1. Introduction
SARS-COV-2 has been associated with increased morbidity and mortality in cancer patients [
1,
2], indicating the need for a prompt preventive intervention in this fragile population. We conducted an observational study looking for predictors of poor antibody response to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients, including psychological factors. In our initial study focused on clinical predictors of antibody response, we showed that chemotherapy, targeted therapy, hormone therapy, lymphocyte count< 1x10
9/L, and increasing age predicted poor seroconversion (vaccine failure) after two doses of BNT162b2 in up to 20% of patients [
3]. While these clinical factors are obviously important, the psychological and behavioral characteristics of vaccine recipients also matter. There is evidence that psychosocial factors influence antibody response to immunity and vaccination, including covid-19 [
4]. Evidence also indicates the influence of psychosocial factors on immunity [
5] and more specifically the antibody response to vaccination [
6,
7]. In addition, psychosocial factors such as social cohesion have been shown to be relevant to COVID- 19 vaccine antibody response [
8]. However, the effect of psychological factors on antibody response to COVID-19 vaccine in an immunocompromised population of cancer patients under active treatment is unknown.
In the present study, we determined the influence of psychological distress, anxiety and depressive symptoms on immunogenicity of vaccines in cancer patients undergoing different treatments through evaluation of rates of anti-spike immunoglobulin G (IgG) antibody positivity following vaccination with the BNT162b2 SARS-COV-2. Specifically, our prospective cohort study aimed at assessing which psychological disorders predict a poor seroconversion (a proxy of lack of vaccine efficacy, [
3]) and markers of vaccine activation such as D-dimer [
9,
10]in this frail population. D-dimer is a fibrin degradation product which is a prognostic factor for worse outcomes in COVID-19 patients [
9] and a predictor of vaccine-induced thrombocytopenia and thrombosis [
10], suggesting its elevation is a marker of activation of coagulation cascade following COVID-19 vaccine. . Moreover, since women tend to stabilize their distress during follow-up, whereas men tend to worsen, especially because of physical and emotional problems [
11], we aimed at assessing if there was a different sex related distress response to vaccination.
2. Materials and Methods
2.1. Study Design and Participants
The main study and subject characteristics have previously been shown [
3]. Briefly, we conducted a prospective, observational cohort study to assess the antibody titer reactogenicity to BNT162b2 SARS-COV-2 vaccine (Pfizer-BioNTech) in cancer patients on active treatment. Inclusion criteria were patients with malignancy aged ≥ 18 years, cancer treatment ongoing or ended within the last 6 months and lymphocytes count ≥ 0.5x10
9/L (500/uL) based on risk of infections in subjects on chronic immunosuppressive therapy with lymphopenia< 0.6x10
9/L [
12]. We categorised patients based on last systemic treatment (Chemotherapy, Hormone therapy, biological therapy and Immunotherapy). Patients with last treatment ≥180 days before the vaccine administration were considered as untreated.
Participants underwent a clinical visit and blood sample collection: 1) at baseline before the first vaccine dose [visit 1], 2) 21 days after the first vaccine dose [visit 2], 3) 42 days after visit 1 [visit 3], and 4) 6 months after visit 1 [visit 4]. The trial was registered at ClinicalTrials.gov ID: NCT04932863 and approved by the National Institute for Infectious Diseases, Rome, and the local Ethical Committee. Participants were recruited at Galliera Hospital, Genoa, from March 15 to July 21, 2021, and all signed an informed consent.
2.2. Procedures
Vaccine treatment consisted of 30 μg of BNT162b2 (0.3 ml volume per dose) delivered in the deltoid muscle in 2 doses, 21 days apart. We pooled treatments in five groups to facilitate comparisons: active surveillance (no treatment), chemotherapy, hormone therapy, targeted therapy/monoclonal antibodies and immune checkpoint inhibitors.
