Introduction: The 1842-1872 MEH Revolution
Sara Walker, a theoretical physicist and astrobiologist, is quoted as expressing in a NYT piece on her new book, “Life as No One Knows It: The Physics of Life’s Emergence,” that “She was struck by how hard it was to explain life with standard physics theories” [[
1]]. Schrödinger famously attempted, if not to explain, to provide one necessary condition of life based on standard physics that life entails negentropy, [[
2]], or free energy. This paper is a critical examination about the nature of sustainability. While an examination of life is outside its purview, the paper argues that the nature of sustainability necessitates inclusion of life in its consideration (the sustainability of a planet without life is not a topic of great interest), and it does involve detailed investigation of Schrödinger’s negentropy as one of its focuses.
This paper is a part of the announcement of a Special Issue, “Thermodynamic analysis and its theoretical system for sustainable energy sources,” by the journal Energies: It begins with a comment on what sustainable “energy” is. Which is then followed in a two-parts body: a first part on the meaning of the entropy law as the law of transformation; a second part on the path toward sustainability as a transition not of energy but of nexus, from the efficiency nexus to an autotrophic nexus. The two parts are organized as follows: The paper gives first a historical background of fire’s roles in human existence and the beginning of the theory of fire and heat in Sects. 2 and 3—and then explains why, beginning with Sect. 4, the free energy centric theory of thermodynamics is deficient in providing solutions and answers to the sustainability problem, a deficiency that can be overcome by a new thermodynamics erected on an entropy premise. Followed in Sects. 5 and 6 is the second part, with arguments developed from the entropy premise, the paper puts forth a thesis that the usefulness of the free energy concept is limited to using the term in two narrower senses, which is then followed with conclusion that the renewable energy regime defined by an “autotrophic” nexus is pivotal to the sustainability of the Earth, our living planet.
The original concept of energy and the notion of conservation of energy came from mechanical science, both as inferences of a science erected on the primary concept of force (in this case, the singular primary concept). With the advent of the science of heat or thermodynamics, the new science was erected on dual primary concepts, force
and “energy as a generalized concept” [[
3]: p. 327; [
4]]. The new phenomenon that thermodynamics explicated is the “energy consumption”-driven phenomena, the governing law of which is the first law of thermodynamics, “
Energy can be neither created nor destroyed; only the form in which energy exists can be transformed from one form into another” [[
5]: p.44]. The law statement is a sweepingly powerful statement evidencing that Willaim Thomson was correct when he claimed that there was a new primary concept in physics beyond force [4: p. 58]. “But is it energy? More precisely, what does energy consumption mean? Since energy can be neither created nor destroyed, what is consumed is not energy but some form of energy, energy of one form is consumed to become energy of another form. So, the
operative part of the first law statement is ‘
the form in which energy exists can be transformed from one form into another.’ Since energy form and the direction of energy transformations are the purview of the second law of thermodynamics, this first law statement is not a statement of the first law per se but a statement of the combined first and second laws with its essence being in fact the second law” [[
6]: p.2].
The origin of the first law was the theorem of the mechanical equivalent of heat (MEH), the formulation of MEH and the history of its development has been recounted in an excellent paper by the historian of science, Kipnis [[
7]], which aims to provide an account of ‘How the principle of energy conservation evolved between 1842 and 1870’ by examining ‘about 200 papers and books on topics relevant to PEC and published within three decades since the first communications of Mayer and Joule’ [7: p. 2011]. We have, reflecting Kipnis’ investigation, referred to the event of the period as the 1842-72 MEH revolution [6: p.3].
The most interesting finding, for the purpose of our discussion, is “The list of names [from analyzing these 200 papers and books] also provided a few surprises, the greatest of which was a prominent presence of people who were not supposed to be there at all, namely, Carnot, Clapeyron, and Holtzmann who are known for their contribution to thermodynamics but not PEC” [7: p. 2012] wrote Kipnis. That is, the discourse of PEC, the first law, cannot be separated from that of the second law of thermodynamics, the same realization that is derived from analyzing the statement of the first law of thermodynamics: the in-separated-ness of energy and entropy in the first law of thermodynamics statement is a direct reflection of the in-separated-ness of energy and entropy in the historical evolution of the two concepts.
The in-separated-ness of energy and entropy should be made even stronger to be primacy of entropy over energy. Since the notion of energy conservation is one that was carried over from mechanical science and generalized, instead of as the law of energy conservation, the new law of thermodynamics that is truly new is as the law of energy degradation, which was formulated in 1852 by Thomson [[
8]: pp.511-514]. As Henry Adams noted
The second law was briefly stated by Thomson in a paper " On a Universal Tendency in Nature to the Dissipation of Mechanical Energy," published in October 1852, which is now as classic as Kepler's or Newton's Laws, and quite as necessary to a scientific education [[
9]: 3].
In this paper, Thomson not only introduced, for the first time, free energy, the concept, but also, by asserting the universal dissipation of mechanical energy and free energy in addition to their spontaneous dissipation, posited the conversion doctrine of free energy, the doctrine.
Adams identified the Thomson’s version of the second law, both the concept and the doctrine, as the core of thermodynamics, the new physics for scientific education for general public (though, for physicists and advanced students the version of the law should be the entropy law; more on this in the immediately following and throughout of the paper). We do not talk about energy by itself. When we talk about energy, it is always energy in the sense of free energy. This is what Schrödinger means when he talks about negentropy in the context of biological organisms. This is also what Georgescu-Roegen means when he talks about, “there is a continuous and irrevocable qualitative degradation of free into bound energy” [[
10]: p. 6] in the context of economic and industrial units.
Talking about free energy of organism units and economic units greatly facilitates the thread of our narrative. But we shall argue that the consideration of the sustainability of organism units and economic units entails the consideration of the whole system of the units as well as the consideration of the subtle difference of energy degradation, Thomson’s second law, from inexorable growth of entropy, i.e., Clausius’ second law. This difference is of the greatest significance for our cultural and social discourses: without making the distinction, Kelvin’s (Thomson’s) worldview remains the certified worldview of the learned society as Adams noted that the central theme of his essay was,
The violent contradiction between Kelvin's Degradation and Darwin's Elevation was so profound, — so flagrant, — so vital to mankind… [8: p. 39]
This certified worldview is also the worldview that led Georgescu-Roegen to his tenet, “there is a continuous and irrevocable qualitative degradation of free into bound energy” [10: p. 6]. It will be argued that differentiation of energy degradation from entropy growth amounts to demarcating free energy, the concept, from the conversion doctrine of free energy, the doctrine.
Two previous papers, [
3,
6], proposed a new theoretical system, Unified Classical Thermodynamics (UCT), defined by rejecting the conversion doctrine of energy physics (orthodox thermodynamics). This paper, the third of the trilogy, provides the crucial rationale for the rejection of the conversion doctrine of free energy because the doctrine precludes orthodox thermodynamics from successful conceptual differentiation of caloric into energy, entropy, and heat.
The new theoretical system has advantages in its application to framing the problem of the sustainability of a whole system—providing the solution to Adams’ Contradiction between degradation and elevation and Georgescu-Roegen’s entropy pessimism.
The “Second-Fire,” the Role of Heat in the Sustainability of Human
We trace the necessity of energy transition to the acceleration of human use of fire since the eighteenth and nineteenth centuries. Before the acceleration, the story of fire and the myth of Prometheus has already been integral to the story of
Homo sapiens. The fire historian Stephen Pyne structures his history of fire in three stages [[
11]]: “first-fire” is the natural fire, a natural phenomenon that existed before the appearance of humans; “second-fire” is the anthropogenic fire; “third-fire” is the industrial fire. Pyne makes a compelling case that Earth is a fire planet, telling an epic history of the evolutionary and ecological roles of the first-fire. The term “Pyrocene” is proposed to provide a narrative of how humans, with the development of the anthropogenic second-fire, have been in the second stage of this history interacting with fire.
