1. Introduction
Breast cancer has the highest occurrence rate in female cancer patients, accounting for about 16.7% of new cases, and is also the primary reason of mortality [
1,
2]. Triple-negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer, known for its elevated risk of disease progression and reduced survival rates [
3]. While chemotherapy remains the cornerstone of therapy for early-stage and advanced TNBC, alongside traditional methods like surgery and radiotherapy [
4,
5], its effectiveness is often hampered by the adverse effects of chemotherapy medications and the development of drug resistance resulting from repeated drug administration [
6]. Hence, there is a pressing necessity to explore a more promising therapeutic strategy for addressing TNBC while minimizing adverse effects.
The combination of chemotherapy with various anticancer strategies, such as immunotherapy [
7], photothermal therapy [
8], photodynamic therapy [
9], has drawn much attention because it breaks through limited effectiveness of single-agent chemotherapy [
10]. Among them, PDT kills tumor cells by converting molecular oxygen into highly toxic ROS under laser irradiation with photosensitizer, which has the advantages of spatiotemporal controllability and non-invasiveness [
11]. The utilization of active ingredients from traditional Chinese medicines, such as rhein, has shown promising therapeutic outcomes in cancer treatment [
12,
13,
14]. Rhein, an anthraquinone compound primarily derived from Polygonum multiflorum Thunb and rhubarb [
15], has inhibitory effects on cancer cell proliferation, including tongue cancer cells (SSC-4) [
16], lung cancer cells (A-549) [
17], nasopharyngeal carcinoma cells [
18], and promyelocytic leukemia cells (HL-60) [
19]. Recent research has demonstrated significant inhibitory effects of rhein on the growth of 4T1 breast cancer xenografts in mice, showing its potential in cancer therapy [
20]. Rhein achieves this by influencing the potential of the mitochondrial membrane and inducing cell death through mitochondrial mediated apoptosis pathway [
21,
22,
23]. However, Rhein has a low bioavailability due to its hydrophobicity, which limits its clinical application.
Recently, Nanomedicines without carriers, created by the self-assembly of chemotherapy drugs and photosensitizers, could accumulate preferentially at tumor sites due to the EPR effect and showed great synergistic therapeutic effects in a variety of cancers [
24,
25]. Compared with traditional nanomedicines, carrier-free nanomedicines exhibit a high drug loading capacity and simple preparation process, thereby avoiding carrier-induced toxicity and immunogenicity [
26]. However, the efficacy of these nanodrugs is hindered by factors such as tumor vascular heterogeneity and high interstitial pressure, resulting less than 1% of the injected dose reaching the tumor tissue [
27]. Enhancing the accumulation of nanoparticles in tumors is crucial for efficacy of combination therapy for TNBC.
A variety of strategies have been developed to enhance drug delivery efficiency, such as the design of tumor microenvironment-adjustable or size-variable nanomedicine to promote penetration of nanomedicines by disrupting the tumor's dense matrix [
28] or changing the size of nanoparticles [
29]. The surface properties of nanoparticles have also been optimized to prolong blood circulation and increase tumor targeting and cross-cell transport abilities [
30]. However, these strategies rely heavily on the design of nanocarriers, which are complex in synthesis, low in drug loading rates, and can exhibit significant toxicity and side effects, limiting their clinical application. As a common means of clinical diagnosis and treatment, ultrasound (US) offers advantages such as being non-invasive, simple, and capable of deep tissue penetration, making it a useful tool for promoting nano-drug delivery. It has been reported that US irradiation could increase the permeability of tumor blood vessels and promote the penetration depth and drug concentration of nanodrugs in tumor cells, thereby achieving ideal therapeutic effects [
31,
32,
33].
Herein, a carrier-free nanodrug (designated as RC NPs) for chemo-photodynamic therapy of breast cancer was prepared by self-assembly between the chemotherapeutic agent (Rhe) and photosensitizer (Ce6) (
Scheme 1A). The synthesized RC NPs exhibited a high drug loading rate and demonstrate favorable dispersion stability. After intravenous injection, RC NPs showed significant tumor accumulation and deep penetration upon US irradiation (
Scheme 1B). Upon internalization by tumor cells, Ce6 could produce a large number of ROS under laser irradiation for PDT, while rhein could reduce mitochondrial membrane potential and induce cell apoptosis through the mitochondrial-dependent apoptosis pathway, thus effectively realizing the combined PDT and chemotherapy. Our study demonstrated that this US combined with carrier-free nanodrug treatment strategy could effectively increase the drug concentration in the tumor tissue and achieve better therapeutic effects for chemo-photodynamic therapy in breast cancer with high biosafety.
2. Materials and Methods
2.1. Materials
Chlorin e6 (Ce6) was bought from Frontier Scientific (Logan, Utah). Rhein was bought from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT), annexin V-FITC/PI cell apoptosis kit and Calcein-AM/PI cell viability/cytotoxicity detection kit were bought from Yeasen Biotechnology Company. (Shanghai, China). 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and Mitochondrial membrane potential detection kit (JC-1) were bought from Beyotime Biotechnology Co., Ltd. (Shanghai, China). All additional chemicals were of analytical quality and used with no other processing.
2.2. Preparation and Characterization of RC NPs
The nanoprecipitation approach was used to create RC NPs through the self-assembly of rhein and Ce6 at a 3:1 molar ratio. Initially, rhein and Ce6 powders were dissolved in dimethyl sulfoxide (DMSO), and then solutions of rhein (solution A) and Ce6 (solution B) with a final concentration of 20 mM were prepared, respectively. Subsequently, 45 μL of solution A and 15 μL of solution B were mixed and stirred for 4 h. The obtained solution was then dispersed in 2 ml of ultrapure water under sonication, and subjected to overnight stirring. The next day, a dialysis bag (MWCO: 3500 Da) was used for dialysis for 12 h to remove the unassembled free drugs. Finally, the nanodrugs in the dialysis bag were centrifuged at 1,200 g for 10 min to collect the supernatant. The RC NPs solution was stored at 4 ℃ for later usage.