At the beginning of the clinical visit, the distress thermometer in the previous week was compiled as previously described [
13]. The National Comprehensive Cancer Network indicates that all cancer survivors should undergo a distress measurement as the sixth vital parameter to prevent more serious psychological disorders, including anxiety, depressive symptoms, and coping disturbances [
14]. The instrument is a self-reported tool using a single-item tool using a 0 (no distress) to 10 (extreme distress)– point Likert scale resembling a thermometer. Additionally, the patient is prompted to identify sources of distress using a 39-item supplemental list of potential sources of distress, including the following domains: emotional, physical, practical, family, and spiritual/religious problems. DT scores are categorized into two levels, 0–4, low, 5-10, high, according to the National Comprehensive Cancer Network 2013 [
15]. The DT has demonstrated adequate reliability and has been translated and validated into numerous languages, including Italian [
16]. Anxiety and depressive symptoms were evaluated using the Hospital Anxiety and Depression Scale [
17]. The scoring system for both anxiety and depressive symptoms was 0-7 = Normal, 8-10 = Borderline elevated, 11-21 = Elevated. The antibody titer was initially measured by the LIAISON® SARS-CoV-2 S1/S2 IgG, a chemiluminescent immunoassay (CLIA) for the quantitative detection of IgG antibodies against the S1/S2 dimeric domains of the SARS-CoV-2 spike protein in the human serum [
18,
19]. The analyzer calculates SARS-CoV-2 S1/S2 IgG antibody concentrations as arbitrary units (AU/mL; assay range 3.8-400 AU/ml) and grades the results. The threshold of seroconversion was ≥25 AU/mL according to our lab procedures due to previous preliminary study of correlation between level of antibodies and concomitant T cell response that further proved immunization.
During the study, a more sensitive LIAISON® SARS-CoV-2 Trimeric Spike IgG assay expressed in BAU/mL [
20] became available and was introduced at 6 months and compared with the dimeric method.
Since there was no significant difference on the proportion of non-responders at 6 months between the dimeric and trimeric detection methods (<25 AU/mL and 33.8 BAU/mL, respectively, Table 3), results of the primary endpoint at 6 months were calculated with the cut-off of the more sensitive trimeric method [
20].
D-dimer was measured with an automated, latex enhanced turbidimetric immunoassay [HemosIL® D-Dimer HS 500, Instrumentation Laboratory (IL), as previously described [
21]. The primary objective was to assess if psychological factors including distress, anxiety and depressive symptoms were associated with poor antibody titer reactogenicity (cut-off levels <25 AU/mL or <33.8 BAU/mL) to BNT162b2 vaccine at 6 months (primary endpoint). Secondary endpoints were the repeated measure analysis of antibodies measured with the dimeric method at 6 months and the effect of psychological variables on biomarkers of vaccine response such as D-dimer.
2.3. Statistical Analysis
Median and interquartile range (IQR) for continuous variables and absolute and relative frequencies as summary measures of categorical variables were calculated. Fishers-Exact tests, Wilcoxon Rank tests or the Kruskal-Wallis rank sum test were performed based on the nature of the variables.
Multivariable logistic was applied to identify independent factors associated with vaccine failure at 6 months. Multivariable mixed effects models for repeated measures analysis were adopted both to analyze changes in time of IgG response, D-dimer, and DT and to identify independent factors associated with these outcomes. Normal distribution of residuals from fully adjusted models were graphically checked and log transformation was adopted when it was needed to achieve normality. Odds ratio (OR) and percentages of IgG non-responders are presented with 95% confidence intervals.
Distress was categorized as ‘High’ or “Low” considering 5 as cut-off point. Bar plots were presented to describe the percentages of responders by type of distress. Independent variables were included in the model collapsing the categories when the frequencies were too low (e.g: stage IV vs I-III) and categorizing continuous variables such as age, considering the median value, to have more clinically interpretable estimates.
Boxplot were generated to compare patient characteristics in terms of change of outcomes in time from baseline to 6 weeks and 6 months.
All analyses were carried considering two cut-off points for responders at 33.8 BAU/mL and <25 AU/mL. All p-values were two-sided with 5% significance level. Analyses were carried out using the R studio (R version 4.2.3) software.
3. Results
From March 15, 2021, to July 21, 2021, 407 patients were screened for vaccination and offered to participate in the study of whom 320 agreed to participate, 290 were assessable at 42 days and 218 at 6 months, as shown in the CONSORT statement in
Supplemental Figure S1.
