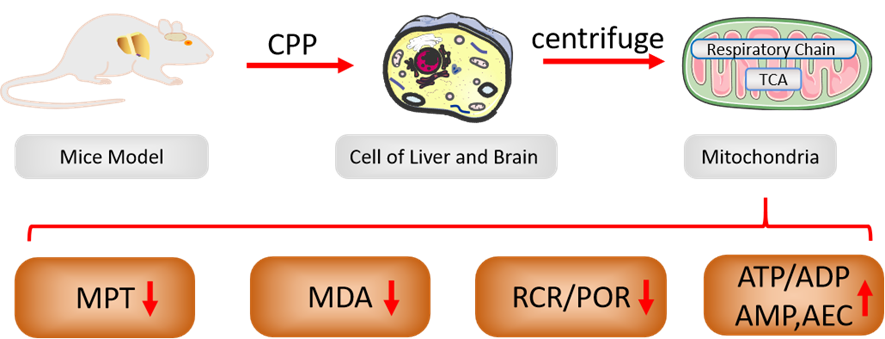
Introduction
In the field of traditional Chinese medicine (TCM), “Qi” (vital energy) is deemed as the fundamental essence of vitality, embodying the capacity to strengthen the physique, invigorate the spleen, tonify the lung, nourish the blood and engender liquid(Li, 2012; Li et al., 2009). The concept of “Qi” plays a central role in understanding energy-dependent body functions. It refers to the vital energies or “minute substances” that circulate within the body, encompassing both their physical presence and their functional significance. Viewing “Qi” from another perspective, it can be considered an expression of the operational state of various organs, which in turn is regulated by complex neuroendocrine mechanisms and energy transformation processes(Ko & Chiu, 2006). Modern medicine believes that the biochemical unit of “Qi” is adenosine 5'-triphosphate (ATP). The mitochondrion, serving as the cellular powerhouse for ATP synthesis, effectively becomes the intrinsic source of “Qi” within the cell. Mitochondria are the driving force behind life, as mitochondrial oxidative phosphorylation (OXPHOS) provides the main source of energy in the cell. Mitochondria are crucial for both life and death of eukaryotic cells(Cadassou & Jordheim, 2023; Strzyz, 2020). Accordingly, mitochondria represent potential and susceptible hotspots for damage, as the mitochondrion plays a central role in many of the metabolic processes or pathways altered in tumorigenesis(Cadassou & Jordheim, 2023; Schirrmacher, 2020). Unfortunately, the mitochondria are prone to damage due to the reactive oxygen species (ROS) generation during energy transforming process or external stimuli under normal physiological conditions or stressful conditions, when ROS generation exceeds the capacity of antioxidant defenses, oxidative stress ensues and has been implicated in cellular degradation during aging as well as in a variety of disease states, resulting in the impairment in “Qi” generation(Ko & Chiu, 2006; Lu et al., 2024). The mitochondrial protection is therefore of crucial importance for “Qi-invigoration”.
According to TCM, the "Qi-invigorating" herbs mainly include Panax ginseng, Codonopsis pilosula, Astragali radix, and Schisandra chinensis, etc.(Huang et al., 2018; Ko & Chiu, 2006; Kwan et al., 2019; Li et al., 2021; Liu et al., 2023). Among these, P. ginseng, a commonly optimal “Qi-invigorating” herb, can combat oxidative stress, affect energy metabolism, and enhance mitochondrial function(Yang et al., 2008). However, P. ginseng commonly remains expensive as it requires the high growth environment, long growth cycle, and its harvest is difficult, which limits the widespread use of P. ginseng. Alternatively, Codonopsis pilosula (CP), as a substitute for the more expensive P. ginseng, has received widespread praise in China, because it possesses some pharmacological activities that P. ginseng also provides. CP belonging to Campanulaceae family, commonly known as “Dangshen” in China, is a perennial species of flowering plant native to Northeast Asia, which is a traditional Chinese tonic medicine with uses for thousands of years(Guo et al., 2024). CP contains a diverse range of pharmacologically active natural compounds, such as polysaccharides, saponins, alkaloids, flavonoids, volatile oil, lignans, terpenoids, etc., and polysaccharides are the main components(Guo et al., 2024; Liu et al., 2023; Ma et al., 2024; Sun et al., 2019). Traditional quality control and evaluation of CP are performed by measuring its polysaccharides content. C. pilosula polysaccharide (CPP) has anti-tumor, anti-stress, anti-oxidation, enhanced immune function and other activities, making it a key ingredient in CP's holistic health-promoting effects(Chu et al., 2024; Liu et al., 2023; Ma et al., 2024). However, there is very few reports have systematically measured cell mitochondrial bioenergetics for replenishing “Qi” after CPP treatment.
Our previous studies showed that P. ginseng polysaccharide (PGP) was capable of mitigating mitochondrial injury and swelling, consequently enhancing ATP levels and the adenylate energy charge (AEC) in liver cells under chronic hypoxia conditions(Li et al., 2009). Other subsequent studies have corroborated these findings, PGP was also found to be related to its ability to promote neuronal mitophagic activity, and the structural degeneration of mitochondria were all ameliorated(Wang et al., 2014; Zhang et al., 2023). These results indicate that PGP protects mitochondria by inhibiting mitochondrial swelling and improving energy status(Li et al., 2009; Wang et al., 2014). Both P. ginseng and CP contain large amounts of polysaccharide that is one of their main and key active components. Therefore, we guessed that CPP has an analogous effect to those of PGP. To this end, we investigated the protective effects of CPP on mitochondria and ascertain regulation of energy metabolism, further revealed the influence of Qi-invigoration on mitochondrial function and delineated its underlying mechanism of action, laying the foundation for exploring the essence of “Qi” in the context of traditional Chinese medicine.
Materials and Methods
Animals and Materials
Male Kunming mice, weighing 22±2.0 g each, were purchased from Shenzhen Top Biotechnology Co., LTD. All mice were cared for according to the Guiding Principles in the Care and Use of Animals. The experiment was approved by the Institutional Animal Care and Use Committee of Shenzhen Top Biotechnology. Rodent laboratory chow and tap water were available ad libitum during the period. Spherisorb C18 reversed-phase chromatographic column (4.6 mm×250 mm, 5 µm particle size) was produced by Dalian Institute of Chemistry and Physics, Chinese Academy of Sciences. Adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), 2-Thiobarbituric acid (TBA), 1,1,3,3-tetraethoxypropane (TEP), L-glutamic acid, and DL-malate were from Sigma Chemical (St Louis, MO, USA). N-2-Hydroxyethylpiperazine-N’-2-ethane sulfonic acid (HEPES) was from Merck (Darmstadt, Germany). Coomassie Brilliant Blue G-250 (CBBG-250) was purchased from Fluka (Bushs SG, Switzerland). Bovine serum albumin (BSA) was from Boehringer Mannheim Corp. (Indianapolis, IN, USA). Tris(hydroxymethyl)aminomethane (Tris) was from Gibco BRL (Grand Island, NY, USA). All other chemicals and solvents used in the study were of analytical grade made in China. The plant materials of the roots of C. pilosula were harvested from the region of Large Xing’an Mountains, which is located in the most northern border of China, Heilongjiang province and were identified according to the identification standard of Pharmacopeia of the People’s Republic of China. The plant materials were thoroughly air-dried and finely powdered.
