1. Background
Dysnatremias can cause increased morbidity and contribute to poor outcomes, including increased mortality rates and higher medical costs [
1,
2]. The pathophysiology of these clinical disorders is complex, and clinicians often have difficulty in both the diagnosis and management of these patients [
3,
4]. Focused treatment is critical, since these electrolyte disorders can cause neurologic complications and rapid changes in plasma sodium concentrations can lead to severe brain injury [
5]. Treatment is often guided by formulas that can predict changes in sodium concentration after a hypertonic saline infusion or after a correction of a water deficit [
2]. Important issues in understanding clinical disorders with abnormal serum sodium concentrations include the distribution of sodium in tissues, the evaluation of patients with hyponatremia and hypernatremia, and the initial approaches to the management of these patients.
This discussion depends on several important ideas, including sodium balance, serum sodium concentration, and sodium storage. Sodium balance is a dynamic process involving sodium intake, distribution, storage, and excretion. Sodium storage involves several compartments in the body in addition to extracellular fluid which have an uncertain size and an uncertain sodium content. Clinicians depend on clinical information to make estimates of volume status and laboratory tests to measure electrolytes to make decisions regarding the management of dysnatremias. There is often uncertainty in these decisions.
2. Introduction
The body regulates effective plasma osmolality by carefully controlling water intake and excretion using an osmoregulatory system, that maintains sodium in a range of 135-145 mmol/L[
5]. The percentage of body water in men and women corresponds to about 60% of their body weight or about 42 liters. Water is the most common solvent in which electrolytes are present in varying concentrations in the body, and total body water (TBW) includes both the intracellular compartment (55-65% of TBW) and the extracellular compartment (35-45% of TBW) [
6,
7,
8]. The differences between extracellular fluid, which contains a large percentage of sodium, chloride, and bicarbonate ions plus nutrients, such as glucose, fatty acids, and amino acids, and intracellular fluid, which contains a large percentage of potassium, magnesium, and phosphate ions, were determined decades ago [
6,
7].
Changes in sodium concentrations can have direct effects on its distribution and storage. For example, a 5% increase of serum sodium concentration can increase endothelial cells rigidity by 25% and cause cellular dysfunction [
9]. Deterioration of the endothelial glycocalyx that is mostly composed by heparan sulfate also occurs with high concentrations of sodium [