Counting only energetic/entropic consideration, a rudimentary list of three necessities for human survival are food, fire (the second-fire) for heat, light, and cooking, and various modes of transportation. Let us limit to for the time being the consideration of the latter two of the three. For thousands of years, humans depended on the second-fire for heat, light, and cooking while on animals’ power and on wind and waterpower for transportation.
The required scientific knowledge for the applications of these technologies were quite different. For thousands of years, the applications of fire were represented in terms of the idea of the efficiency of the devices, e.g., the efficiency of furnaces. This, however, was more in the tradition of craftmanship than that of the modern tradition of science and engineering since the seventeenth century.
The science of heat in the new tradition of science and engineering emerged in the 18th century and early 19th century with the doctrine of heat conservation, i.e., the doctrine of caloric. We may consider this science in two phases, the early phase of Black, Laplace, Lavoisier, and Fourier and the later phase of Carnot, more on the later in Sect. 3. “Fourier treated heat transfer as a workless dissipation, while, for Carnot, heat gradient was the source of dissipation-less work” [5: p.4]. We may, therefore, refer to the first phase as the workless-dissipation phase and the latter the dissipation-less-work phase. Here, in this section, on the fact that the new science of heat emerged in the background of mechanical science, it is noted of epistemological similarities between the science of workless-dissipation, represented by the 1822 Fourier’s The Analytical Theory of Heat, and mechanical science.
It should be noted that the required scientific knowledge for the application of wind and waterpower, before the seventeenth century, was also in the tradition of craftmanship. This tradition underwent a transformation to becoming mechanics or mechanical science with the idea that mechanical phenomena can be described analytically in terms of the locomotion of mechanical entities. The term “locomotion” was used by G-R [
10]: for instance, in the case of microscopic molecular mechanical phenomena it refers to the positions and momentums of every molecule that are determined by
equations of motion (EOMs). Under the idealization of mechanical phenomena, EOMs predict a reversible trajectory, therefore, a deterministic reversible motion of no qualitative change. These are the hallmarks of
objective knowledge.
While idealized mechanical phenomena are reversible, the phenomenon of heat is irreversible. Irreversibility defines the fundamental difference between the phenomena of locomotion and the phenomena of heat. However, the real differences, both physical and epistemological ones, manifest themselves when we study in Sects. 3 and 4 the reversible-idealization of the dissipation-less-work and the reversible-like versions of it—for which qualitative changes in trajectories are not determined by EOMs.
Whereas for workless dissipation considered in this section, Fourier’s
The Analytical Theory of Heat led to an EOM for spontaneous heat transfer phenomena, [
5,
6],
Note that this equation includes the constitutive laws, Fourier’s law of heat conduction, , and Stokes’ law of viscosity for . With these constitutive laws, Eq. (1), though customarily referred to as the energy equation, is in fact a governing differential equation – i.e., an equation of motion – representing both the first law and the second law: the constitutive laws in (1) collectively are the second law.
While the irreversible world of heat and the reversible world of mechanical particles are different physically, the two worlds when the irreversible world is limited to spontaneous changes are epistemologically similar. Both are deterministically governed by equations of motion. For the reversible mechanical world, the deterministic trajectory means there is no qualitative change. For the irreversible world of heat, the deterministic trajectory moves toward a predictable internal equilibrium state.
Energy Physics, from the Doctrine of Caloric to the Conversion Doctrine
At the very end of the second stage, a transition from the anthropogenic second-fire into the industrial third-fire stage emerged with the practice of burning fossil (lithic) biomass. Pyne prefers to use the term “industrial combustion” to describe the third-fire, to emphasize that the Enlightenment scientific approach to fire phenomena led to the disappearance of the phenomena with all their complexity into the neatly categorized processes (mixing, ignition, combustion) and components (fuel reactants, oxidizer, input chamber, furnace). The scientific approach to fire phenomena turning it into combustion processes made it possible to scale up third-fire into unsustainable industrial combustion.
The scientific approach began in the 1840s when Mayer, Joule, and Helmholtz attacked the caloric doctrine of heat conservation. This was one of the greatest scientific events in the nineteenth century beginning with the hypothesis of the mechanical equivalent of heat in 1842-43 by Mayer and Joule, which was followed with three decades of contribution by large number of scientists and engineers.[
7] The event is referred to as the 1842-72 MEH revolution (see
Table 1) leading to the unification of the mechanical world and the world of heat phenomena. And the firm establishment of the hypothesis into the law that in the interactions of heat and work it is energy, rather than heat, which is conserved. We call this version of thermodynamics
energy physics.
Whereas heat was the driving force in Caloric theory, there has been a widely shared view in energy physics that the “equivalence” in MEH implies “causation,” therefore, the supplanting of heat conservation with energy conservation implies that energy, more precisely free energy (see
Table 1), is the driving force in energy physics.
The key in the development of the concept of free energy is the second law formulated by Thomson in a paper "On a Universal Tendency in Nature to the Dissipation of Mechanical Energy” [8: p. 511-14]. In the paper, barely of four pages, Thomson noted that while energy can never be destroyed, free energy, a derived term from energy, dissipates or degrades “irrecoverably from the control of man sources of power which…might have been rendered available” (cited in [[
12]]: Appendix II).
The concept of free energy or available energy is one of the most productive but widely misunderstood concepts. A goal of the paper is to point out where the misunderstandings as a general concept are, and to put forward a thesis that the usefulness of the concept is limited to using the term in two narrower senses: as available energy in equilibrium thermodynamics and as free reactive enthalpy (defined in Sect. 5) in engineering thermodynamics.
Thomson’s introduction of available energy preceded Clausius’ introduction of entropy and formulation of the entropy law as the second law. Gibbs followed Clausius noting, “It is an inference naturally suggested by the general increase of entropy which accompanies the changes occurring in any isolated material system that when the entropy has reached a maximum, the system will be in a state of equilibrium” [[
13]]. From this starting point, Gibbs developed his equilibrium thermodynamics theory making extensive use of available energy’s spontaneous tendency as thermodynamic potentials. “Although Gibbs never once mentioned Thomson in his work, he was indebted, I believe, to Thomson's concept of dissipation of energy via the good offices of Maxwell and his Theory of Heat” noted Daub [[
14]].
There is a fundamental difference between the inexorable tendency of entropy to increase and the tendency of available energies toward their optimal stable-equilibrium values. Entropy increase is ironclad, inexorable, and universal, whereas tendency toward equilibrium is spontaneous but not universal. If the tendency toward stable-equilibrium is universal, the far-from-equilibrium state of a system is impossible, and life is impossible.
However, when Thomson closed the 1852 paper with general conclusion 2 of his three general conclusions, he was very much thinking along the notion of inexorable tendency of free energy as he wrote,
General conclusion 2. Any restoration of mechanical energy, without more than an equivalent of dissipation, is impossible in inanimate material processes, and is probably never effected by means of organized matter, either endowed with vegetable life or subjected to the will of an animated creature.[8:514]
That is, mechanical and free energy not only dissipate spontaneously but also universally. In one stroke, Thomson introduced the concept of free energy and asserted that the universal tendency of free energy dissipation was the driving force of all thermodynamic processes.