The main subject and tumor characteristics of the 218 patients are summarized in
Table 1. Median age was 68.2 years, approximately 60% were females and had metastatic disease, over 20% were treated >6 months ago, one third were on (neo)adjuvant treatment and two thirds were on 1
st-3
rd line of treatment. Overall, 31 subjects (14.2%) had no antibody response at 6 months. The predictors of vaccine failure at 6 months are summarized in
Table 2. In addition to metastatic disease stage, the only independent significant variable was high distress (OR=2.46, 95% CI, 1.05-5.77, p=0.04,
Table 2). The proportion of non-responders was similar between the dimeric and the most sensitive trimeric assay method
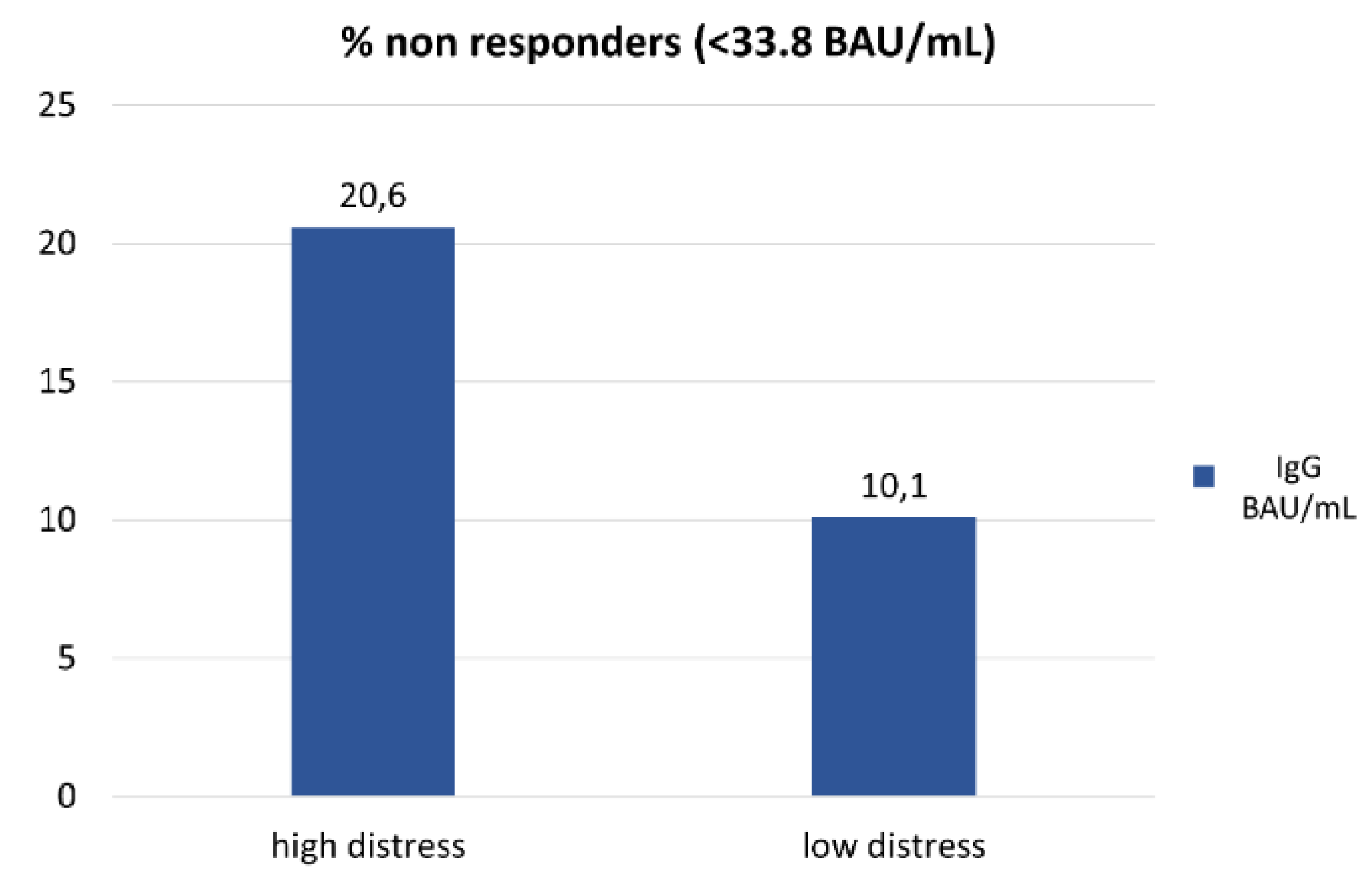
(Supplemental Table S1). Among non-responders, the proportion of subjects with high distress versus low distress was twofold higher (21% vs 10%, respectively, p=0.04,
Figure 1).
