1. Introduction
Type 2 diabetes mellitus (T2DM) and morbid obesity are well-known clinical conditions that continue to raise significant global health concerns [
1,
2,
3,
4]. Obesity is defined as the excessive accumulation of fat in various parts of the body or organs. It is a chronic, progressive, and relapsing disease with multifactorial causes, leading to adverse metabolic and psychosocial health consequences. One of the primary causes of obesity is an imbalance between excess stored energy and energy expenditure, which disrupts nutrient signaling and results in insufficient energy use. The evaluation of adiposity involves measuring height, weight, body mass index (BMI), waist circumference, and body fat percentage. Obesity diagnosis is primarily based on BMI thresholds, which take into account body weight and fat distribution patterns, though BMI alone is now considered insufficient for a full assessment, as obesity is a multifactorial condition.
According to data from 2017 to 2020, 42.4% of adults in the U.S. have a BMI greater than 30 kg/m², while 20.9% of young people fall into the same category. Furthermore, the age-adjusted prevalence of severe obesity (BMI > 40 kg/m²) is 9.2% [
1,
2]. Currently, only around 30% of the adult population in the U.S. has a normal BMI (18-25 kg/m²). When race and ethnicity are considered, the highest rates of obesity are found among Black women, Native Americans, and Hispanics. It is predicted that by 2023, approximately 50% of the U.S. adult population will be obese, with 25% experiencing severe obesity [
2].
In Brazil, the prevalence of obesity has risen by 72% in the last 13 years, from 11.8% in 2006 to 20.3% in 2019. It is estimated that 55.4% of the Brazilian population has a BMI over 25 kg/m², with 47.1% of these being men and 53.9% women. Patients with a BMI over 30 kg/m² account for 19.8% of the population [
3]. By 2030, it is projected that approximately 14% of men and 20% of women globally will develop clinical obesity. Furthermore, 18% of individuals are expected to have a BMI over 30 kg/m², 6% a BMI over 35 kg/m², and 2% a BMI over 40 kg/m² [
3].
Obesity is closely associated with T2DM, a condition that has been described as a global pandemic. T2DM presents one of the greatest challenges to human health, with its rapid global spread. The prevalence of T2DM increased from 5.7% of the population in 1985-1989 to 8.7% between 2005-2011. In 2015, the International Diabetes Federation reported that approximately 415 million people worldwide were living with T2DM, a figure projected to rise to 642 million by 2040 [
1,
2,
3,
4].
T2DM is characterized by varying degrees of pancreatic beta-cell dysfunction and insulin resistance, and its development may also be influenced by hormonal systems such as the incretin axis [
5,
6]. The relationship between obesity and T2DM appears to be strongly connected to the increased concentration of adipocytes, where insulin resistance is more pronounced. Although the connection between adipose tissue and the synthesis of matrix metalloproteinases (MMPs) remains unclear, a deficiency of MMPs in adipose tissue is thought to contribute to the development of T2DM. Both diabetes mellitus (DM) and obesity are primary risk factors for cardiovascular diseases. While recent studies have highlighted the significant role of extracellular matrix metalloproteinases (MMPs) in atherosclerosis, little is known about the effects of hyperglycemia on MMP regulation in vascular cells [
7].
Bariatric surgery, particularly Roux-en-Y gastric bypass (RYGB) and vertical gastrectomy (VG), is one of the most effective treatments for morbid obesity, significantly reducing weight and improving clinical comorbidities, including T2DM [
5,
6,
7,
8]. MMPs are calcium- and zinc-dependent proteases involved in extracellular matrix (ECM) synthesis, basement membrane degradation, and tissue growth factor stimulation, all of which contribute to adipogenesis and adipose tissue expansion [
9,
10].
MMPs were first identified in the early 1960s for their role in ECM protein degradation [
10]. As members of the metzincin superfamily of proteases, MMPs have evolved into a family of endopeptidases called matrixins. MMPs are highly homologous multidomain metalloproteinases that break down various ECM proteins through their zinc-dependent catalytic activity. The MMP family is classified into six subgroups based on their substrate specificity and homology: collagenases (MMP-1, MMP-8), gelatinases (MMP-2, MMP-9), stromelysins (MMP-3, MMP-11), matrilysins (MMP-7, MMP-26), and membrane-type MMPs (MMP-14, MMP-15, MMP-16, MMP-17, MMP-24) [
9,
11,
12,
13].