That is, in one stroke, Thomson introduced free energy, the concept, and the tenet of conversion of free energy, the conversion doctrine. The latter was presented as a self-evident assertion. These are the dual foundations of energy physics (see
Table 1).
The conversion doctrine has had the unfortunate consequences of encouraging thinking of free energy as some kind of energy. Marc Henry, the polymath, has this to say,
… one should really avoid the common error of thinking that by adding the word “free” before the word “energy”, one still refers to energy changes. It should rather be realized that “free energies” are in fact entropies [see
Table 1 in this article making the same point], ... Besides forgetting that thermodynamics is a science of the whole universe, there is also the fact that entropy changes
are masqueraded in Gibbs’ formulation as energy changes after multiplication by the temperature of the thermostat. Such a manipulation, pushes to the belief for unaware people that a thermodynamic system tries, upon spontaneous evolution, to minimize its energy, as in reality he tries to maximize the entropy of the universe! ([[
15]]: p.61)
Rather than preserving the subtlety of the free energy concept, the conversion doctrine encourages a simple-minded interpretation of free energy as, “
body’s internal energy or enthalpy, subtracted by energy that is not available.” Numerous examples of that interpretation being nonsensical are given in [
3], the first paper of a trilogy of papers that this paper is one of which.
The conversion doctrine also linked the MEH theorem, with the interpretation of “equivalence” as “
transformed from one form into another”, to the standard statement of the first law—the statement that Job and Lankau claimed in a paper with an eye-catching title, “How harmful is the first law?” [[
16]]
In the following, we shall carry out a development of ideas following Marc Henry’s argument (he makes the case for life phenomenon, but the same can be made for what makes sustainability of “fire phenomena” possible). His argument: “as far as life phenomenon is concerned, one should not rely on energy and the first law, but only on the second law stating that for any kind of evolution a single non-ambiguous and universal criterion should be used:
... So, among all the possible extensive variables that could be associated to a macrostate, entropy and not energy should be the privileged one ...” [15: 58] The same argument has been made in [
3] for “fire phenomena” and engineering processes. We shall argue beginning in Sect. 4.2. followed into Sect. 6 that with the replacement of Kelvin’s degradation with Clausius’ entropy-growth, Adams’ contradiction between degradation and elevation and Georgescu-Roegen’s
entropy pessimism will be resolved.
3. The Premise of “Entropy Growth Drives all Macroscopic processes,” from Carnot to Clausius to Gibbs
The in-separated-ness of energy and entropy in the first law of thermodynamics statement is a direct reflection of the in-separated-ness of energy and entropy in the historical evolution of the two concepts. This is a reflection of the fact that the original term, heat, has been used to describe both the process of energy and that of entropy that, while “work is energy in transit,” “heat is energy and entropy in transit” [5: pp.105-106]. But this is also the consequence of the conversion doctrine that solidifies linking the MEH to the standard statement of the first law, negating, as we argue below, the methodological foundation of energy physics, conceptual differentiation. We begin in this section with a discussion of the relationship between the conversion doctrine, the conceptual foundation of energy physics, and conceptual differentiation, its methodological foundation.
4.1. The EN Thermo School
There is a school of thermodynamics, which has been referred to as the EN Thermo (Experientially Natural form of Thermodynamics) school, [
6]. The term is adopted from its original source [[
17]]. The EN Thermo school is a conceptual scheme that survived the MEH Revolution. It gave up “heat as substance” doctrine but preserved the concept of heat in the general sense.
The EN Thermo school regrets energy physics’ assigning heat narrowly as
. The school questions the meaning of “equivalence” of the MEH theorem.[
16] It prefers to use heat in its original, general sense of the caloric theory holding, in that sense, that heat is
the driver of all macroscopic processes (
Table 1).
Objecting the assignment of heat by energy physics as
and questioning the meaning of “equivalence”, the EN Thermo school wrote, “… the name of an existing quantity [heat, or caloric] was taken away from this quantity and given to another one. However, the old quantity was not given a new name, resulting in its disappearance from the scene… Thus, the beautiful analogy [of Carnot heat engine/pump] to the water wheel … was no longer recognizable…” [[
18]: p.9]. Of course, everyone knows that Rudolf Clausius later between 1854 and 1865 rediscovered “the old quantity” and gave it the name of entropy. But the so-called entropy, the school argues: “corresponds almost perfectly with what is colloquially called heat” [18:11]; or it “resembles an imaginatively constructed
Extensive Quantity of Heat (EQH)” [17:1]. So, the story of caloric and work before 1850 became for energy physics the story of heat, entropy, and energy after 1850 ([
16]:
Table 1).
In the narrative of the development of energy physics centered around the MEH revolution, a missing piece in the narrative is a new conceptual scheme that Carnot initiated and Clausius followed through, as Tisza described, by “ ‘splitting’ the concept of caloric into heat quantity, energy, and entropy” [[
19]: 22]. The ”splitting” or conceptual differentiation (CD) is necessary because for the development and evaluation of any conceptual scheme, “we need clear instructions for the manipulation of these concepts, including their connection with experiment” [19: 22]. CD provides the framework making such instructions and definition-formulations possible.
Both Thomson and Clausius recognized CD’s key role in their theories, energy physics and the mechanical theory of heat, [[
20]], respectively. They together succeeded in the formulation of the first law and the entropy law (Clausius) providing instructions and definitions for “heat quantity, energy, and entropy.” Clausius’ treatment will be briefly given in Sect. 4.2. Thomson’s treatment is briefly described in Sect. 3.
In Thomson’s treatment, it is noted here that Thomson self-sabotaged his own treatment when he put forth his universal dissipation of free energy, the conversion doctrine: The conversion doctrine linked the MEH theorem, with the interpretation of “equivalence” as “transformed from one form into another”, to the standard statement of the first law rendering the statement hopelessly short from a clear-cut CD. That is, the conversion doctrine (the energy conversion doctrine), the conceptual foundation of energy physics, and CD, the methodological foundation of thermodynamics, are mutually contradictory.
When the EN Thermo school preserves the premise of “heat” as
the driving force, it chooses a conceptual scheme that is against the implication of the statement of the first law that energy, or free energy, is the driver of
all macroscopic processes (see
Table 1). By asking how harmful the first law is, the EN Thermo directs its critique against the first law statement but not the first law itself. Since it is the conversion doctrine that linked the MEH theorem to the Statement, the assertion against the Statement amounts to assertion against the conversion doctrine, as it noted in
Table 2.
For energy physics as it is noted in
Table 2, while its achievement in providing the definitions of energy and entropy remains intact, its implementation of CD resulting in the formulation of two laws was marred by the mutual contradiction between the conversion doctrine and CD.
The EN Thermo school did not, however, take advantage of its critique of conversion doctrine against energy physics’ position of depriving the second law of the claim as the law of transformation. Instead, the school is non-committal with CD as a methodological tool: rather than restoring the second law to its privileged position of the law of transformation, it begins with the premise of heat as
the driving force (“heat as a force of nature” in [
17]).
With the EN Thermo school’s motto, “heat as entropy” (heat is related to entropy as, in [
18], “entropy is introduced as a measure of heat” and “entropy largely corresponds to what is colloquially understood by heat”), we invite the school to participate in the discussion of the following questions:
If heat is a force of nature with entropy as a measure of which, whether heat or entropy is the primary concept?
If entropy is the primary concept, how is the theory different from orthodox thermodynamics?
If heat is the primary concept, how is heat defined? That is, can heat be defined in terms of a precise set of measures? If it can, what such a set will be?