Preparation of C. pilosula Polysaccharide (CPP)
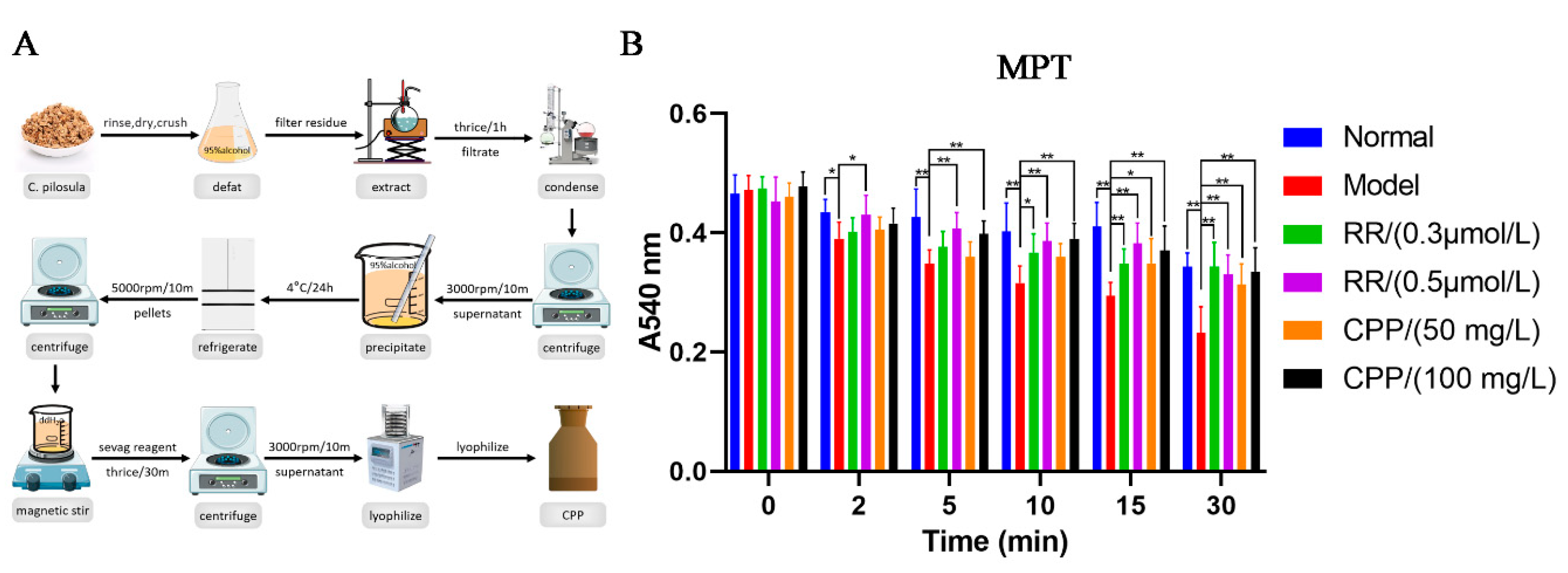
CPP was isolated by hot-water extraction and ethanol precipitation according to the method of Yang, et al. (Yang et al., 2008)with slight modifications. The dried materials of C. pilosula were defatted with 95% alcohol and then extracted thrice with distilled water (g/mL=1:10) for 1 hour each in a boiling water bath. The filtrate was collected after filtration with gauze, mixed and condensed to 1 g crude drug/mL under a reduced pressure and then centrifuged at 3000 rpm for 10 min. The supernatant was collected and 3 volume of 95% alcohol was added slowly by stirring to precipitate the polysaccharide, and then kept at 4 °C for 24 h and finally the polysaccharide pellets were obtained by centrifugation at 5000 rpm for 10 min. The polysaccharide pellets were completely dissolved in appropriate volume of distilled water, deproteinated with Sevag reagent (CHCl3:n-BuOH=4:1, v/v) for 30 min under the magnetic force stirring and the procedure was repeated 3 times, and then centrifuged to remove insoluble material. Finally, the supernatant was lyophilized in the freeze-dry apparatus to give CPP with a brown fluffy shape. The polysaccharides content (92.3%) in extracts was determined using the phenol-sulfuric acid method.
Chronic Hypoxia Model
Model group and CPP group mice were exposed to hypoxia (10.5% O2, 89.5% N2) for 10 days in specially constructed plastic cages. The cages were sealed at the top by plastic covers. Small openings were made in the top covers to allow inflow and outflow of gases and to accommodate water bottles. The oxygen content in the chambers was monitored using a Clark O2 electrode inserted through an opening in the top cover. Total gas flow was set at about 1.5 L/min to maintain 10.5% O2 in the cage and prevent excessive accumulation of moisture and ammonia. Soda lime was put into the chambers to absorb the CO2 which was breathed out by mice. Cages were opened daily to change bedding and food, a CPP group mouse was administered a dose of CPP (200, 300 mg /kg/day) by oral gavage and a model group mouse an equivalent volume of normal saline. Normal group mice were housed in standard open cages (21% O2) and given normal saline. All the mice were maintained with free access to food and drinking water.
Isolation of Liver Mitochondria
Mitochondria were isolated by differential centrifugation using a modified protocol of Fink et al.(Fink et al., 2005). Mice were dislocated, the livers were excised immediately, placed in precooled normal saline to wash the blood on the surface, then placed in an ice-cold isolation medium (containing 0.25 M sucrose, 0.5 mM EDTA and 3 mM HEPES, pH 7.4) and were homogenized with a motor-driven Teflon pestle in wet ice. Following homogenization, samples were centrifuged at 1,000×g for 10 min. A Beckman JA-25.50 rotor and Beckman Coulter Avanti J-E centrifuge were used in this and all other centrifugation steps at 4 °C. Supernatants were removed and centrifuged at 10,000×g for 10 min. The pellets were washed twice in the isolation medium, and respun at 10,000×g for 10 min each. After the final wash, mitochondria were resuspended in the same medium and stored in ice until use. Protein determinations were carried out by Bradford method using BSA as a standard.
Evaluation of Mitochondrial Permeability Transition
Liver mitochondria were isolated and resuspended (0.25 mg of protein/mL) in an incubation medium (250 mM sucrose, 1 mM Pi-Tris, 10 mM Tris-MOPS, 5 mM glutamate-Tris, 2.5 mM malate-Tris, pH 7.4, 25 °C). 150 µM Ca2+ was added followed by CPP or ruthenium red (0.3 or 0.5 µM) (the model group was excluded). Experiments were started by the addition of 0.5 mg of mitochondrial protein. The final volume was 2 mL. MPT was monitored as the absorbance (A) decrease of the mitochondrial suspension at 540 nm at 0, 2, 5, 10, 15, and 30 min(He et al., 2000; Walter et al., 2000).
