Submitted:
28 October 2024
Posted:
29 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Density Gradient Theory And Peng-Robinson Equation Of State.
3. Sources Of Data
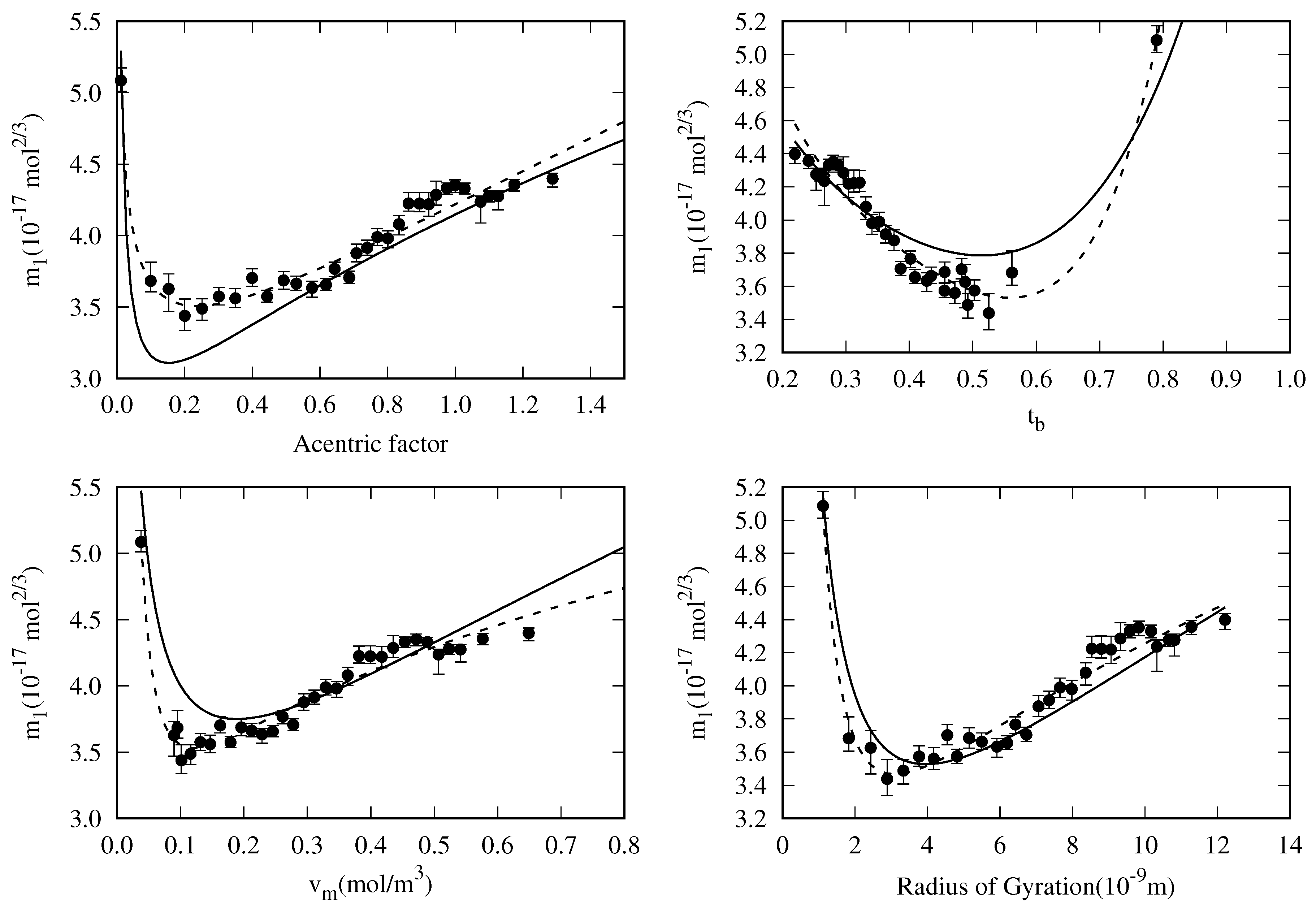
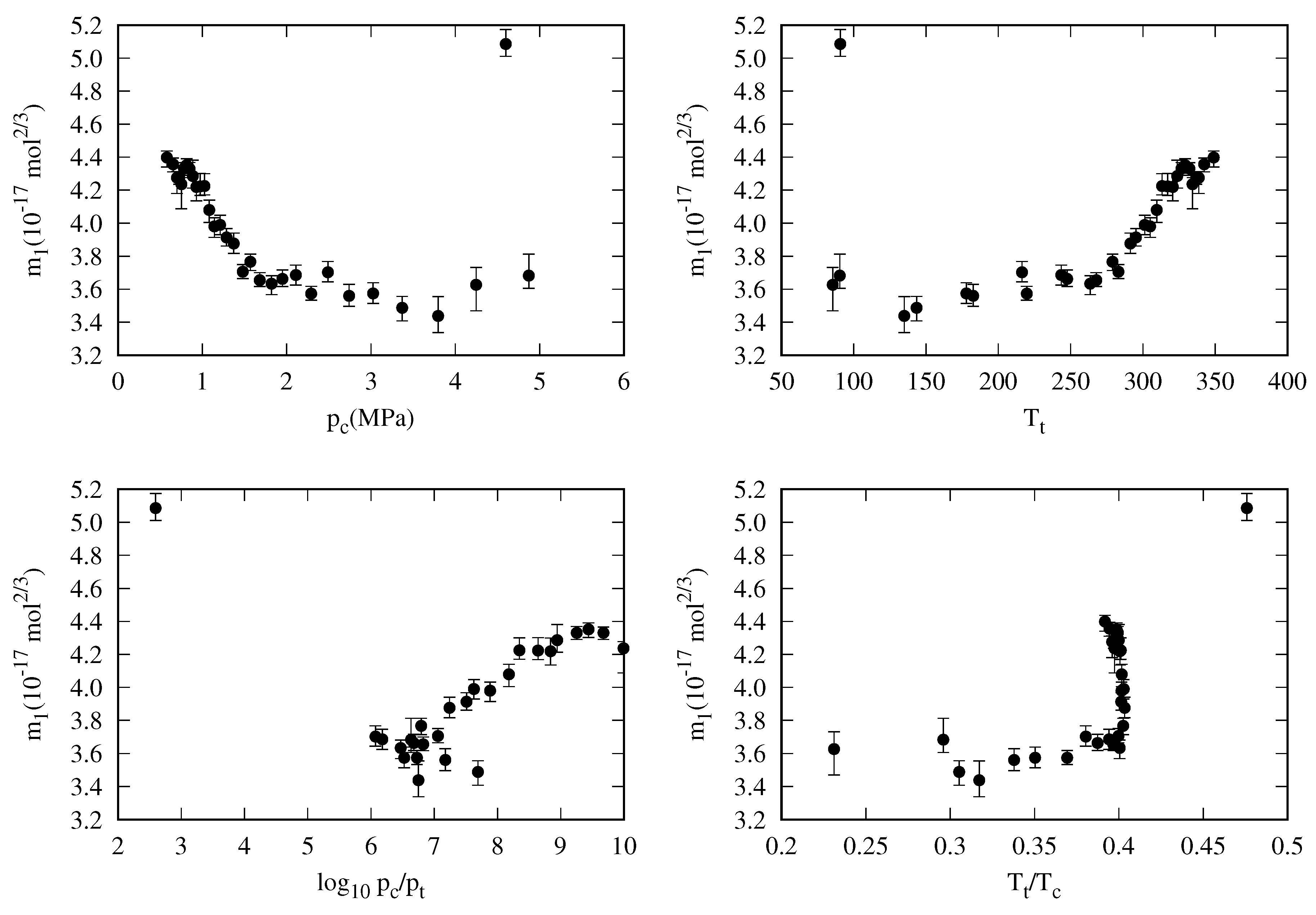
4. New Correlation For The Reduced Influence Parameter
- i.
- decreases almost linearly in the range [], so it could be fitted to a linear expression:where is the slope and is the value of the reduced influence parameter at the triple point temperature.
- ii.