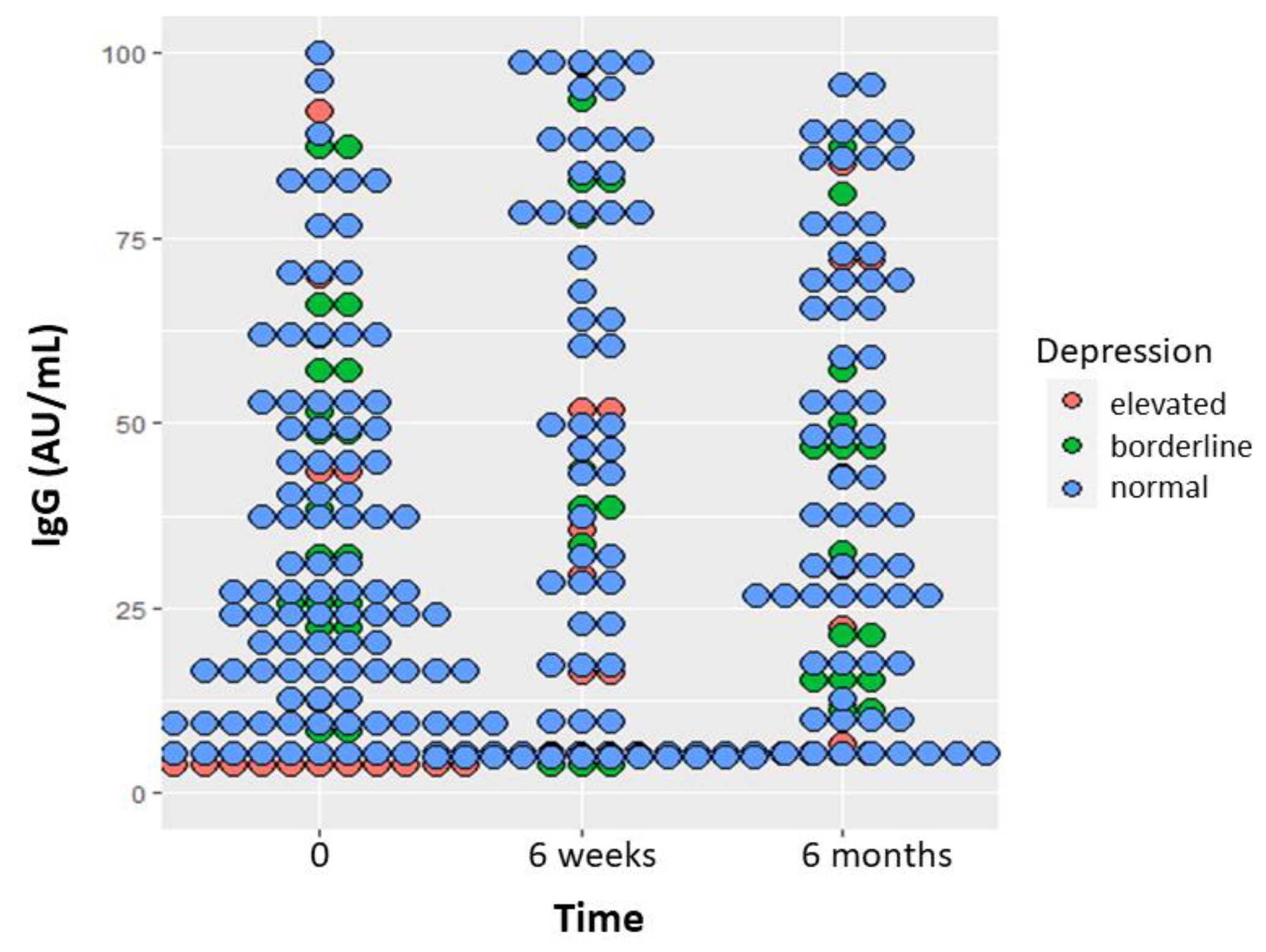
Repeated measure analysis of IgG response dotplot measured with the dimeric method at 0, 6 weeks and 6 months indicates that participants with high depressive symptoms level at baseline had lower antibody response during the 6-month time (p=0.003,
Figure 2). Current and former smokers had lower antibody levels than never smokers (p=0.0004, data not shown). Patients with age above the median of 68.2 years also had lower antibody levels (p<0.0001, data not shown). In addition, women with elevated anxiety level at baseline had lower D-dimer levels at 6 months (p=0.03, Figure 3). The proportion of high distress remained stable during the 6-month observation period: 25.9% at baseline, 24.8% at 6 weeks and 25.0% at 6 months. At baseline, women with high distress were 34.4% versus 23.8 in men (p=0.08). The median level of distress in women was 2 at baseline and did not change in the 6 months observation period, whereas it increased from 1 to 2 in men
(Supplemental Figure S2). Repeated measure analysis showed that high distress at baseline (p=0.0001) and higher education (p=0.04) explained high distress at 6 months (
Supplemental Figure.3), but this effect was limited to women only (p=0.046).
As a sensitivity analysis we also assessed COVID antibodies as a continuous variable, but we did not find any significant association with distress (P=0.93 and 0.27 with AU and BAU, respectively). We tried also to assess the association of smoking with response to vaccine including the variable in the model, but it was not significant and results did not change. The adjustment for baseline level of antibody did not change the results either (data not shown).