Mouse Brain Homogenate Lipid Peroxidation Assay
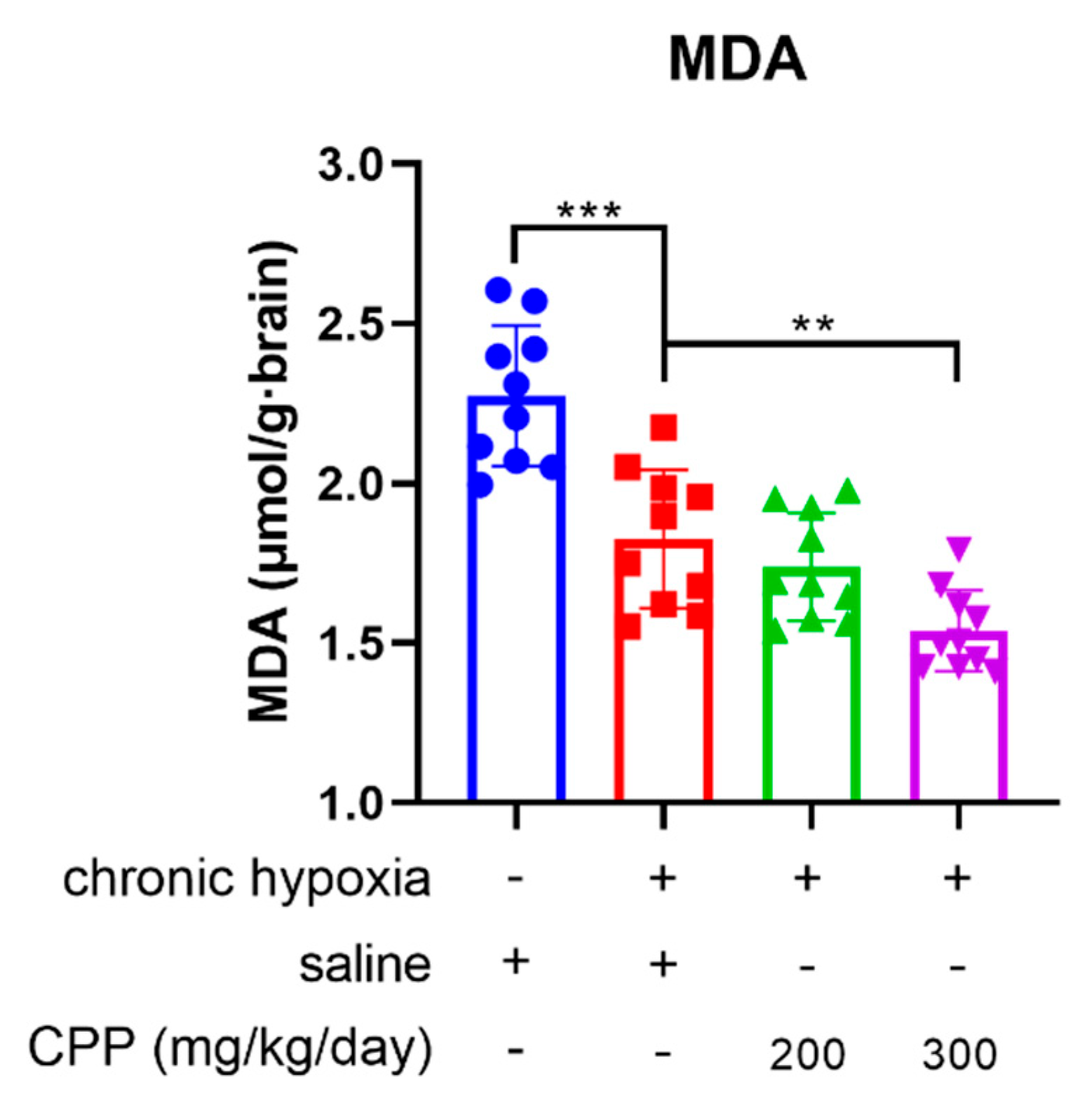
Mice were killed via dislocation, and brains were rapidly removed, weighed and made into 10% homogenates with normal saline at 0℃. Lipid peroxidation was monitored in terms of malondialdehyde (MDA) using thiobarbituric acid (TBA) colorimetry(Ohkawa et al., 1979). Briefly, to 0.4 mL homogenate was added 1.5 mL 20% [v/v] acetic acid buffer (pH 3.5), 0.2 mL of 8.1% [w/v] sodium dodecyl sulphate, 1 mL of 0.67% TBA (w/v) and 0.4 mL water, the tubes were incubated at 95℃ for an hour, cooled with running tap water, and were extracted with 5 mL n-butanol. After centrifugation (2,000×g, 10 min), the absorbance of the butanol phase was read at 532 nm. MDA were determined by linear regression analysis of a standard aliquot using 1,1,3,3-tetraethoxypropane as a standard.
Measurement of Liver Mitochondrial Respiratory Function
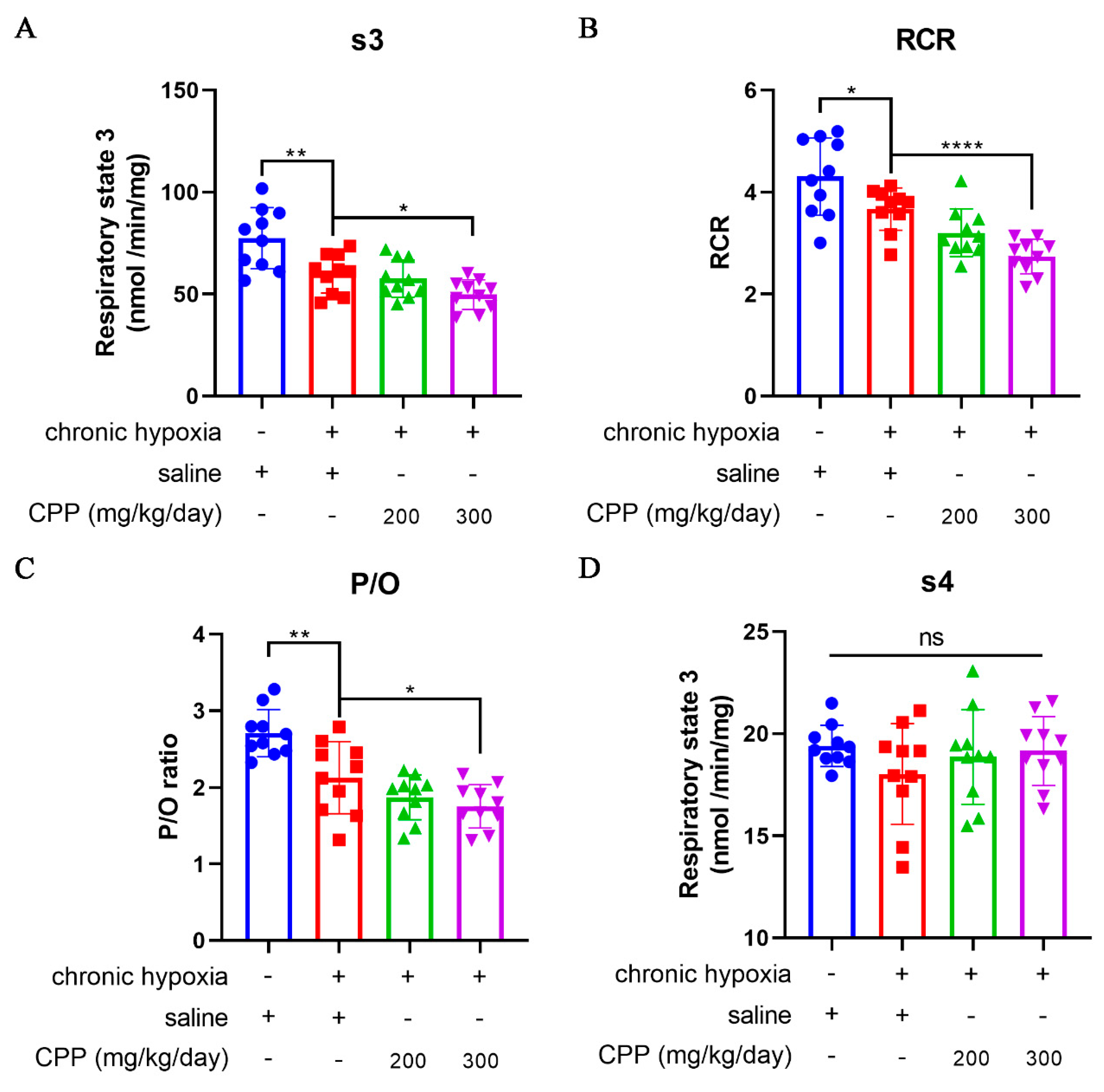
Respiratory function of liver mitochondria was measured using the method described by Estabrook. Oxygen consumption was measured at 30°C in a closed, stirred, and thermostatted glass vessel equipped with a Clark-type oxygen electrode in 2.0 mL respiration buffer (pH 7.4), which consisted of sucrose 225 mM, EDTA 1 mM, MgCl2 5 mM, KCl 15 mM, KH2PO4 15 mM, Tris 50 mM, L-glutamic acid 5 mM, DL-malate 10 mM, and mitochondrial protein 5 mg/mL. Respiratory state 3 (s3) was the oxygen (O2) consumption by mitochondria in the presence of substrate after the addition of 0.25 mM ADP (a potent stimulator of mitochondrial respiration). Respiratory state 4 (s4) was the oxygen consumption when all the ADP has been phosphorylated. s3 and s4 can be calculated according to the OXPHOS curve. Respiration rates were expressed in nanomoles atom O per minute per milligram of protein. Respiratory control ratio (RCR) was the ratio of s3 to s4 respiration. P/O ratio is the number of ADP molecules phosphorylated per oxygen atom reduced.