In 2001, Bouloumié et al. first described MMP-2 and MMP-9 production by human adipocytes and pre-adipocytes, identifying them as potential regulators of adipocyte differentiation [
14]. MMPs have been widely studied in other medical fields and are recognized as biomarkers for oxidative stress-related diseases, including coronary heart disease and heart failure. Plasma levels of MMPs can be detected in peripheral blood or histopathologically identified in cell membranes. MMP levels are significantly increased in patients with T2DM and obesity [
14,
15,
16].
It has been hypothesized that obesity and T2DM increase plasma MMP concentrations, especially gelatinases and matrilysins. Therefore, bariatric surgery may reduce MMP plasma levels, and serum MMP concentrations could potentially serve as markers of surgical efficacy in controlling metabolic diseases [
18,
19,
20]. Several studies have shown that bariatric surgery significantly reduces morbidity and mortality in obese patients and improves cardiovascular risk factors [
12,
15]. However, few studies have explored changes in MMP-2 and MMP-9 levels after bariatric surgery [
21,
22,
23,
24,
25].
This study aims to determine plasma concentrations of MMP-2 and MMP-9, as well as clinical and laboratory parameters related to obesity throughout the follow-up period, to investigate the associations between these markers and the effects of weight loss in patients undergoing bariatric surgery.
2. Materials and Methods
2.1. Patient Recruitment and Grouping
This prospective, controlled study was conducted at São Camilo Hospital – Pompeia Unit, São Paulo, Brazil. The study included adult patients of both sexes diagnosed with morbid obesity and type 2 diabetes mellitus (T2DM) who had undergone bariatric surgery. Eligible patients met the World Health Organization (WHO) diagnostic criteria for morbid obesity, defined as a BMI > 40 kg/m² or BMI > 35 kg/m² with comorbidities, and were aged between 18 and 65 years. Additionally, patients had to have been diagnosed with T2DM for at least six months and have undergone either Roux-en-Y gastric bypass (RYGB) or vertical gastrectomy (VG). Only patients with glycosylated hemoglobin (HbA1c) levels greater than 6.5% and who had been receiving regular follow-up care with appropriate treatment were included in the study.
Patients with a history of malignant neoplasms within the last five years, human immunodeficiency virus (HIV) infection, cardiovascular diseases, pulmonary embolism, acute or chronic renal failure, viral hepatitis, liver cirrhosis, inflammatory bowel diseases, or a history of alcohol abuse or illicit drug use were excluded.
The study evaluated patients who had undergone RYGB or VG, with weight loss assessed using anthropometric data (weight, height, and BMI). Plasma levels of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) were measured at three time points: one week before surgery (M0), 3 months after surgery (M3), and 12 months postoperatively (M12) (Appendix 1). Patients were followed for 12 months and were grouped based on the surgical technique: RYGB (Group 1) or VG (Group 2). Blood samples for laboratory analysis were collected from the median cubital vein between 8:00 a.m. and 11:00 a.m., and measurements included plasma levels of MMP-2, MMP-9, glycemia, and other routine tests used in the follow-up of bariatric patients.
2.2. MMPs Data
Peripheral blood samples were collected in Eppendorf pressure tubes for the determination of MMP-2 and MMP-9 levels. These levels were measured using the enzyme-linked immunosorbent assay (ELISA) method, specifically the Sandwich-ELISA technique, followed by spectrophotometric analysis. The plasma/serum concentration of MMPs was determined by calculating optical density (OD) compared to the standard deviation of the ELISA kit.
The following ELISA kits were used: Human MMP-2 (Matrix Metalloproteinase 2) EL-H1445 and Human MMP-9 (Matrix Metalloproteinase 9) EL-H6075, both from ELABSCIENCE (California, LA, USA). The ELISA was conducted using a microplate pre-coated with a specific antibody for human MMP-2. Standards or samples were added to the wells of the micro-ELISA plate, which combined with the antibody. A biotinylated detection antibody specific for human MMP-2 and an Avidin-Horseradish Peroxidase (HRP) conjugate were successively added to each well, followed by incubation. After the incubation period, unbound components were washed away. A substrate reagent was then added, resulting in a blue color in wells containing human MMP-2, the biotinylated detection antibody, and Avidin-HRP conjugate. The enzyme-substrate reaction was halted with the addition of stop solution, turning the wells yellow. OD was measured by spectrophotometry at a wavelength of 450 nm ± 2 nm. The OD value was proportional to the concentration of human MMP-2. Concentrations in the samples were calculated by comparing sample OD values to a standard curve.