Thereafter, the particle size and polydispersity index (PDI) of samples were detected using dynamic light scattering. The nanoparticles' morphology was examined using transmission electron microscopy (TEM), while its stability was assessed by incubation in water, PBS, and 10% FBS over varying durations.
2.3. Exploration of Self-Assembly Mechanism
To explore the noncovalent interactions for the formation of RC NPs, RC NPs were incubated with 0.2% SDS and varying contents of NaCl solution (0, 0.5, 1.0, and 1.5 M), and then a Shinadu UV-2600 spectrophotometer was utilized in order to accurately detect the fluctuations in the UV-vis absorption spectra. Additionally, the spectral changes of RC NPs dispersed in water and DMSO were also examined.
2.4. Cell Culture
The HUVEC cells were cultured in DMEM medium with 1% antibiotics and 10% FBS, while mouse breast cancer cells (4T1) were cultured in RPMI-1640 medium with the same supplements. The cells were cultivated in an environment containing 5% carbon dioxide at a temperature of 37 ℃.
2.5. Cellular Uptake
In 24-well culture plates with a cover glass at the bottom, 4T1 cells were seeded and left to culture overnight. Subsequently, it was cultured in medium containing RC NPs and Ce6 (where Ce6 concentration was 5 μM) for 1, 2, 4, and 6 h, respectively. The cells were carefully rinsed with PBS after incubation and fixed for 20 minutes with 500 μL of 4% paraformaldehyde solution. Following the fixation process, the cell nuclei were stained with an anti-fluorescence quenching agent that contained DAPI. The intracellular fluorescence was then visualized using confocal microscopy (CLSM) (LSM 880, Carl Zeiss). Meanwhile, the intracellular fluorescent intensity was examined based on flow cytometry. 4T1 cells were seeded in 6-well plates and left to incubate overnight. Subsequently, these cells were exposed to RC NPs and Ce6 for 1, 2, 4, and 6 h. Flow cytometry was employed to measure the intracellular fluorescence following cell collection (BD LSRFortessa X-20).
2.6. Intracellular ROS Generation
DCFH-DA was applied as a fluorescent probe to monitor intracellular ROS generation. Initially, after seeding 4T1 cells into 24-well plates, they were cultured for 12 hours and then given different treatments, including PBS, Rhe, RC NPs, PBS+L, Ce6+L, and RC NPs+L. For each treatment, Ce6 (0.5 μM) and rhein (2 μM) concentrations were used, and the cells were left to grow for 4 hours. Subsequently, cells were rinsed with PBS and cultured in basic media containing DCFH-DA (10 μM) for 30 min. The light groups were then irradiated via a 630 nm laser panel (120 mW/cm2) for 1 minute. At last, intracellular ROS was visualized by fluorescence inverted microscopy (FIM) (Axio Observer).
2.7. In Vitro Cytotoxicity
For cytotoxicity assessment, the MTT assay was employed. To investigate dark toxicity, 4T1 cells were seeded in 96-well plates and left to incubate for 12 hours. Following this, the cells were incubated for another 24 hours with fresh media that contained Ce6 and rhein at varying concentrations (0 to 0.5 μM and 0 to 2 μM, respectively). Then, 20 μL of MTT solution was applied to every well and incubated for 4 h in darkness. After removing the original medium, add 200 μL of DMSO solution to each well, and the OD at 490 nm was detected via microplate reader (Thermo, USA). Similar to the dark toxicity assay, the phototoxicity assay involved irradiating the sample with a 630 nm laser panel (120 mW/cm2) for 1 min at 4 h after drug addition, followed by an additional 20 h of incubation.
2.8. Live/Dead Cell Staining Assay
4T1 cells were seeded in 24-well plates and left to incubate for 12 hours. The medium was replaced by fresh medium containing Rhe, RC NPs, and Ce6 (Ce6: 0.5 μM, rhein: 2 μM). After incubation for 4 h, the light groups (PBS+L, Ce6+L, and RC NPs+L) were exposed to a 630 nm laser panel (120 mW/cm2) for 1 min, and then the incubation was continued for 1 h. Finally, the cells in various groups were treated with Calcein-AM (2 μM) and PI (4.5 μM) for 30 minutes. Intracellular fluorescence was visualized using FIM (Axio Observer).
2.9. Apoptosis Assay
Annexin V-FITC/PI method was employed to analyze apoptosis in 4T1 cells based on flow cytometry. The 4T1 cells were seeded in 6-well plates and left to incubate overnight, then fresh medium containing Rhe, RC NPs, and Ce6 (rhein: 2 μM, Ce6: 0.5 μM) was added, following 4 h of incubation. Among them, the light groups (PBS+L, Ce6+L, and RC NPs+L) were irradiated via laser panel (120 mW/cm2) for 1 min and continued incubation for 1 h. Finally, the cells were stained with Annexin V-FITC and PI based on relevant kit instructions, and the fluorescence was then measured.
2.10. Detection of Mitochondrial Membrane Potential
When mitochondria are damaged, their membrane potential decreases, the changes were assessed using JC-1 staining, and with the decrease of mitochondrial membrane potential, mitochondrial fluorescence gradually changes from red to green fluorescence. 4T1 cells were incubated for 12 hours in 24-well plates. After dividing cells into 6 groups, various drug formulations were added: (1) PBS, (2) Rhe, (3) RC NPs, (4) PBS+L, (5) Ce6+L, and (6) RC NPs+L (rhein: 2 μM, Ce6: 0.5 μM). After 4-hour incubation, cells in the light groups were exposed to laser irradiation for 1 minute, followed by a 1-hour incubation period before conducting JC-1 staining. Finally, the changes in mitochondrial membrane potential were identified using CLSM.
