1. Introduction
Trichophyton rubrum is the most common agent of dermatophytosis worldwide [
1], primarily causing cutaneous infections in keratin-rich structures, such as nails and skin. This fungus secretes proteolytic enzymes that degrade keratinized structures, which are metabolized and used as a nutritional source during infection [
2,
3].
T. rubrum senses and adapts to the host environment to survive and thrive during infection, responding appropriately to environmental cues and the cellular stresses they induce. To this end, the fungus has evolved diverse adaptation mechanisms, including a complex interplay between signaling molecules and stress response pathways [
4].
Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses, leading to ROS levels that exceed the antioxidant capacity required to maintain the intracellular redox environment in a reduced state, ultimately resulting in cellular damage. ROS are generated as byproducts of normal cellular metabolism in response to environmental factors. Therefore, the ability of fungi to detect environmental signals, perform robust signal transduction, and trigger accurate responses is fundamental for their survival [
5,
6].
The systems responsible for removing or detoxifying ROS include superoxide dismutase, catalase, glutathione, and thioredoxins, with glutathione being the primary antioxidant system [
6]. Additionally, glutathione is involved in iron metabolism owing to its requirement in Fe-S cluster assembly, sensing and regulation of iron levels, iron trafficking, and biosynthesis of iron cofactors [
7,
8].
However, literature on how fungi respond to oxidative stress is scarce. The prominent roles of three significant modulators have been highlighted: the glycerol high osmolarity (HOG) pathway, the yeast activation protein 1-like basic-leucine zipper (bZIP) transcription factor, and the response regulator and transcription factor SKN7 [
5,
6]. The HOG pathway, which is analogous to the p38 and Jun N-terminal kinase pathways in mammals, is highly conserved across the fungal kingdom, regardless of the species niche. This pathway plays a prominent role in adaptation to various stresses in fungal environments, including antifungals, antimicrobial peptides, and osmotic and oxidative stresses, and contributes to virulence [
5,
9]. Transcription factors can also orchestrate a robust signaling pathway by either activating or suppressing molecular responses based on the specific biological context to which the fungus is exposed [
10,
11]. The bZIP family of transcription factors plays a role in stress adaptation in fungi. Among these, the transcription factor activator protein 1 (Ap1) is a major oxidative stress response regulator and is homologous to the mammalian Jun/Fos bZIP transcription factors. Ap1 is activated via oxidation by redox-sensitive glutathione peroxidase 3 in
Saccharomyces cerevisiae or thioredoxin peroxidase 3 in
S. pombe [
5,
6]. Oxidized Ap1 accumulates in the nucleus and triggers expression of oxidative stress-related genes. Additionally, Ap1 interacts with the transcription factor SKN7 to enhance the transcription of oxidative stress-related genes [
12].
Regarding transcription factors involved in regulating oxidative stress, previous reports have demonstrated that the APSES transcription factor StuA may play a role in this response [
13,
14]. However, the mechanism by which StuA regulates the expression of oxidative stress-related genes remains unclear. Additionally, this transcription factor is multifaceted as a critical regulator of metabolism-, adhesion-, and pathogenicity-related genes [
13,
15,
16,
17].
Mining RNA sequencing data from the mutant strain Δ
stuA during growth in keratin [
13], we identified that some genes involved in oxidative stress were downregulated in the mutant strain compared with the wild-type (WT). Based on these findings, we hypothesized that StuA plays a role in the regulation of oxidative stress-related genes. Therefore, this study aimed to evaluate the transcriptional regulation of oxidative stress pathways, specific genes, and metabolic aspects, focusing on catalase and glutathione detoxification systems in WT and Δ