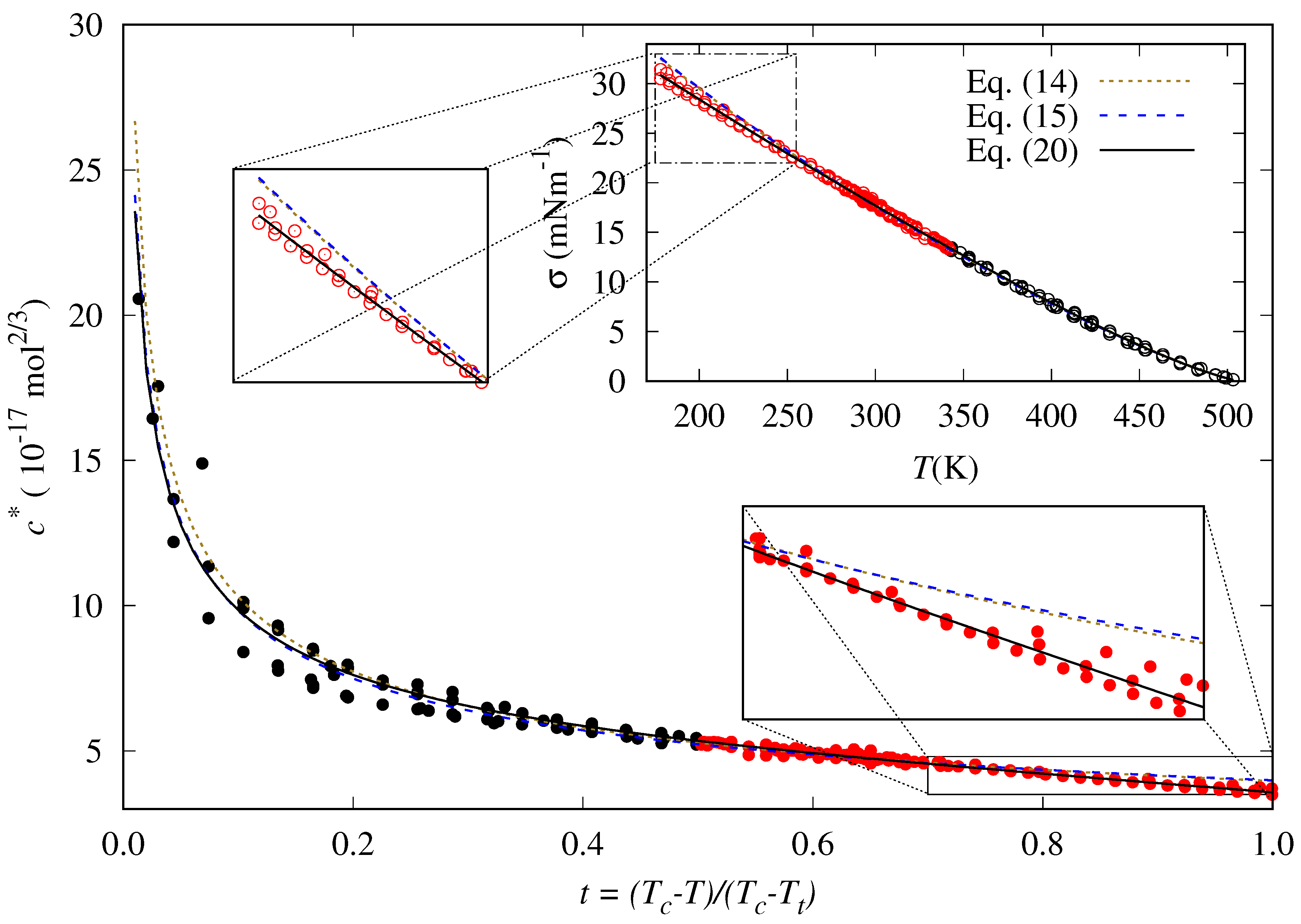
- Near the critical point (), tends to infinity. To reproduce this behavior, an analytical expression as the one proposed by Zuo and Stenby [33], has to be used:
5. Results and Discussion
5.1. Adjustable Coefficients For The Specific Correlation
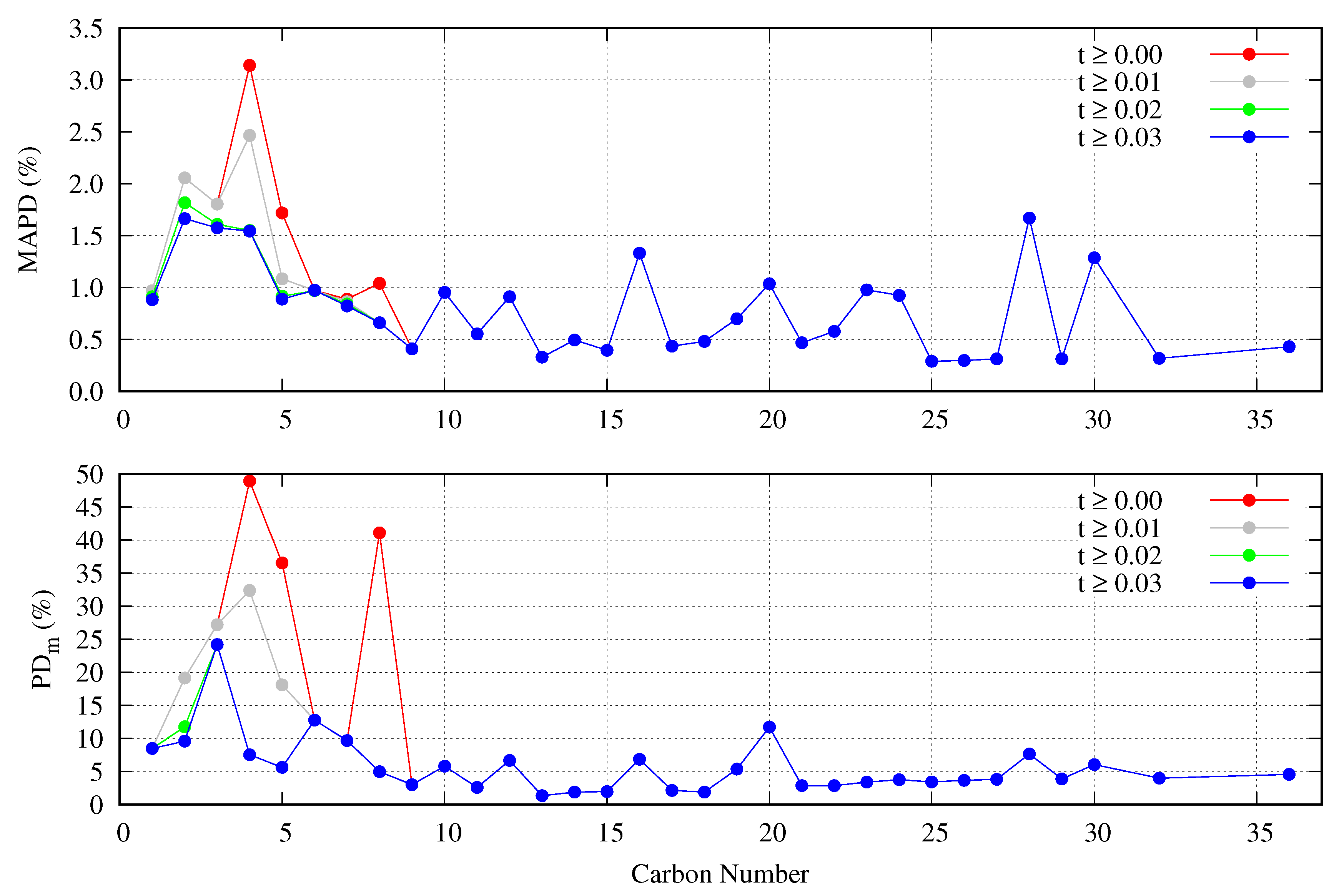
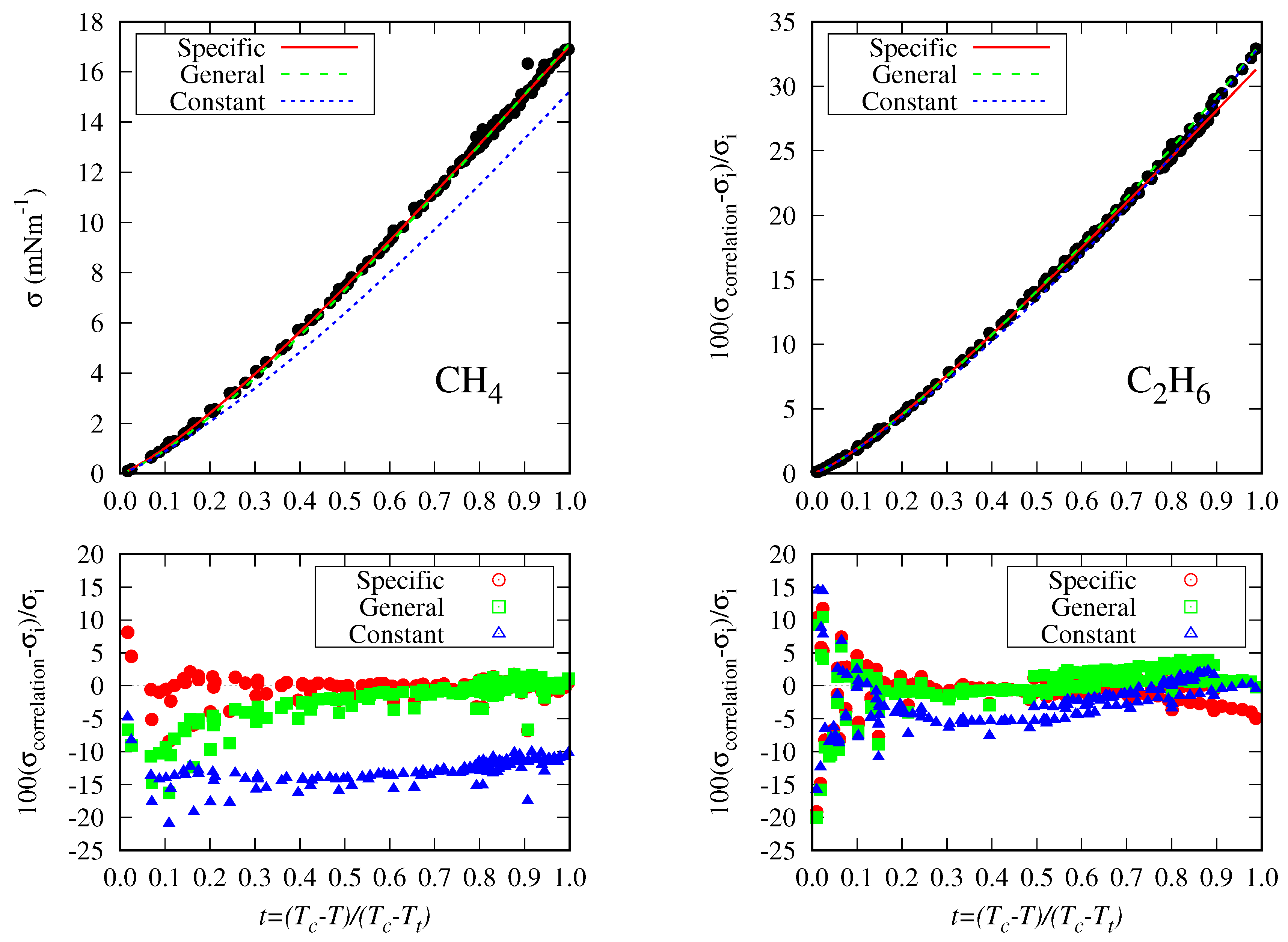
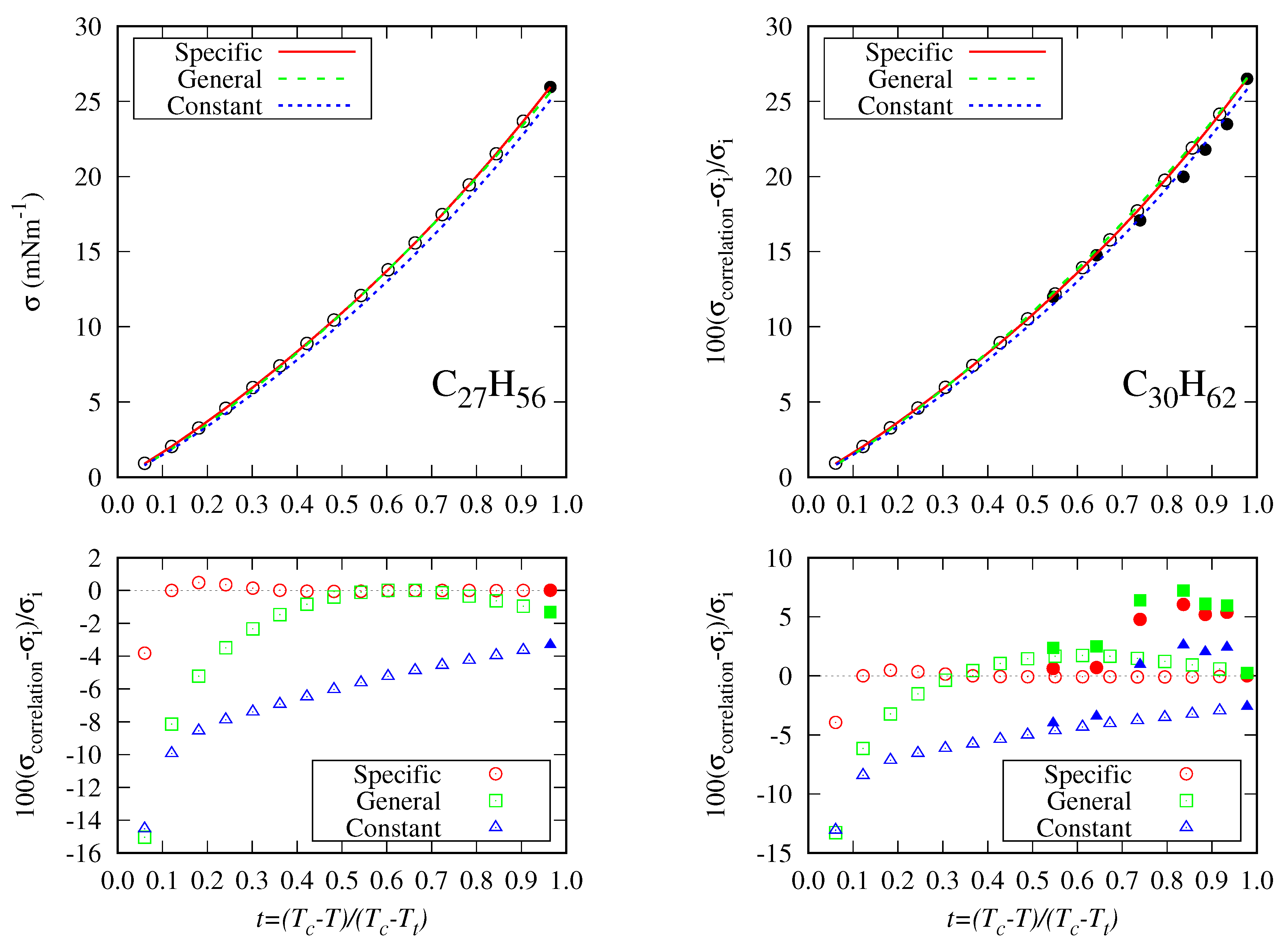