2.11. Ultrasonic Facilitated Penetration Detection In Vitro
To verify that the US could promote RC NPs to penetrate through tumor blood vessels, HUVEC cells were employed to simulate the tumor blood vessel barrier in vitro. Initially, HUVEC cells were seeded and incubated in a transwell upper chamber (2×104/well), while 4T1 cells were seeded in 24-well plates. When HUVEC cells had grown and covered the bottom of the upper chamber, they were transferred to 24-well plates in which 4T1 cells were cultured. After that, new medium with RC NPs (Ce6: 5 μM) was put into the upper chamber. It was then exposed to US irradiation (1.0 MHz, 0.3 W/cm2) for 1 minute. The transwell insert was then removed and continued incubation for 4 hours. The group that did not get US treatment was designated as the control group. Finally, 4T1 cells in 24-well plates were harvested, and flow cytometry was applied to detect the passage of RC NPs through the vascular barrier after US irradiation.
Also, to make sure that US improved the penetration of RC NPs in vitro, 3D tumor spheres were made to show how cell-cell and cell-matrix interactions affect the penetration of RC NPs. Initially, 70 μL of 2.5% agarose solution was added to a 96-well plate when hot. After sufficient cooling, the surface formed a depression to prevent cell adhesion. Subsequently, 150 μL of medium containing 4T1 cells (500 cells/well) was added to each well, shaken evenly, and placed in an incubator. Half of the medium was replaced every other day. After 2 weeks, the 3D tumor spheres with a diameter of about 200 μm were identified selected, and administered with fresh medium containing RC NPs (Ce6: 5 μM). The US treatment group received US irradiation at a frequency of 1.0 MHz with a duty cycle of 50% and intensity of 0.6 W/cm2 for 2 minutes. Subsequently, the drugs were incubated with the tumor spheres for 4 hours. Tumor spheres were finally washed with PBS and transferred to confocal laser small dishes. Fluorescence in the tumor spheres was observed using CLSM.
2.12. Hemolysis Test of RC NPs
The female BALB/c mice utilized in the research were purchased from the Animal Experiment Center of Southern Medical University. The animal studies were conducted in accordance with the guidelines of the ethics committee of the Animal Experiment Centre of Southern Medical University (No. 00251499).
In order to carry out animal experiments on RC NPs, it is necessary to first verify the safety of nanodrugs through hemolysis experiments. Healthy BALB/c mice were anesthetized, and fresh blood was sampled via heart puncture. Pure erythrocytes were obtained after centrifugation and washing with PBS, and 4% (v/v) red blood cell suspension was prepared. A mixture was created by combining 250 μL of red blood cell suspension with a solution of RC NPs at varying concentrations (Ce6: 5, 10, 20, 25, and 50 μM). After centrifugation, 100 μL of supernatant was transferred to a 96-well plate. All samples were then analyzed for absorbance at 545 nm using a microplate reader. (Positive control group: red blood cell suspension mixed with ultrapure water, negative control group: red blood cell suspension mixed with PBS).
2.13. Fluorescence Imaging In Vivo
To create tumor-bearing mice models, subcutaneous injections of 4T1 cell suspension (2×106 cells) were administered to the right subdermal dorsal region of BALB/c nude mice. Once the tumor volume reached around 200 mm3, the mice were divided into two groups and injected with 0.1 ml of Ce6 and RC NPs solution (Ce6 dose: 3 mg/kg), respectively. Isoflurane gas anesthesia was then applied, followed by performing in vivo fluorescence imaging of tumor-bearing mice at specified time intervals (0, 2, 4, 6, 12, and 24 h) using IVIS Lumina II (USA). We euthanized the mice 24 hours after injection and collected their tumors for later experiments.
To confirm the ability of US to enhance the penetration and accumulation of RC NPs in vivo tumors, a BALB/c nude mouse model with bilateral 4T1 tumors was constructed. When the tumor volume had grown to approximately 200 mm3, the RC NPs solution (Ce6 dose: 1.5 mg/kg) was injected. After 30 min, the right tumor was irradiated with US (1.0 MHz, 50 duty cycle, 0.6 W/cm2) for 10 min. The left tumor was not irradiated with US as a control. 6 h later, the anesthetized mice underwent in vivo fluorescence imaging to assess and compare the distribution of drug fluorescence in the tumors on both sides. Afterward, the tumors on both sides were excised for frozen sections and imaged using CLSM.
2.14. Antitumor Study In Vivo and Biosafety Assay
4T1 cells were injected into the right subdermal dorsal region to create a tumor-bearing animal model. Treatment began after the tumor volume was about 90 mm3. They were divided into 7 groups (n = 5), namely PBS, PBS+US+L, Rhe, RC NPs, Ce6+L, RC NPs+L, and RC NPs+US+L. Based on the reported dosage (2 mg/kg) of carrier-free nanomaterials associated with Ce6 utilized in animal studies, we administered a dose of 1.5 mg/kg to each mouse for preliminary trials, resulting in a favorable therapeutic outcome. The molar ratio of rhein to Ce6 in the produced RC NPs is around 2:1, resulting in a comparable rhein dosage of approximately 2.9 mg/kg. The mice received intravenous injections of the specified drugs (Ce6 dose: 1.5 mg/kg, rhein dose: 2.9 mg/kg). The US treatment groups were exposed to US (1.0 MHz, 50 duty cycle, 0.6 W/cm2) for 10 min after 30 minutes post-injection, while the light group was irradiated via laser with an intensity of 0.5 mW/cm2 for 10 minutes, 6 hours post-injection. Each group of mice received therapy three times: on the day of treatment and on days 2 and 4 following the initial treatment. On the 14th day, we euthanized the mice and collected the major organs and tumor tissues for HE staining or TUNEL immunofluorescence detection.
Healthy female BALB/c mice were divided into three groups (n = 3): the PBS group, the 1-day after administration group, and the 7-day after administration group. The mice were administered with RC NPs solution (Ce6 dose: 3 mg/kg) at predetermined time points, and blood was gathered for routine blood biochemical tests.
2.15. Statistical analysis
The experiment's quantitative data were presented as mean ± standard deviation (m ± SD), and statistical differences between the groups were analyzed using ANOVA (SPSS 23). A p-value of < 0.05was considered significant, with the following criteria: NS (no significant difference), *, **, and *** representing p < 0.05, p < 0.01, and p < 0.001, respectively.