Measurement of ATP, ADP, and AMP in Liver Cells by HPLC
Briefly, determination of ATP, ADP, and AMP in liver cells, which was carried out with our previous method(Li et al., 2009), by gradient RP-HPLC (reversed-phase high performance liquid chromatography) with ultraviolet detector at room temperature and with mobile phase at a rate of 0.8 mL/min. Mobile phases used for the gradient system were buffer A (0.05 M KH2PO4-K2HPO4, pH 6.0) and buffer B, consisting of buffer A plus 10% methanol (v/v). Gradient elution procedure: buffer A was used as mobile phase between 0 and 3 min, buffer A was changed from 100% to 0% and buffer B from 0% to 100% between 3 and 6 min, buffer B was mobile phase between 6 and 9 min, buffer A was the mobile phase after 9 min, all the running time was 12 min, the detection wavelength was set at 254 nm. ATP, ADP and AMP contents in liver cells was calculated by computing the peak area of standard solutions of nucleotides with known concentrations. Total adenylate pool (TAP) and adenylate energy charge (AEC) were calculated by the following formulas respectively: TAP = [ATP] + [ADP] + [AMP], AEC = ([ATP] + 0.5[ADP])/TAP.
Statistical Analysis
Data were expressed as means ± SEM and statistical differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc multiple comparisons test using the statistical software package SPSS 16.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Results were considered statistically significant at the probability (P) values < 0.05 level.
Results
CPP Inhibited Ca2+-Induced Liver Mitochondrial Permeability Transition (MPT) In Vitro
As is well known, mitochondrial energy metabolism is a complex system in where biochemical reactions are coupled to membrane electrophysiology, the normal function of mitochondria is highly dependent on their fluidity and integrity. The opening of the mitochondrial permeability transition pore (MPTP) significantly reduces mitochondrial membrane potential, further damaging mitochondrial function and resulting in the impairment in “Qi” generation(Li, 2012; Li et al., 2009) Mitochondrial calcium overload can trigger the opening of the MPTP, causing uncoupling of OXPHOS, swelling of the mitochondria due to water influx, and rupture of the mitochondrial outer membrane(Bauer & Murphy, 2020; Carraro & Bernardi, 2023; Gustafsson & Gottlieb, 2008). Here, we extracted CPP from
C. pilosula using combine hot-water extraction with ethanol precipitation (
Figure 1A), and then isolated the mice liver mitochondria by differential centrifugation and constructed the Ca
2+-induced MPTP openness model. The MPTP can be monitored via mitochondrial permeabilization to sucrose based on the changes of absorbance at 540 nm(Bauer & Murphy, 2020; Carraro & Bernardi, 2023; Gustafsson & Gottlieb, 2008). The harvested mitochondria treated with CPP or Ca
2+ blocker ruthenium red (RR) were challenged with a Ca
2+ load of 150 µM. In model group, Ca
2+ can decrease significantly the absorbance of 540 nm on 2 min, which caused a detectable MPTP, indicating the rapid and large amplitude mitochondrial swelling induced by Ca
2+ (
Figure 1B). Interestingly, the effects of Ca
2+ on MPTP were completely blocked by 0.5 µM RR and partially blocked by 0.3 µM RR (
Figure 1B). Fortunately, CPP also obviously inhibited Ca
2+ induced MPTP openness, and the inhibitory potency was stronger when the incubation time was longer and the concentration of CPP was higher (
Figure 1B). No significant difference was observed between CPP group (100 mg/L) and the normal group (
Figure 1B), suggesting CPP can completely resist the toxicity of Ca
2+ overload on mitochondria, allowing mitochondria to maintain normal function and replenish “Qi” in TCM concept.
The Effects of CPP on Liver Mitochondrial Respiratory Function In Vivo
The liver plays an important role in metabolism to maintain energy levels and structural stability of the body. The mitochondria isolated from hepatocytes are widely used in many biochemical studies. To assay the CPP protection of mitochondrial respiratory function, we detected the respiratory state 3 (s3, O
2 consumption rate after adding ADP) and the respiratory state 4 (s4, O
2 consumption rate after conversion ADP to ATP via oxidative phosphorylation reaction) of liver mitochondria from chronic hypoxia mice model, and then calculated the respiratory control ratio (RCR) values and P/O ratio (the number of moles of Pi consumed for each oxygen atom reduced to H
2O, POR). Compared to normal group mice, hypoxic mice (model group) showed a significant decrease in s3, RCR and POR (
Figure 3A–C), suggesting a decrease in oxygen consumption rate and a robust reduction in the rate of ATP generation through ADP phosphorylation during the exposure in chronic hypoxia. CPP could further reduce these parameters (
Figure 3A–C), whereas there was no significant effect on s4 (P >0.05) (
Figure 3D).