stuA strains during growth in keratin and under oxidative stress induced by hydrogen peroxide. Our results demonstrated, for the first time, the importance of StuA in a specific class of glutathiones, enhancing our understanding of oxidative stress response mechanisms in
T. rubrum and underscoring the pivotal role of transcription factors, such as StuA, in fungal pathogenesis.
3. Discussion
Regulation of the oxidative stress response is crucial for pathogen survival and thrive in the host. Transcription factors play an essential role in modulating the expression of genes involved in detoxifying ROS and managing oxidative stress pathways, thereby maintaining cellular redox homeostasis.
In this study, we investigated the role of the transcription factor StuA in the regulation of oxidative stress response pathways in T. rubrum. Our findings revealed, for the first time, that StuA significantly influences the expression of genes involved in oxidative stress and other related transcription factors. Several genes, including those involved in oxidative stress pathways, are identified in T. rubrum as encoding hypothetical proteins. Therefore, we conducted an orthological analysis to identify potential orthologs of these genes in T. rubrum based on other dermatophytes.
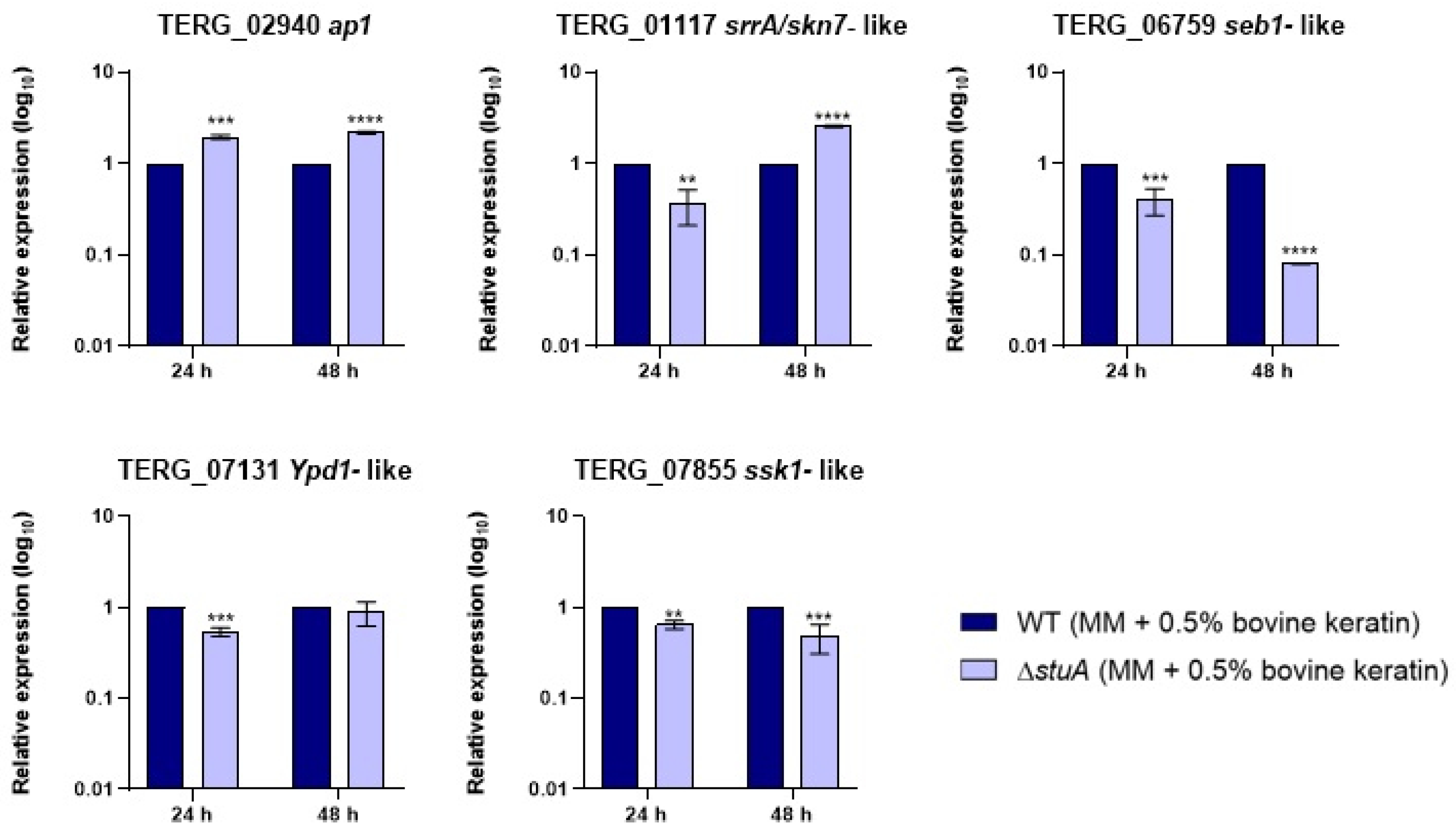
The absence of StuA substantially impaired the expression of TERG_07855, a gene that encodes the response regulator (ssk1), which is crucial for activating Hog1. The Hog1 pathway, one of the most evolutionarily conserved pathways in eukaryotes, forms the backbone of stress signaling via a two-component system-dependent mechanism. Although not present in mammals, the eukaryotic two-component system involves a multistep histidine-aspartate phosphorylation mechanism consisting of a hybrid histidine kinase and a histidine-containing phosphotransfer protein, such as TERG_07131, which encodes the
Ypd1 gene. Histidine-containing phosphotransfer mediates histidine kinase and the response regulator
ssk1, leading to a cascade of mitogen-activated protein kinase phosphorylation and subsequent Hog1 activation [
5,
18]. Our results suggest that even if
Ypd1 expression is not significantly affected by StuA deletion during fungal growth in keratin or hydrogen peroxide exposure, the cascade of mitogen-activated protein kinase phosphorylation leading to Hog1 activation may be compromised because of the lower transcript levels of
ssk1 in the mutant strain than in the WT strain under all conditions evaluated. Additionally, reduced levels of
hog1 transcripts in the
T. rubrum Δ
stuA mutant during fungal cultivation in keratin [
16] suggest that StuA may play a critical role in modulating the oxidative stress response by influencing key components of this pathway.
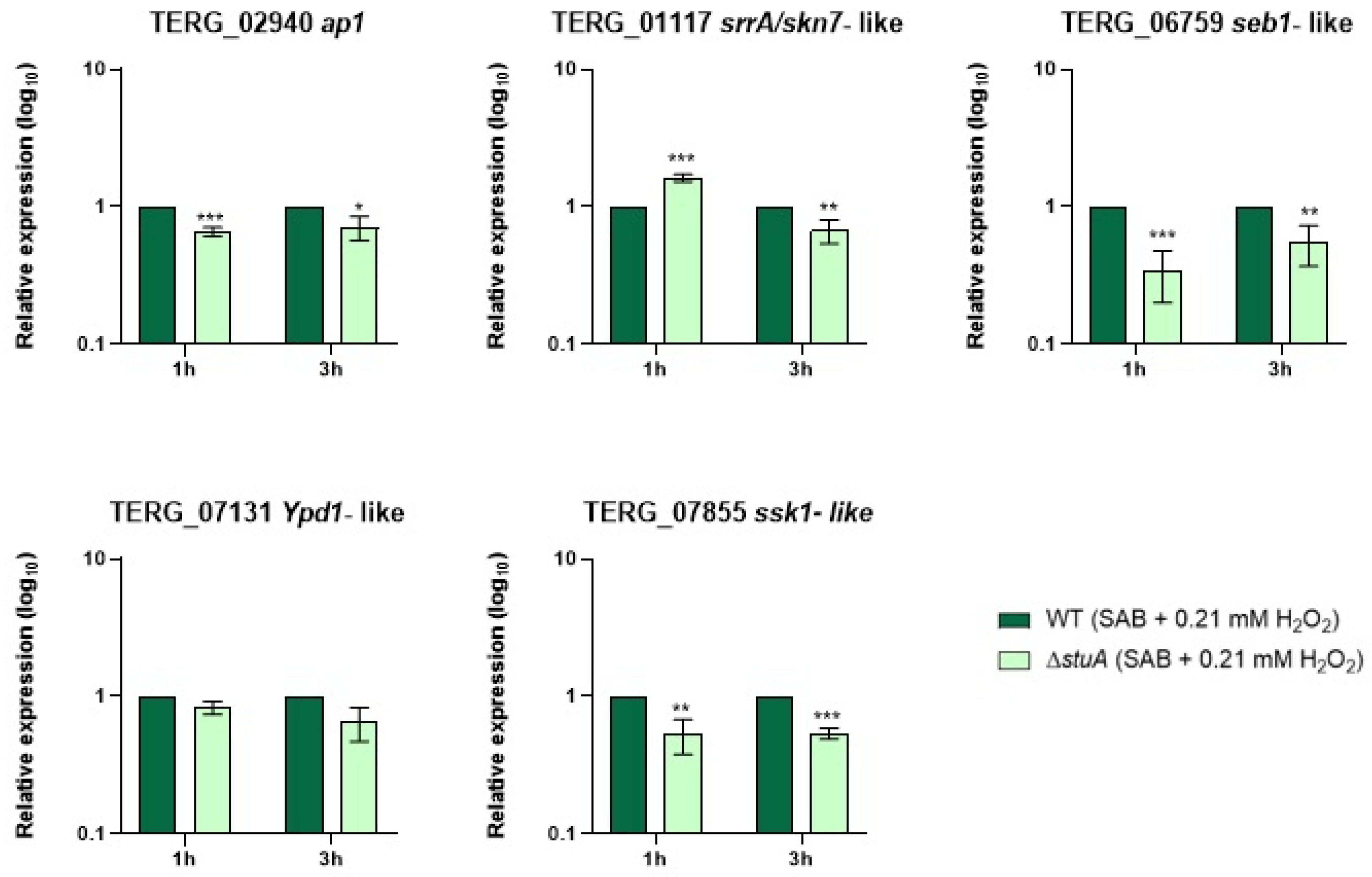
Our research reveals a complex interplay between transcription factors in response to environmental stress. We found that transcript levels of the
ap1 coding gene (TERG_02940) were downregulated in the Δ
stuA strain when exposed to oxidative stress compared with the WT strain. The opposite effect was observed during cultivation in keratin-containing medium. Ap1 can also interact with other transcription factors, such as Skn7, which is involved in the antioxidant response. Approximately half of the genes regulated by Ap1 require interaction with Skn7, whereas the other half are independent of Skn7 [
19]. The exact mechanism of Skn7 activation via phosphorylation remains unclear. However, once activated, Skn7 cooperates with oxidized Ap1 to regulate oxidative stress-related genes [
5]. In the present study, we showed that the skn7 coding gene (TERG_01117) did not show a consistent pattern of regulation by StuA, fluctuating between upregulation and downregulation across different conditions and time points.