5.2. Accuracy Of The Proposed Specific Model
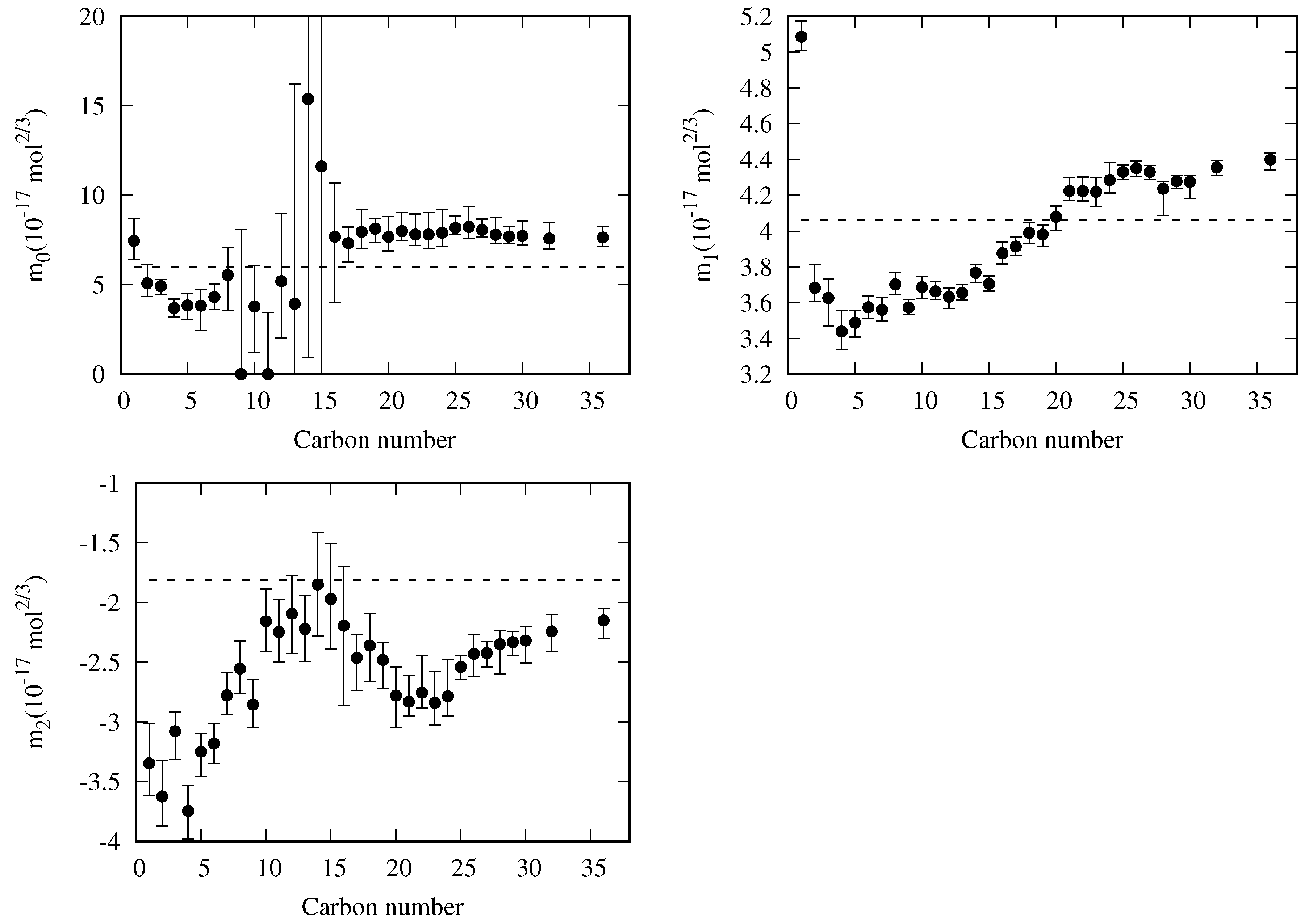
5.3. General Correlation
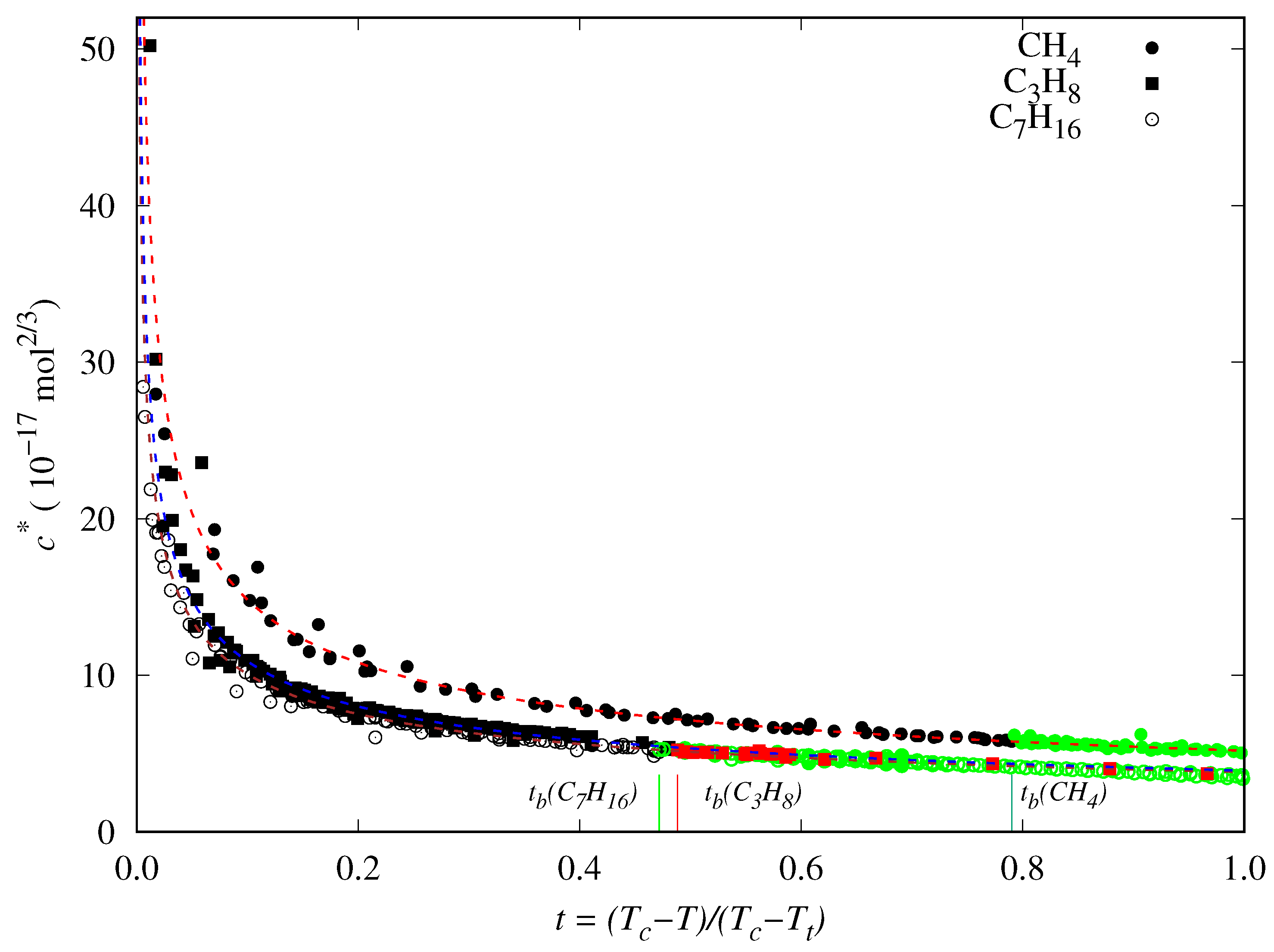
- The DIPPR data predicted using Sugden’s correlation will not be considered in the new global correlation, and only data in the range will be considered in the coefficient determination of the global correlation. The number of available data and fitting data for each fluid are compiled in Table 5.