4.1. Equivalence and Co-Existence; TET vs. the Second Fundamental Theorem
The energy physics narrative, the highlight of which was the MEH Revolution, misses one pivotal event, the 1824 publication of Carnot’s magnum opus [[
21]]. MEH asserts equivalence between heat and work. “Equivalence” can be equivalence in a broad sense with implication of implied nature of transformation from heat into work, or that in a strictly narrow sense of “closure condition” without involving any nature of transformation. The closure condition of MEH is the fact that any production of work of a given amount is accompanied by the disappearance of heat of a corresponding amount, and vice versa. By starting with the material doctrine of heat, Carnot has been criticized that his treatment of heat-work interaction violated the
energy conservation closure condition.
But it is possible to interpret Carnot not through the lens of equivalence of heat and work as a study of interaction of heat and work, but a study of the interaction of “heat transmission” and “transformation of heat into work.”
Equivalence in a broad sense with implication of implied nature of transformation from heat into work is derived from the premise of “heat could be converted into work.” Carnot’s idea is described by Kipnis, “…neither Carnot and Clapeyron nor Holtzmann and Thomson thought before 1850 that heat could be converted into work. Apparently, before 1850 they assumed a certain association between heat and work, such that the two existed independently of one another but could influence each other. For instance, Carnot’s supposition that work was created by a mere transfer of heat by expanding gas, in fact, implied such a coexistence” [7: p.2032, Sect. 9]. Carnot began not with a premise of “heat could be converted into work,” but with a premise of “co-existence of heat transmission and heat-work transformation.” This is exactly how Clausius updated Carnot’s theory of heat engine.
Clausius first adopted the following names: he referred to the MEH theorem in the narrow sense of “closure condition” as the First Fundamental Theorem and an updated Carnot’s existence theorem as the Second Fundamental Theorem. The preamble of the Second Fundamental Theorem is the assumption that there exist two kinds of dissymmetric or irreversible transformations in nature, transformations of natural direction or what Clausius referred to as positive direction, and those of unnatural direction or negative direction. The Second Fundamental Theorem, as stated by Clausius, is the assertion,
all transformations occurring in nature may take place in a certain direction, which I have assumed as positive, by themselves, that is, without compensation; but that in the opposite, and consequently negative direction, they can only take place in such a manner as to be compensated by simultaneously occurring positive transformations [20: p.364].
In the 1854 Fourth Memoir, [
20], he began with
“… the limiting case to investigate quantitatively the details of cyclic processes involving transformations in reversible coexistence in a six-step cycle (his invention of a modified Carnot cycle) [20: Figure in p.119]. He was able to devise a system of assigning for each transformation its equivalence-value and referred to the condition of their reversible coexistence as the condition of equivalence, the condition that “algebraical sum [of equivalence-values of the transformations of a reversible cyclical process] is zero” [20: pp.127-129]. This case of reversible cyclical process was appropriately referred to as the theorem of the equivalence of transformations [TET].
“The second fundamental theorem and TET are two different theorems, the former asserts the idea of coexistence, first introduced by Carnot, and the latter the idea of equivalence, the quantitative expression of Carnot’s idea that has been made to be consistent with the equivalence theorem [the First Fundamental Theorem].
“Clausius’ extraordinary insight was marred with one problem, he never used the terms, coexistence. This is reflected in the fact that he has not consistently been making clear the distinction between the second fundamental theorem and TET. In fact, while he mentioned both terms in the Fourth Memoir, the Memoir treated both terms synonymously with the same theorem-statement, the TET statement as a replacement statement as given in [20: pp.125-126 (bottom of p. 125 and top of p.126)]. The Fourth Memoir is all about TET.
“Only by Sixth Memoir, there as he noted,
In a memoir published in the year 1854…I deduced a theorem which is closely allied to, but does not entirely coincide with, the one first deduced by S. Carnot… I have called it the Theorem of the Equivalence of Transformations. I did not, however, there communicate the entire theorem in the general form [20: p.218]
—Clausius began writing about the statement of a theorem in the general form as a distinctive statement from the TET statement calling it the Second Fundamental Theorem. Clausius then followed with the treatment of the second fundamental theorem in the Seventh Memoir and the Ninth Memoir; the above statement of the Second Fundamental Theorem is from the Ninth.
“In a nutshell, while TET is deservedly famous, it is the coexistence theorem that gives rise to the second law for engineering thermodynamics. Whereas TET, serving beautifully as the foundation for equilibrium thermodynamics, is not sufficient by itself to be the foundation for engineering thermodynamics. Because they highlighted TET over the role of the coexistence theorem, Clausius himself and Gibbs, who followed him, did not carry out the obvious extension of their approach to make their theories applicable to energy physics and engineering thermodynamics. Nor did they attempt to unify the two separate sciences, engineering thermodynamics and Gibbsian equilibrium thermodynamics. The extension and unification have been carried out by stressing the role of coexistence theorem in a recent paper on Unified Classical Thermodynamics (UCT)” (quoted from [6: pp.8-9]).
Evidence that the Second Fundamental Theorem, which Clausius considered, together with the First Fundamental Theorem, as the bedrock of the mechanical theory of heat, has been overlooked in energy physics and engineering thermodynamics can be found in a recent review paper [[
22]]. The paper, entitled “What Is the Real Clausius Statement of the Second Law of Thermodynamics?”, declares “the theorem of the equivalence of transformations is the real Clausius Statement of the second law of thermodynamics.” In the paper, the Second Fundamental Theorem is nowhere to be found.
Clausius’ six-step cycle [20: 119] has been interpreted into a five-step cycle, referred to as the Carnot/Clausius cycle [[
23]: Fig. 7; 3: Fig. 2]. With the Second Fundamental Theorem restored to its privileged position, the high-temperature heat energy
as a “driving force” of a Carnot cycle is now supplanted with a heat transmission of
from
to
in [23: Fig. 7; 3: Fig. 2] as the driving force of the Carnot/Clausius cycle. More precisely, the driving force is the entropy growth in association with the heat transmission of a
high temperature source-system to an environment at
.
That is, it can be generalized to the consideration of a “driving force” in association with a source-system, in an environment at
, whether it is a
high temperature source-system, or a composite system considered in Section 6 of reference [
3] (two such systems considered there: Equations (41) and (42)), or in Fig. 7 of Sect. 7 of reference [
3].
“We referred to, in this generalization, the ‘driving force’ as Entropy Growth Potential, EGP ([
5]: Sections 8.3 to 8.5). The value of EGP is determined by the total entropy growth or entropy production of the source-system and the environment the system interacts with (referred to as “source-system” + “the environment-reservoir” =
universe),
“That is,
is the total entropy growth of the Universe in a spontaneous event. Correspondingly, there is a reversible event. It has been argued in [
5] that the two events define a set of infinite possibilities (the set is referred to as Poincare range) that share “a property common to all possibilities” ([[
24]]; also see [
5]: p. 197).
and naming it entropy growth potential, we acknowledge EGP to be the common property of all possibilities within the set of a Poincare range.
“While the entropy growth of each event is different from other events, every event in the set has the same entropy growth potential, which represents the maximum (potential) useful work of every event in the set, corresponding to,
The actual useful work produced by each specific event is less than the maximum useful work.
“For the case of the Carnot–Clausius cycle, (4) takes the form of
Note that in this case,
as a function of
, in accordance with (2) and (3), equals to
It follows that the general expression, (5), reduces to the special expression,
Which is the Carnot formula.