2.3. Bioinformatics and Statistical Analysis
Descriptive and inferential statistical methods were employed to analyze the sample of 45 morbidly obese diabetic patients who underwent RYGB (n = 24) or VG (n = 21). Qualitative variables were presented as absolute and relative frequency distributions, while quantitative variables were summarized using measures of central tendency and variation.
For the inferential analysis, the following statistical methods were applied:
The Shapiro-Wilk test was used to assess the normality of quantitative variables.
Fisher’s exact test was applied to compare the categorical variable of gender between the RYGB and VG groups.
The Mann-Whitney U test was used to compare quantitative variables between the RYGB and VG groups.
Longitudinal comparisons between the time points (M0, M3, and M12) were conducted using the Friedman test.
The alpha error was set at 5% for rejecting the null hypothesis. Statistical processing was performed using Bioestat version 5.3 and SPSS version 27 (IBM, New York, NY, USA).
3. Results
3.1. Characteristics of the Study Population
The groups differed significantly by gender (p = 0.0018*). However, there was no significant difference in age between the RYGB and VG groups (p = 0.6409) (
Table 1).
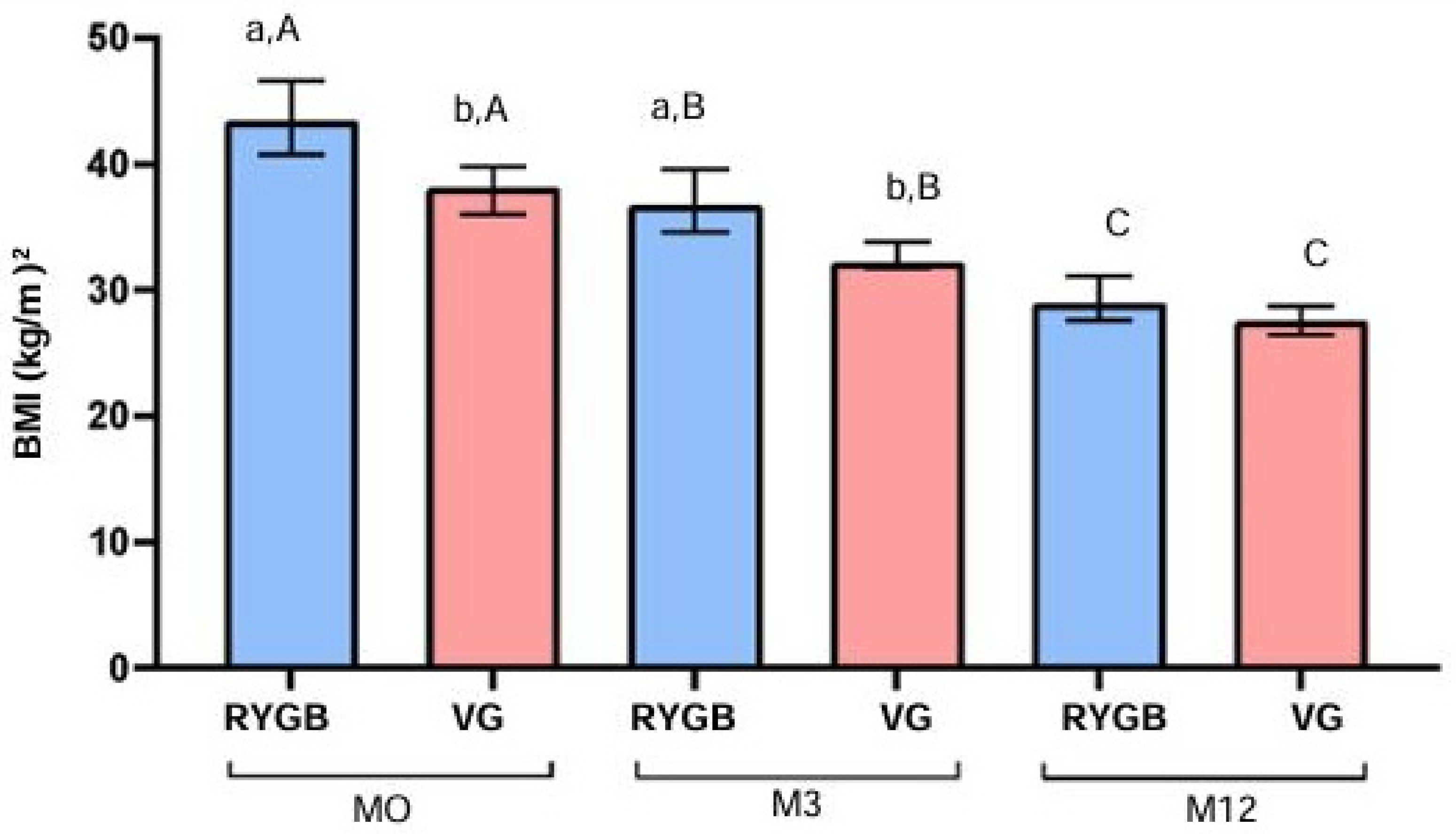
3.2. The BMI Analysis
At baseline (M0), there was a significant difference in BMI (kg/m²) between the RYGB and VG groups (p = 0.0003). This difference remained significant at the 3-month follow-up (M3) (p = 0.0022). By the 12-month follow-up (M12), the difference between groups was not statistically significant (p = 0.0571).