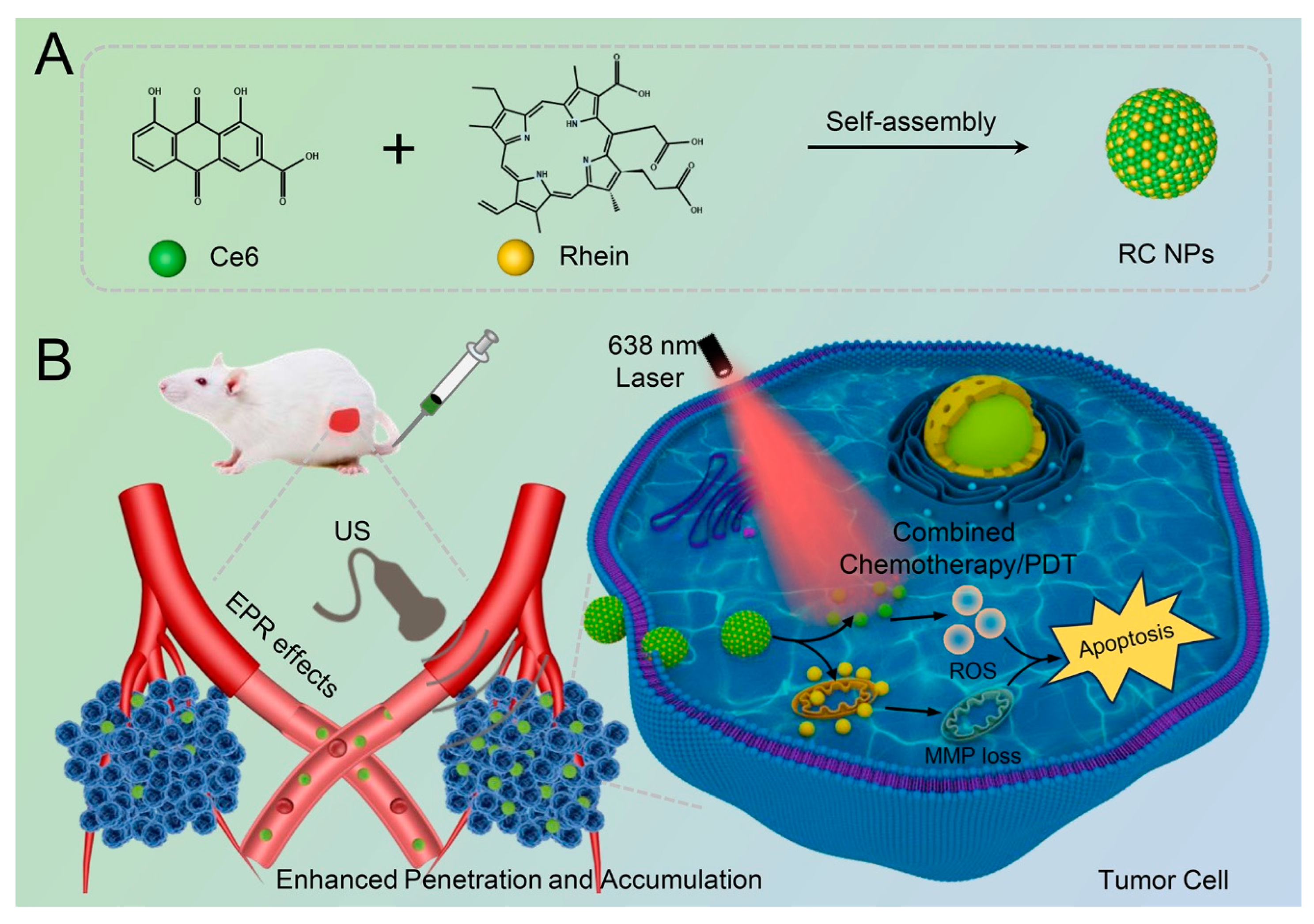
Scheme 1.
Schematic illustration of the preparation process of RC NPs and its antitumor therapeutic mechanism. (A) The chemical structures of rhein and Ce6, as well as the self-assembled RC NPs. (B) The process of using RC NPs in tumor therapy. After intravenous injection, the tumor site was irradiated with US to promote the penetration and accumulation of RC NPs in the tumor tissue, followed by laser irradiation to achieve the combined effects of chemotherapy and photodynamic therapy.
Scheme 1.
Schematic illustration of the preparation process of RC NPs and its antitumor therapeutic mechanism. (A) The chemical structures of rhein and Ce6, as well as the self-assembled RC NPs. (B) The process of using RC NPs in tumor therapy. After intravenous injection, the tumor site was irradiated with US to promote the penetration and accumulation of RC NPs in the tumor tissue, followed by laser irradiation to achieve the combined effects of chemotherapy and photodynamic therapy.