- There are some fluids (see Figure 1) for which a considerable number of fitting data are available (i.e., n-heptane and n-hexane with 357 and 269 data, respectively), whereas in other cases the number of data is one (n-hexatriacontane and others). To have a suitable general correlation not biased by the data availability, the adjustable coefficients will be obtained by minimizing the overall mean absolute percentage deviation (), defined as:where is the mean absolute percentage deviation of the fluid k, defined in Equation (25), but taking from Table 5, and with the number of fluids (32 in these case). Thus, the coefficients of the general correlation will be determined with data, with a weighing scheme depending on the fitting data of each fluid.
| CN | MAPD/ | MD/ | / | ||
|---|---|---|---|---|---|
| (%) | (%) | (%) | |||
| 1 | 127/126 | 12.99/13.05 | -12.99/-13.05 | +20.95/+20.95 | 0.11/ 0.11 |
| 2 | 163/160 | 3.48/3.28 | -2.33/-2.29 | 15.80/14.41 | 0.01/ 0.02 |
| 3 | 193/191 | 2.14/2.00 | -0.49/-0.34 | 22.59/22.10 | 0.01/ 0.06 |
| 4 | 126/118 | 5.42/2.59 | 2.35/-0.70 | 75.48/+15.04 | 0.01/ 0.03 |
| 5 | 149/143 | 3.53/2.06 | 3.00/ 1.51 | 62.01/17.12 | 0.01/ 0.02 |
| 6 | 270/269 | 1.82/1.76 | 1.20/ 1.14 | 17.35/16.20 | 0.01/ 0.04 |
| 7 | 363/357 | 2.94/2.71 | 2.90/ 2.68 | 20.39/17.97 | 0.01/ 0.05 |
| 8 | 196/194 | 2.41/2.00 | 2.17/ 1.76 | 45.07/5.72 | 0.01/ 1.00 |
| 9 | 78 | 3.52 | 3.52 | 6.93 | 0.99 |
| 10 | 149 | 3.80 | 3.74 | 9.71 | 0.12 |
| 11 | 60 | 4.36 | 4.36 | 10.43 | 0.30 |
| 12 | 100 | 4.98 | 4.98 | 12.18 | 0.91 |
| 13 | 48 | 4.30 | 4.30 | 5.86 | 0.99 |
| 14 | 49 | 3.60 | 3.39 | 5.72 | 0.97 |
| 15 | 40 | 4.19 | 4.19 | 5.37 | 0.95 |
| 16 | 127/117 | 2.40/2.31 | 1.52/ 1.90 | 7.31/7.17 | 0.08/ 1.00 |
| 17 | 44/ 34 | 1.56/1.06 | -0.13/ 0.80 | 7.16/2.15 | 0.07/ 0.78 |
| 18 | 39/ 29 | 1.57/0.55 | -0.92/ 0.34 | 9.37/1.40 | 0.07/ 1.00 |
| 19 | 23/ 12 | 2.85/1.20 | -2.49/-0.51 | 10.41/10.20 | 0.07/ 0.34 |
| 20 | 38/ 25 | 2.89/1.43 | -2.78/-1.26 | 17.69/17.69 | 0.33/ 0.33 |
| 21 | 28/ 14 | 5.86/4.78 | -5.86/-4.78 | 14.00/7.35 | 0.07/ 0.49 |
| 22 | 32/ 19 | 5.16/4.05 | -5.16/-4.05 | 13.18/6.99 | 0.07/ 0.54 |
| 23 | 36/ 22 | 5.05/3.74 | -5.05/-3.74 | 13.93/6.97 | 0.06/ 0.52 |
| 24 | 36/ 22 | 5.60/4.43 | -5.56/-4.36 | 14.37/7.22 | 0.06/ 0.61 |
| 25 | 15/ 1 | 6.92/3.48 | -6.92/-3.48 | 14.70/3.48 | 0.06/ 0.95 |
| 26 | 31/ 19 | 5.40/3.96 | -5.40/-3.96 | 14.97/4.45 | 0.06/ 0.81 |
| 27 | 16/ 1 | 6.45/3.32 | -6.45/-3.32 | 14.52/3.32 | 0.06/ 0.96 |
| 28 | 24/ 9 | 4.42/2.57 | -3.35/ 0.29 | 13.13/4.23 | 0.06/ 0.78 |
| 29 | 16/ 1 | 5.41/2.60 | -5.41/-2.60 | 12.95/2.60 | 0.06/ 0.97 |
| 30 | 22/ 7 | 4.64/2.57 | -3.91/-0.30 | 13.07/4.01 | 0.06/ 0.55 |
| 32 | 25/ 12 | 4.92/3.64 | -4.92/-3.64 | 12.61/4.23 | 0.06/ 0.85 |
| 36 | 18/ 1 | 5.67/3.48 | -5.67/-3.48 | 13.36/3.48 | 0.06/ 1.00 |
| - | 2681/2427 | 75.48/22.10 | |||
| OMAPD/ | OMD/ | ||||
| 32 | 4.38/3.35 | -1.38/-0.53 |
| Name | Symbol | Units | |
|---|---|---|---|
| 0 | No dependency (constant) | - | - |
| 1 | Critical Pressure | Pa | |
| 2 | Critical Temperature | K | |
| 3 | Acentric factor | - | |
| 4 | Critical compressibility factor | - | |
| 5 | Critical Volume | L | |
| 6 | Melting temperature | K | |
| 7 | Triple point temperature | K | |
| 8 | Normal boiling point temperature | K | |
| 9 | Logarithmic ratio between and | - | |
| 10 | Liquid molar volume at 298.15 K and 101325 Pa | L | |
| 11 | Radius of Gyration | RG | m |
| 12 | Dipole moment | Cm | |
| 13 | Molar weight | kg | |
| 14 | Reduced triple point temperature | - | |
| 15 | Reduced normal boiling temperature | - | |
| 16 | Pseudo compressibility factor | ) | - |
| 17 | Reduced boiling temperature | - |
| Adjustable coefficients | RG | |||||
|---|---|---|---|---|---|---|
| ( | 5.01227 | 4.91156 | 5.37325 | 6.48294 | 4.99955 | |
| ( | 4.40431 | 1.09159 | 0.662073 | 3.03929 | 0.625431 | |
| 0.885059 | 0.53364 | 0.595946 | 0.254804 | 0.452929 | ||
| ( | 1.08825 | 2.68771 | 4.84453 | 5.0875 | 3.52179 | |
| 0.519495 | 0.401163 | 0.542305 | 4.98139 | 0.405698 | ||
| ( | -2.92951 | -2.6442 | -2.05972 | -2.20715 | -3.45186 | |
| Statistical figures for the fitting set | ||||||
| (%) | 1.78 | 1.94 | 1.99 | 2.16 | 2.18 | |
| (%) | 0.04 | -0.02 | -0.13 | 0.16 | 0.24 | |
| (%) | 23.93 | 24.37 | 23.76 | 21.44 | 24.35 | |
| Statistical figures for the whole set | ||||||
| OMAPD(%) | 2.26 | 2.52 | 2.78 | 2.68 | 2.57 | |
| MD(%) | -0.45 | -0.79 | -1.20 | -0.37 | 0.11 | |
| PDm(%) | 65.02 | 63.50 | 68.36 | 81.76 | 64.95 | |
| CN | MAPD/ | MD/ | / | ||
|---|---|---|---|---|---|
| (%) | (%) | (%) | |||
| 1 | 127/126 | 2.49/2.45 | -2.20/-2.16 | 16.28/16.28 | 0.11/ 0.11 |
| 2 | 163/160 | 2.58/2.35 | 0.09/ 0.25 | 20.01/10.71 | 0.01/ 0.04 |
| 3 | 193/191 | 1.84/1.65 | 0.27/ 0.48 | 26.76/23.93 | 0.01/ 0.06 |
| 4 | 126/118 | 4.54/2.19 | 1.53/-1.02 | 65.02/10.03 | 0.01/ 0.03 |
| 5 | 149/143 | 2.42/1.25 | 1.75/ 0.54 | 51.44/11.26 | 0.01/ 0.02 |
| 6 | 270/269 | 1.72/1.69 | -0.37/-0.41 | 12.64/12.64 | 0.07/ 0.07 |
| 7 | 363/357 | 1.36/1.22 | 0.96/ 0.81 | 13.96/13.96 | 0.05/ 0.05 |
| 8 | 196/194 | 1.40/1.08 | -0.34/-0.68 | 35.68/4.47 | 0.01/ 0.19 |
| 9 | 78 | 0.54 | 0.38 | 3.76 | 0.54 |
| 10 | 149 | 1.55 | 0.97 | 8.52 | 0.12 |
| 11 | 60 | 1.77 | 1.77 | 11.63 | 0.30 |
| 12 | 100 | 2.37 | 2.34 | 7.96 | 0.91 |
| 13 | 48 | 1.66 | 1.66 | 4.26 | 0.69 |
| 14 | 49 | 0.96 | 0.61 | 3.76 | 0.72 |
| 15 | 40 | 1.67 | 1.67 | 4.58 | 0.74 |
| 16 | 127/117 | 1.77/1.73 | -0.24/-0.09 | 8.64/7.67 | 0.08/ 0.46 |
| 17 | 44/ 34 | 1.15/0.84 | -0.68/-0.39 | 8.56/2.20 | 0.07/ 0.78 |
| 18 | 39/ 29 | 1.50/1.08 | -1.24/-0.74 | 10.54/2.65 | 0.07/ 0.99 |
| 19 | 23/ 12 | 2.06/1.44 | -1.54/-0.64 | 11.62/7.08 | 0.07/ 0.34 |
| 20 | 38/ 25 | 2.48/1.97 | -2.18/-1.52 | 14.55/14.55 | 0.33/ 0.33 |
| 21 | 28/ 14 | 3.80/2.81 | -3.80/-2.81 | 14.97/3.63 | 0.07/ 0.49 |
| 22 | 32/ 19 | 3.15/2.48 | -3.15/-2.48 | 13.98/3.32 | 0.07/ 0.63 |
| 23 | 36/ 22 | 2.79/1.90 | -2.53/-1.47 | 14.82/3.23 | 0.06/ 0.64 |
| 24 | 36/ 22 | 3.02/2.20 | -2.92/-2.04 | 15.08/3.94 | 0.06/ 0.80 |
| 25 | 15/ 1 | 3.64/2.25 | -3.64/-2.25 | 15.32/2.25 | 0.06/ 0.95 |
| 26 | 31/ 19 | 2.32/1.39 | -2.31/-1.36 | 15.59/2.09 | 0.06/ 0.93 |
| 27 | 16/ 1 | 2.53/1.31 | -2.53/-1.31 | 15.04/1.31 | 0.06/ 0.96 |
| 28 | 24/ 9 | 3.33/4.91 | 1.05/ 4.91 | 13.58/8.81 | 0.06/ 0.78 |
| 29 | 16/ 1 | 2.22/0.07 | -0.92/ 0.07 | 13.24/0.07 | 0.06/ 0.97 |
| 30 | 22/ 7 | 3.07/4.39 | 0.83/ 4.39 | 13.29/7.22 | 0.06/ 0.84 |
| 32 | 25/ 12 | 1.64/0.55 | -0.11/ 0.55 | 12.66/0.97 | 0.06/ 0.87 |
| 36 | 18/ 1 | 2.88/1.30 | 0.45/ 1.30 | 13.31/1.30 | 0.06/ 1.00 |
| Overall | 2681/2427 | 65.02/23.93 | |||
| OMAPD/ | OMD/ | ||||
| 32 | 2.26/1.78 | -0.45/0.04 |
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Adamson, A.; Gast, A. Physical Chemistry of Surfaces; Interscience publishers: New York, 1967; Vol. 150. [Google Scholar]
- Myers, D. Surfaces, Interfaces, and Colloids: Principles and Applications; John Wiley & Sons, Inc., 1999.
- Liu, H. Science and Engineering of Droplets: Fundamentals and Applications; Noyes Publications, 2000.
- Hartland, S. Surface and Interfacial Tension: Measurement, Theory and Applications; Marcel Dekker, 2004.
- Schramm, L.L. Emulsions, foams, and suspensions: Fundamentals and applications; John Wiley & Sons, 2006.
- Neumann, A.W.; David, R.; Zuo, Y. Applied Surface Thermodynamics; Vol. 151, CRC Press, 2010.
- Ashgrid, N. Handbook of Atomization and Sprays. Theory and Applications; Springer, 2011.
- Ejim, C.; Fleck, B.; Amirfazli, A. Analytical study for atomization of biodiesels and their blends in a typical injector: Surface tension and viscosity effects. Fuel 2007, 86, 1534–1544. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; Hughes, T.J.; Johns, M.L.; Marsh, K.N.; May, E.F. Surface tension and critical point measurements of methane+ propane mixtures. The Journal of Chemical Thermodynamics 2017, 111, 173–184. [Google Scholar] [CrossRef]