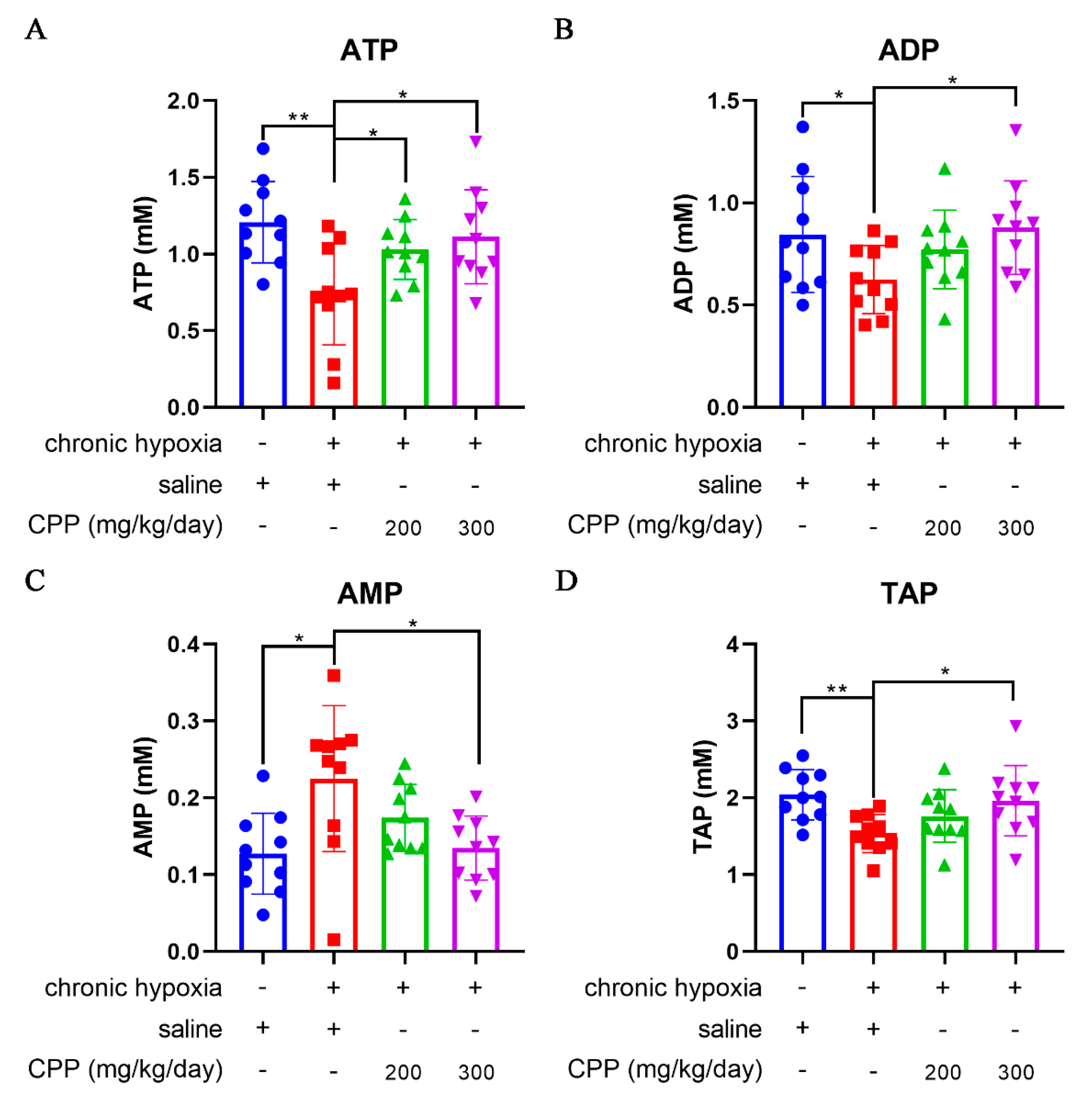
Effects of CPP on Energy State of Mice Hepatocyte under Chronic Hypoxia In Vivo
ATP is a direct supplier of energy required within cells. Insufficient supply of ATP can have serious adverse consequences on energy dependent metabolic pathways, and energy depletion can lead to cell death. Abovementioned studies showed that CPP can reduce the O
2 consumption rate of mitochondria under hypoxic conditions, but it is unclear whether this process will affect the generation of mitochondrial ATP. Our research identified that hypoxia led to a marked fall in cellular ATP and ADP levels (
Figure 4A,B), and a rise in cellular AMP levels (
Figure 4C) associated with decreases in ATP/ADP and ATP/AMP ratios, the changes in ATP/ADP ratio might significantly influence the mitochondrial membrane potential (∆Ψ
m). The cellular AMP/ATP ratio was monitored as a biomarker or an index of metabolic stress, through the action of adenylate kinase (AK), any decrease in the cellular ATP/ADP ratio is converted into a decrease in the ATP/AMP ratio. Hypoxia elicits a marked decrease in the ATP/AMP ratio, the ATP/AMP ratio reduced from 10.46 under normoxia to 3.22 under hypoxic conditions, whereas the ATP/ADP ratio reduced from 1.39 to 1.18 (
Table 1). These indicate that the hypoxia significantly altered cellular energy state.
Adenylate energy charge (AEC) represents a linear measure of the metabolic energy stored in the adenine nucleotide system. TAP is a measure of the cell energy status, TAP levels and AEC in liver cells of model group were observed to be decreased compared with normal group in our study (
Figure 4D,
Table 1). Furthermore, the AMP level in the model group remained 2-fold higher than in the normal group (
Figure 4C). Treatment with CPP (300 mg/ kg/day) could increase ATP, ADP, TAP levels and ATP/ADP, ATP/AMP ratio, AEC of liver cells compared to the model group (
Figure 4A–D,
Table 1). The data showed CPP to be an enhancer of ATP production under hypoxia-induced anti-ATP circumstance, while ATP levels were drastically lowered by hypoxia but CPP stimulated an increased output of ATP. Notably, ATP/AMP ratio in CPP (300 mg/ kg/day) group is increased over 2-fold than the model group (
Table 1), indicating that CPP-treated mice mitochondria exhibited higher production capacity efficiency under chronic hypoxia, despite the lower O
2 consumption rate.
Discussion
Mitochondria are important and pivotal regulators of cell death, responding to a wide variety of stress signals, including loss of growth factors, hypoxia, oxidative stress, and DNA damage, Mitochondria are also considered the pacemakers of tissue aging due to the continuous production of free radicals, oxygen, and nitrogen free radicals and related reactive species, and to the selective oxidative damage that leads to mitochondrial dysfunction. “Qi” is the most vital force for retaining the physiological functions of the human body in TCM. Modern TCM science has established a certain connection between nourishing “Qi” and mitochondrial protection(Huang et al., 2019; Li, 2012; Li et al., 2009; Valcarcel-Jimenez et al., 2017). For instance, the Qi-invigorating P. ginseng extracts improved the tolerance of mitochondria to oxidative damage through increasing TAP, mitochondrial membrane potential (MMP) and antioxidant capacity reflected by superoxide dismutase (SOD) and glutathione (GSH) (Huang et al., 2019; Li et al., 2021). As a substitute of costly P. ginseng, CP is also known for its ability to nourish “Qi”. Among them, CPP is the primary active components of CP, which is a typically acidic heteropolysaccharide, including arabinose, glucose, rhamnose, galactose, mannose, glucuronic acid and galacturonic acid in the mole percentages of 13.9, 29.8, 4.6, 14.0, 2.2, 1.2 and 34.3% (mol%) respectively(Yang et al., 2008). Although a recent study demonstrated that CPP protected mitochondrial membrane integrity of the sheep sperm after preservation at 4°C(Wang et al., 2024), it is still unclear whether CPP can also achieve the“Qi-invigoration” through mitochondrial protection in TCM theory. The integrity of mitochondria is a prerequisite for the production of energy through oxidative phosphorylation (OXPHPS). Furthermore, mitochondrial calcium overload might trigger the opening of the MPTP, causing uncoupling of OXPHOS, swelling of the mitochondria due to water influx, and rupture of the mitochondrial outer membrane(Bauer & Murphy, 2020; Gustafsson & Gottlieb, 2008). Our study demonstrated that CPP can inhibit MPTP openness during Ca2+-challenge. CPP can scavenge superoxide anion and hydroxyl radicals, enhancing the SOD activity(Feng & Zhang, 2020; Ma et al., 2024). The inhibition of CPP on MPTP might be closely related to its scavenging activity on ROS and the inhibition on lipid peroxidation, this indicates that CPP may protect mitochondria by scavenging ROS and antioxidation properties.