“Instead of looking at
, [the Carnot/Clausius account] identifies the dual roles the heat reservoir plays, as a heat sink for the
EGP driving force as shown by (6), and as a heat source reservoir for the heat extraction mechanism made possible by the driving force as shown by (5). The reason for the decrease in heat rejected from the Carnot/Clausius cycle in association with a lower heat reservoir temperature is the combined result of a stronger increase in EGP, the driving force in
, and a proportional decrease in extracted heat resulting from a lower heat reservoir temperature
in (5), rather than of a lower heat reservoir temperature favoring the heat extraction process” (quoted from [
3]: pp.338-39).
We close the entropy law analysis of the paper with an interim summary: this paper’s new finding is that the conversion doctrine of free energy precludes orthodox thermodynamics from successful conceptual differentiation—with the new finding, the paper serves as a review of some earlier contributions to UCT; with the rejection of the doctrine, UCT carries out successfully differentiation between the
two DOE questions, to its logical conclusion [
3]; thereby, entropy growth is the universal driving force for all macroscopic processes, including reversible-like processes indeterminately [
6]; the quantitative determination of the driving force is facilitated by the concept of
EGP [
5]; every reversible-like process requires a heat reservoir, which serves as a heat source intrinsically and, optionally when the process is powered by fuel combustion, as a heat sink for actualizing EGP [23, 3].
In the rest of the paper, it’ll address the full meaning of free energy; critically examine what makes “true” reversible processes, or more precisely, what kind of processes that really matter; carry out a brief discourse on Schrödinger’s precondition of life; as well as a broad discourse on the sustainability of a living planet.
Free Reaction Enthalpy Sustaining Heterotrophic Organisms
Another way for summarizing the interim summary is that a basic equation for energy-consumption processes is:
Or, as a balance equation for energy-consumption processes,
A surprising finding in reference [
3] is that Carnot cycle is not a truly reversible cycle: “Thermodynamics began with a focus on the relation between heat and work and with Carnot’s innovation of investigating this relation in terms of reversible processes. … However, this historical background of thermodynamics contains, by linking heat and the discussion of reversibility so closely, a misleading notion of the true nature of reversibility. Any discussion of heat necessitates the involvement of heat release that is intrinsically irreversible. ‘Reversible’ use of heat, such as in the Carnot cycle or the Carnot/Clausius cycle, only idealizes the part involving heat transmission, leaving the irreversible heat release hidden from consideration” [3:339].
Correspondingly, for source-systems of fossil-fuels, the Carnot cycle, though its theoretical role in the theory of thermodynamics is beyond question, is not the idealization for efficient heat engines. The notion of an isothermal heat addition step in a Carnot cycle is totally impractical. Instead, the most efficient heat engine cycle is the combined cycle of a top gas turbine in combination with a bottom steam turbine with
thermal efficiency surpassing 62%. Its idealized reference is the entropy growth potential of a fossil-fuel source-system undergoing a two-step process: an irreversible spontaneous combustion followed with a heat transmission that is coupled with transformation of heat into work. In this case,
, the LHS of (9), takes the form, [3: Eq.(50)],
With a superiorly efficient heat exchanger and a bottom cycle with perfectly designed condenser, the third and second terms of the RHS of (9) are,
in (10) represents minimum required heat-rejection for fossil fuel powered cycles. That is, the unavailable part of fossil fuel enthalpy.
Therefore, we may refer to it as
free flame enthalpy [3: p. 339] or
free reaction enthalpy. That is, we adopt the definition of
free reaction enthalpy (fre), and the special version of the balance equation (9) as (10a) and (12) respectively,
Where is given in accordance with (11).
In reference [
3], numerous reversible transformation examples are shown that the universal feature of harnessing entropy growth is heat extraction instead of heat disposal. In every example of reversible transformation,
is manifested as heat extracted from a heat reservoir. To the extent that “free energy” has been associated with the maximum amount of energy in a source-system that is available for a reversible transformation, after it is subtracted of the unavailable part, the term is a misnomer. It is not energy that is unavailable or available, but
heat that is made
available by entropy growth.
Only for the practice of combustion application of fossil-fuels, in which words such as “available” (free) and “enthalpy” (energy) have meaning. The qualifier “flame” or “reaction” reminds us of intrinsic irreversibility involved in such practice. We shall argue that Georgescu-Roegen’s assertion, “there is a continuous and irrevocable qualitative degradation of free into bound energy,” as a law of physics is mistaken. Irrevocable degradation of free energy is, instead, the consequence of technological choice of prodigious combustion applications of fossil-fuels.
We now direct our question away from mechanical work to the question raised by Schrödinger, “How does the living organism avoid decay? The obvious answer is: by eating, drinking, breathing and (in the case of plants) assimilating. The technical term is called metabolism” [
2]. He then offered a physics interpretation of the answer, “What an organism feeds upon is negative entropy. Or, to put it less paradoxically, the essential thing in metabolism is that the organism succeeds in freeing itself from all the entropy it cannot help produce while alive.”
There are two ways to read “an organism feeds on negative-entropy”: what an organism “feeds” on, and that the possibility of life is the availability of “negentropy.” We consider the first way first.
“What an organism feeds on” addresses how heterotrophic organisms obtain food-energy because they cannot make their own food. For this purpose, the balance equation for the energy-consumption problem of work production, (12), should be re-applicable to the food-energy-consumption problem of heterotrophs: as consumption of
free reaction enthalpy for biomass growth as,
It is undoubtedly true that “Microbial metabolism consists of irreversible processes and therefore generates entropy continuously within the cell” [[
25]: p.206]. What is assumed in writing the balance equation (13) is the
fre hypothesis,
The irreversible microbial metabolic processes of heterotrophs, which feed on either autotrophs or other heterotrophs or both, can be demarcated into spontaneous irreversible reaction that stands alone, and transformations of positive kind that serve as compensation for (are coupled with) transformations of negative kind synthesizing and maintaining cell biomass.
The hypothesis is motivated by the speculation that the evolution of technologies and that of biological world bear similarity. For the purpose of this paper (which addresses the issue of sustainability), the hypothesis leads to balancing groupings in (13). The groupings in (13), which are different from that in reference [
25], enable the application of “engineering thermodynamic analyses” in the paper to the investigation of the sustainability question.
Reversible-like Transformations Without Heat Sink: Autotrophic Artifacts
The paper articulates a thermodynamic view that there is similarity between engineering artifacts and biological organisms, the former are energy consumers, and the latter are food-energy consumers, heterotrophs, as well as autotrophs, i.e., organisms that can make their own food. This section considers similarity between engineering artifacts and biological organisms in the broader sense that “engineering artifacts” can be made to be “autotrophic” as well.