Within the RYGB group, BMI decreased significantly over time (p < 0.0001), with a reduction from a median of 41.9 kg/m² at M0 to 39.5 kg/m² at M3 and 28.4 kg/m² at M12. This represents a 32.2% reduction in BMI after 12 months. Similarly, in the VG group, BMI also decreased significantly over time (p < 0.0001), with a reduction from a median of 38.2 kg/m² at M0 to 32.0 kg/m² at M3 and 27.6 kg/m² at M12, representing a 27.7% reduction in BMI after 12 months (
Figure 1).
3.3. MMP Analysis
MMP-2 levels did not differ significantly between the RYGB and VG groups at M0 (p = 0.4528), M3 (p = 0.7074), or M12 (p = 0.4949). Similarly, there were no significant differences in MMP-9 levels between the two groups at M0 (p = 0.2192), M3 (p = 0.5390), or M12 (p = 0.2279) (
Table 2).
3.4. MMP-2 Analysis
In the RYGB group, MMP-2 levels decreased significantly over time (p < 0.0001), from a median of 17.7 ng/mL at M0 to 14.2 ng/mL at M3 and 5.1 ng/mL at M12. This represents a 71.2% reduction in MMP-2 levels after 12 months. Similarly, in the VG group, there was a significant reduction in MMP-2 levels over time (p < 0.0001), from a median of 22.9 ng/mL at M0 to 13.8 ng/mL at M3 and 9.5 ng/mL at M12, representing a 58.5% reduction in MMP-2 levels after 12 months (
Figure 2).
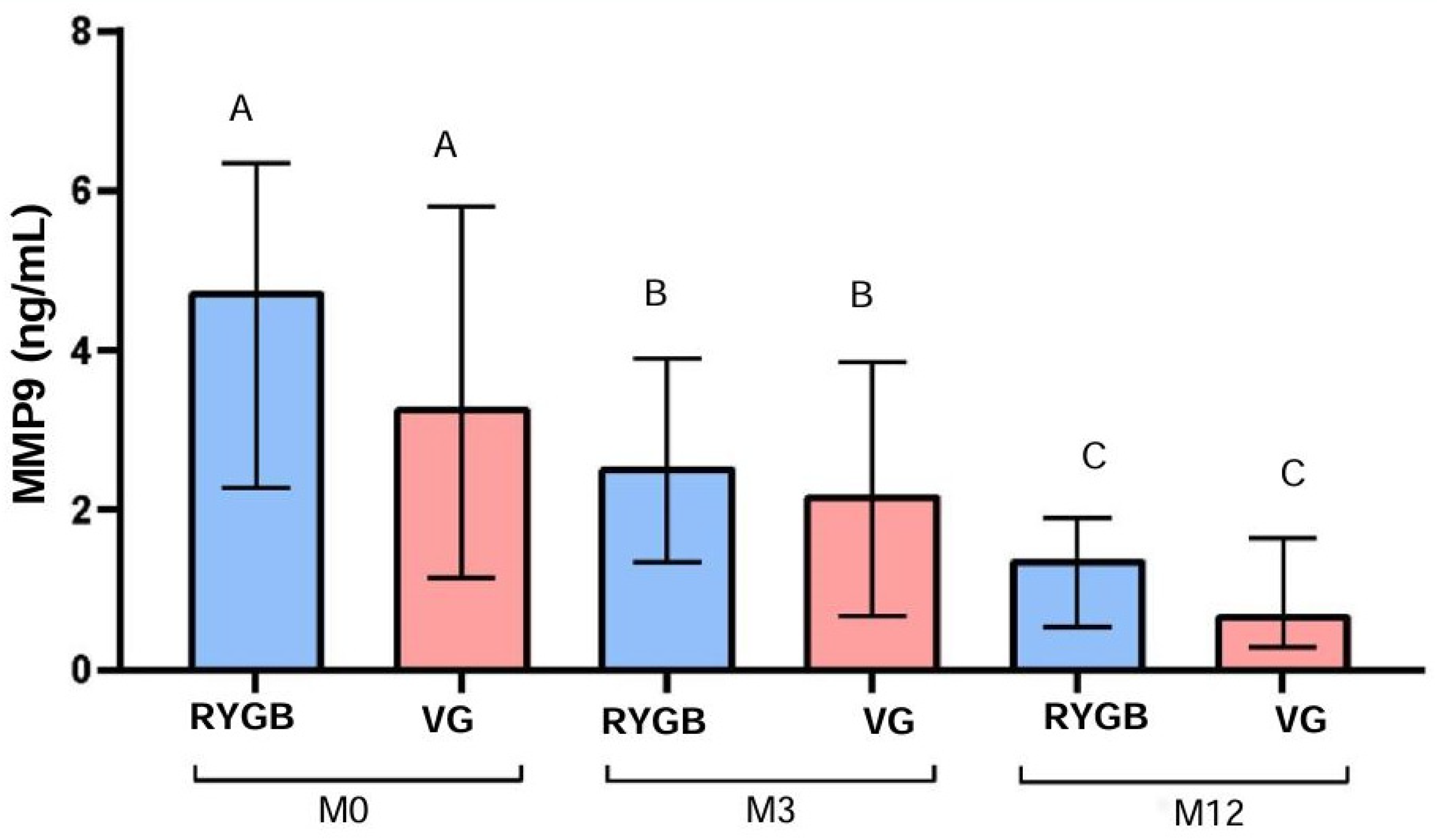
3.5. MMP-9 Analysis
