Kaur, R.; Salwan, R. Streptomyces: host for refactoring of diverse bioactive secondary metabolites. 3 BIOTECH 2021, 11, doi:10.1007/s13205-021-02872-y. [CrossRef]
- Ferraiuolo, S.B.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. APPLIED MICROBIOLOGY AND BIOTECHNOLOGY 2021, 105, 551-568, doi:10.1007/s00253-020-11064-2. [CrossRef]
- Lacey, H.J.; Rutledge, P.J. Recently Discovered Secondary Metabolites from Streptomyces Species. MOLECULES 2022, 27, doi:10.3390/molecules27030887. [CrossRef]
- Nakae, K.; Kojima, F.; Sawa, R.; Kubota, Y.; Igarashi, M.; Kinoshita, N.; Adachi, H.; Nishimura, Y.; Akamatsu, Y. Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons. JOURNAL OF ANTIBIOTICS 2010, 63, 41-44, doi:10.1038/ja.2009.109. [CrossRef]
- Wang, L.; Zhu, M.; Zhang, Q.; Zhang, X.; Yang, P.; Liu, Z.; Deng, Y.; Zhu, Y.; Huang, X.; Han, L.; et al. Diisonitrile Natural Product SF2768 Functions As a Chalkophore That Mediates Copper Acquisition in Streptomyces thioluteus. ACS CHEMICAL BIOLOGY 2017, 12, 3067-3075, doi:10.1021/acschembio.7b00897. [CrossRef]
- Werneburg, M.; Busch, B.; He, J.; Richter, M.E.A.; Xiang, L.; Moore, B.S.; Roth, M.; Dahse, H.-M.; Hertweck, C. Exploiting Enzymatic Promiscuity to Engineer a Focused Library of Highly Selective Antifungal and Antiproliferative Aureothin Analogues. JOURNAL OF THE AMERICAN CHEMICAL SOCIETY 2010, 132, 10407-10413, doi:10.1021/ja102751h. [CrossRef]
- Busch, B.; Hertweck, C. Evolution of metabolic diversity in polyketide-derived pyrones: Using the non-colinear aureothin assembly line as a model system. PHYTOCHEMISTRY 2009, 70, 1833-1840, doi:10.1016/j.phytochem.2009.05.022. [CrossRef]
- Herrmann, A.; Roesner, M.; Werner, T.; Hauck, S.M.; Koch, A.; Bauer, A.; Schneider, M.; Brack-Werner, R. Potent inhibition of HIV replication in primary human cells by novel synthetic polyketides inspired by Aureothin. SCIENTIFIC REPORTS 2020, 10, doi:10.1038/s41598-020-57843-9. [CrossRef]
- Kang, M.-K.; Kim, J.-H.; Liu, M.-J.; Jin, C.-Z.; Park, D.-J.; Kim, J.; Sung, B.-H.; Kim, C.-J.; Son, K.-H. New discovery on the nematode activity of aureothin and alloaureothin isolated from endophytic bacteria Streptomyces sp. AE170020. SCIENTIFIC REPORTS 2022, 12, doi:10.1038/s41598-022-07879-w. [CrossRef]
- Kim, S.J.; Cantrell, C.L.; Avula, B.; Chen, J.; Schrader, K.K.; Santo, S.N.; Ali, A.; Khan, I.A. Streptomyces distallicus, a Potential Microbial Biolarvicide. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 2022, doi:10.1021/acs.jafc.2c03537. [CrossRef]