No significant associations between high and low psychological symptoms and cancer sites were found
(Supplemental Table S2).
4. Discussion
Cancer patients develop severe COVID-19 with higher mortality [
1,
2] and were therefore considered a highly frail population requiring priority in accessing vaccination. The immunogenicity of cancer patients is also lower than the general population after contracting SARS-CoV-2 infection [
22] so that the ability to develop a response to the vaccine was also expected to be different in cancer patients compared to healthy subjects, who attained a 95% efficacy with BNT162b2 vaccine [
23]. Our initial report showed that chemotherapy, targeted therapy/monoclonal antibodies, hormone therapy, lymphocyte count<1×10
9/L, and increasing age predicted poor seroconversion at 6 weeks after two doses of BNT162b2 in up to 20% of patients [
3], indicating the need for further vaccine dose and long-term follow-up.
The present study was designed to determine if psychosocial factors predicted failure to BNT162b2 vaccine at 6 months in a cohort of patients under treatment for solid cancers to guide better strategies to increase vaccine efficacy in non-responders. Our main finding indicates that a high level of psychological distress is associated with a 20% failure rate to COVID-19 vaccine versus 10% in the remaining patients with low distress, a statistically significant difference.
Our findings also indicate that patients with depressive symptoms have a lower D-dimer response to vaccination. Plasma D-dimer elevation is associated with worse outcomes in COVID-19 patients [
9,
24] and is a marker of activation of coagulation cascade following COVID-19 vaccine. Patients with depressive symptoms have therefore a lower response to vaccination.