- West, Z.J.; Yamada, T.; Bruening, C.R.; Cook, R.L.; Mueller, S.S.; Shafer, L.M.; DeWitt, M.J.; Zabarnick, S. Investigation of water interactions with petroleum-derived and synthetic aviation turbine fuels. Energy & fuels 2018, 32, 1166–1178. [Google Scholar]
- Ameli, F.; Hemmati-Sarapardeh, A.; Schaffie, M.; Husein, M.M.; Shamshirband, S. Modeling interfacial tension in N2/n-alkane systems using corresponding state theory: Application to gas injection processes. Fuel 2018, 222, 779–791. [Google Scholar] [CrossRef]
- Ahmad, W.; Vakilinejad, A.; Aman, Z.M.; Vakili-Nezhaad, G.R. Thermophysical study of binary systems of tert-amyl methyl ether with n-hexane and m-xylene. Journal of Chemical & Engineering Data 2019, 64, 459–470. [Google Scholar]
- Chaparro, G.; Cartes, M.; Mejía, A. Vapor–liquid equilibrium at 94 kPa and surface tension at 298.15 K for hexane+ ethanol+ cyclopentyl methyl ether mixture. Fuel 2020, 279, 118415. [Google Scholar] [CrossRef]
- Mejía, A.; Cartes, M.; Chaparro, G. Isobaric vapor–liquid equilibrium and isothermal surface tension for hexane+ cyclopentyl methyl ether binary mixture: Experimental determinations and theoretical predictions. Fluid Phase Equilibria 2020, 520, 112654. [Google Scholar] [CrossRef]
- Klein, T.; Lenahan, F.D.; Kerscher, M.; Rausch, M.H.; Economou, I.G.; Koller, T.M.; Fröba, A.P. Characterization of long linear and branched alkanes and alcohols for temperatures up to 573.15 K by surface light scattering and molecular Dynamics Simulations. The Journal of Physical Chemistry B 2020, 124, 4146–4163. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, G.; Zhang, X.; Yin, J.; Ma, S. Experimental investigation and correlations of thermophysical properties for bio-aviation kerosene surrogate containing n-decane with ethyl decanoate and ethyl dodecanoate. The Journal of Chemical Thermodynamics 2020, 150, 106201. [Google Scholar] [CrossRef]
- Shardt, N.; Wang, Y.; Jin, Z.; Elliott, J. Surface tension as a function of temperature and composition for a broad range of mixtures. Chemical Engineering Science 2021, 230, 116095. [Google Scholar] [CrossRef]
- Aleiferis, P.G.; van Romunde, Z. An analysis of spray development with iso-octane, n-pentane, gasoline, ethanol and n-butanol from a multi-hole injector under hot fuel conditions. Fuel 2013, 105, 143–168. [Google Scholar] [CrossRef]
- Prak, D.J.L.; Trulove, P.C.; Cowart, J.S. Density, viscosity, speed of sound, surface tension, and flash point of binary mixtures of n-hexadecane and 2, 2, 4, 4, 6, 8, 8-heptamethylnonane and of algal-based hydrotreated renewable diesel. Journal of Chemical & Engineering Data 2013, 58, 920–926. [Google Scholar]
- Luning Prak, D.J.; Cowart, J.S.; Trulove, P.C. Density, viscosity, speed of sound, bulk modulus, and surface tension of binary mixtures of n-heptane+ 2, 2, 4-trimethylpentane at (293.15 to 338.15) K and 0.1 MPa. Journal of Chemical & Engineering Data 2014, 59, 3842–3851. [Google Scholar]
- Luning Prak, D.J.; Luning Prak, P.J.; Cowart, J.S.; Trulove, P.C. Densities and viscosities at 293.15–373.15 K, speeds of sound and bulk moduli at 293.15–333.15 K, surface tensions, and flash points of binary mixtures of n-hexadecane and alkylbenzenes at 0.1 MPa. Journal of Chemical & Engineering Data 2017, 62, 1673–1688. [Google Scholar]
- Luning Prak, D.J.; Mungan, A.L.; Cowart, J.S.; Trulove, P.C. Densities, viscosities, speeds of sound, bulk moduli, surface tensions, and flash points of binary mixtures of ethylcyclohexane or methylcyclohexane with n-dodecane or n-hexadecane at 0.1 MPa. Journal of Chemical & Engineering Data 2018, 63, 1642–1656. [Google Scholar]
- Luning Prak, D.J.; Fries, J.M.; Gober, R.T.; Vozka, P.; Kilaz, G.; Johnson, T.R.; Graft, S.L.; Trulove, P.C.; Cowart, J.S. Densities, viscosities, speeds of sound, bulk moduli, surface tensions, and flash points of quaternary mixtures of n-dodecane (1), n-butylcyclohexane (2), n-butylbenzene (3), and 2, 2, 4, 4, 6, 8, 8-heptamethylnonane (4) at 0.1 MPa as potential surrogate mixtures for military jet fuel, JP-5. Journal of Chemical & Engineering Data 2019, 64, 1725–1745. [Google Scholar]
- Luning Prak, D.J.; Cowart, J.S.; Simms, G.R. Physical Properties of Binary Mixtures of n-Dodecane and Various Ten-Carbon Aromatic Compounds (2-Methyl-1-phenylpropane, 2-Methyl-2-phenylpropane, 2-Phenylbutane, and 1, 3-Diethylbenzene): Densities, Viscosities, Speeds of Sound, Bulk Moduli, Surface Tensions, and Flash Points at T=(293.15–333.15) K and 0.1 MPa. Journal of Chemical & Engineering Data 2020, 65, 3941–3954. [Google Scholar]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Reports 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Vijande, J.; Pineiro, M.; García, J.; Valencia, J.; Legido, J. Density and surface tension variation with temperature for heptane+ 1-alkanol. Journal of Chemical & Engineering Data 2006, 51, 1778–1782. [Google Scholar]
- Estrada-Baltazar, A.; López-Lázaro, J.d.l.S.; Iglesias-Silva, G.; Barajas-Fernández, J. Density and surface tension of binary mixture of 1-nonanol+ n-octane,+ n-nonane, and+ n-decane from (293.15 to 323.15) K at P= 0.1 MPa. The Journal of Chemical Thermodynamics 2020, 150, 106225. [Google Scholar] [CrossRef]
- Mejía, A.; Segura, H.; Wisniak, J.; Polishuk, I. Association and molecular chain length effects on interfacial behavior. Physics and Chemistry of Liquids 2006, 44, 45–59. [Google Scholar] [CrossRef]
- Oliveira, M.; Marrucho, I.; Coutinho, J.; Queimada, A. Surface tension of chain molecules through a combination of the gradient theory with the CPA EoS. Fluid Phase Equilibria 2008, 267, 83–91. [Google Scholar] [CrossRef]
- Chalbaud, C.; Robin, M.; Lombard, J.; Martin, F.; Egermann, P.; Bertin, H. Interfacial tension measurements and wettability evaluation for geological CO2 storage. Advances in Water Resources 2009, 32, 98–109. [Google Scholar] [CrossRef]
- Mejia, A.; Cartes, M.; Segura, H.; Müller, E. Use of equations of state and coarse grained simulations to complement experiments: Describing the interfacial properties of carbon dioxide+ decane and carbon dioxide+ eicosane mixtures. Journal of Chemical & Engineering Data 2014, 59, 2928–2941. [Google Scholar]
- Cui, J.; Bi, S.; Fröba, A.P.; Wu, J. Viscosity and interfacial tension of n-heptane with dissolved carbon dioxide by surface light scattering (SLS). The Journal of Chemical Thermodynamics 2021, 152, 106266. [Google Scholar] [CrossRef]
- Zuo, Y.X.; Stenby, E. Calculation of interfacial tensions with gradient theory. Fluid Phase Equilibria 1997, 132, 139–158. [Google Scholar] [CrossRef]
- Fu, D.; Li, X.S.; Yan, S.; Liao, T. Investigation of critical properties and surface tensions for n-alkanes by perturbed-chain statistical associating fluid theory combined with density-gradient theory and renormalization-group theory. Industrial & Engineering Chemistry Research 2006, 45, 8199–8206. [Google Scholar]
- Singh, J.; Errington, J. Calculation of phase coexistence properties and surface tensions of n-alkanes with grand-canonical transition-matrix Monte Carlo simulation and finite-size scaling. The Journal of Physical Chemistry B 2006, 110, 1369–1376. [Google Scholar] [CrossRef]
- Biscay, F.; Ghoufi, A.; Goujon, F.; Lachet, V.; Malfreyt, P. Surface tensions of linear and branched alkanes from Monte Carlo simulations using the anisotropic united atom model. The Journal of Physical Chemistry B 2008, 112, 13885–13897. [Google Scholar] [CrossRef]