10]. A model for sodium transferring out of the vascular space across the endothelium involves two barriers; the first is the endothelial glycocalyx, which selectively buffers sodium ions with its negatively charged proteoglycans, and the second is the endothelial plasma membrane, which contains sodium channels [
9]. These two barriers regulate the distribution of sodium into interstitial fluid and other compartments.
3. Sodium and Skin Storage
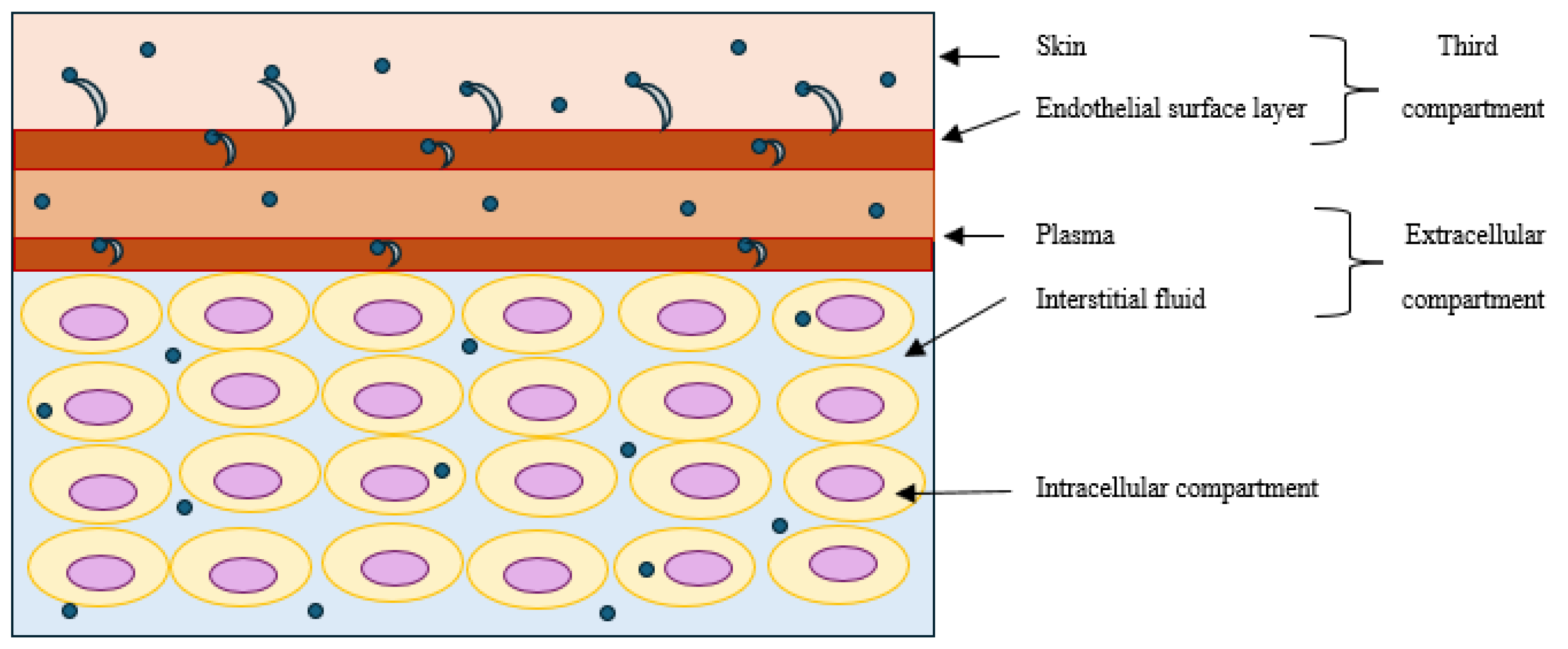
In addition to the traditionally described compartments, sodium can also be stored without water retention in the skin and on endothelial surfaces (Figure 1), and these two sites form a dynamic third compartment [
5,
11]. Recent studies have demonstrated that the skin can function as a “kidney-like” countercurrent system [
12]. In conditions with low sodium concentrations, sodium ions diffuses paracellularly through the glycocalyx of blood vessels into the skin; in contrast, in conditions with high sodium concentrations, sodium can damage the glycocalyx and activate vascular epithelial sodium channels (ENac), which allow transfer of sodium from the vasculature into the skin [
13]. Warner and colleagues found large gradients of sodium, potassium, and chloride in the stratum corneum of the skin using electron probe analysis and analytical electron microscopy [
14]. The relevance of sodium measurements in basic physiology and its significance has led to the development of tools, such as the sodium-MRI (also known as
23Na magnetic resonance imaging or
23Na-MRI), which produces images using signals from endogenous sodium nuclei as a substitute for proton measurements for the accurate assessment of sodium in various tissues [
15]. Kopp found that the use of
23Na magnetic resonance imaging can determine the sodium content or concentration in the skin; these values vary with age and sex, and patients with refractory hypertension often have increased tissue sodium storage [
16].
The negatively charged glycosaminoglycans in the skin are likely involved in sodium storage; Titze and his coinvestigators found concentrations of sodium in skin up to 180-190 mmol/L in experimental studies in rats and found that increasing skin sodium occurred with increasing glycosaminoglycans content in the skin [
17]. Sodium may affect the immunological responses in the body [
18,
19]. Sodium content has been analyzed in infected tissues, and it was found that cutaneous sodium stores increased immune-mediated host defense by increasing nitric oxide secretion and controlling the disease in protozoan infections [
18]. Studies by Selvarajah suggest that the skin can buffer dietary sodium loads and reduce the hemodynamic changes in blood pressure, stroke volume, and peripheral resistance during increased salt intake, especially in men [
20].