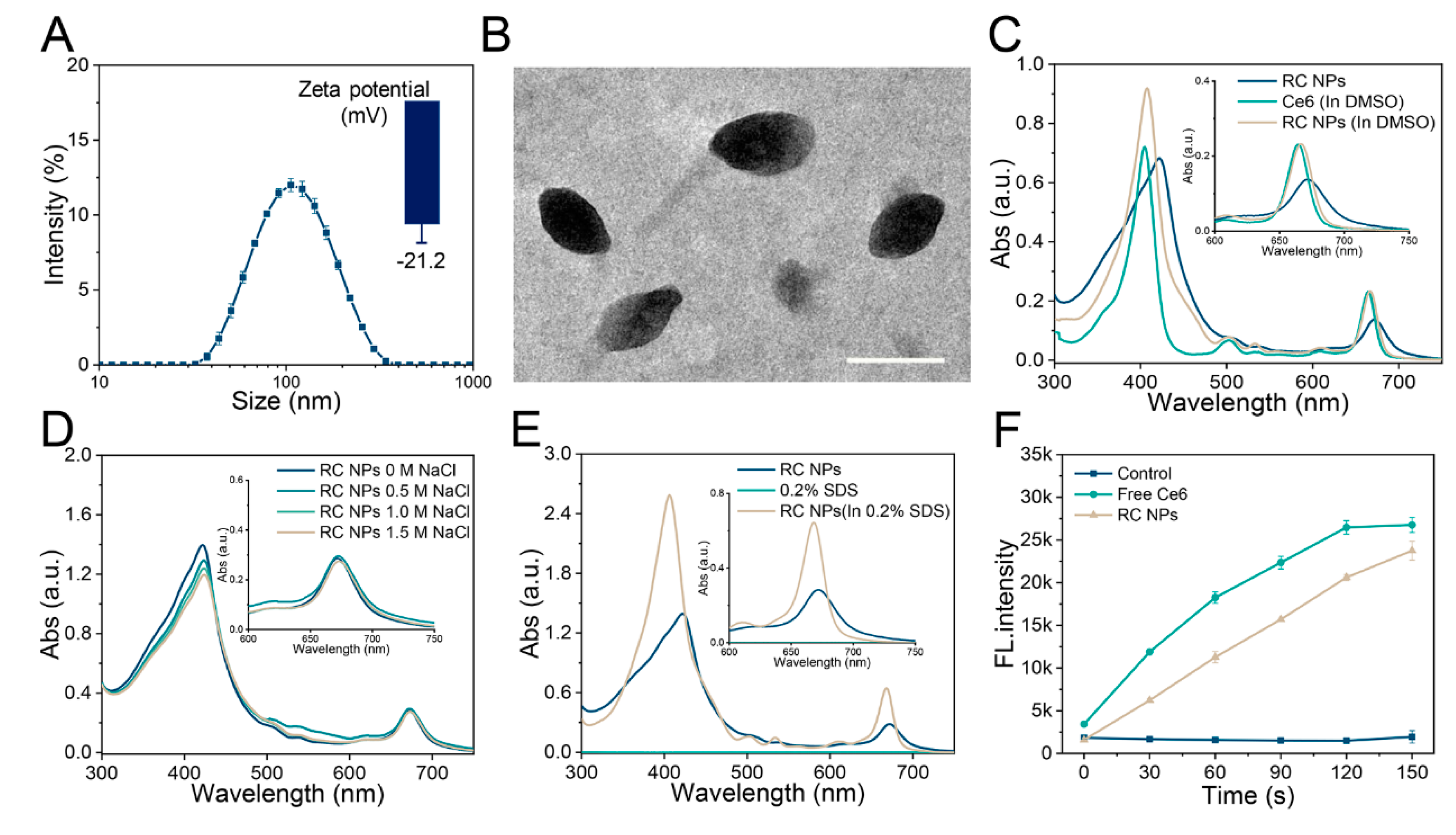
Figure 1.
Characterizations of RC NPs. (A) Particle size distribution and zeta potential of RC NPs at the feeding ratio of 3:1. (B) TEM image of RC NPs. Scale bar: 100 nm. UV−vis absorbance spectrum of RC NPs in the presence or absence of (C) DMSO, (D) NaCl, and (E) SDS (0.2%, w/v). (F) ROS production in various solutions with laser irradiation, measured by multifunctional microplate reader using DCFH as a probe. The error bars indicate means ± SD, n = 3.
Figure 1.
Characterizations of RC NPs. (A) Particle size distribution and zeta potential of RC NPs at the feeding ratio of 3:1. (B) TEM image of RC NPs. Scale bar: 100 nm. UV−vis absorbance spectrum of RC NPs in the presence or absence of (C) DMSO, (D) NaCl, and (E) SDS (0.2%, w/v). (F) ROS production in various solutions with laser irradiation, measured by multifunctional microplate reader using DCFH as a probe. The error bars indicate means ± SD, n = 3.
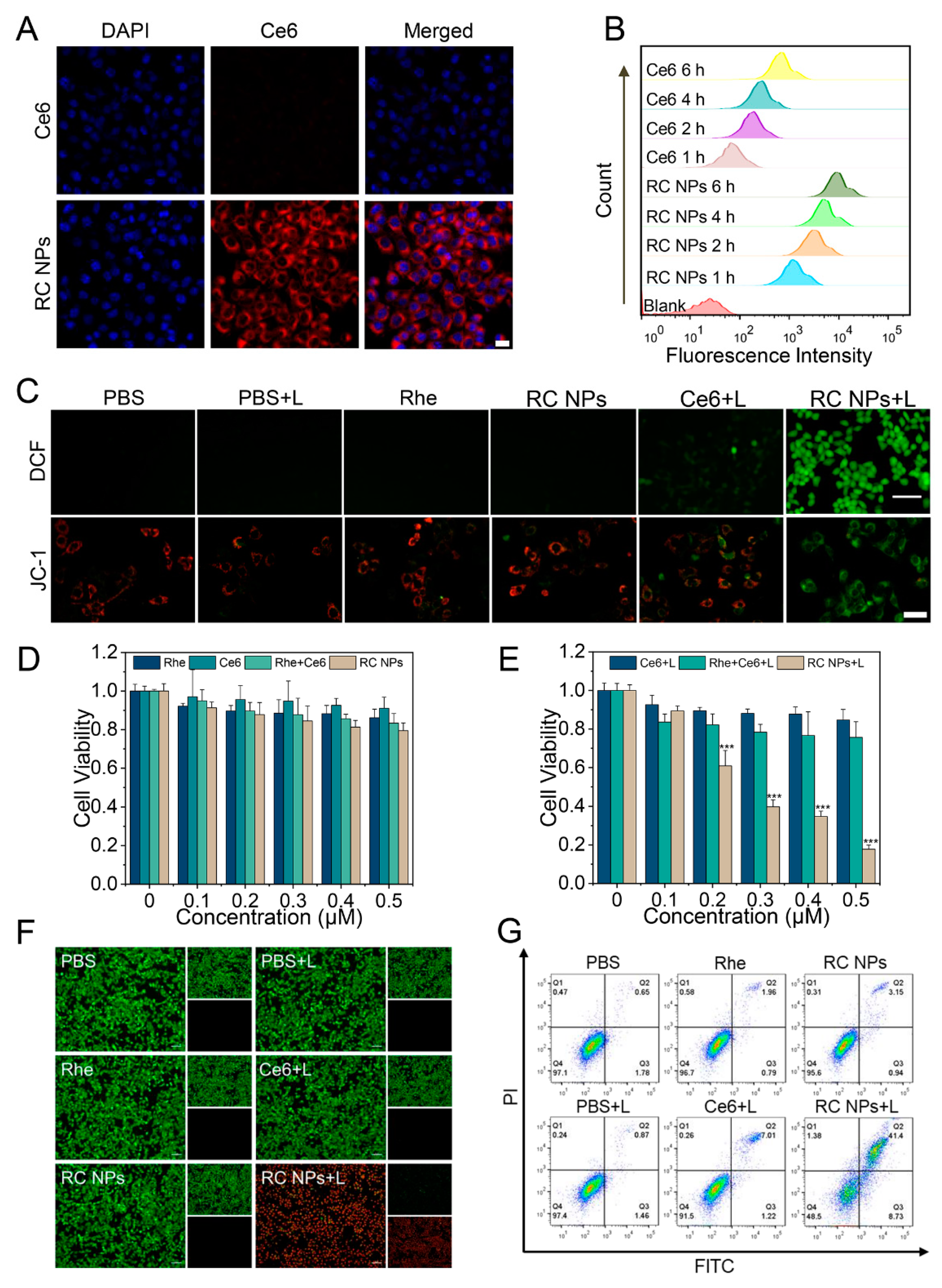
Figure 2.