Analysis of a Δ
ap1 strain of
T. rubrum indicated that Ap1 acts as a negative regulator of specific virulence attributes. However, deletion of
ap1 did not affect susceptibility to oxidative stress induced by hydrogen peroxide [
20]. To the best of our knowledge, there is no evidence of an interaction between Ap1 and StuA. Based on our findings, we propose that StuA acts as a positive regulator of Ap1 during oxidative stress exposure to hydrogen peroxide and a negative regulator of Ap1 during keratin decomposition.
Additionally, the gene encoding the C2H2 transcription factor Seb-1 (TERG_06759) was consistently downregulated in the Δ
stuA strain compared with the WT strain, suggesting that StuA positively regulates this transcription factor. Although not specifically a part of the oxidative stress response pathway, Seb-1 binds to the Stress Response Element under heat stress. In
Neurospora crassa, a Δ
seb1 mutant strain was more sensitive to oxidative stress during hydrogen peroxide exposure [
21], and Seb-1 is also involved in oxidative stress sensitivity in
Valsa mali [
22]. However, there is no evidence that StuA directly regulates or cooperates with Seb1 or other transcription factors. Additionally, no consensus-binding site for StuA was identified in any of the genes involved in the oxidative stress pathways evaluated in this study, suggesting that this regulation might be indirect or mediated through other transcription factors not addressed here.
Considering the obtained results regarding genes involved in oxidative stress pathways, we hypothesize that the ΔstuA mutant strain may be more susceptible to oxidative stress effects during hydrogen peroxide exposure owing to the reduced transcript levels of essential genes in the main AP1-SKN7 and HOG1 oxidative stress response pathways.
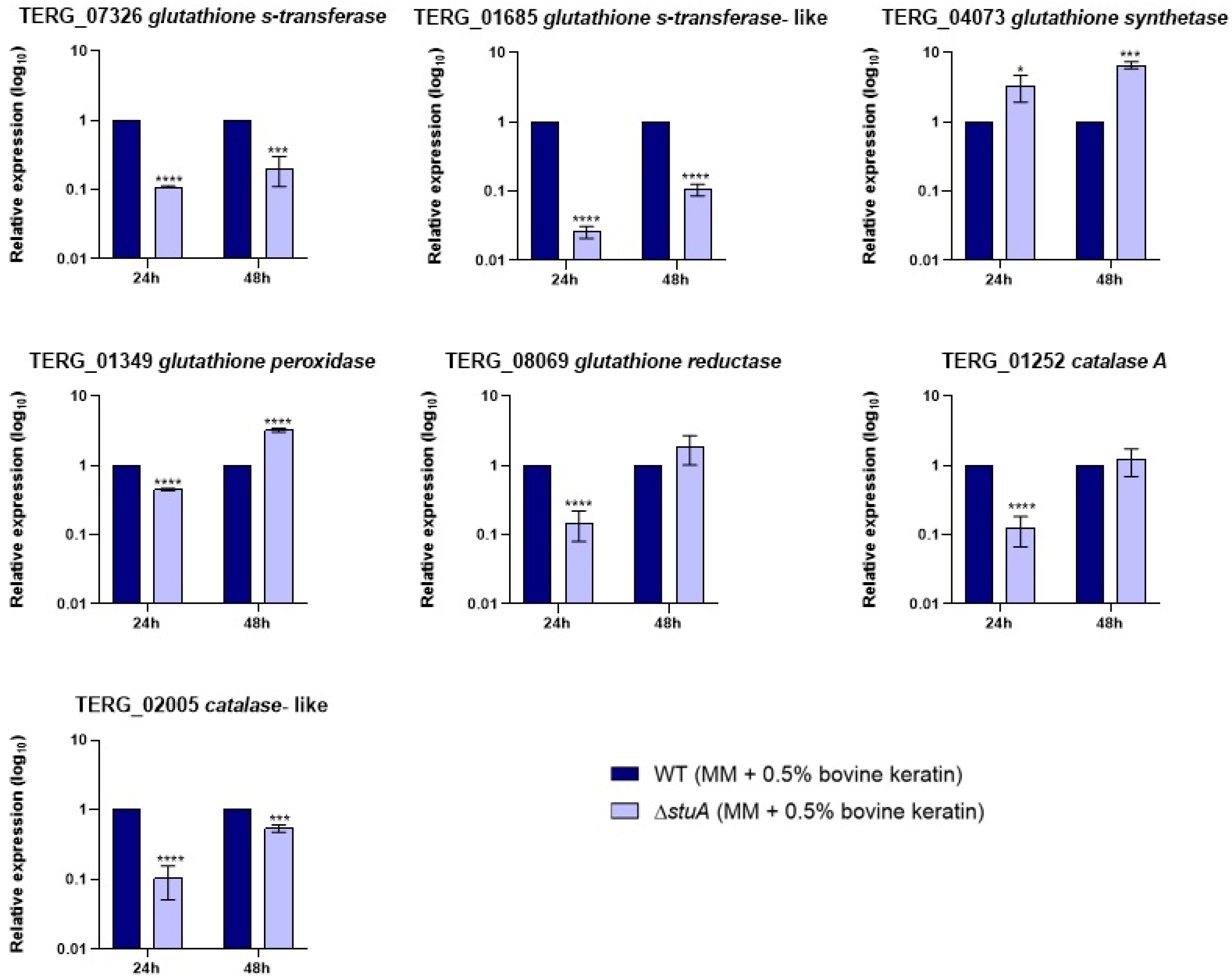
Regarding antioxidant enzyme-coding genes, we focused on evaluating the transcript levels of the glutathione family and catalases. Our results suggest that StuA can indirectly regulate the transcriptional response of TERG_02005, a catalase-like gene, as the transcript levels were lower in the mutant strain than in the WT under all conditions evaluated. In contrast, the catalase A-coding gene (TERG_01252) did not show a consistent regulation pattern, alternating between down and upregulation depending on time and culture conditions. The regulatory mechanisms of specific gene transcription factors are complex and involve several factors. Similarly, previous RNA sequencing data of the Δ
stuA strain during growth in keratin showed that catalase genes in
T. rubrum were downregulated [
13].