- Fu, X.; Liu, S.; Ru, J.; Tang, B.; Zhai, Y.; Wang, Z.; Wang, L. Biological control of potato late blight by Streptomyces sp. FXP04 and potential role of secondary metabolites. BIOLOGICAL CONTROL 2022, 169, doi:10.1016/j.biocontrol.2022.104891. [CrossRef]
- Feng, S.; Tang, S.; Jian, Y.; Huang, X.; Jin, L.; Zhu, Z.; Dong, P.; Li, Z. Complete Genome Sequence Data of a Novel Streptomyces sp. Strain A2-16, a Potential Biological Control Agent for Potato Late Blight. PLANT DISEASE 2022, 106, 723-726, doi:10.1094/PDIS-04-21-0858-A. [CrossRef]
- Abbasi, S.; Safaie, N.; Sadeghi, A.; Shamsbakhsh, M. Tissue-specific synergistic bio-priming of pepper by two Streptomyces species against Phytophthora capsici</i>. PLOS ONE 2020, 15, doi:10.1371/journal.pone.0230531. [CrossRef]
- Ji, S.; Tian, Y.; Li, J.; Xu, G.; Zhang, Y.; Chen, S.; Chen, Y.; Tang, X. Complete genome sequence of Bacillus cereus Z4, a biocontrol agent against tobacco black shank, isolated from the Western Pacific Ocean. MARINE GENOMICS 2023, 72, doi:10.1016/j.margen.2023.101071. [CrossRef]
- Rocio Suarez-Moreno, Z.; Marcela Vinchira-Villarraga, D.; Isabel Vergara-Morales, D.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth Promotion and Biocontrol Properties of Three Streptomyces spp. Isolates to Control Bacterial Rice Pathogens. FRONTIERS IN MICROBIOLOGY 2019, 10, doi:10.3389/fmicb.2019.00290. [CrossRef]
- Bae, S.-J.; Mohanta, T.K.; Chung, J.Y.; Ryu, M.; Park, G.; Shim, S.; Hong, S.-B.; Seo, H.; Bae, D.-W.; Bae, I.; et al. Trichoderma metabolites as biological control agents against Phytophthora pathogens. BIOLOGICAL CONTROL 2016, 92, 128-138, doi:10.1016/j.biocontrol.2015.10.005. [CrossRef]
- Liu, Z.; Tian, J.; Yan, H.; Li, D.; Wang, X.; Liang, W.; Wang, G. Ethyl acetate produced by Hanseniaspora uvarum is a potential biocontrol agent against tomato fruit rot caused by Phytophthora nicotianae. FRONTIERS IN MICROBIOLOGY 2022, 13, doi:10.3389/fmicb.2022.978920. [CrossRef]
- Zheng, T.-w.; Liu, L.; Nie, Q.-w.; Hsiang, T.; Sun, Z.-x.; Zhou, Y. Isolation, identification and biocontrol mechanisms of endophytic bacterium D61-A from Fraxinus hupehensis against Rhizoctonia solani. BIOLOGICAL CONTROL 2021, 158, doi:10.1016/j.biocontrol.2021.104621. [CrossRef]
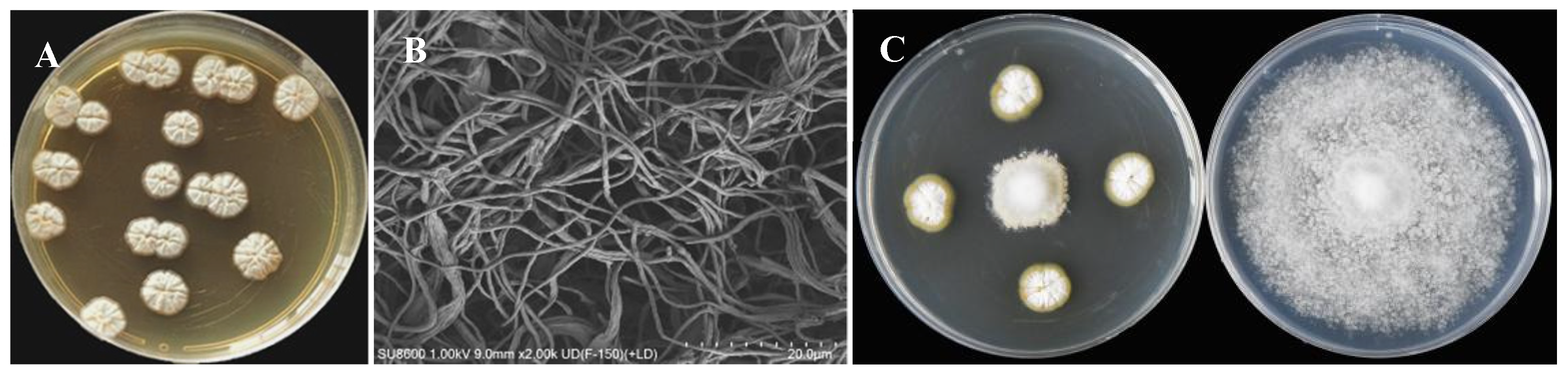


| Pathogens | Mycelial inhibition(%) |
|---|---|
| Phytophthora colocasiae | 83.74±0 |
| Phytophthora cinnamomi | 72.87±0.46 |