Cellular uptake and in vitro antitumor evaluation. (A) CLSM images of 4T1 cells incubated with RC NPs and Ce6 for 4 h. Scale bar: 20 μm. (B) FCM analysis of the intracellular uptake of RC NPs and Ce6 in 4T1 cells at different time points. (C) FIM images of intracellular ROS production and CLSM images obtained from 4T1 cells stained with JC-1 after different treatments. Scale bars: 10 μm and 20 μm, respectively. (D) Cell viability in different treatment groups at gradient concentrations of Ce6 and Rhe in the dark, and (E) under laser irradiation. (F) FIM images of live/dead cell staining and (G) apoptotic analysis of 4T1 cells after treatment with Rhe, Ce6, or RC NPs in the presence or absence of laser irradiation. Scale bar: 10 µm. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
Figure 2.
Cellular uptake and in vitro antitumor evaluation. (A) CLSM images of 4T1 cells incubated with RC NPs and Ce6 for 4 h. Scale bar: 20 μm. (B) FCM analysis of the intracellular uptake of RC NPs and Ce6 in 4T1 cells at different time points. (C) FIM images of intracellular ROS production and CLSM images obtained from 4T1 cells stained with JC-1 after different treatments. Scale bars: 10 μm and 20 μm, respectively. (D) Cell viability in different treatment groups at gradient concentrations of Ce6 and Rhe in the dark, and (E) under laser irradiation. (F) FIM images of live/dead cell staining and (G) apoptotic analysis of 4T1 cells after treatment with Rhe, Ce6, or RC NPs in the presence or absence of laser irradiation. Scale bar: 10 µm. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
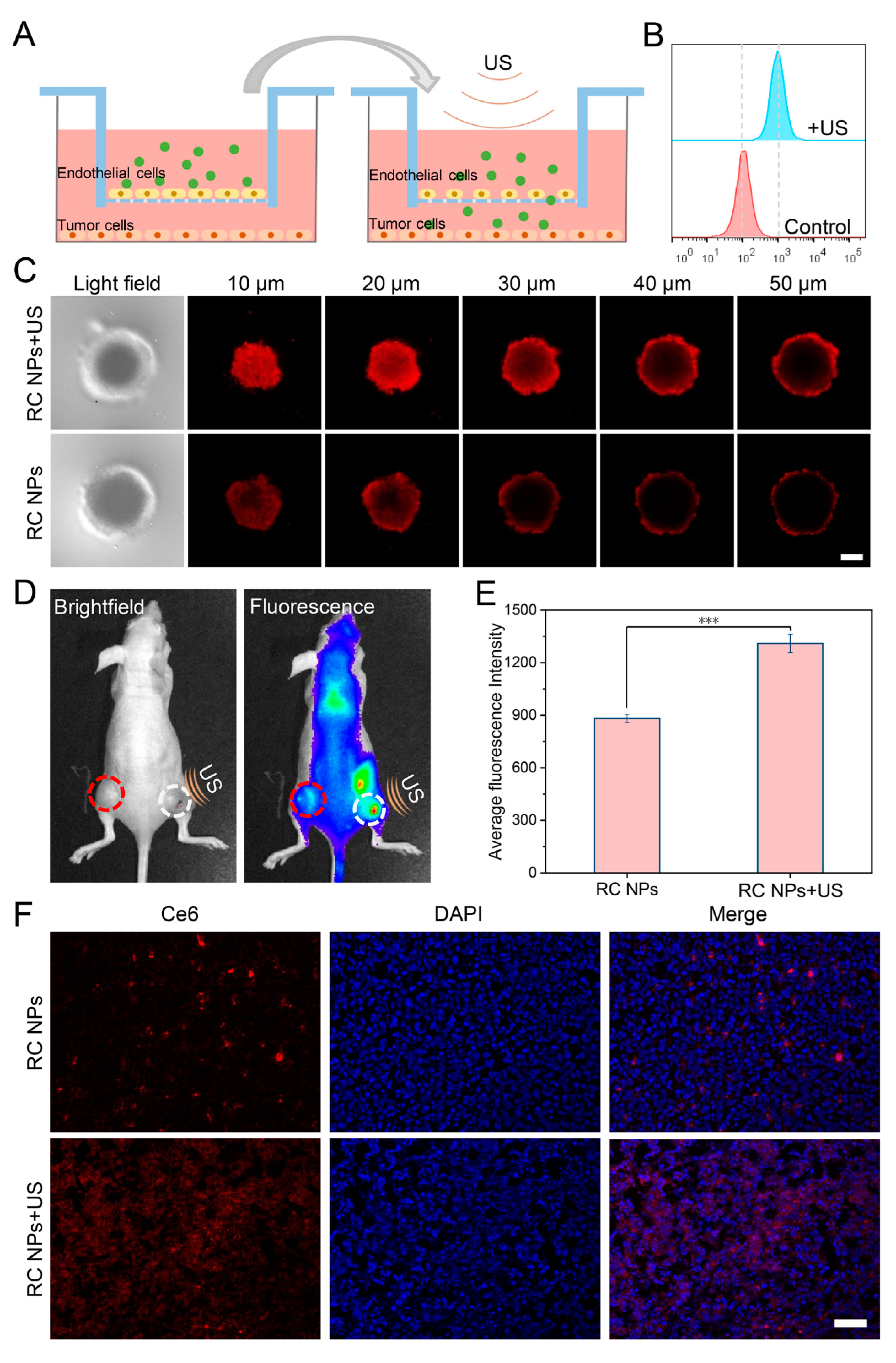
Figure 3.