Increased sodium content in the skin may contribute to other disorders. Patients with type 2 diabetes mellitus (DM) have higher skin sodium content than patients with primary hypertension, and increased tissue sodium content is independently associated with hypertrophic vascular remodeling in patients with type 2 DM [
21,
22]. Sodium accumulation in patients with chronic kidney disease (CKD) is associated with higher blood pressures and left ventricular hypertrophy [
23]. Sodium content in the skin have also been studied in dermatological and immunological disorders, but studies have not defined a standard reference range for skin sodium concentrations or content [
24,
25,
26,
27,
28,
29].
4. Sodium Homeostasis and Cardiovascular Function
Sodium ingestion and absorption occur periodically with food during the day. These boluses undergo distribution to maintain extracellular volume, basic cellular functions, and cardiovascular stability. In addition, sodium stores in the skin, muscle, and bone maintain reserves to offset temporary imbalances, including inadequate intake and excessive intake.
The transport of sodium through membranes is key since Na
+/K
+ ATPase maintains a sodium gradient that participates in several sodium dependent transport mechanisms used in the regulation of glucose uptake, excitatory neurotransmitters, calcium signaling, acid-base balance, renal salt reabsorption, and plasma osmolality [
30]. These essential functions are present in a steady state, but they can also increase in response to sudden stimuli.
Circulatory stability depends on regulatory mechanisms with an afferent limb, based on volume and stretch detectors distributed in the vascular bed and an efferent effector limb (
Table 1). Adjustments in the effector mechanisms occur in response to afferent stimuli and modify vascular tone and cardiac output [
8].
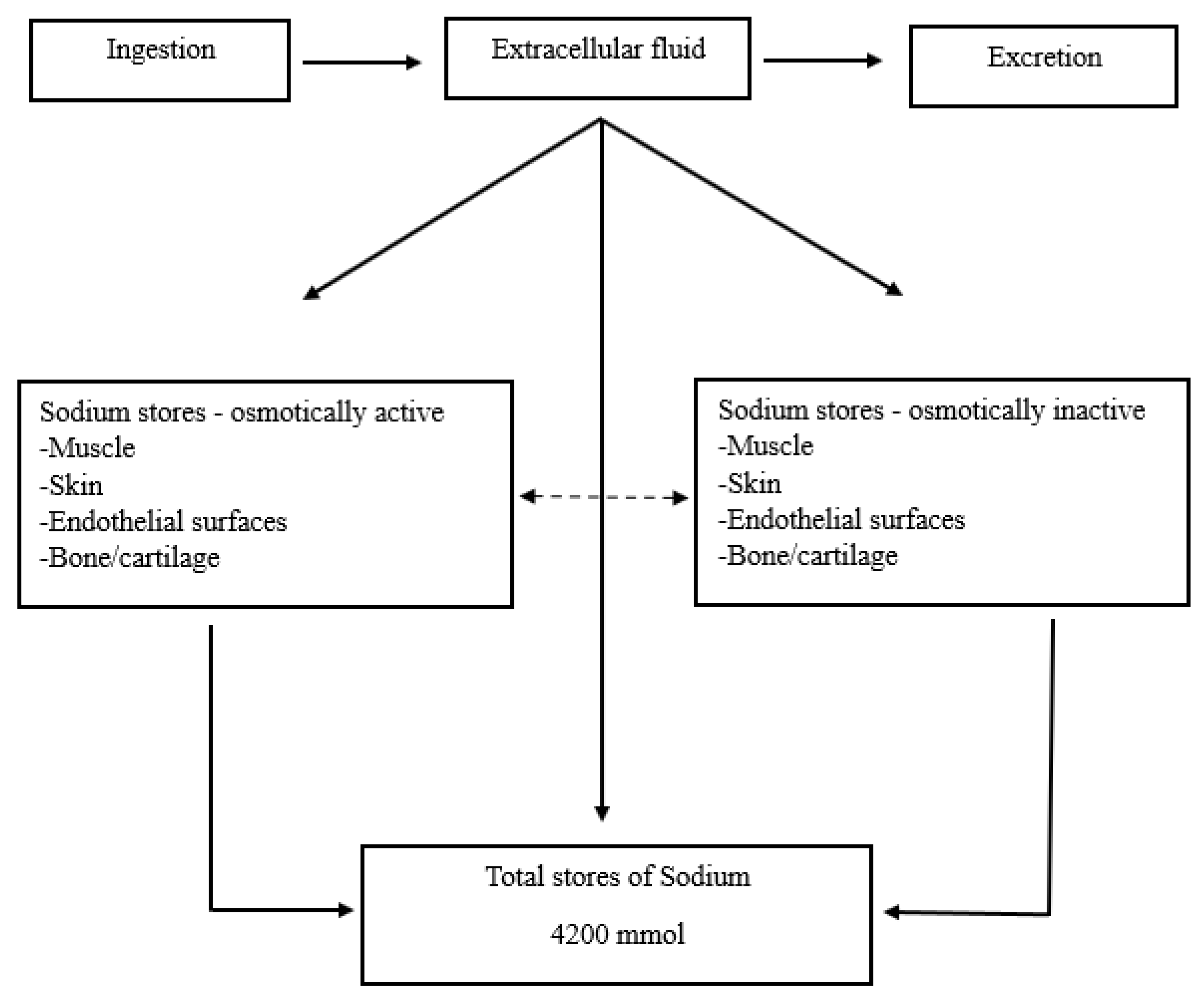
Plasma sodium concentrations reflect an average dietary intake of 90 to 250 mEq per day. Sodium excretion usually matches sodium intake, and on an average diet, urine sodium excretion will range between 80 and 180 mEq per day (Figure 2) [
31]. Long term sodium balance has been studied, and a sodium reservoir exists which can store significant amounts of sodium in an osmotically inactive form. This conclusion is based on prospective studies for 135 days in healthy subjects who had increased total body sodium content but no weight gain [
32]. Another study reported by Rakova included men in a controlled long-term simulated space-flight program for 105 and 205 days and found rhythmic sodium excretory and retention patterns that were independent of blood pressure, body water, and salt intake [
33].