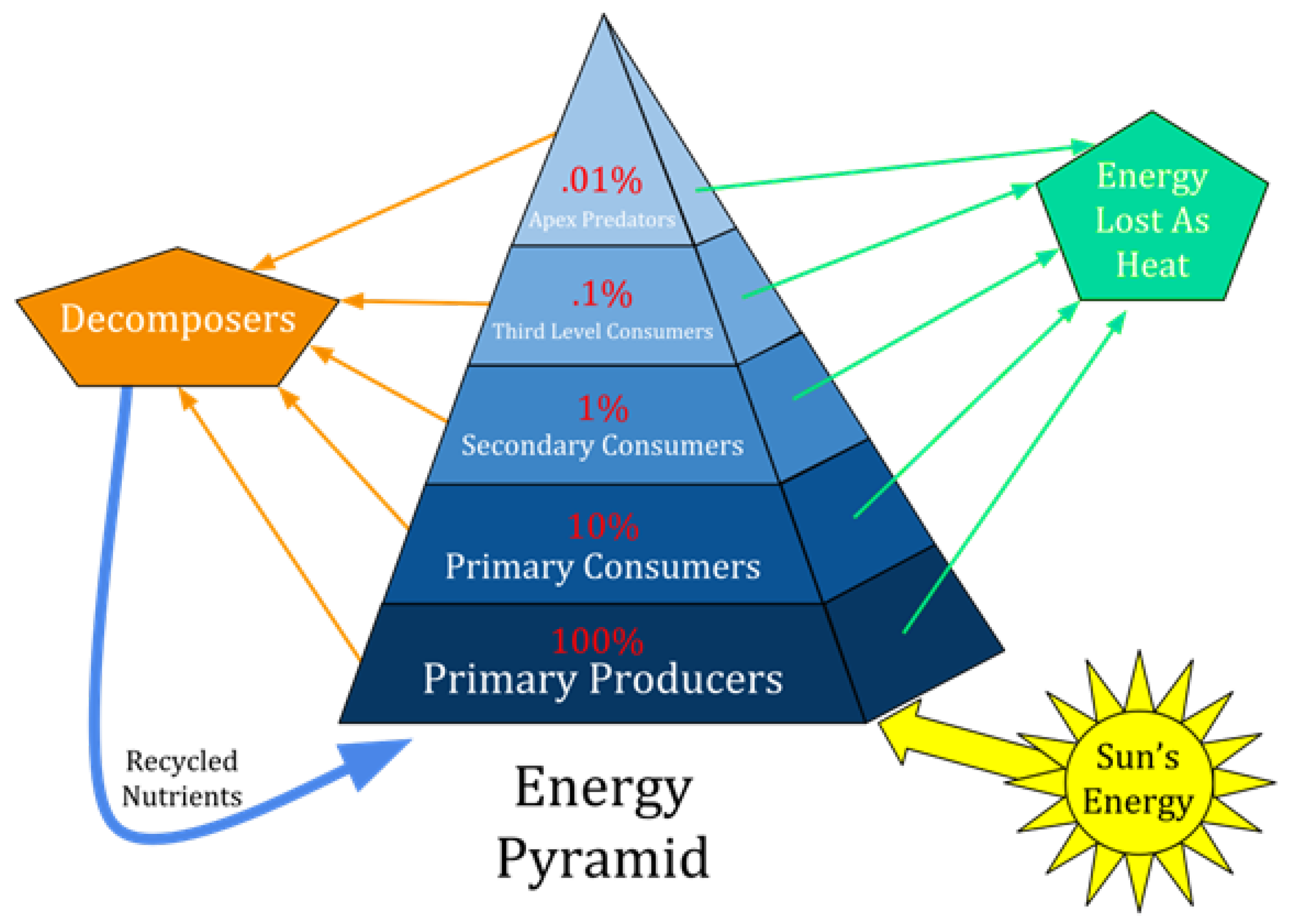
Similarity between engineering artifacts and biological organisms has been, in fact, accepted implicitly all along. Ecological processes have been described as “energy transfer processes” from one trophic level to a next, higher, trophic level as shown in
Figure 1 (reproduced from [[
26]]). The so-called energy transfer processes, however, are based on the energetic understanding of orthodox thermodynamics rest on the idea of transfer of free energy as a reversible concept,
and the tenet of conversion doctrine of free energy.
The latter necessitates that dissipation of free energy is universal. Or the implication that dissipations at each trophic level follow the same pattern. This view is reflected in the statement,
“The concept of energy loss between trophic levels can be illustrated using an energy pyramid diagram [see
Figure 1]. Each step of the pyramid represents a different trophic level, starting with primary producers at the bottom. The size of each step represents the rate of energy flow through each trophic level. The steps decrease in size as you travel up the pyramid because energy is lost at every level in the food chain … Ultimately, only 10 percent of energy is transferred from one trophic level to the next” [[
27]].
Note that
Figure 1 shows for autotroph the same “energy lost as heat,” in pattern though not in size, as that for each of the heterotrophic levels.
In the entropic understanding of UCT, entropy growth (see
Table 2) is the universal driving force. Under the fre hypothesis, fre as the proxy of entropy growth is the driving force for the maintenance and growth of organisms at each heterotrophic level—at each level, energy lost as heat is given as
with
corresponding to its minimum value. It is worth noting that since Carnot and Schrödinger made their seminal contributions to the ideas of
reversible transformation and
negentropy, respectively, the two contributions have been viewed as general conceptual significances. But we argued in reference [
3] and argue here that the two contributions are in fact of specific in the qualified sense rather than general in the strict sense.
Carnot heat engine, which has been referred to as the Carnot/Clausius account, is reversible only if irreversibility of combustion is ignored. A strictly reversible heat engine has been referred to as the Carnot/Clausius/Gibbs account, ([
3]: p. 334 and Fig. 7), in which the finite-affinity driven combustion is transformed into an infinitesimal-affinity driven “reversible reaction.” In contrast, real heat engines are designed in reference to the idealization of free reaction enthalpy. Whereas the former, a strictly reversible heat engine, requires a heat reservoir only as a reservoir of heat source not as a heat sink, the latter do require a reservoir as a heat sink, as a result of combustion irreversibility.
We argue, similarly here, that negentropy as food-energy to be fed to organisms is interpreted as
fre for heterotrophic organisms, rather than as a universal concept. Due to irreversible reactions, such energy flow through heterotrophic levels results in energy lost as heat. If energy flow across the autotrophic, i.e., the producers, level is understood in terms of the same energetic sense, the energy pyramid in
Figure 1 is not sustainable entropically because no “entropy export” mechanism is explicitly incorporated in the pyramid [[
28]]. By stressing the availability of “negentropy” as the condition of possibility of life, Schrödinger implicitly acknowledged “any kind of biological thinking should be centered on the concept of entropy of the whole universe and not on energy,” an assertion made by Henry in more recent time of 2021 [15: p. 66]. A proper understanding of how primary producers use sun’s energy requires, rather than simply as
sun’s energy fed to primary producers, the consideration of the
entropic interactions of sun’s energy with the Earth surface (part that radiantly interacting with sun and cosmos) where primary producers are populated, and cosmos.
Considering surface (part that radiantly interacting with sun and cosmos) of the whole Earth as a system, change in a system’s entropy may be expressed as a sum of two parts per the Brussels School, [[
29]: 88]
is system entropy exchange,
system entropy growth or system entropy production. Consider the case of a time-rate of (15),
For the Earth system, system entropy exchange is associated with the energy inflow from the Sun,
, and Earth’s outgoing infrared radiative flow,
,
With the assumption of thermal steady state (TSS) and negligible internal heat source,
. It follows,
Correspondingly, for a TSS Earth,
For an
assumed entropically steady state Earth (
), the entropy growth of the Earth is in balance with the entropy exchange of the Earth,
Which, according to A Treatise [5: Sects. 8.3 and 8.4], is the entropy growth
potential (
EGP,
) of the Earth,
With that denotation, (16) is rewritten as
This is the entropy balance of the Earth system, in which is given by (18), which is dependent of sun’s astrophysical condition and the geometric relationship between sun and Earth. But the value of , while it is related to sun’s condition and the sun and Earth geometric relationship in accordance with (17), is dependent of geophysical conditions of surface layer at various surface localities as well.
That is, that the value of as given by (17) is its value during Earth’s natural existence with geophysical processes absent of life. The assumption made in this discussion is that Earth’s natural existence with geophysical processes absent of life is in a state of entropic steady state.