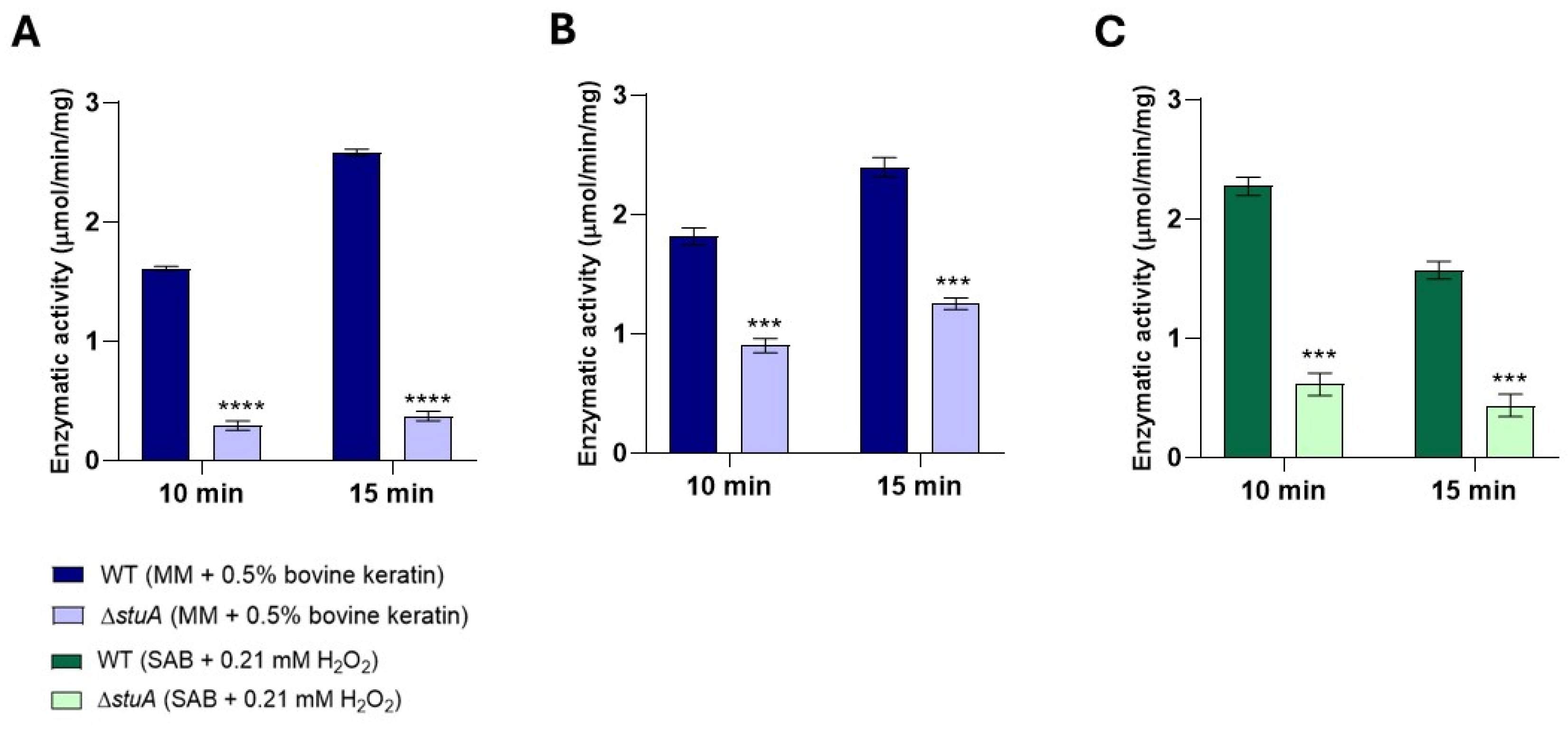
Additionally, our analysis of intracellular catalase enzymatic activity showed reduced activity in the mutant strain in protein extracts during fungal growth in keratin for 24 and 48 h and in the protein extract after fungal exposure to hydrogen peroxide for 3 h. These findings further confirm the reduced levels of transcription and enzymatic activity of catalases in mutants lacking the transcription factor StuA, which has significant implications for our understanding of the critical role of this transcription factor in regulating catalase activity and managing oxidative stress responses [
14,
16,
23].