Oxidizing agents and ROS lead to the opening of MPTP, whose immediate consequence is the collapse of ∆Ψm, besides increasing matrix volume, leading to major modifications of mitochondrial function and structure that eventually jeopardize the maintenance of cell viability. And lipid peroxides (such as MDA) can increase membrane permeability, leading to mitochondrial swelling. The present results showed that CPP was a potent inhibitor of MDA, which could decrease lipid peroxides extent of brain. Previous reports CPP can scavenge superoxide anion and hydroxyl radicals, enhance the SOD activity and decrease MDA content in mouse brain. Mitochondrial oxidative stress has been implicated in cell death(Orrenius et al., 2007), high levels of pro-oxidants produced by mitochondria can induce apoptosis by changing cellular redox status, depleting reduced glutathione (GSH), reducing ATP levels, and decreasing reducing equivalents such as NADH and NADPH(Orrenius et al., 2007). Either Qi deficiency or hypoxia can decrease ATP, TAP and AEC, increase AMP content, and these severe cases cause the excessive decrease of ATP levels, leading to rapid cell necrosis. Our investigation found that CPP (300 mg/kg/day) can further decrease state 3 respiration, RCR, and P/O ratio of liver mitochondria compared to model group. We thus think that the treatment of CPP reduces the energy consumption of the body during hypoxia. When hypoxia occurs, the utilization rate of oxygen and ATP production efficiency are significantly improved, and the production of oxygen byproduct ROS is significantly inhibited during electron transfer, thus plays a role in nourishing “Qi”.
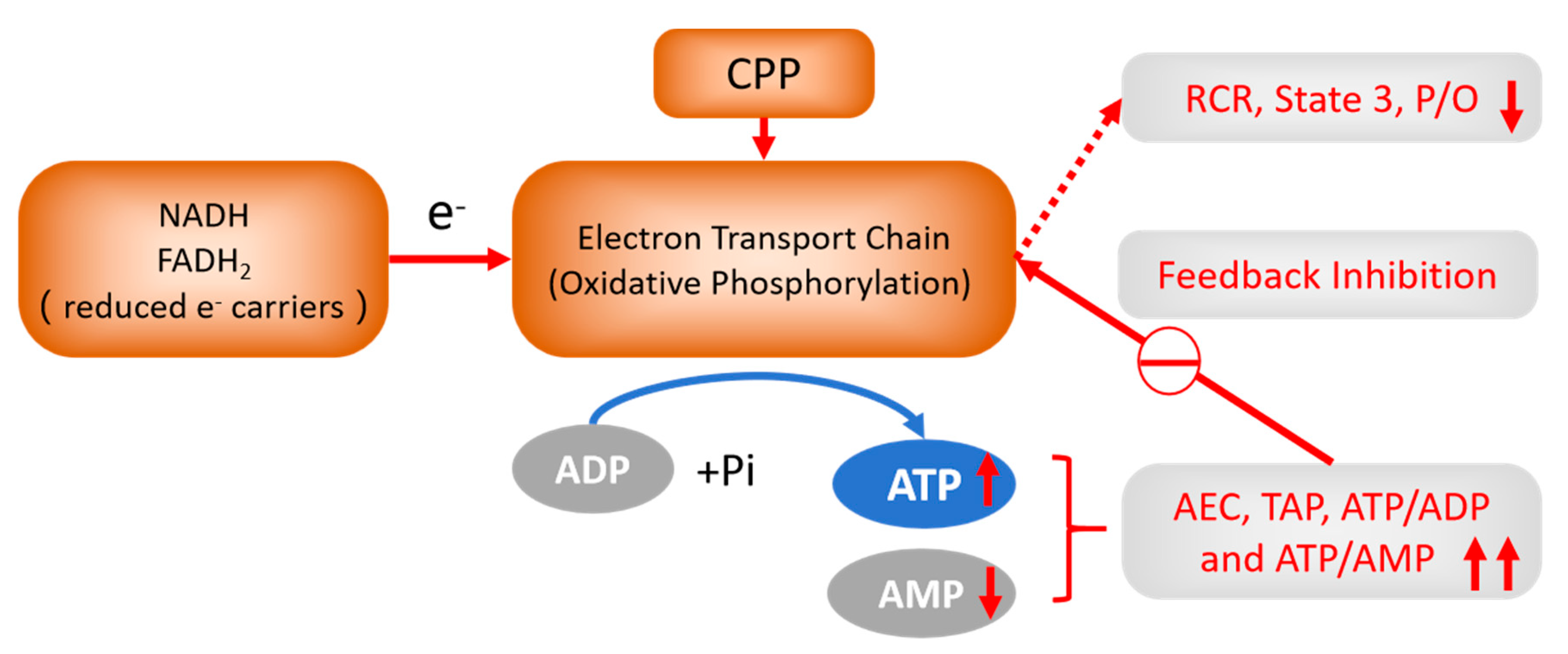
Recently, a second mechanism of respiratory control has been found in eukaryotes, which is based on the intramitochondrial ATP/ADP ratio, with a high ratio inhibiting OXPHOS through allosteric binding of ATP to a subunit of Complex Ⅳ, with this inhibition being reversed by elevated ADP concentrations. Cell energy metabolism be further regulated by AEC. We also discovered that CPP could increase the levels of ATP and TAP in hypoxic liver cells, reduce AMP levels, increase ATP/ADP, ATP/AMP ratios and AEC (i.e., increase cellular bioenergetics). We consider this is the result of feedback inhibition on OXPHOS by improving mitochondrial energy metabolism and bioenergetic level. Critical mitochondrial functions, including ATP synthesis, ion homeostasis, metabolites transport, ROS production, and cell death are highly dependent on Δψm(Solaini et al., 2007). The mitochondrial energy state can retro-regulate the nuclear-encoded energy genes. The changes in mitochondrial respiratory chain activity are followed by changes in "energy-state messengers" which include ROS (such as the diffusive H2O2), mitochondrial and cytosolic calcium, NADH/NAD+, ATP/ADP, GTP, AMP, cyclic AMP (cAMP), Δψm and ΔpH(Benard et al., 2010). In addition, an increase in Δψm, whether caused by impaired OXPHOS or by an overabundance of nutrients relative to ADP, will result in aberrant electron migration in the electron transport chain and elevated ROS production(Wallace, 2005). From both an economic and health point of view, multicellular organisms require a mechanism that does not rely on the Mitchell theory (chemiosmotic theory), while maintaining the Δψm at reasonably low levels (120–140 mV), thereby optimizing the efficiency of OXPHOS. Surprisingly, the German scientist Kadenbach et al.(Kadenbach et al., 2010)proposed a new mechanism that did not rely on the Mitchell theory, in which high ATP/ADP ratio feedback inhibits CcO (complex Ⅳ), and maintains a low Δψm value, thereby preventing ROS generation and maintaining high efficiency of OXPHOS — the mechanism represents the new extension of Mitchell theory, known as the “The second mechanism of respiratory control”.