- Müller, E.; Mejía, A. Interfacial properties of selected binary mixtures containing n-alkanes. Fluid Phase Equilibria 2009, 282, 68–81. [Google Scholar] [CrossRef]
- Maghari, A.; Najafi, M. On the calculation of surface tensions of n-alkanes using the modified SAFT-BACK-DFT approach. Journal of Solution Chemistry 2010, 39, 31–41. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Rasa, H.; Naghibi, S. Experimental and theoretical study of surface tension of n-pentane, n-heptane, and some of their mixtures at different temperatures. The Journal of Chemical Thermodynamics 2010, 42, 110–113. [Google Scholar] [CrossRef]
- Mohsen-Nia, M. Measurement and modelling of surface tensions of systems containing n-hexadecane, n-heptane and n-pentane. Physics and Chemistry of Liquids 2011, 49, 608–614. [Google Scholar] [CrossRef]
- Müller, E.A.; Mejia, A. Comparison of united-atom potentials for the simulation of vapor–liquid equilibria and interfacial properties of long-chain n-alkanes up to n-C100. The Journal of Physical Chemistry B 2011, 115, 12822–12834. [Google Scholar] [CrossRef]
- Breure, B.; Peters, C. Modeling of the surface tension of pure components and mixtures using the density gradient theory combined with a theoretically derived influence parameter correlation. Fluid Phase Equilibria 2012, 334, 189–196. [Google Scholar] [CrossRef]
- Mejía, A.; Herdes, C.; Müller, E. Force fields for coarse-grained molecular simulations from a corresponding states correlation. Industrial & Engineering Chemistry Research 2014, 53, 4131–4141. [Google Scholar]
- Garrido, J.; Mejía, A.; Piñeiro, M.; Blas, F.; Müller, E. Interfacial tensions of industrial fluids from a molecular-based square gradient theory. AIChE Journal 2016, 62, 1781–1794. [Google Scholar] [CrossRef]
- Garrido, J.M.; Cartes, M.; Mejía, A. Coarse-grained theoretical modeling and molecular simulations of nitrogen+ n-alkanes:(n-pentane, n-hexane, n-heptane, n-octane). The Journal of Supercritical Fluids 2017, 129, 83–90. [Google Scholar] [CrossRef]
- Farzi, N.; Yazdanshenas, Z. Surface tension prediction of n-alkanes by a modified Peng-Robinson equation of state using the Density Functional Theory. Physical Chemistry Research 2017, 5, 569–583. [Google Scholar]
- Chaparro, G.; Mejía, A. Phasepy: A Python based framework for fluid phase equilibria and interfacial properties computation. Journal of Computational Chemistry 2020, 41, 2504–2526. [Google Scholar] [CrossRef]
- Miqueu, C.; Mendiboure, B.; Graciaa, A.; Lachaise, J. Modelling of the surface tension of pure components with the gradient theory of fluid interfaces: A simple and accurate expression for the influence parameters. Fluid Phase Equilibria 2003, 207, 225–246. [Google Scholar] [CrossRef]
- Cachadiña, I.; Hernández, A.; Mulero, Á. Surface tension of esters. Temperature dependence of the influence parameter in density gradient theory with Peng-Robinson equation of state. Case Studies in Thermal Engineering 2022, 36, 102193. [Google Scholar] [CrossRef]
- Sastri, S.; Rao, K. A simple method to predict surface tension of organic liquids. The Chemical Engineering Journal and the Biochemical Engineering Journal 1995, 59, 181–186. [Google Scholar] [CrossRef]
- Zuo, Y.X.; Stenby, E. Corresponding-states and parachor models for the calculation of interfacial tensions. The Canadian Journal of Chemical Engineering 1997, 75, 1130–1137. [Google Scholar] [CrossRef]
- Romero-Martinez, A.; Trejo, A. Surface tension of pure hydrocarbons. International Journal of Thermophysics 1998, 19, 1605–1614. [Google Scholar] [CrossRef]
- Miqueu, C.; Broseta, D.; Satherley, J.; Mendiboure, B.; Lachaise, J.; Graciaa, A. An extended scaled equation for the temperature dependence of the surface tension of pure compounds inferred from an analysis of experimental data. Fluid Phase Equilibria 2000, 172, 169–182. [Google Scholar] [CrossRef]
- Knotts, T.; Wilding, W.; Oscarson, J.; Rowley, R. Use of the DIPPR database for development of QSPR correlations: Surface tension. Journal of Chemical & Engineering Data 2001, 46, 1007–1012. [Google Scholar]
- Queimada, A.; Marrucho, I.M.; Coutinho, J. Surface tension of pure heavy n-alkanes: A corresponding states approach. Fluid Phase Equilibria 2001, 183, 229–238. [Google Scholar] [CrossRef]
- Queimada, A.J.; Silva, F.A.; Caço, A.I.; Marrucho, I.M.; Coutinho, J.A. Measurement and modeling of surface tensions of asymmetric systems: heptane, eicosane, docosane, tetracosane and their mixtures. Fluid Phase Equilibria 2003, 214, 211–221. [Google Scholar] [CrossRef]
- Queimada, A.J.; Marrucho, I.M.; Stenby, E.H.; Coutinho, J.A. Generalized relation between surface tension and viscosity: A study on pure and mixed n-alkanes. Fluid Phase Equilibria 2004, 222, 161–168. [Google Scholar] [CrossRef]
- Queimada, A.; Miqueu, C.; Marrucho, I.; Kontogeorgis, G.; Coutinho, J. Modeling vapor–liquid interfaces with the gradient theory in combination with the CPA equation of state. Fluid Phase Equilibria 2005, 228, 479–485. [Google Scholar] [CrossRef]
- Delgado, E.; Diaz, G. A molecular structure based model for predicting surface tension of organic compounds. SAR and QSAR in Environmental Research 2006, 17, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Pazuki, G.; Nikookar, M.; Sahranavard, L. Prediction of surface tension of pure hydrocarbons by an artificial neural network system. Petroleum Science and Technology 2011, 29, 2384–2396. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Use of artificial neural network-group contribution method to determine surface tension of pure compounds. Journal of Chemical & Engineering Data 2011, 56, 2587–2601. [Google Scholar]
- Gharagheizi, F.; Eslamimanesh, A.; Tirandazi, B.; Mohammadi, A.H.; Richon, D. Handling a very large data set for determination of surface tension of chemical compounds using quantitative structure–property relationship strategy. Chemical Engineering Science 2011, 66, 4991–5023. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Eslamimanesh, A.; Sattari, M.; Mohammadi, A.; Richon, D. Development of corresponding states model for estimation of the surface tension of chemical compounds. AIChE Journal 2013, 59, 613–621. [Google Scholar] [CrossRef]
- Roosta, A.; Setoodeh, P.; Jahanmiri, A. Artificial neural network modeling of surface tension for pure organic compounds. Industrial & Engineering Chemistry Research 2012, 51, 561–566. [Google Scholar]
- Mulero, A.; Parra, M.; Cachadiña, I. The Somayajulu correlation for the surface tension revisited. Fluid Phase Equilibria 2013, 339, 81–88. [Google Scholar] [CrossRef]
- Aleem, W.; Mellon, N.; Sufian, S.; Mutalib, M.; Subbarao, D. A model for the estimation of surface tension of pure hydrocarbon liquids. Petroleum Science and Technology 2015, 33, 1908–1915. [Google Scholar] [CrossRef]
- Randová, A.; Bartovská, L. Group contribution method: Surface tension of linear and branched alkanes. Fluid Phase Equilibria 2016, 429, 166–176. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, H.; Tian, J. Lielmezs–Herrick correlation for the temperature-dependent surface tension of hydrocarbons. International Journal of Modern Physics B 2016, 30, 1650154. [Google Scholar] [CrossRef]
- Farzi, R.; Esmaeilzadeh, F. Prediction of surface tension of pure hydrocarbons using Esmaeilzadeh-Roshanfekr equation of state and group contribution method. Fluid Phase Equilibria 2016, 427, 353–361. [Google Scholar] [CrossRef]
- Aleem, W.; Mellon, N. New relation between viscosity and surface tension for pure hydrocarbon liquids. Petroleum Science and Technology 2017, 35, 338–344. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Bayat, M. Prediction of surface tension of liquid normal alkanes, 1-alkenes and cycloalkane using neural network. Chemical Engineering Research and Design 2018, 137, 154–163. [Google Scholar] [CrossRef]