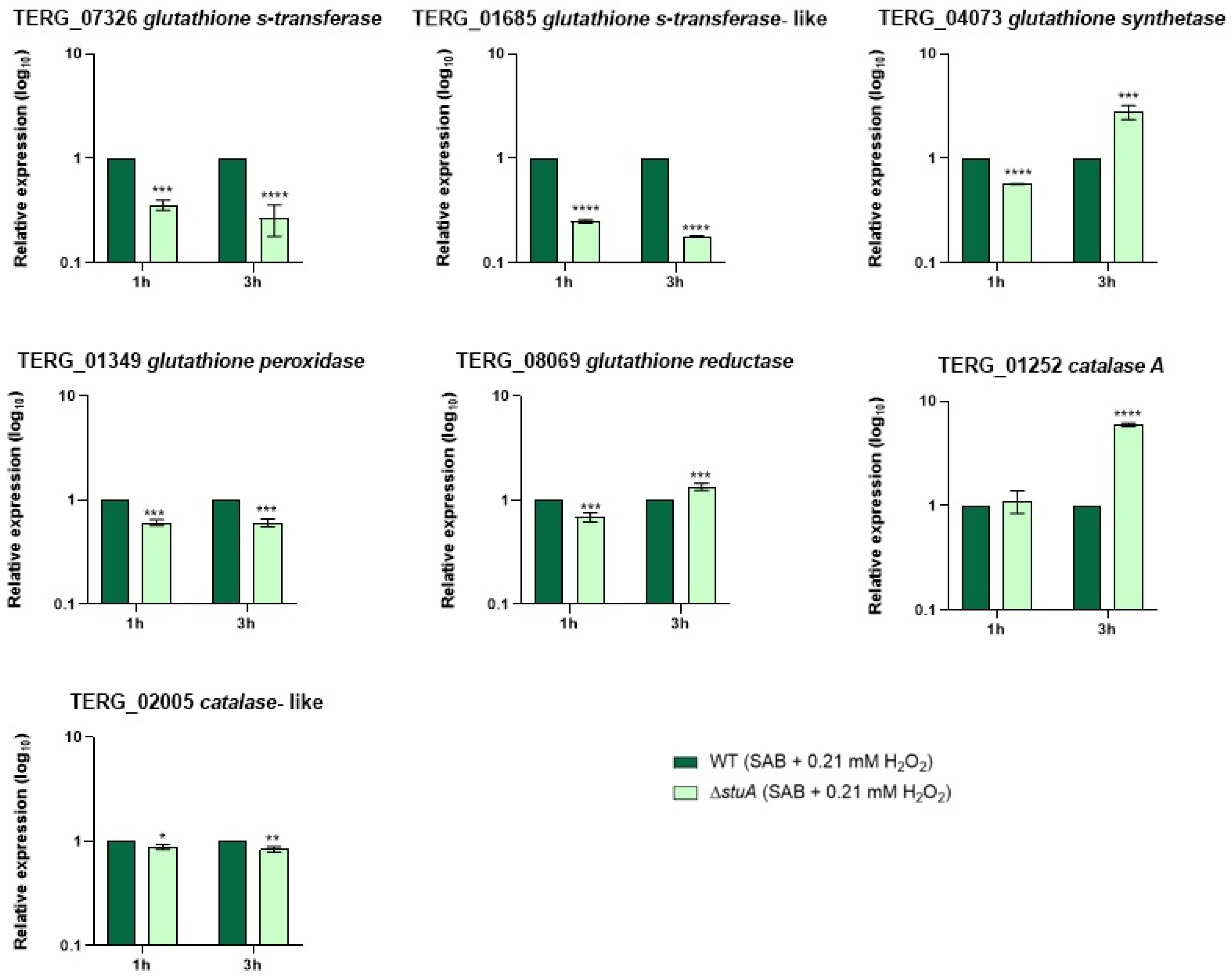
Our findings also indicate that the absence of StuA affects the transcriptional regulation of multiple glutathione-related genes. Specifically, StuA appears to act as both a positive and negative regulator of transcript levels for genes encoding glutathione synthetase (TERG_04073), which is responsible for glutathione synthesis, and glutathione reductase, which maintains reduced glutathione levels in cells for cellular homeostasis, depending on time and culture conditions. The absence of StuA diminished the transcription of the glutathione peroxidase gene (TERG_01349) in the presence of hydrogen peroxide, suggesting that the ability to reduce peroxide in cells could be compromised in the mutant strain.
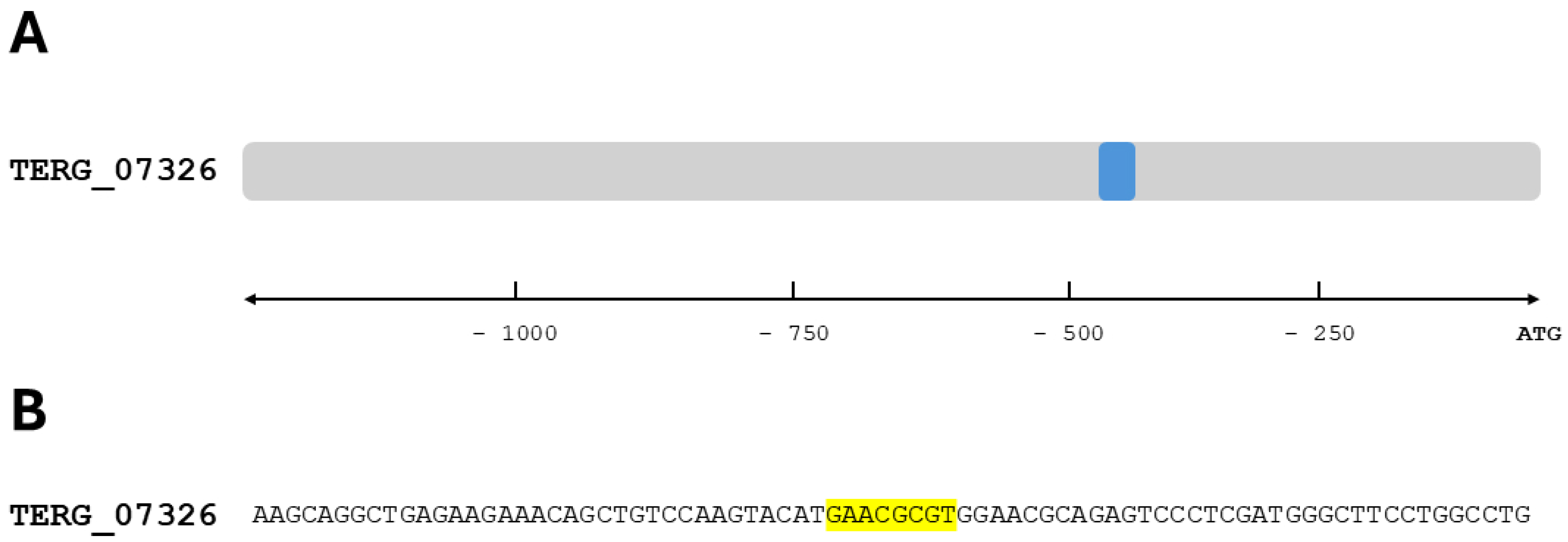
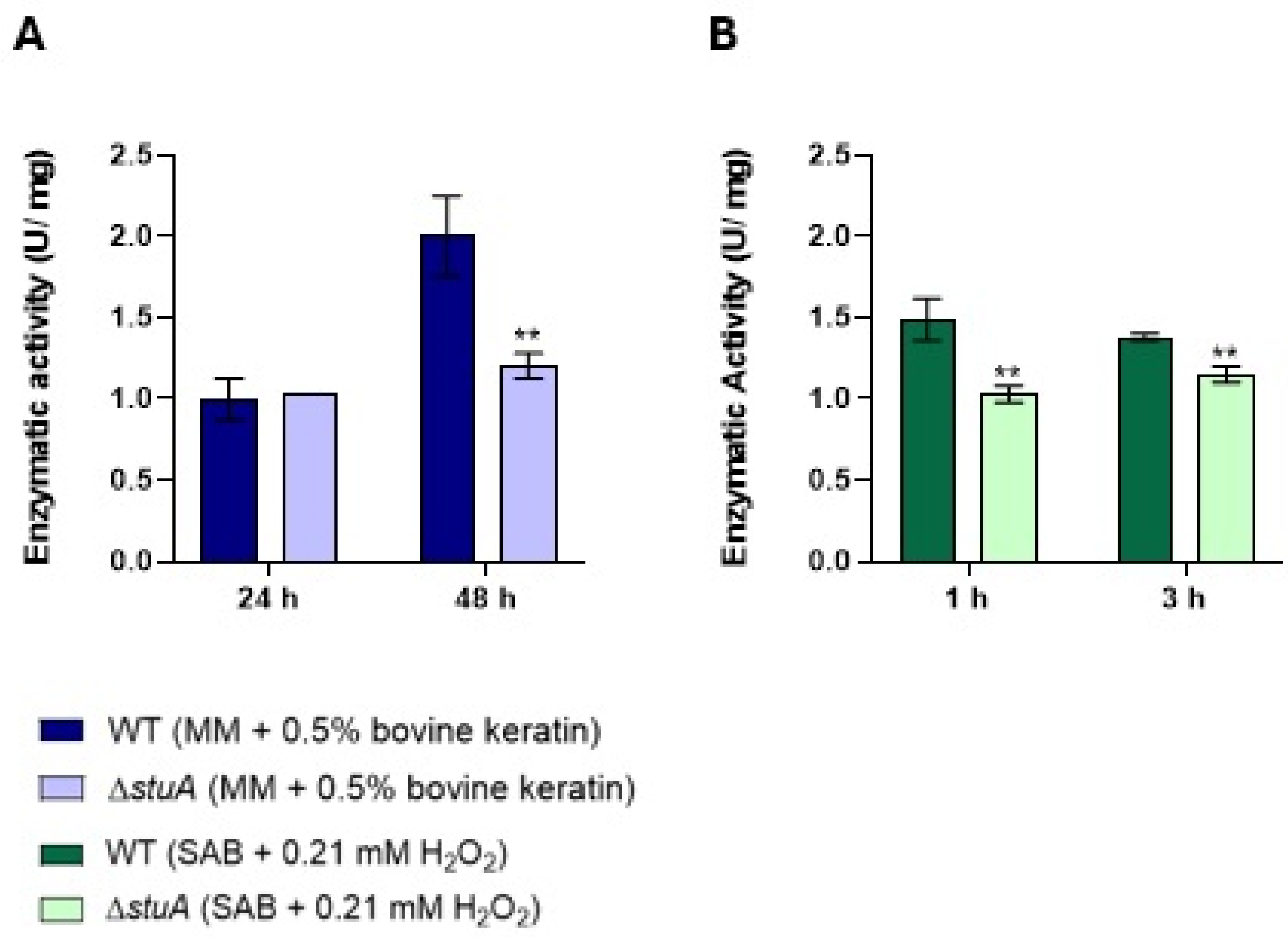
Our study showed that the absence of StuA significantly affected a specific class within the glutathione family. This led to a downregulation of both glutathione S-transferase coding genes (TERG_07326 and TERG_01685) in the ΔstuA strain compared with the WT strain across all conditions evaluated in this study. Furthermore, we identified a consensus StuA-binding site in TERG_07326, suggesting direct regulation by this transcription factor. Analysis of the enzymatic activity of this class of enzymes also revealed diminished activity in the mutant strain.
Glutathione S-transferases (GSTs) are multifunctional enzymes primarily involved in detoxification. They catalyze the conjugation of glutathione to various toxic compounds, facilitating their excretion from the cells. Additionally, GSTs are involved in cell signaling, regulation of apoptosis, and resistance to chemotherapeutic drugs [