| Phytophthora palmivora | 75.63±0.27 |
| Phytophthora capsici | 61.27±0.18 |
| Phytophthora vignae | 88.43±0.31 |
| Phytophthora sojae | 56.70±0.26 |
| Phytophthora melonis | 70.53±0.15 |
| Colletotrichum gloeosporioides | 62.78±0.60 |
| Magnaporthe grisea | 85.87±0 |
| Fusarium oxysporum | 43.21±0.16 |
| Fusarium graminearum | 44.96±0.31 |
| Pestalotiopsis palmarum | 58.95±0.16 |
| Database | Number of annotated functional proteins | Proportion/% |
|---|---|---|
| eggNOG | 5,549 | 70.54 |
| GO | 4,598 | 58.45 |
| KEGG | 2,385 | 30.32 |
| Nr | 7,531 | 95.73 |
| Pfam | 5,793 | 73.64 |
| Swiss-prot | 3,440 | 43.73 |
| TrEMB | 7,510 | 95.46 |
| All database | 7,549 | 95.96 |
| Region | Type | From | To | Most similar known cluster |
Similarity |
|---|---|---|---|---|---|
| Clusters 1 | Terpene,NRPS,NRPS-like | 60,185 | 117,028 | antipain | 100% |
| Clusters 2 | T1PKS | 169,015 | 216,487 | himastatin | 8% |
| Clusters 3 | T1PKS | 242,821 | 288,100 | petrichorin | 5% |
| Clusters 4 | NRPS,T1PKS | 473,674 | 553,694 | tallysomycin | 55% |
| Clusters 5 | Terpene | 578,046 | 600,082 | tylactone | 6% |
| Clusters 6 | Terpene | 610,891 | 631,859 | - | |
| Clusters 7 | Lassopeptide,T1PKS | 638,187 | 696,947 | ulleungdin | 75% |
| Clusters 8 | Thiopeptide | 748,305 | 808,571 | geosmin | 100% |
| Clusters 9 | T1PKS,NRPS | 816,029 | 911,078 | peucechelin | 20% |
| Clusters 10 | T1PKS,NRPS-like | 930,265 | 1,105,264 | conglobatin | 26% |
| Clusters 11 | NRPS | 1,122,162 | 1,166,496 | diisonitrile antibiotic SF2768 | 100% |
| Clusters 12 | T1PKS,phenazine | 1,194,939 | 1,257,441 | aureothin | 100% |
| Clusters 13 | NRPS,T1PKS | 1,295,327 | 1,467,303 | sceliphrolactam | 48% |
| Clusters 14 | NRPS-like,arylpolyene | 1,547,967 | 1,590,881 | lankacidin C | 20% |
| Clusters 15 | RiPP-like | 1,632,931 | 1,644,841 | - | |
| Clusters 16 | Lassopeptide | 1,746,532 | 1,769,080 | lagmysin | 80% |
| Clusters 17 | Terpene | 1,779,551 | 1,800,171 | - | |
| Clusters 18 | T1PKS | 1,890,303 | 1,957,177 | cremimycin | 10% |
| Clusters 19 | NI-siderophore | 2,265,382 | 2,295,133 | - | |
| Clusters 20 | Terpene,NRPS | 2,725,486 | 2,770,746 | pyrroloformamide | 37% |
| Clusters 21 | Thioamide-NRP,T1PKS | 3,661,290 | 3,719,169 | 5-isoprenylindole-3-carboxylate β-D-glycosyl ester | 33% |
| Clusters 22 | Butyrolactone,T3PKS | 4,656,145 | 4,697,411 | neocarzinostatin | 8% |
| Clusters 23 | Terpene | 4,924,188 | 4,945,345 | ebelactone | 5% |
| Clusters 24 | T3PKS | 5,734,254 | 5,775,303 | violapyrone B | 28% |