- Yaws, C.L.; Gabbula, C. Yaws’ Handbook of thermodynamic and physical properties of chemical compounds; Knovel, 2003.
- Mulero, A.; Cachadiña, I.; Bautista, D. Recommended correlations for the surface tension of n-alkanes. Journal of Physical and Chemical Reference Data 2021, 50, 023104. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D. A new two-constant equation of state. Industrial & Engineering Chemistry Fundamentals 1976, 15, 59–64. [Google Scholar]
- Robinson, D.; Peng, D. The characterization of the heptanes and heavier fractions. Gas Processors Association Report. Research Report RR-28, (booklet only sold by the GPA, Gas Processors Association). 1978; 1–366. [Google Scholar]
- Cahn, J.; Hilliard, J. Free energy of a nonuniform system. I. Interfacial free energy. The Journal of Chemical Physics 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Carey, B.; Scriven, L.; Davis, H. Semiempirical theory of surface tensions of pure normal alkanes and alcohols. AIChE Journal 1978, 24, 1076–1080. [Google Scholar] [CrossRef]
- Oliveira, M.; Coutinho, J.; Queimada, A. Surface tensions of esters from a combination of the gradient theory with the CPA EoS. Fluid Phase Equilibria 2011, 303, 56–61. [Google Scholar] [CrossRef]
- Hernández, A. Interfacial behavior prediction of alcohol+ glycerol mixtures using gradient theory. Chemical Physics 2020, 534, 110747. [Google Scholar] [CrossRef]
- Hernández, A.; Khosharay, S. Investigation on the Surface Tension and Viscosity of (dimethylsulfoxide+alcohol) Mixtures by Using Gradient Theory and Eyring’s Rate Theory. International Journal of Thermophysics 2020, 41, 1–22. [Google Scholar] [CrossRef]
- Hernández, A.; Zabala, D. Modeling of the Interfacial Behavior of Carbon Dioxide+ Methyl Myristate, Carbon Dioxide+ Palmitate, and Carbon Dioxide+ Methyl Myristate+ Methyl Palmitate Mixtures Using CPA-EOS and Gradient Theory. International Journal of Thermophysics 2021, 42, 1–21. [Google Scholar] [CrossRef]
- Biglar, F.; Hernández, A.; Khosharay, S. Modeling of the Interfacial Behavior of CO2 + H2O and H2S + H2O with CPA EOS and Gradient Theory. International Journal of Thermophysics 2021, 42, 1–19. [Google Scholar] [CrossRef]
- Yang, A.; Fleming III, P.; Gibbs, J. Molecular theory of surface tension. The Journal of Chemical Physics 1976, 64, 3732–3747. [Google Scholar] [CrossRef]
- Liang, X.; Michelsen, M.; Kontogeorgis, G. A density gradient theory based method for surface tension calculations. Fluid Phase Equilibria 2016, 428, 153–163. [Google Scholar] [CrossRef]
- Garrido, J.; Polishuk, I. Toward development of a universal CP-PC-SAFT-based modeling framework for predicting thermophysical properties at reservoir conditions: inclusion of surface tensions. Industrial & Engineering Chemistry Research 2018, 57, 8819–8831. [Google Scholar]
- Chow, Y.; Eriksen, D.; Galindo, A.; Haslam, A.; Jackson, G.; Maitland, G.; Trusler, J. Interfacial tensions of systems comprising water, carbon dioxide and diluent gases at high pressures: Experimental measurements and modelling with SAFT-VR Mie and square-gradient theory. Fluid Phase Equilibria 2016, 407, 159–176. [Google Scholar] [CrossRef]
- Cornelisse, P.; Peters, C.; de Swaan Arons, J. Application of the Peng-Robinson equation of state to calculate interfacial tensions and profiles at vapour-liquid interfaces. Fluid Phase Equilibria 1993, 82, 119–129. [Google Scholar] [CrossRef]
- Larsen, P.; Maribo-Mogensen, B.; Kontogeorgis, G. A collocation method for surface tension calculations with the density gradient theory. Fluid Phase Equilibria 2016, 408, 170–179. [Google Scholar] [CrossRef]
- Pérez-López, J.; González-Ortiz, L.; Leiva, M.; Puig, J. Estimation of surface tension of pure fluids using the gradient theory. AIChE journal 1992, 38, 753–760. [Google Scholar] [CrossRef]
- Zuo, Y.X.; Stenby, E. A linear gradient theory model for calculating interfacial tensions of mixtures. Journal of Colloid and Interface Science 1996, 182, 126–132. [Google Scholar] [CrossRef]
- Mejía, A.; Segura, H.; Wisniak, J.; Polishuk, I. Correlation and prediction of interface tension for fluid mixtures: An approach based on cubic equations of state with the Wong-Sandler mixing rule. Journal of Phase Equilibria and Diffusion 2005, 26, 215–224. [Google Scholar] [CrossRef]
- Mejía, A.; Segura, H.; Vega, L.; Wisniak, J. Simultaneous prediction of interfacial tension and phase equilibria in binary mixtures: An approach based on cubic equations of state with improved mixing rules. Fluid Phase Equilibria 2005, 227, 225–238. [Google Scholar] [CrossRef]
- Oliveira, M.; Freitas, S.; Llovell, F.; Vega, L.; Coutinho, J.A. Development of simple and transferable molecular models for biodiesel production with the soft-SAFT equation of state. Chemical Engineering Research and Design 2014, 92, 2898–2911. [Google Scholar] [CrossRef]
- Haarmann, N.; Reinhardt, A.; Danzer, A.; Sadowski, G.; Enders, S. Modeling of Interfacial Tensions of Long-Chain Molecules and Related Mixtures Using Perturbed Chain-Statistical Associating Fluid Theory and the Density Gradient Theory. Journal of Chemical & Engineering Data 2019, 65, 1005–1018. [Google Scholar]
- Avaullee, L.; Trassy, L.; Neau, E.; Jaubert, J.N. Thermodynamic modeling for petroleum fluids I. Equation of state and group contribution for the estimation of thermodynamic parameters of heavy hydrocarbons. Fluid Phase Equilibria 1997, 139, 155–170. [Google Scholar] [CrossRef]
- Lopez-Echeverry, J.; Reif-Acherman, S.; Araujo-Lopez, E. Peng-Robinson equation of state: 40 years through cubics. Fluid Phase Equilibria 2017, 447, 39–71. [Google Scholar] [CrossRef]
- Jaubert, J.N.; Mutelet, F. VLE predictions with the Peng–Robinson equation of state and temperature dependent kij calculated through a group contribution method. Fluid Phase Equilibria 2004, 224, 285–304. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I.; Parra, M. Recommended correlations for the surface tension of common fluids. Journal of Physical and Chemical Reference Data 2012, 41, 043105. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I. Recommended correlations for the surface tension of several fluids included in the REFPROP program. Journal of Physical and Chemical Reference Data 2014, 43, 023104. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I.; Sanjuán, E. Recommended correlations for the surface tension of aliphatic, carboxylic, and polyfunctional organic acids. Journal of Physical and Chemical Reference Data 2016, 45, 033105. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I.; Bautista, D. Recommended Correlations for the Surface Tension of n-Alkanes. Journal of Physical and Chemical Reference Data 2021, 50, 023104. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I.; Vegas, A. Recommended correlations for the surface tension of 80 esters. Journal of Physical and Chemical Reference Data 2021, 50, 033106. [Google Scholar] [CrossRef]
- Mulero, A.; Cachadiña, I.; Vegas, A. Recommended correlations for the surface tension of aromatic, polyfunctional and glyceride esters. Journal of Physical and Chemical Reference Data 2022, 51, 023102. [Google Scholar] [CrossRef]
- Rowley, R.; Wilding, W.; Oscarson, J.; Knotts, T.; Giles, N. DIPPR®Data Compilation of Pure Chemical Properties; AIChE, New York, NY, 2020. [CrossRef]
- DETHERM, Thermophysical Properties of Pure Substances & Mixtures. DECHEMA, Gesellschaft für Chemische Techink und Biotechnologie e.V., 2018. Accessed 2018.