US-enhanced penetration of RC NPs. (A) Schematic illustration of US-promoted penetration of RC NPs across the vascular barrier. (B) Flow cytometric analysis of NPs uptake by tumor cells across the vascular barrier after US irradiation. (C) Z-stack images of RC NPs penetrating inside 3D tumor spheres with or without US irradiation. Scale bar: 100 μm. (D) Brightfield (left) and fluorescence (right) images of mice bearing bilateral 4T1 tumors at 6 hours after intravenous administration (white circle on the right indicates US irradiation treatment, red circle on the left serves as the control), and (E) the corresponding quantitative fluorescence analysis. (F) CLSM images of frozen sections of 4T1 tumors treated with US irradiation or not. Scale bar: 50 μm. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
Figure 3.
US-enhanced penetration of RC NPs. (A) Schematic illustration of US-promoted penetration of RC NPs across the vascular barrier. (B) Flow cytometric analysis of NPs uptake by tumor cells across the vascular barrier after US irradiation. (C) Z-stack images of RC NPs penetrating inside 3D tumor spheres with or without US irradiation. Scale bar: 100 μm. (D) Brightfield (left) and fluorescence (right) images of mice bearing bilateral 4T1 tumors at 6 hours after intravenous administration (white circle on the right indicates US irradiation treatment, red circle on the left serves as the control), and (E) the corresponding quantitative fluorescence analysis. (F) CLSM images of frozen sections of 4T1 tumors treated with US irradiation or not. Scale bar: 50 μm. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
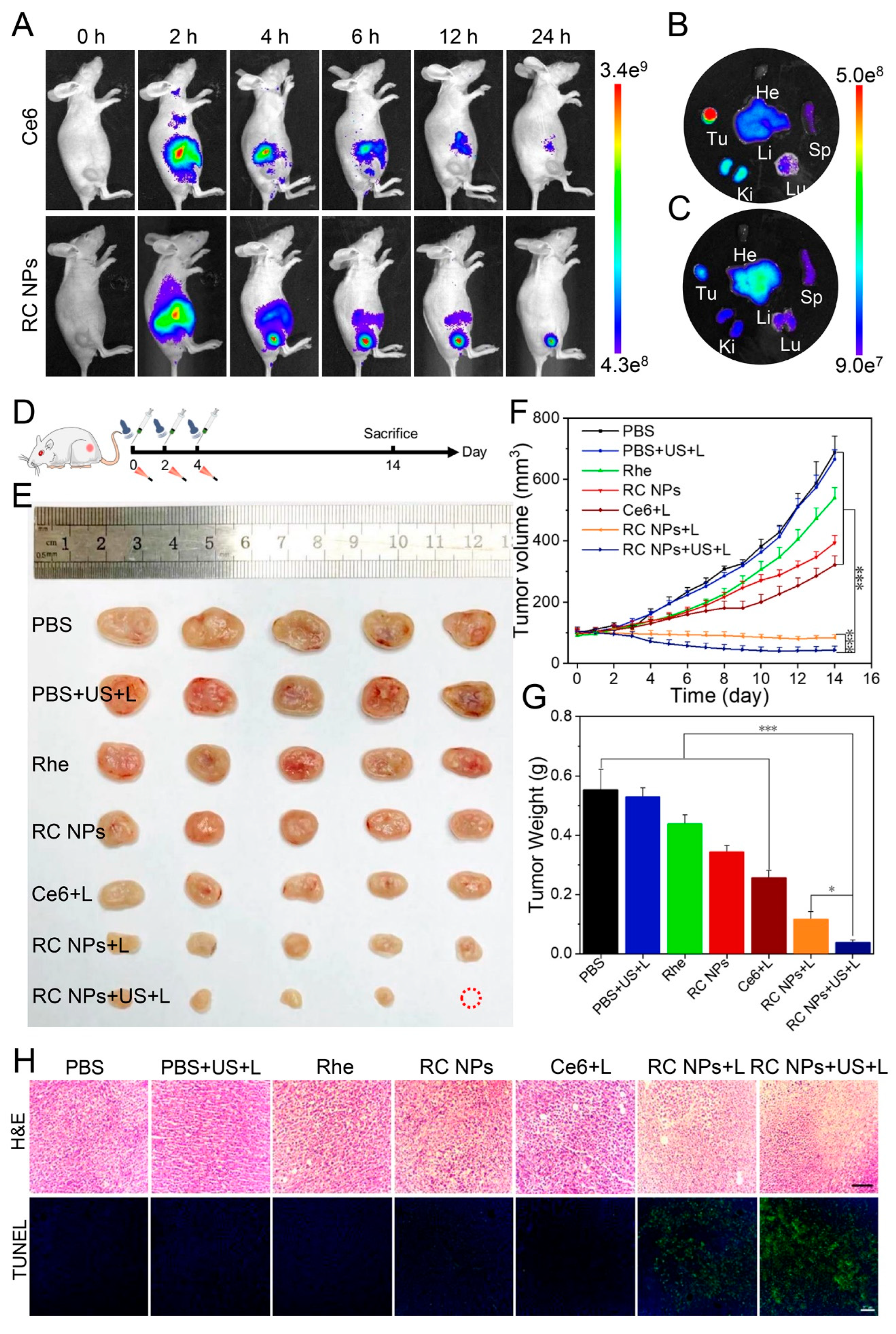
Figure 4.