| Clusters 25 | NI-siderophore | 6,121,786 | 6,151,618 | kinamycin | 19% |
| Clusters 26 | RiPP-like | 6,210,115 | 6,220,963 | - | |
| Clusters 27 | T3PKS,phenazine | 6,247,692 | 6,295,310 | endophenazine | 47% |
| Clusters 28 | T2PKS | 6,367,291 | 6,439,815 | allocyclinone | 43% |
| Clusters 29 | Phenazine,NRPS | 6,617,890 | 6,676,804 | streptophenazine | 24% |
| Clusters 30 | Indole | 6,688,763 | 6,712,068 | AT2433-A1 | 14% |
| Clusters 31 | NAPAA,terpene | 6,721,859 | 6,774,236 | hopene | 76% |
| Clusters 32 | NRPS-like | 6,831,419 | 6,873,260 | kitacinnamycin | 7% |
| Clusters 33 | NRPS-like | 6,898,154 | 6,961,149 | cyphomycin | 5% |
| Clusters 34 | Other,terpene | 6,985,496 | 7,026,419 | ECO-0501 | 4% |
| Clusters 35 | Other | 7,182,203 | 7,223,099 | griseusin | 15% |
| Clusters 36 | RiPP-like | 7,298,812 | 7,310,096 | - | |
| Clusters 37 | NRPS,T1PKS,terpene | 7,364,647 | 7,465,253 | hexacosalactone A | 18% |
| Clusters 38 | NRPS-like,CDPS | 7,500,628 | 7,543,702 | guanipiperazine | 80% |
| Clusters 39 | T1PKS,NRPS | 7,647,760 | 7,783,943 | qinichelins | 83% |
| Clusters 40 | T3PKS,T1PKS,furan | 7,816,344 | 7,894,681 | paerucumarin | 60% |
| Pathogens | Regression equation | EC50 (μg/mL) | Correlation coefficient | |
|---|---|---|---|---|
| Phytophthora vignae | y = 1.3297x + 4.8299 | 1.3427 | 0.9864 | |
| Phytophthora sojae | y = 1.6772x + 4.6219 | 1.6806 | 0.9985 | |
| Phytophthora palmivora | y = 1.7603x + 4.1724 | 2.9521 | 0.9920 | |
| Phytophthora nicotiana | y = 0.8279x + 4.8283 | 1.6120 | 0.9952 | |
| Phytophthora melonis | y = 1.1621x + 4.5614 | 2.3846 | 0.9994 | |
| Phytophthora colocasiae | y = 1.7260x + 4.4184 | 2.1725 | 0.9687 | |
| Phytophthora cinnamomi | y = 1.6358x + 5.0999 | 0.8688 | 0.9998 | |
| Phytophthora capsici | y = 1.7841x + 4.1978 | 2.8159 | 0.9933 | |
| Pestalotiopsis palmarum | y = 1.3937x + 2.8532 | 34.6959 | 0.9895 | |
| Magnaporthe grisea | y = 1.1531x + 3.9087 | 8.8389 | 0.9807 | |
| Fusarium oxysporum | y = 1.1694x + 3.0012 | 51.2052 | 0.9956 | |
| Fusarium graminearum | y = 1.5994x + 3.1874 | 13.5908 | 0.9958 | |
| Colletotrichum gloeosporioides | y = 1.7642x + 3.1930 | 10.5738 | 0.9938 | |
| Treatment | Incidence rate(%) | Disease index | Control effect (%) |
|---|---|---|---|
| CK | 0 | / | / |
| Pn | 100.00 | 52.45 | / |
| T-1 | 30.00 | 8.33 | 84.12 |
| T-2 | 46.67 | 18.43 | 64.86 |
| Me-2 | 36.67 | 11.99 | 77.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