24]. Accordingly, mutants with deletions in the GST gene of various fungi show increased sensitivity to xenobiotics and different stressors [
8]. For instance, in
S. pombe,
gst1Δ,
gst2Δ, and
gst3Δ mutants were sensitive to the antifungal fluconazole, indicating the role of GSTs in mediating drug resistance [
8]. In
S. cerevisiae,
gtt1Δ
gtt2Δ, and
gtt1Δ
gtt2Δ mutants displayed increased sensitivity to heat shock or growth defects at high temperatures. In
Aspergillus nidulans, the
gstAΔ mutant was sensitive to heavy metals [
8,
25]. To the best of our knowledge, no previous studies have investigated the role of StuA in regulating glutathione family genes. However, it is plausible to hypothesize that a deficiency in the conjugation and excretion of toxic compounds in the glutathione-mediated detoxification system may occur in the mutant strain, which could increase its sensitivity to toxic compounds and oxidative stress.
Deficiency of the glutathione-mediated detoxification system leads to an imbalance in iron metabolism. Abnormal iron metabolism causes iron overload, leading to the Fenton reaction and increasing the generation of ROS [
7,
26,
27]. Iron acquisition is essential during the infectious process of dermatophytosis because this metal is vital for many cellular functions, including respiration and energy production. Iron plays crucial roles in the survival and virulence of pathogens during dermatophyte infections. Dermatophytes such as
T. rubrum need to acquire iron from the environment to sustain their growth and pathogenicity, mainly when infecting keratinized tissues such as the skin and nails [
28,
29].