MMP-9 levels also decreased significantly in the RYGB group over time (p < 0.0001), from a median of 4.7 ng/mL at M0 to 2.5 ng/mL at M3 and 1.4 ng/mL at M12, resulting in a 70.2% reduction after 12 months. In the VG group, MMP-9 levels decreased significantly as well (p < 0.0001), from a median of 3.3 ng/mL at M0 to 2.2 ng/mL at M3 and 0.7 ng/mL at M12, representing a 78.8% reduction after 12 months (
Figure 3).
4. Discussion
Obesity results from lipid accumulation in adipose tissue, which triggers extracellular matrix (ECM) remodeling to accommodate tissue growth. Our study demonstrated that bariatric surgery effectively facilitates significant and sustained weight loss, as reflected in the reduction of BMI. This supports existing literature showing that bariatric surgery is the most effective long-term treatment for weight loss and improvement of comorbidities associated with obesity, including type 2 diabetes mellitus (T2DM).
Oxidative stress and tissue inflammation in obesity lead to pathological ECM expansion, characterized by collagen deposition and increased lipid accumulation. These processes contribute to adipocyte necrosis and macrophage infiltration, which exacerbates insulin resistance. Matrix metalloproteinases (MMPs), a group of zinc-dependent endoproteases, play a critical role in ECM remodeling, tissue repair, angiogenesis, and immune response modulation. Their dysregulation in obesity has been associated with insulin resistance, tissue inflammation, and impaired glucose uptake.