Mitochondria regulate the life and death of cells by manipulating and regulating several factors, including bioenergetics, mitochondrial permeability transition pore (MPTP), and mitochondrial redox-status(Das & Maulik, 2005; Guo, 2021; Papa, 1996). Mitochondria consume >90% of the oxygen utilized in cells, and are the major site of ROS production, Mitochondria produce significant amounts of cellular ROS via aberrant O2 reactions during electron transport, which is stringently controlled in physiological conditions, and the majority of ROS remain confined inside intact mitochondria, they appear to be more susceptible to bear the brunt of the free radical damage observed in cells during aging, which is associated with a decline in respiratory function. This can be a result of an escalating cycle, whereby damaged mitochondria leak more free radicals to inflict additional self-damage, as well as damage to the rest of the cell, the rate of mitochondrial respiration and ROS formation is substantially influenced by the coupling state of the mitochondria, and mitochondrial metabolism can be both advantageous and detrimental to these processes, which keep a balance that the dualities of mitochondria is adaptive homeostasis mechanism(Navarro & Boveris, 2007; Valcarcel-Jimenez et al., 2017). CPP has been shown to decrease the oxygen-consuming rate and RCR of liver mitochondria in present study, thereby increase hypoxia tolerance and prolong the survival time of mice. CPP was able to improve mitochondrial function by enhancing cellular bioenergetics. We consider that this effect is interpreted as a reduction in the standard metabolic rate, representing a form of protective adaptation mechanism. Qi deficiency patients, which exhibit increased susceptibility to fatigue and diminished energy levels, need nutritional supplements, adequate rest, and should reduce energy consumption, CPP can just achieve “Qi-invigoration” by mitigating the associated symptoms. According to the current study, we propose that CPP can improve mitochondrial bioenergetics, while higher levels of bioenergy will feedback inhibit OXPHOS. The study enriched Kadenbach’s “New extension of the Mitchell Theory” through multiple bioenergetic parameters; this not only provides the strongest evidence for the contention that “The second mechanism of respiratory control represents new extension of the Mitchell Theory for OXPHOS”, but also proposes how to improve bioenergetic levels of cells by Qi-invigorating CP intervention. A theoretical basis and novel ideas were provided for Qi-invigoration, using mitochondrial bioenergetics as a target.
Conclusion
In summary, we proved that CPP had the pharmacological activities of antihypoxia, antioxidation and mitochondrial protection, and concluded that improving mitochondrial energy metabolism and bioenergetic levels via CPP administration are the possible biological mechanisms to invigorate Qi (
Figure 5).
Author Contributions
Y.M.Z., H.L., L.G. and X.T.L. conceptualized original idea. H.L. and X.T.L. conceived and designed the study. H.L., L.T.Z., and H.C.H performed the experiments and analyzed the data. H.L., L.G., X.T.L. and G.A.F. wrote and edited the manuscript. L.G., X.T.L., Y.Z.Z. and Y.M.Z. corrected the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (T2250710184 to YM Zhang), Shenzhen Science and Technology Plan Platform and Carrier Special Project (ZDSYS20220303153551001 to YM Zhang), and the Fundamental Research Funds for the Central Universities in China (C10030105 to XT Li).
Consent for Publication
All authors have given approval to the final version of the manuscript.
Availability of Data and Materials
The data used in the article can be obtained from the author of the article through formal channels.
Acknowledgments
Not applicable.
Competing Interests
The authors declared no competing interests.
Ethics Approval and Consent to Participate
The animal experiments received approval from the Institutional Animal Care and Use Committee of Shenzhen Top Biotechnology and were fully compliant with the guidelines for animal care and use. All efforts were made to minimize animal suffering.
Abbreviations
| CP |
codonopsis pilosula |
| CPP |
C. pilosula polysaccharide |
| P. G |
panax ginseng |
| PGP |
P. ginseng polysaccharide |
| TCM |
raditional Chinese medicine |
| MPT |
mitochondrial permeability transition |
| MPTP |
mitochondrial permeability transition pore |
| MDA |
malondialdehyde |
| OXPHOS |
oxidative phosphorylation |
| ATP |
adenosine triphosphate |
| ADP |
adenosine diphosphate |
| AMP |
adenosine monophosphate |
| AEC |
adenylate energy charge |
| TBA |
thiobarbituric acid |
| TAP |
total adenylate pool |
| TEP |
tetraethoxypropane |
| RCR |
respiratory control ratio |
| POR |
the number of moles of Pi consumed for each oxygen atom reduced to H2O |
| ROS |
reactive oxygen species |
| SOD |
superoxide dismutase |
| s3 |
respiratory state 3 |
| s4 |
respiratory state 4 |
| GSH |
glutathione |
| RP-HPLC |
reversed-phase high performance liquid chromatography |
| ANOVA |
one-way analysis of variance |
| LSD |
least significant difference |
| PUFA |
polyunsaturated fatty acids |
References
- Bauer, T. M., & Murphy, E. (2020). Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ Res, 126(2), 280-293. [CrossRef]
- Benard, G., Bellance, N., Jose, C., Melser, S., Nouette-Gaulain, K., & Rossignol, R. (2010). Multi-site control and regulation of mitochondrial energy production. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1797. [CrossRef]
- Cadassou, O., & Jordheim, L. P. (2023). OXPHOS inhibitors, metabolism and targeted therapies in cancer. Biochem Pharmacol, 211, 115531. [CrossRef]
- Carraro, M., & Bernardi, P. (2023). The mitochondrial permeability transition pore in Ca(2+) homeostasis. Cell Calcium, 111, 102719. [CrossRef]
- Chu, R., Zhou, Y., Ye, C., Pan, R., & Tan, X. (2024). Advancements in the investigation of chemical components and pharmacological properties of Codonopsis: A review. Medicine (Baltimore), 103(26), e38632. [CrossRef]
- Cui, K., Gong, L., Wang, K., Wang, Y., Huang, L., Liu, B., Li, Q., Zhang, Q., Fei, B., & Huang, Z. (2022). Ferroptosis-Associated Molecular Features to Aid Patient Clinical Prognosis and Therapy Across Human Cancers. Front Immunol, 13, 888757. [CrossRef]
- Dai, D.-F., Hsieh, E. J., Liu, Y., Chen, T., Beyer, R. P., Chin, M. T., MacCoss, M. J., & Rabinovitch, P. S. (2012). Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovascular Research, 93(1), 79-88. [CrossRef]