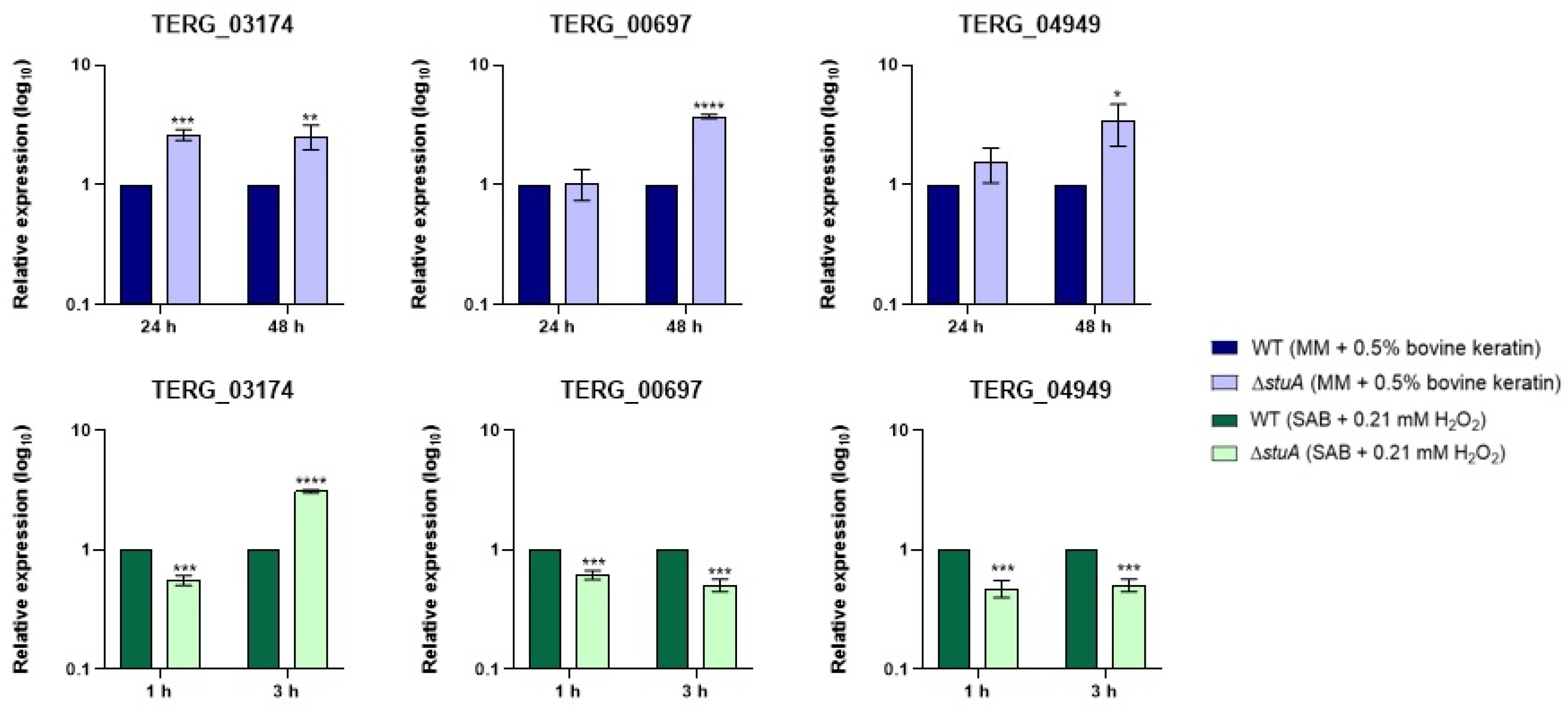
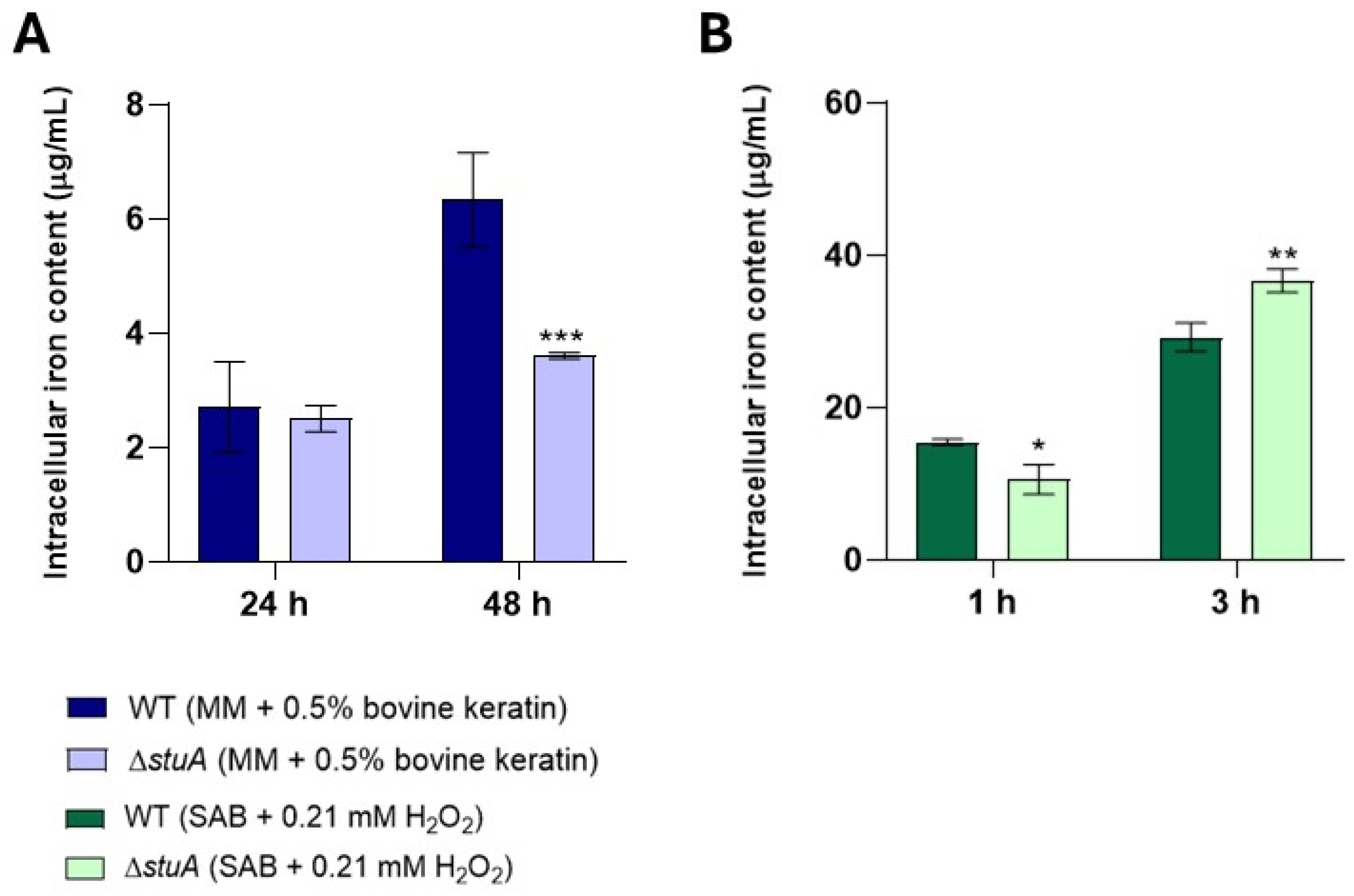
Our results showed that genes encoding siderochrome iron transporters and a nonribosomal siderophore peptide synthase, which are essential for iron acquisition, transport, and metabolism, were upregulated in the mutant strain during fungal growth in keratin compared with the WT strain. The WT strain presented higher intracellular iron content under the same growth conditions. It is possible to hypothesize that during growth in keratin, the absence of StuA may lead to the upregulation of genes involved in iron metabolism as a compensatory strategy to enhance the uptake of this essential metabolite and mitigate the harmful effects of the mutation. However, despite this upregulation, there was no accumulation of intracellular iron, suggesting that iron transport and distribution within mutant cells may also be compromised.