In our study, we observed significant reductions in MMP-2 and MMP-9 levels postoperatively in both the RYGB and VG groups. These findings align with previous studies suggesting that elevated MMP levels in obesity contribute to ECM remodeling and metabolic dysfunction. While some studies have reported conflicting results regarding MMP activity in obesity, our data supports the notion that bariatric surgery effectively reduces MMP levels and improves metabolic outcomes.
Compared to the VG group, patients undergoing RYGB showed a more pronounced reduction in MMP levels, although the difference was not statistically significant. Both surgical techniques, however, effectively reduced MMP-2 and MMP-9 levels after 12 months, suggesting that the impact of bariatric surgery on MMP regulation is not dependent on the specific surgical technique. This is consistent with findings by Wu et al., who reported no significant differences in MMP values between RYGB and VG techniques.
Our results align with those of Lee et al., who also found significant reductions in MMP-2 and MMP-9 levels following bariatric surgery in diabetic patients. These findings further reinforce the association between weight loss, MMP reduction, and improved insulin sensitivity in patients with T2DM. Additional studies, such as those by Roumans et al. and García-Prieto et al., have demonstrated the role of MMPs in ECM remodeling and insulin resistance, supporting the importance of MMP regulation in metabolic health.
The potential role of integrins in MMP-mediated metabolic pathways has also been highlighted in rodent studies, where integrins were shown to modulate glucose transporter activity and influence insulin sensitivity. This mechanism was further corroborated by findings that integrin genetic alterations contribute to ECM remodeling and metabolic dysregulation in obese individuals.
Our study contributes to the growing body of evidence suggesting that MMPs, particularly MMP-2 and MMP-9, are important biomarkers in obesity and metabolic diseases. The reduction in MMP levels following bariatric surgery may serve as an indicator of improved ECM function and metabolic health in patients with T2DM. However, further research is needed to confirm these findings and explore the potential of MMPs as therapeutic targets for the management of obesity-related complications, including cardiovascular disease and T2DM.
5. Conclusions
This study demonstrates that oxidative stress markers, particularly MMP-2 and MMP-9, significantly decrease after bariatric surgery in both RYGB and VG groups. These findings suggest that bariatric surgery is an effective intervention for controlling metabolic conditions such as T2DM. Although no significant differences were found between the two surgical techniques in terms of MMP reduction, both RYGB and VG effectively reduced MMP levels over time. This highlights the potential role of MMPs as important biomarkers for ECM remodeling and metabolic improvements post-surgery. Further studies are warranted to explore the association of MMP reduction with cardiovascular risk and chronic disease management in obese patients.
Author Contributions
Conceptualization, J.K.A.G. and R.M.; methodology, J.K.A.G. and M.A.F.R.J.; software, J.K.A.G.; validation, M.A.F.R.J., J.; formal analysis, J.K.A.G.; investigation, J.K.A.G. and R.M.; resources, J.K.A.G.; data curation, J.K.A.G.; writing—original draft preparation, J.K.A.G.; writing—review and editing, J.K.A.G. and M.A.F.R.J.; visualization, J.K.A.G.; supervision, J.W.; project administration, J.K.A.G.; funding acquisition, J.K.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Research Ethics Committee (CAAE: 58869422.9.0000.0062) of the São Camilo Hospital - Pompeia Unit, São Paulo/SP - Brazil.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Dataset available on request from the authors