Once with the appearance of life in the form of autotroph: while
remains the same because of an assumed unchanging sun and geometric relationship between sun and Earth,
in (16a), on the other hand, will be different dependent on specific life processes of local surface layers subject to the second-law-constraint,
Note that the second law asserts that entropy growth is positive definite,
. But the law does not address whether the
rate of growth can be speeded up or slowed down. Physically speaking, any value is possible within the range,
Note that Eq. (17) is the maximum limit of the inequality,
. It has been argued in Progress in entropy principle [[
30]] that entropy growth rate can be slowed that any value of which in the range of (19) is compatible with the laws of physics.
Let the slowdown in entropy growth rate by autotrophic photosynthesis be represented by a positive
. The history of Earth’s entropy is accordingly, according to (16a),
,
The actual evaluation of and is outside the scope of the paper and outside the expertise of the author, who has no training in chemistry and biology, as well. The point made by the paper is that there is nothing in the laws of physics that precludes decrease in the planetary entropy of the Earth with the appearance of autotrophs (plants and algae/phytoplankton). The key to this decrease is the consequence of sun’s energy “entering” the autotrophic level as manifested as entropy growth potential giving rise to extraction of heat from the planetary heat reservoir, the opposite of “energy lost as heat.”
With autotroph enabled “extraction of heat from the planetary heat reservoir,” heterotrophs and energy transfer across the heterotrophic levels can be supported. The cumulative “energy lost as heat” across the heterotrophic levels contributes to the reduction of but still leaves a positive in (20).
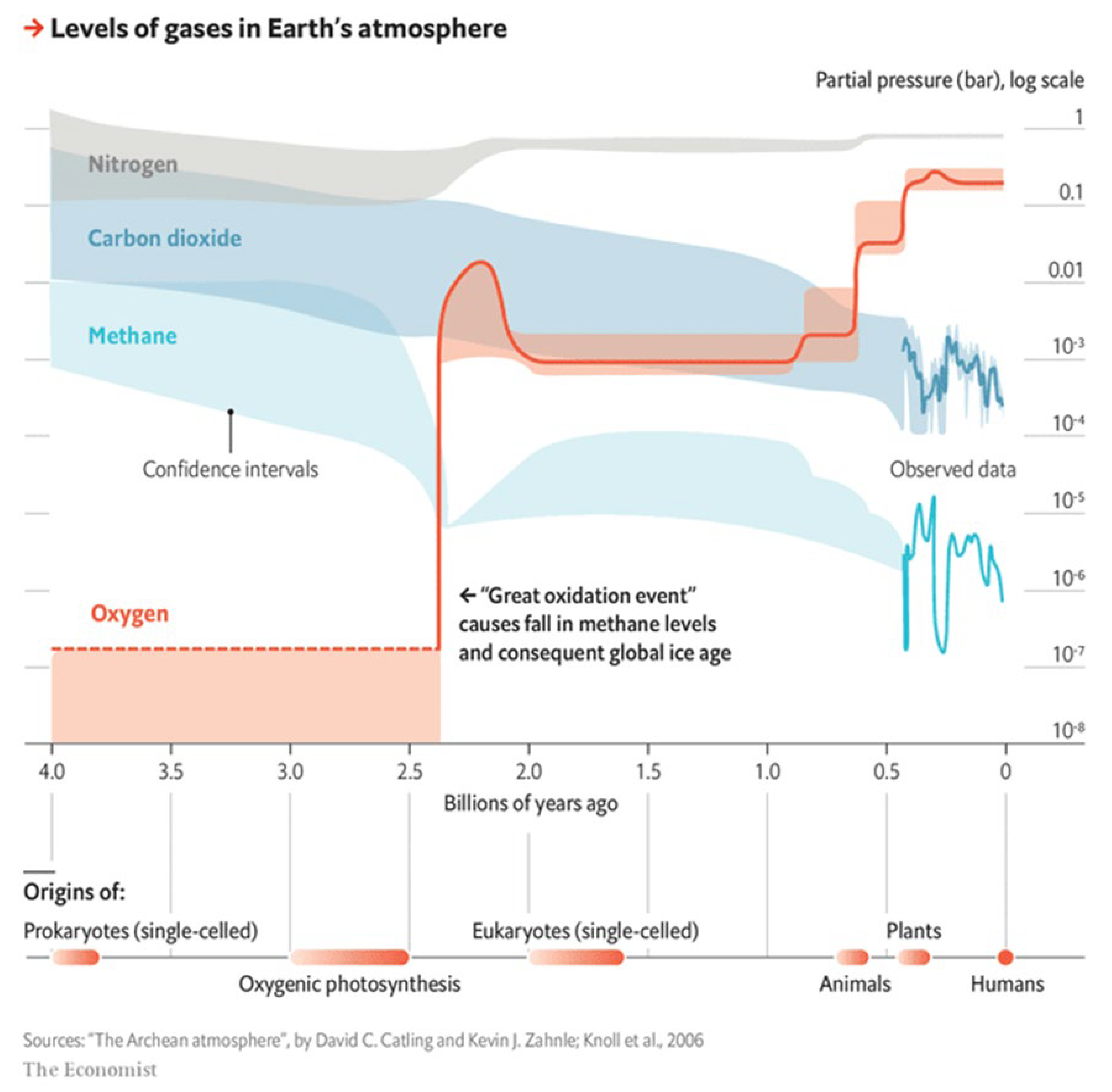
Evidence of a history of such entropy-growth delay, thus decrease in Earth entropy, is the build-up of atmospheric Oxygen and the general trend of Carbon dioxide decline as shown in
Figure 2, which is consistent with the thermodynamic-state history of the Earth moving away from thermodynamic equilibrium toward radical disequilibrium. This trend continued generally during the Age of Gaia [[
31]] until the onset of the Anthropocene.
This brief discussion of the difference between energy flow across autotrophic level and energy flows across heterotrophic levels explicates Adam’s Contradiction between degradation (increase in Earth’s entropy) and elevation (decrease in Earth’s entropy).
The scientific approach to fire phenomena turning it into combustion processes made it possible to scale up third-fire into industrial combustion. We have suggested that the entropy law, with indeterminateness as an integral part of the law [
6], does not
determine the impossibility of sustainable human economic activities, including the second-fire. As a fire planet, Earth will continue to exist with the first-fire and the second-fire as necessary events for their evolutionary and ecological roles. What cannot continue is the continuation of human economic development based on industrial combustion due to its unsustainable
scaling-upward-ness.
First-fire, second-fire, energy transfer across heterotrophic levels are processes involving spontaneous, irreversible tendency that all involve energy lost as heat. The invention of the third-fire was thought to be undergirded by reversibility idealization: for the first time in human history, humans discovered a new way of using fire, a reversible way of using the third-fire in addition to the second-fire for heat, light, and cooking. It turns out that the Carnot reversibility is not reversible strictly speaking.
More importantly, searching for reversibility has led to striving for efficiency. That has been spectacularly successful in the ingenious hand of humans: beginning with the Agricultural Revolution, human ingenuity had transcended the limit of Apex Predators of the Energy Pyramid resulting in a different Energy Paradigm—that, instead of pyramid limit, is characterized with the efficiency nexus of human-ingenuity-efficiency-gain/scaling-upward-ness(rebound-effect)/greater-commutive-energy-lost-as-heat.