- Das, D. K., & Maulik, N. (2005). Mitochondrial function in cardiomyocytes: target for cardioprotection. Curr Opin Anaesthesiol, 18(1), 77-82. [CrossRef]
- Enoki, M., Shinto, S., Matsuoka, Y., Otsuka, A., Kaidzu, S., Tanito, M., Shibata, T., Uchida, K., Ohira, A., Yamato, M., & Yamada, K. I. (2017). Lipid radicals cause light-induced retinal degeneration. Chem Commun (Camb), 53(79), 10922-10925. [CrossRef]
- Feng, G., & Zhang, X. F. (2020). Production of a codonopsis polysaccharide iron complex and evaluation of its properties. Int J Biol Macromol, 162, 1227-1240. [CrossRef]
- Fink, B. D., Reszka, K. J., Herlein, J. A., Mathahs, M. M., & Sivitz, W. I. (2005). Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria. Am J Physiol Endocrinol Metab, 288(1), E71-79. [CrossRef]
- Guo, H., Lou, Y., Hou, X., Han, Q., Guo, Y., Li, Z., Guan, X., Liu, H., & Zhang, C. (2024). A systematic review of the mechanism of action and potential medicinal value of codonopsis pilosula in diseases. Front Pharmacol, 15, 1415147. [CrossRef]
- Guo, L. (2021). Mitochondria and the permeability transition pore in cancer metabolic reprogramming. Biochem Pharmacol, 188, 114537. [CrossRef]
- Gustafsson, A. B., & Gottlieb, R. A. (2008). Heart mitochondria: gates of life and death. Cardiovascular Research, 77(2), 334-343. [CrossRef]
- He, L., Poblenz, A. T., Medrano, C. J., & Fox, D. A. (2000). Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem, 275(16), 12175-12184. [CrossRef]
- Huang, Y., Kwan, K. K. L., Leung, K. W., Wang, H., Kong, X. P., Dong, T. T. X., & Tsim, K. W. K. (2018). The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds. Int J Mol Sci, 19(6). [CrossRef]
- Huang, Y., Kwan, K. K. L., Leung, K. W., Yao, P., Wang, H., Dong, T. T., & Tsim, K. W. K. (2019). Ginseng extracts modulate mitochondrial bioenergetics of live cardiomyoblasts: a functional comparison of different extraction solvents. J Ginseng Res, 43(4), 517-526. [CrossRef]
- Kadenbach, B., Ramzan, R., Wen, L., & Vogt, S. (2010). New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta, 1800(3), 205-212. [CrossRef]
- Ko, K. M., & Chiu, P. Y. (2006). Biochemical basis of the "Qi-invigorating" action of Schisandra berry (wu-wei-zi) in Chinese medicine. The American Journal of Chinese Medicine, 34(2), 171-176. [CrossRef]
- Kwan, K. K. L., Huang, Y., Leung, K. W., Dong, T. T. X., & Tsim, K. W. K. (2019). Danggui Buxue Tang, a Chinese Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Modulates Mitochondrial Bioenergetics in Cultured Cardiomyoblasts. Front Pharmacol, 10, 614. [CrossRef]
- Li, M., Chen, Y., Cai, Z., Teng, J., Feng, Q., Chen, Y., Wang, L., Li, C., Tang, B. Q., & Bai, X. (2021). Exploring the Biochemical Basis of the Meridian Tropism Theory for the Qi-Invigorating Traditional Chinese Medicine Herb Panax ginseng. J Evid Based Integr Med, 26, 2515690X20983249. [CrossRef]
- Li, X.-T. (2012). An Approach to the Nature of Qi in TCM-Qi and Bioenergy. IntechOpen. https://torl.biblioboard.com/content/0359a39f-97d0-495e-b621-dfd4f6329172?organizationId=1f7368e7-f10b-49a1-8ced-2d9476279974.
- Li, X. T., Chen, R., Jin, L. M., & Chen, H. Y. (2009). Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. The American Journal of Chinese Medicine, 37(6), 1139-1152. [CrossRef]
- Liu, M., Zhang, G., Zhou, K., Wen, J., Zheng, F., Sun, L., & Ren, X. (2023). Structural characterization, antioxidant activity, and the effects of Codonopsis pilosula polysaccharides on the solubility and stability of flavonoids. J Pharm Biomed Anal, 229, 115368. [CrossRef]
- Lu, Y., Gao, L., Zhang, W., Zeng, Y., Hu, J., & Song, K. (2024). Caffeic acid phenethyl ester restores mitochondrial homeostasis against peritoneal fibrosis induced by peritoneal dialysis through the AMPK/SIRT1 pathway. Ren Fail, 46(1), 2350235. [CrossRef]
- Ma, K., Yi, X., Yang, S. T., Zhu, H., Liu, T. Y., Jia, S. S., Fan, J. H., Hu, D. J., Lv, G. P., & Huang, H. (2024). Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo. Int J Biol Macromol, 265(Pt 2), 130988. [CrossRef]
- Navarro, A., & Boveris, A. (2007). The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol, 292(2), C670-686. [CrossRef]
- Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95(2), 351-358. [CrossRef]
- Orrenius, S., Gogvadze, V., & Zhivotovsky, B. (2007). Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol, 47, 143-183. [CrossRef]
- Papa, S. (1996). Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta, 1276(2), 87-105. [CrossRef]
- Schirrmacher, V. (2020). Mitochondria at Work: New Insights into Regulation and Dysregulation of Cellular Energy Supply and Metabolism. Biomedicines, 8(11). [CrossRef]
- Solaini, G., Sgarbi, G., Lenaz, G., & Baracca, A. (2007). Evaluating mitochondrial membrane potential in cells. Biosci Rep, 27(1-3), 11-21. [CrossRef]
- Strzyz, P. (2020). Immortalizing switch to OXPHOS. Nat Rev Mol Cell Biol, 21(11), 658-659. [CrossRef]
- Sun, Q. L., Li, Y. X., Cui, Y. S., Jiang, S. L., Dong, C. X., & Du, J. (2019). Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages. Int J Biol Macromol, 130, 556-563. [CrossRef]
- Valcarcel-Jimenez, L., Gaude, E., Torrano, V., Frezza, C., & Carracedo, A. (2017). Mitochondrial Metabolism: Yin and Yang for Tumor Progression. Trends Endocrinol Metab, 28(10), 748-757. [CrossRef]
- Wallace, D. C. (2005). A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet, 39, 359-407. [CrossRef]
- Walter, L., Nogueira, V., Leverve, X., Heitz, M. P., Bernardi, P., & Fontaine, E. (2000). Three classes of ubiquinone analogs regulate the mitochondrial permeability transition pore through a common site. J Biol Chem, 275(38), 29521-29527. [CrossRef]
- Wang, J., Sun, C., Zheng, Y., Pan, H., Zhou, Y., & Fan, Y. (2014). The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Arch Pharm Res, 37(4), 530-538. [CrossRef]
- Wang, Y., Zhao, Y., Chen, H., Lu, T., Yang, R., Weng, X., & Li, W. (2024). Effect of Codonopsis pilosula polysaccharide on the quality of sheep semen preservation at 4 degrees C. Anim Biosci, 37(6), 1001-1006. [CrossRef]
- Yang, X., Zhao, Y., Ruan, Y., & Yang, Y. (2008). Development and application of a capillary electrophoretic method for the composition analysis of a typical heteropolysaccharide from Codonopsis pilosula NANNF. Biol Pharm Bull, 31(10), 1860-1865. [CrossRef]
- Zhang, S., Liu, F., Li, J., Jing, C., Lu, J., Chen, X., Wang, D., Cao, D., Zhao, D., & Sun, L. (2023). A 4.7-kDa polysaccharide from Panax ginseng suppresses Abeta pathology via mitophagy activation in cross-species Alzheimer's disease models. Biomed Pharmacother, 167, 115442. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).