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. NEJM 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J. Cardiovascular and Metabolic Effects of Obesity. Clin. Exp. Pharmacol. Physiol. 2008, 35, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.; Addepalli, V. Matrix metalloproteinases in diabesity. Diabesity 2015, 1, 18–20. [Google Scholar] [CrossRef]
- Kang, J.H.; Le, Q.A. Effectiveness of bariatric surgical procedures. Medicine 2017, 96, e8632. [Google Scholar] [CrossRef] [PubMed]
- Kailaki, C.; Liatis, S.; le Roux, C.W.; Kokkinos, A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC End Disord. 2017, 17, 50. [Google Scholar] [CrossRef]
- Wang, W.; Fann, C.S.J.; Yang, S.H.; Chen, H.H.; Chen, C.Y. Weight loss and metabolic improvements in obese patients undergoing gastric banding and gastric banded plication: A comparison. Nutrition 2019, 57, 290–9. [Google Scholar] [CrossRef]
- Huang, H.H.; Lee, W.J.; Chen, S.C.; Chen, T.F.; Lee, S.D.; Chen, C.Y. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. JCM 2019, 8, 815. [Google Scholar] [CrossRef]
- Medeiros, N.I.; Gomes, J.A.S.; Fiuza, J.A.; Sousa, G.R.; Almeida, E.F.; Novaes, R.O.; Rocha, V.L.S.; Chaves, A.T.; Dutra, W.O.; Rocha, M.O.C.; et al. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Scientific Reports 2019, 9, 14170. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Koh, Y.; Jaoude, J. Matrix metalloproteinases in exercise and obesity. Vasc. Health Risk Manag. 2016, 14, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Migliore, R.; Gentile, J.K.A.; Franca, F.T.; Kappaz, G.T.; Bueno-De-Souza, P.M.S.; Assef, J.C. Impact of Bariatric Surgery on the Inflammatory State Based on CPR Value. ABCD 2018, 31, e1402. [Google Scholar] [CrossRef]
- Rydlova, M.; Holubec, L.; Ludvikova, M.; Kalfert, D.; Franekova, J.; Povysil, C.; Ludvíková, M. Biological Activity and Clinical Implications of the Matrix Metalloproteinases. Anticancer. Res. 2008, 28, 1389–1397. [Google Scholar]
- Bouloumié, A.; Sengenès, C.; Ghyslaine Portolan Galitzky, J.; Lafontan, M. Adipocyte Produces Matrix Metalloproteinases 2 and 9. Diabetes. 2001, 50, 2080–2086. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Martens, E.; Van den Steen, P.E.; Opdenakker, G. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [CrossRef]
- Geurts, N.; Opdenakker, G.; Van den Steen, P.E. Matrix metalloproteinases as therapeutic targets in protozoan parasitic infections. Pharmacol. Ther. 2012, 133, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Ferrari, I.; D’Angelo, A.; Tinelli, C.; Sibilla Ciccarelli, L.; Ciccarelli, L.; Piccinni, M.N.; Gravina, A.; Ramondetti, F.; Maffioli, P. Matrix Metalloproteinase-2 and -9 Levels in Obese Patients. Endothelium 2008, 15, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Thorp, E.B. Contrasting Inflammation Resolution during Atherosclerosis and Post Myocardial Infarction at the Level of Monocyte/Macrophage Phagocytic Clearance. Front. Immunol. 2012, 12, 39. [Google Scholar] [CrossRef]
- Hopps, E.; Rosalia Lo Presti Montana, M.; Noto, D.; Averna, M.; Caimi, G. Gelatinases and Their Tissue Inhibitors in a Group of Subjects with Metabolic Syndrome. J. Investig. Med. 2013, 61, 978–983. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kamanna, V.S.; Kashyap, M.L. Niacin decreases leukocyte myeloperoxidase: ECMhanistic role of redox agents and Src/p38MAP kinase. Atherosclerosis 2014, 235, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing Inflammation and Oxidative Stress in Cardiovascular Disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.C.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring Myeloperoxidase Activity in Biological Samples. Johnson R, editor. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.A.; Al-Jarrah, M.I.; Ibrahim, K.S.; Alzoubi, K.H. Level and significance of plasma myeloperoxidase and the neutrophil to lymphocyte ratio in patients with coronary artery disease. Exp. Ther. Med. 2014, 8, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm. Res. 2012, 61, 789–807. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield JW 2nd DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Lotan, Y.; Zhang, J.; Rosenthal, T.R.; Rogers, J.T.; Adams-Huet, B.; Sakhaee, K.; Moe, O.W. Triglycerides in the Human Kidney Cortex: Relationship with Body Size. PLoS ONE 2014, 9, e101285-5. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Ayala, J.E.; Lee-Young, R.S.; Zhang, Z.; James, F.D.; Neufer, P.D.; et al. Diet- induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes 2011, 60, 416–426. [Google Scholar] [CrossRef]