Industrial combustion was the cause of requiring a heat reservoir serving as a heat sink for heat disposal. “The universal feature of harnessing entropy growth is heat extraction instead of heat disposal.” With the supplant of combustion with entropy growth phenomena sans combustion, significant heat disposal, or energy lost as heat, can be eliminated. That is, economic growth can be supported by “autotrophic” engineering artifacts. Instead of the above fossil-fuel nexus, we have the “autotrophic” nexus of human-ingenuity-deployment-of-renewables/scaling-upward-ness-for-economic-growth/elimination-of-heat-disposal.
How do we know that the new nexus will work. It will work because of the three constants we can count on: that the world never runs out of energy, that entropy always grows as long as sun exists and lives, that human ingenuity never falters.
Conclusions, Towards a Post-Pyrocene World
Sapiens by Yuval Noah Harari is a book on the history of humans, including cultures, religion, and economic development. Graham Mann in a Summary and Notes [[
32]] sums up the book with this comment, “Seventy thousand years ago,
Homo sapiens was still an insignificant animal minding its own business in a corner of Africa. In the following millennia it transformed itself into the master of the entire planet and the terror of the ecosystem. Today it stands on the verge of becoming a god, poised to acquire not only eternal youth, but also the divine abilities of creation and destruction. Unfortunately, the Sapiens regime on earth has so far produced little that we can be proud of. We have mastered our surroundings, increased food production, built cities, established empires and created far-flung trade networks. But did we decrease the amount of suffering in the world?” [
32].
Agricultural Revolution, the discovery of fire, and the Scientific Revolution of objective knowledge had a lot to do with acquiring these divine abilities. When Mayer, Joule, and Kelvin discovered free energy and its conversion doctrine, these new discoveries added to the power of objective scientific knowledge with dynamite leading to the fossil-fuel nexus, which has given rise to unprecedented economic growth. But the Sapiens, as Sisyphus in the Myth of Sisyphus, is condemned to eternally push a boulder up a hill, only for it to roll back down each time—exposing the futility of economic growth for growth’s sake—because of his misplaced faith in the Mayer/Joule/Kelvin’s flawed second law.
In a trilogy of papers, we argue that the entropy law, the law that entropy growth is the universal driving force of all macroscopic processes, cannot be encapsulated as objective knowledge: its encapsulation entails the idea of entropic indeterminateness [
6]. We have referred to thermodynamics based on this new entropy law as Unified Classical Thermodynamics (UCT). One finding of UCT is that reversible-like processes necessitate heat reservoirs and that such a heat reservoir functions intrinsically as a reservoir of heat to be extracted while optionally as a heat sink. In this Carnot/Clausius/Gibbs entropy paradigm, the reservoir serves as a heat sink only when combustion processes are involved.
The fossil-fuel/efficiency nexus is the manifestation of the massive human power rests on the quick-sand of Mayer/Joule/Kelvin’s energy paradigm. The salvation of Homo sapiens from his Sisyphus fate is to be found in the autotrophic nexus of human-ingenuity-deployment-of-renewables/scaling-upward-ness-for-economic-growth/elimination-of-heat-disposal. Energy transition is the transition, not of energy, but of nexus.
References
- Zimmer C (July 31, 2024) “A Test for Life Versus Non-Life,” New York Times.
- Schrödinger (1944) What is Life? (Cambridge University Press).
- Wang L-S (2024). ”Unified Classical Thermodynamics: Primacy of Dissymmetry over Free Energy,”. Thermo 2024, 4, 315–345. [CrossRef]
- Harman, P M (1982). Energy, Force, and Matter: The Conceptual Development of Nineteenth-Century Physics (Cambridge Univ. Press).
- Wang L-S (2020). A Treatise of Heat and Energy (Springer Nature).
- Wang L-S (2024) “Conceptual differentiation of heat,” Qeios ID: 8692A4. Available online: https://www.qeios.com/read/8692A4.2.
- Kipnis N Thermodynamics and Mechanical Equivalent of Heat. Sci & Educ 2014, 23, 2007–2044.
- Thomson W (Lord Kelvin) (1911) Mathematical and Physical Papers of William Thomson 1:1–571. (Cambridge Univ Press).
- Adams H (1910) A Letter to American Teachers of History. pp.1-206 (Washington).
- Georgescu-Roegen N (1971) The Entropy Law and the Economic Process (Harvard Univ. Press).
- Pyne S J (2021) The Pyrocene: How We Created an Age of Fire, and What Happens Next (Univ. of Calif.
- Smith, C.W. “William Thomson and the Creation of Thermodynamics: 1840–1855. ” Arch. Hist. Exact Sci. 1977, 16, 231–288. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the equilibrium of heterogeneous substances. In The Collection of the Scientific Papers of J W Gibbs, Volume 1: Thermodynamics: 354–371; Dover Publications: New York, NY, USA, 1961. [Google Scholar]
- Daub E E “Entropy and dissipation,”. Historical Studies in the Physical Sciences 1970, 2, 321–354.
- Henry M “Thermodynamics of life,”. Substantia 2021, 5, 43–71.
- Job G and Lankau T “How harmful is the first law? Ann N Y Acad of Sci 2003, 988, 171–181. [CrossRef] [PubMed]
- Fuchs H U, D’Anna M, and Corni F “Entropy and the Experience of Heat. ” Entropy 2022, 24, 646.
- Herrmann F and Pohlig M “Which Physical Quantity Deserves the Name ‘Quantity of Heat’? ” Entropy 2021, 23, 1078. [CrossRef] [PubMed]
- Tisza L (1966; 1977 paperback edition) Generalized Thermodynamics (The MIT press).
- Clausius R. The Mechanical Theory of Heat, with Its Applications to the Steam-Engine and to the Physical Properties of Bodies. (John van Voorst: London, UK, 1867): pp. 1–374: (Fourth Memoir, 111–135; Sixth Memoir, 215–256; Ninth Memoir, 327–374).
- Carnot, S. (1960). Reflections on the Motive Power of Fire. [Reprinted from Reflections on the Motive Power of Fire and Other Papers, edited by E. Mendoza. (New York: Dover Publications (1960)].
- Xue and Guo “What Is the Real Clausius Statement of the Second Law of Thermodynamics? ” Entropy 2019, 21, 926. [CrossRef]
- Wang L-S (2022) “Clausius’ thermodynamics, a theory of motive power from disorganized energy,” Qeios ID: G43182.3.
- Poincaré, H. Science and Hypothesis; The Science Press: Lancaster, PA, USA, 1913; pp. 122–123. [Google Scholar]
- von Stockar U and Liu J-S (1999) “Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth,” Biochimica et Biophysica Acta 1999, 1412, 191–211.
- Wikipedia-Ecological pyramid - Wikipedia.
- National geographic-Energy Flow and the 10 Percent Rule (nationalgeographic.org).
- Henry M and Schwartz L (2019) “Entropy export as the driving force of evolution,”. Substantia. An International Journal of the History of Chemistry 2019, 3 (Suppl. 3), 29–58.
- Kondepudi, D. and Prigogine, I. (1998) Modern Thermodynamics: From Heat Engines to Dissipative Structures, John Wiley & Sons, New York.
- Wang L-S “Progress in Entropy Principle, as Disclosed by Nine Schools of Thermodynamics, and Its Ecological Implication,”. International Journal of Design & Nature and Ecodynamics 2021, 16, 359–372.
- Lovelock J (1988). The Ages of Gaia: A Biography of Our Living Earth (Norton & Company: New York).
- Mann G (accessed Sept. 1, 2024) “Sapiens by Yuval Noah Harari: Summary & Notes,” Sapiens by Yuval Noah Harari: Summary & Notes (grahammann.net).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).