- Wohlfarth, C.; Wohlfarth, B. Surface Tension of Pure Liquids and Binary Liquid Mixtures; Springer, 1997.
- Wohlfarth, C. Surface Tension of Pure Liquids and Binary Liquid Mixtures, Supplement to IV/16; Springer, 2008.
- Wohlfarth, C.; Lechner, M. Surface Tension of Pure Liquids and Binary Liquid Mixtures: Supplement to Volume IV/24; Springer, 2016.
- Rowley, R.; Wilding, W.; Oscarson, J.; Knotts, T.; Giles, N. DIPPR®Data Compilation of Pure Chemical Properties; AIChE, New York, NY, 2022. [CrossRef]
- Sugden, S. VI. The variation of surface tension with temperature and some related functions. Journal of the Chemical Society Transactions 1924, 125, 32–41. [Google Scholar] [CrossRef]
- Cornelisse, P. The Gradient Theory Applied, Simultaneous Modelling of Interfacial Tension and Phase Behaviour. PhD thesis, Technische Universiteit Delft, 1997.
- Soreide, I. Improved phase behavior predictions of petroleum reservoir fluids from a cubic equation of state. Dr. Techn. Thesis 1989. [Google Scholar]
- Press, W.; Teukolsky, S.; Vetterling, W.; Flannery, B. Numerical Recipes, 3rd edition: The art of scientific computing; Cambridge University Press, 2007.
- Poling, B.; Prausnitz, J.; O’Connell, J. The properties of gases and liquids; Vol. 5, McGraw-Hill, New York, 2001.
- Di Nicola, G.; Pierantozzi, M. A new scaled equation to calculate the surface tension of ketones. Journal of Thermal Analysis and Calorimetry 2014, 116, 129–134. [Google Scholar] [CrossRef]
- Di Nicola, G.; Coccia, G.; Pierantozzi, M. Surface tension of silanes: A new equation. Fluid Phase Equilibria 2016, 418, 88–93. [Google Scholar] [CrossRef]
- Di Nicola, G.; Coccia, G.; Pierantozzi, M. A new equation for the surface tension of carboxylic acids. Fluid Phase Equilibria 2016, 417, 229–236. [Google Scholar] [CrossRef]
- Di Nicola, G.; Coccia, G.; Malvagi, L.; Pierantozzi, M. New equation for the liquid viscosity of silanes. Journal of Thermophysics and Heat Transfer 2017, 31, 832–840. [Google Scholar] [CrossRef]


| CN | Fluid | MAPD | MD | ||||||
| ) | (%) | (%) | (%) | ||||||
| 1 | methane | 7.5(12+) | 5.086(88+) | -3.35(34+) | 127/126 | 0.97 | -0.28 | 8.5 | 0.11 |
| 2 | ethane | 5.1(10+) | 3.68(13+) | -3.63(31+) | 163/160 | 2.06 | -0.77 | 19.2 | 0.01 |
| 3 | propane | 4.92(48−) | 3.63(16−) | -3.08(24−) | 193/191 | 1.80 | 0.04 | 27.2 | 0.01 |
| 4 | n-butane | 3.70(51+) | 3.44(12+) | -3.75(23−) | 126/118 | 3.14 | 1.17 | 48.9 | 0.01 |
| 5 | n-pentane | 3.84(76−) | 3.488(80−) | -3.25(21−) | 149/143 | 1.72 | 1.03 | 36.5 | 0.01 |
| 6 | n-hexane | 3.8(14−) | 3.574(64+) | -3.18(17+) | 270/269 | 0.97 | 0.18 | 12.8 | 0.07 |
| 7 | n-heptane | 4.32(74+) | 3.560(68+) | -2.78(20+) | 363/357 | 0.89 | 0.09 | 9.7 | 0.22 |
| 8 | n-octane | 5.5(20−) | 3.702(65+) | -2.55(23+) | 196/194 | 1.04 | 0.43 | 41.1 | 0.01 |
| 9 | n-nonane | 0.0(81+) | 3.573(44+) | -2.86(21+) | 78 | 0.41 | 0.07 | 3.0 | 0.80 |
| 10 | n-decane | 3.8(26−) | 3.686(62−) | -2.16(27+) | 149 | 0.95 | -0.08 | 5.8 | 0.68 |
| 11 | n-undecane | 0.0(34+) | 3.663(53+) | -2.25(27+) | 60 | 0.55 | 0.05 | 2.6 | 0.49 |
| 12 | n-dodecane | 5.2(38+) | 3.633(65−) | -2.09(33−) | 100 | 0.91 | 0.30 | 6.7 | 0.91 |
| 13 | n-tridecane | 4(12+) | 3.655(45+) | -2.22(28+) | 48 | 0.33 | -0.02 | 1.3 | 0.69 |
| 14 | n-tetradecane | 15(14−) | 3.767(53−) | -1.85(44+) | 49 | 0.49 | 0.07 | 1.9 | 0.97 |
| 15 | n-pentadecane | 12(44+) | 3.705(44+) | -1.97() | 40 | 0.40 | -0.01 | 2.0 | 0.67 |
| 16 | n-hexadecane | 7.7() | 3.876(64+) | -2.19(67−) | 127 | 1.33 | 0.12 | 6.8 | 0.40 |
| 17 | n-heptadecane | 7.3(11−) | 3.914() | -2.46() | 44 | 0.44 | 0.08 | 2.1 | 0.78 |
| 18 | n-octadecane | 8.0() | 3.990(59−) | -2.36(30−) | 39 | 0.48 | 0.05 | 1.9 | 0.79 |
| 19 | n-nonadecane | 8.13(79−) | 3.981(67−) | -2.48() | 23 | 0.70 | 0.02 | 5.4 | 0.34 |
| 20 | n-eicosane | 7.7(11+) | 4.079(75−) | -2.78(26−) | 38 | 1.04 | -0.10 | 11.7 | 0.33 |
| 21 | n-heneicosane | 8.0(10+) | 4.225(76+) | -2.83() | 28 | 0.47 | -0.18 | 2.8 | 0.07 |
| 22 | n-docosane | 7.8(12+) | 4.223(78+) | -2.75(31+) | 32 | 0.58 | -0.28 | 2.9 | 0.07 |
| 23 | n-tricosane | 7.8(12+) | 4.219(84−) | -2.84(27+) | 36 | 0.98 | 0.12 | 3.4 | 0.06 |
| 24 | n-tetracosane | 7.9(13+) | 4.286(96+) | -2.79(31+) | 36 | 0.93 | -0.11 | 3.8 | 0.98 |
| 25 | n-pentacosane | 8.17(67+) | 4.330() | -2.54(10−) | 15 | 0.29 | -0.21 | 3.4 | 0.06 |
| 26 | n-hexacosane | 8.2(11+) | 4.352(49−) | -2.43(19−) | 31 | 0.30 | -0.04 | 3.7 | 0.06 |
| 27 | n-heptacosane | 8.07(60+) | 4.331(41−) | -2.42() | 16 | 0.31 | -0.19 | 3.8 | 0.06 |
| 28 | n-octacosane | 7.80() | 4.24() | -2.35(25−) | 24 | 1.67 | 1.26 | 7.7 | 0.78 |
| 29 | n-nonacosane | 7.70(58+) | 4.278() | -2.33() | 16 | 0.31 | -0.18 | 3.9 | 0.06 |
| 30 | n-triacontane | 7.72() | 4.276() | -2.32(19−) | 22 | 1.29 | 0.86 | 6.0 | 0.84 |
| 32 | n-dotriacontane | 7.58(90+) | 4.356() | -2.24(17−) | 25 | 0.32 | -0.09 | 4.0 | 0.06 |
| 36 | n-hexatriacontane | 7.65(58) | 4.399(58) | -2.15() | 18 | 0.43 | -0.13 | 4.6 | 0.06 |
| Overall mean absolute percentage deviation (defined in Eq. (30)) | 0.79 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