- Roumans, N.J.; Vink, R.G.; Fazelzadeh, P.; van Baak, M.A.; Mariman, E.C. A role for leukocyte integrins and extracellular matrix remodeling of adipose tissue in the risk of weight regain after weight loss. Am. J. Clin. Nutr. 2017, 105, 1054–1062. [Google Scholar] [CrossRef]
- Hopps, E.; Rosalia Lo Presti Montana, M.; Noto, D.; Averna, M.; Caimi, G. Gelatinases and Their Tissue Inhibitors in a Group of Subjects with Metabolic Syndrome. J. Investig. Med. 2013, 61, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Papazafiropoulou, A.; Perrea, D.; Moyssakis, I.; Kokkinos, A.; Katsilambros, N.; Tentolouris, N. Plasma levels of MMP-2, MMP-9 and TIMP-1 are not associated with arterial stiffness in subjects with type 2 diabetes mellitus. J. Diabetes Complicat. 2010, 24, 20–27. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Gil-Ortega, M.; Vega-Martín, E.; Ramiro-Cortijo, D.; Martín-Ramos, M.; Bordiú, E.; Sanchez-Pernaute, A.; Torres, A.; Aránguez, I.; Fernández-Alfonso, M.; et al. Beneficial Effect of Bariatric Surgery on Abnormal MMP-9 and AMPK Activities: Potential Markers of Obesity-Related CV Risk. Front. Physiol. 2019, 8, 553. [Google Scholar] [CrossRef]
- Wu, W.C.; Lee, W.J.; Lee, T.H.; Chen, S.C.; Chen, C.Y. Do different bariatric surgical procedures influence plasma levels of matrix metalloproteinase-2, -7, and -9 among patients with type 2 diabetes mellitus? World, J. Diabetes. 2020, 11, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Heo, Y.S.; Park, H.S.; Lee, S.H.; Le, S.K.; Jang, Y.J. Serum SPARC and Matrix Metalloproteinase-2 and Metalloproteinase-9 Concentrations after Bariatric Surgery in Obese Adults. Obes. Surg. 2013, 24, 604–610. [Google Scholar] [CrossRef]
- Zong, H.; Bastie, C.C.; Xu, J.; Reinhard Fassler Campbell, K.P.; Kurland, I.J.; Pessin, J.E. Insulin Resistance in Striated Muscle-specific Integrin Receptor β1-deficient Mice. J. Biol. Chem. 2009, 284, 4679–4688. [Google Scholar] [CrossRef]
- Meakin, P.J.; Morrison, V.L.; Sneddon, C.C.; Terhi Savinko Liisa Uotila Jalicy, S.M.; Gabriel, J.L.; Kang, L.; Ashford, M.L.J.; Fagerholm, S.C. Mice Lacking beta2-Integrin Function Remain Glucose Tolerant in Spite of Insulin Resistance, Neutrophil Infiltration and Inflammation. PLoS ONE 2015, 10, e0138872–2. [Google Scholar] [CrossRef]
- McCulloch, L.J.; Rawling, T.J.; Kajsa Sjoholm Franck, N.; Dankel, S.N.; Price, E.N.; Knight, B.; Liversedge, N.H.; Mellgren, G.; Nystrom, F.; et al. COL6A3 Is Regulated by Leptin in Human Adipose Tissue and Reduced in Obesity. Endocrinology 2015, 156, 134–146. [Google Scholar] [CrossRef]
- Carbone, F.; Adami, G.; Liberale, L.; Bonaventura, A.; Bertolotto, M.; Andraghetti, G.; Scopinaro, N.; Camerini, G.B.; Papadia, F.S.; Cordera, R.; et al. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes Metab. 2019, 45, 356–362. [Google Scholar] [CrossRef]
- Laimer, M.; Kaser, S.; Kranebitter, M.; Sandhofer, A.; Mühlmann, G.; Schwelberger, H.; Weiss, H.; Patsch, J.R.; Ebenbichler, C.F. Effect of pronounced weight loss on the nontraditional cardiovascular risk marker matrix metalloproteinase-9 in middle-aged morbidly obese women. Int. J. Obes. 2005, 29, 498–501. [Google Scholar] [CrossRef]
- Derosa, G.; Ferrari, I.; D’Angelo, A.; Tinelli, C.; Sibilla Ciccarelli, L.; Piccinni, M.N.; Gravina, A.; Ramondetti, F.; Maffioli, P.; et al. Matrix Metalloproteinase-2 and -9 Levels in Obese Patients. Endothelium 2008, 15, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Bruun, J.M.; Skogstrand, K.; Hougaard, D.M.; Christiansen, T.; Richelsen, B. Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism 2009, 58, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).