The concept of psychological stress has been studied for a long time in the field of psycho-oncology for its repercussions not only on the quality of life of the cancer patients but also on their response to treatment and side effects. However, it is important to consider its connection with the affective experiences and the emotional discomfort that are expressed in these stressful events. Several studies highlight the influence of emotional aspects on the functioning of the immune system and consequently the antibody response to vaccines, including the Covid-19 vaccine. For example, it has been observed that the lack of social cohesion, associated with a sense of loneliness has negative effects on immunity [
8,
25,
26]. A meta-analysis revealed a significant negative association between psychological stress and antibody responses to influenza vaccination [
5]. Moreover, prior research suggests that psychological and behavioral interventions can improve vaccine responsiveness, including Covid-19 vaccine [
4]. Individual with depressive symptoms have been shown to have a lower antibody response to COVID-19 vaccination [
27], and patients with mental disorders including major depressive symptoms have lower response to vaccination in general [
28]. In our study, the association between high distress and vaccine failure, high anxiety and decreased D-dimer, elevated depressive symptoms and lower antibody response can represent and offer an objective and biological measurement of what is happening at an organic level in situations of psychological fragility and vulnerability. Since the antibody response or the increase of biomarkers of vaccine activation is lower in people who have high distress, high anxiety and high depressive symptoms, our findings do strengthen the relationship between mind and immune system. In some studies, it is observed that a positive mental attitude in dealing with the disease has a function of 'modulator of the immune system' and is associated with a greater immune response [
29]. In the same way, a healthy lifestyle is beneficial for health and therefore favors a better functioning of the immune system [
30,
31]. These mechanisms describe a general psychic and organic attitude towards life. Conversely, high psychological stress or depressive symptoms related to an inhibition of the functioning of the immune system could be interpreted as a slowdown of psychic functions in line with organic functions.
We have seen how stress, depressive symptoms and anxiety are closely connected to the sphere of affections and how these have a strong impact on the immune system's antibody response capacity and on the positive activation of the organism in general. Taking care of cancer patients cannot therefore be done without taking care of his emotional dimension by offering a containment of the most distressing emotions to help them reorganize his hope and turn towards life-oriented attitudes, including a better vaccine efficacy.
Our findings suggest no significant change of psychological distress over the 6-month vaccine exposure, at variance with prior studies suggesting an amelioration of distress after Covid-19 vaccine in the general population [
32], possibly because in our population advanced stage disease is a much higher distress source than Covid-19. At variance with a previous study [
12], however, we did not find a significant interaction between sex and time of distress level 6 months apart, but only a small trend to a worsening in males. This may be related to a shorter time exposure compared with our prior observation 16 months apart [
12]. Interestingly, women with a high educational level were those at higher risk of elevated distress after 6 months, consistent with the finding of a large cohort where highly educated persons are at higher risk of distress than those with medium/low educational level after adjustment for confounders [
33]. Finally, our study indicates that current and former smokers have lower antibody titers than never smokers after vaccine, in line with the immunosuppressive effect of smoking [
34,
35]. We previously showed that current and former smokers, who are at higher risk for severe SARS-CoV-2 infection, are protected from developing adverse events following immunization [
3]. We did not observe an association between anxiety and adverse events as in a recent study [
36].
An important limitation of this study is related to the small sample size that did not allow us to present analyses stratified by type of cancer and type of treatment.
5. Conclusions
In conclusion, our study provides compelling evidence that elevated levels of psychological distress, along with anxiety and depressive symptoms, are significantly correlated with reduced efficacy of the COVID-19 vaccine in a cohort of cancer patients undergoing active treatment. These findings reinforce the intricate interplay between psychological well-being and immune function in immunocompromised populations. The observed association highlights the critical importance of tailored psychological support interventions for this vulnerable group, with the potential to enhance vaccine responsiveness and overall health outcomes. This underscores the necessity for a holistic approach to patient care, integrating mental health resources to optimize immunization strategies and improve resilience against infectious diseases.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Supplemental Figure S1. Participant flow diagram
; Supplemental Table S1. Comparison of vaccine non-responders between assay methods;
Supplemental Figure S2. Pre and post vaccination level of distress according to sex;
Supplemental Figure S3. Dotplot of repeated measure analysis of distress according to educational level;
Supplemental Table S2. Univariable analyses describing the association between high and low psychological symptoms and cancer sites.
Author Contributions
Conceptualization, GR, ADC, TBW, IMB; methodology, ADC, SG, AC, MO, OD; validation, ADC, MM, IMB; formal analysis, SG, OD, AC, MO; investigation, TBW, NP, MB, CD, MC, AL, MD; resources, LI, FF; data curation, OD, SG; writing—original draft preparation, ADC, TBW, GR, EM; writing—review and editing, IMB, NP, MM; visualization, ADC; supervision, IMB; project administration, MM; funding acquisition, ADC. All authors have read and agreed to the published version of the manuscript.”.