In vivo fluorescence imaging and antitumor therapy of RC NPs. (A) Fluorescence images in vivo at different time points after intravenous administration of RC NPs and Ce6. Fluorescence images of isolated tumor tissues and major organs 24 hours after injection of RC NPs (B) and Ce6 (C), respectively. (D) Schematic illustration of the treatment process. (E) Resected 4T1 tumor tissue, (F) tumor growth curves, and (G) average tumor weight of different treatment groups. (H) H&E and TUNEL staining images of tumor tissues after various treatments. Scale bar: 100 μm. The error bars indicate means ± SD, n = 5, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
Figure 4.
In vivo fluorescence imaging and antitumor therapy of RC NPs. (A) Fluorescence images in vivo at different time points after intravenous administration of RC NPs and Ce6. Fluorescence images of isolated tumor tissues and major organs 24 hours after injection of RC NPs (B) and Ce6 (C), respectively. (D) Schematic illustration of the treatment process. (E) Resected 4T1 tumor tissue, (F) tumor growth curves, and (G) average tumor weight of different treatment groups. (H) H&E and TUNEL staining images of tumor tissues after various treatments. Scale bar: 100 μm. The error bars indicate means ± SD, n = 5, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p >0.05.
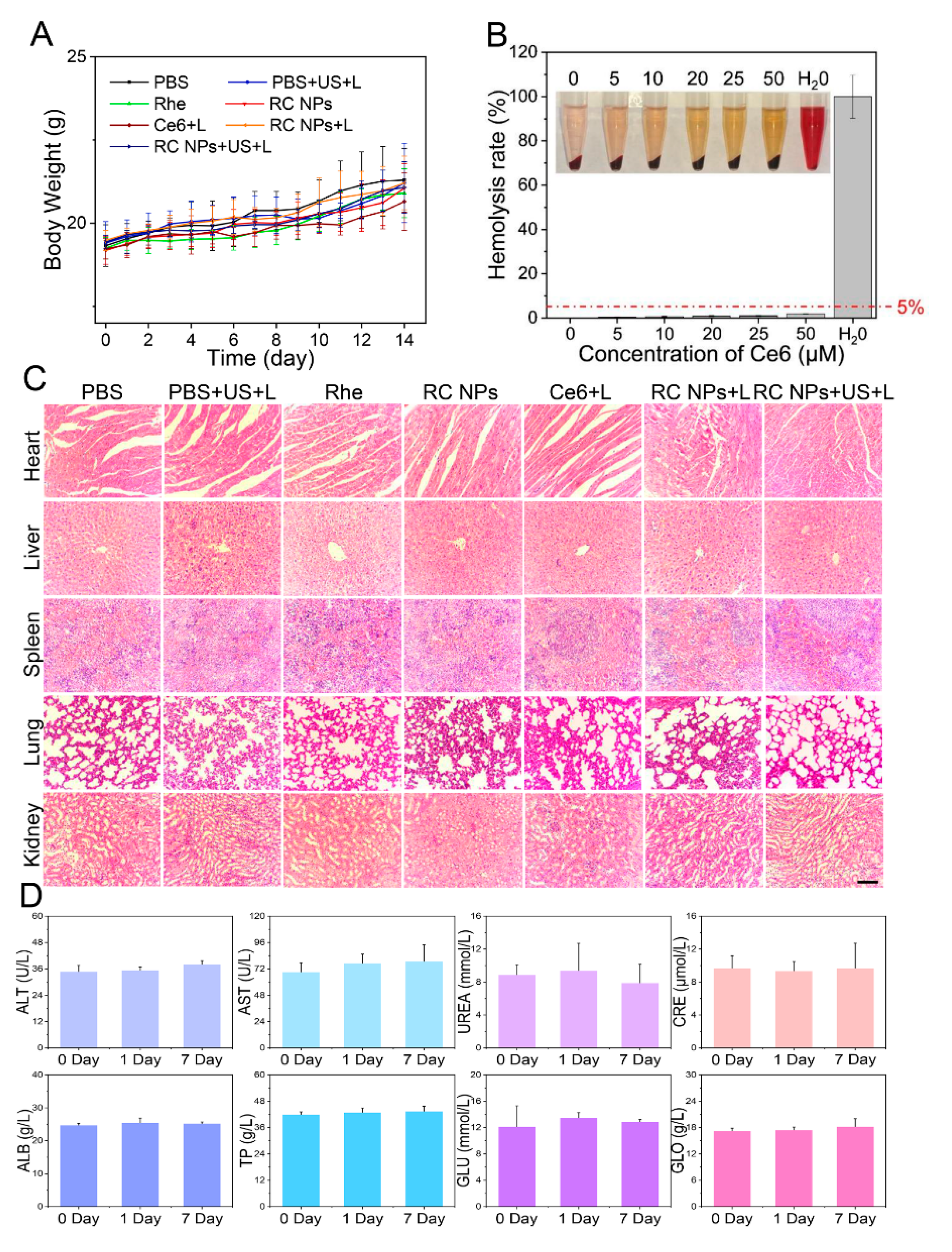
Figure 5.
Biosafety analysis of RC NPs. (A) Body weight changes of mice in different treatment groups over 14 days, n = 5. (B) Changes in the hemolysis rate under different RC NPs concentrations. (C) H&E staining analysis of major organs in different treatment groups. Scale bar: 100 μm. (D) Blood biochemical analysis for the biosafety evaluation of RC NPs. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p> 0.05.
Figure 5.
Biosafety analysis of RC NPs. (A) Body weight changes of mice in different treatment groups over 14 days, n = 5. (B) Changes in the hemolysis rate under different RC NPs concentrations. (C) H&E staining analysis of major organs in different treatment groups. Scale bar: 100 μm. (D) Blood biochemical analysis for the biosafety evaluation of RC NPs. The error bars indicate means ± SD, n = 3, * p <0.05, ** p <0.01, and *** p <0.001; NS indicates p> 0.05.