During exposure to hydrogen peroxide, we observed that most genes involved in iron metabolism were downregulated in the mutant strain compared with those in the WT strain. However, the intracellular iron levels of the mutant strain increased from 3 h onwards, which may indicate that despite the lower transcript levels of iron metabolism genes in the mutant strain, there was an increase in intracellular iron as a strategy to enhance the efficiency of its antioxidants, as iron can act as a cofactor for catalases [
30] which was shown to be impaired in our results. Alternatively, it could also indicate increased cellular intoxication due to a deficiency in GSTs or other components of the glutathione system, potentially leading to significant cellular toxicity.
Figure 1.
Relative expression analysis of genes involved in oxidative stress pathways during fungal growth in keratin (minimal medium (MM) containing 0.5% of bovine keratin) for 24 and 48 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 1.
Relative expression analysis of genes involved in oxidative stress pathways during fungal growth in keratin (minimal medium (MM) containing 0.5% of bovine keratin) for 24 and 48 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 2.
Relative expression analysis of genes involved in oxidative stress pathways during fungal exposure to oxidative stress induction with hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM of hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Figure 2.
Relative expression analysis of genes involved in oxidative stress pathways during fungal exposure to oxidative stress induction with hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM of hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Figure 3.
Relative expression analysis of genes encoding the glutathione family and catalases during fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 and 48 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. *** p < 0.001, and **** p < 0.0001.
Figure 3.
Relative expression analysis of genes encoding the glutathione family and catalases during fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 and 48 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. *** p < 0.001, and **** p < 0.0001.
Figure 4.
Relative expression analysis of genes encoding the glutathione family and catalases during fungal exposure to oxidative stress induction with hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 4.
Relative expression analysis of genes encoding the glutathione family and catalases during fungal exposure to oxidative stress induction with hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 5.
Consensus sequence position for StuA in the promoter region of the gene TERG_07326. The consensus-binding sites were determined by identifying DNA sequences upstream (1,000 bp) that matched the StuA 5’-[A/T]CGCG[T/A]N[A/C]-3’ DNA-binding consensus. (A) Representation of the disposition of the cis-regulatory elements found in the sense strand (blue box). (B) StuA-interaction consensus sequence flanked by 35 adjacent base pairs. Yellow boxes depict the StuA consensus sequence found in the gene promoter.
Figure 5.
Consensus sequence position for StuA in the promoter region of the gene TERG_07326. The consensus-binding sites were determined by identifying DNA sequences upstream (1,000 bp) that matched the StuA 5’-[A/T]CGCG[T/A]N[A/C]-3’ DNA-binding consensus. (A) Representation of the disposition of the cis-regulatory elements found in the sense strand (blue box). (B) StuA-interaction consensus sequence flanked by 35 adjacent base pairs. Yellow boxes depict the StuA consensus sequence found in the gene promoter.
Figure 6.
Intracellular catalase enzymatic activity in the ΔstuA mutant and WT strains. Catalase activity was measured in protein extracts from fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 h (A) and 48 h (B), and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 3 h (C). The activity was assessed at 10- and 15-minute intervals. The statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. *** p < 0.001, and **** p < 0.0001.
Figure 6.
Intracellular catalase enzymatic activity in the ΔstuA mutant and WT strains. Catalase activity was measured in protein extracts from fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 h (A) and 48 h (B), and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 3 h (C). The activity was assessed at 10- and 15-minute intervals. The statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. *** p < 0.001, and **** p < 0.0001.
Figure 7.
Intracellular glutathione S-transferase activity was measured in the ΔstuA mutant and WT strains. The activity was assessed in protein extracts from fungal cultivation in keratin (minimal medium (MM) containing 0.5% bovine keratin) at 24 and 48 h (A) and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) at 1 and 3 h (B). The enzyme activity was measured at 5-minute intervals. The statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. ** p < 0.01.
Figure 7.
Intracellular glutathione S-transferase activity was measured in the ΔstuA mutant and WT strains. The activity was assessed in protein extracts from fungal cultivation in keratin (minimal medium (MM) containing 0.5% bovine keratin) at 24 and 48 h (A) and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) at 1 and 3 h (B). The enzyme activity was measured at 5-minute intervals. The statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. ** p < 0.01.
Figure 8.
Relative expression analysis of genes encoding siderochrome iron transporters (TERG_03174 and TERG_04949) and nonribosomal siderophore peptide synthase (TERG_00697) during fungal growth in keratin (minimal medium (MM) with 0.5% bovine keratin) for 24 and 48 h and during exposure to oxidative stress induced by hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 8.
Relative expression analysis of genes encoding siderochrome iron transporters (TERG_03174 and TERG_04949) and nonribosomal siderophore peptide synthase (TERG_00697) during fungal growth in keratin (minimal medium (MM) with 0.5% bovine keratin) for 24 and 48 h and during exposure to oxidative stress induced by hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h. The WT strain was used as the normalizing condition. Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 9.
Intracellular iron levels in the ΔstuA mutant and WT strains. Intracellular iron levels were measured in protein extracts from fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 and 48 h (A) and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h (B). Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Figure 9.
Intracellular iron levels in the ΔstuA mutant and WT strains. Intracellular iron levels were measured in protein extracts from fungal growth in keratin (minimal medium (MM) containing 0.5% bovine keratin) for 24 and 48 h (A) and from fungal exposure to hydrogen peroxide (Sabouraud medium (SB) with 0.21 mM hydrogen peroxide (H2O2)) for 1 and 3 h (B). Statistical significance was determined using an unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Table 1.
Oxidative stress-related genes with homologs in various Trichophyton species.
Table 1.
Oxidative stress-related genes with homologs in various Trichophyton species.
| Gene ID |
Gene Product Name |
Homologous |
| TERG_01117 |
Stress response transcription factor SrrA/Skn7 |
ARB_07479 (Trichophyton benhamiae) |
| TERG_06759 |
C2H2 transcription factor (Seb1) |
ARB_00366 (Trichophyton benhamiae) |
| TERG_07131 |
Phosphotransmitter protein Ypd1 |
TEQG_03843 (Trichophyton equinum) |
| TERG_07855 |
Response regulator ssk1 |
TRV_02413 (Trichophyton verrucosum) |
| TERG_01685 |
Glutathione S-transferase |
ARB_02229 (Trichophyton benhamiae)
|
| TERG_02005 |
Catalase |
TESG_07337 (Trichophyton tonsurans) |