Funding
This study is supported by E.O. Ospedali Galliera, Genoa, Italy, Associazione WeCare and Lions Club Genoa Sant’Agata, Italy. The European Institute of Oncology, Milan, Italy is partially supported by the Italian Ministry of Health with Ricerca Corrente and 5×1,000 funds. Further support was from a grant by the European Union´s Horizon Europe Research and Innovation Programme under Grant Agreement No 101046016 (Eucare project: EUROPEAN COHORTS OF PATIENTS AND SCHOOLS TO ADVANCE RESPONSE TO EPIDEMICS).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the trial (ClinicalTrials.gov ID: NCT04932863) was approved by the National Institute for Infectious Diseases, Rome, and the local Ethical Committee. Participants were recruited at Galliera Hospital, Genoa, from March 15 to July 21, 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Individual participant data are not publicly available because this requirement was not anticipated in the study protocol. Tania Buttiron Webber, Andrea DeCensi, Oriana D’ecclesiis and Sara Gandini had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Data may be shared upon request for collaborative studies.
Acknowledgments
Thank you to all the people who contributed to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuderer NM, Choueiri TK, Shah DP, et al; Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 Jun 20;395(10241):1907-1918. [CrossRef] [PubMed]
- Lee LYW, Cazier JB, Starkey T, et al.; COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020 Oct;21(10):1309-1316. [CrossRef] [PubMed]
- Buttiron Webber T, Provinciali N, Musso M, et al; A Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur J Cancer. 2021 Dec; 159:105-112. [CrossRef] [PubMed]
- Madison AA, Shrout MR, Renna ME, et al; Psychological and Behavioral Predictors of Vaccine Efficacy: Considerations for COVID-19. Perspect Psychol Sci. 2021 Mar;16(2):191-203. [CrossRef] [PubMed]
- Segerstrom SC, Hardy JK, Evans DR, et al; Vulnerability, distress, and immune response to vaccination in older adults. Brain Behav Immun. 2012 Jul;26(5):747-53. [CrossRef] [PubMed]
- Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 2009 May;23(4):427-33. [CrossRef] [PubMed]
- Whittaker, AC. The Vaccination Model in Psychoneuroimmunology Research: A Review. Methods Mol Biol. 2018; 1781:309-326. [CrossRef] [PubMed]
- Gallagher S, Howard S, Muldoon OT, et al; Social cohesion and loneliness are associated with the antibody response to COVID-19 vaccination. Brain Behav Immun. 2022 Jul; 103:179-185. [CrossRef] [PubMed]
- Li Y, Zhao K, Wei H, et al; Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. 2020 Jul;190(1):e24-e27. [CrossRef] [PubMed]
- Lippi G, Favaloro EJ. Cerebral Venous Thrombosis Developing after COVID-19 Vaccination: VITT, VATT, TTS, and More. Semin Thromb Hemost. 2022 Feb;48(1):8-14. [CrossRef] [PubMed]
- Rondanina G, Siri G, Marra D, et al; Effect of sex on psychological distress and fatigue over time in a prospective cohort of cancer survivors. J Cancer Surviv. 2022 Nov 7. [CrossRef] [PubMed]
- Glück T, Kiefmann B, Grohmann M, et al; Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005 Aug;32(8):1473-80. [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: distress management Version1. 2013.
- Bultz BD, Carlson LE. Emotional distress: the sixth vital sign--future directions in cancer care. Psychooncology. 2006 Feb;15(2):93-5. [CrossRef] [PubMed]
- Donovan KA, Grassi L, McGinty HL, et al; Validation of the distress thermometer worldwide: state of the science. Psychooncology. 2014 Mar;23(3):241-50. [CrossRef] [PubMed]
- Grassi L, Johansen C, Annunziata MA, et al; Italian Society of Psycho-Oncology Distress Thermometer Study Group. Screening for distress in cancer patients: a multicenter, nationwide study in Italy. Cancer. 2013 May 1;119(9):1714-21. [CrossRef] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Sc 1983 Jun;67(6):361-70. [CrossRef] [PubMed]
- Bonelli F, Sarasini A, Zierold C, et al. Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively. J Clin Microbiol. 2020 Aug 24;58(9): e01224-20. [CrossRef] [PubMed]
- National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020 Dec;20(12):1390-1400. [CrossRef] [PubMed]
- Xiong X, Qu K, Ciazynska KA, et al; A thermostable, closed SARS-CoV-2 spike protein trimer. Nat Struct Mol Biol. 2020 Oct;27(10):934-941. [CrossRef] [PubMed]
- Legnani C, Cini M, Scarvelis D, et al; Multicenter evaluation of a new quantitative highly sensitive D-dimer assay, the Hemosil D-dimer HS 500, in patients with clinically suspected venous thromboembolism. Thromb Res. 2010 May;125(5):398-401. [CrossRef] [PubMed]
- Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021 Jun;22(6):765-778. [CrossRef] [PubMed]
- Polack FP, Thomas SJ, Kitchin N, et al.; Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603-2615. [CrossRef] [PubMed]
- Lippi G, Mullier F, Favaloro EJ. D-dimer: old dogmas, new (COVID-19) tricks. Clin Chem Lab Med. 2022 Jul 14;61(5):841-850. [CrossRef] [PubMed]
- Muscatell, KA. Social psychoneuroimmunology: Understanding bidirectional links between social experiences and the immune system. Brain Behav Immun. 2021 Mar; 93:1-3. [CrossRef] [PubMed]
- Slavich, GM. Social Safety Theory: A Biologically Based Evolutionary Perspective on Life Stress, Health, and Behavior. Annu Rev Clin Psychol. 2020 May 7; 16:265-295. [CrossRef] [PubMed]
- Kaneko H, Tsuboi H. Depressive symptoms predict antibody titers after a second dose of the SARS-CoV-2 BNT162b2 vaccine among hospital workers in Japan. Brain Behav Immun. 2023 Jan; 107:414-418. [CrossRef] [PubMed]
- Xiao K, Gillissie ES, Lui LMW, Ceban F, Teopiz KM, Gill H, Cao B, Ho R, Rosenblat JD, McIntyre RS. Immune response to vaccination in adults with mental disorders: A systematic review. J Affect Disord. 2022 May 1; 304:66-77. [CrossRef] [PubMed]
- Prather AA, Marsland AL, Muldoon MF, et al; Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain Behav Immun. 2007 Nov;21(8):1033-7. [CrossRef] [PubMed]
- de Frel DL, Atsma DE, Pijl H, et al; The Impact of Obesity and Lifestyle on the Immune System and Susceptibility to Infections Such as COVID-19. Front Nutr. 2020 Nov 19; 7:597600. [CrossRef] [PubMed]
- Gasmi A, Kumar Mujawdiya P, Noor S, Piscopo S, Résimont S, Menzel A. Increasing Efficacy of Covid-19 Vaccines by Lifestyle Interventions. Arch Razi Inst. 2022 Oct 31;77(5):1527-1538. [CrossRef] [PubMed]
- Perez-Arce F, Angrisani M, Bennett D, et al; COVID-19 vaccines and mental distress. PLoS One. 2021 Sep 8;16(9): e0256406. [CrossRef] [PubMed]
- Molarius A, Granström F. Educational differences in psychological distress? Results from a population-based sample of men and women in Sweden in 2012. BMJ Open. 2018 Apr 28;8(4): e021007. [CrossRef] [PubMed]
- Qiu F, Liang CL, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget. 2017 Jan 3;8(1):268-284. [CrossRef] [PubMed]
- Sopori, M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002 May;2(5):372-7. [CrossRef] [PubMed]
- Chen L, Liang H, Liu L, Qiu W, Su L, Yang H. The association between adverse events of COVID-19 vaccination and anxiety and willingness to receive a booster dose. Hum Vaccin Immunother. 2023 Dec 31;19(1):2176643. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).