Total body sodium content is regulated by the interaction of several effector systems, each of which prevents large variations in response to large variations in sodium intake [
34,
35]. Arginine vasopressin (AVP) is one of the two major physiological mechanisms in place to maintain effective plasma osmolality within normal ranges of ± 5% tolerance (280-295 mOsm/kg) and thirst is the second one. Changes in plasma osmolality of as little as 1-2% stimulate AVP secretion which in turn increases urine osmolality and decreases urine volume. ECF volume homeostasis involves regulation of both thirst and AVP resulting in control of natriuresis and sodium ingestion [
36]. Borst described the relationship between the fluid balance of the body and circulation [
37]. He explained that either fluid excess or depletion can change the volume of circulating blood; hence, a positive fluid balance will increase renal fluid output, and a negative balance reduces fluid excretion. Therefore, sodium homeostasis and blood pressure regulation are tightly intertwined and depend in part on minute-to-minute fluctuations in renal function [
38].
5. Edema and Sodium
Disorders in sodium regulation can result in edema. For this to happen, two factors require consideration: an increase of extracellular fluid and an imbalance of intravascular fluid exchange [
39]. Sodium disturbances and water imbalance can occur in different contexts such as the following.
Heart Failure: This disorder is a pathological state of increased extracellular volume with sodium overload. The two main components of hyponatremia are the result of impaired water excretion (dilutional) and depletion hyponatremia from diuretics and potassium loss. Sodium retention develops in congestive heart failure secondary to reductions in renal blood flow produced by α and β-catecholamines, ADH hormone, endothelins, and angiotensin II, activation of the tubuloglomerular feedback system, which enhances proximal sodium absorption, activation of apical sodium channels (ENaC) by aldosterone and vasopressin, and resistance to atrial natriuretic peptide in the inner medullary collecting duct [
40].
Chronic Kidney Disease: Since sodium is the principal osmotically active ion in extracellular fluid, its concentration varies with fluid volume. Rapid changes in sodium concentration can cause hyponatremia if there is an excess of water intake or other conditions in which the kidneys cannot dilute urine and excrete free water [
41]. The ability to dilute or concentrate urine is decreased in patients with eGFRs between 15 and 60 ml/min [
42].
Nephrotic syndrome: The contraction of blood volume has a primary role in causing sodium retention through the activation of compensatory hormonal mechanisms [
43]. These patients have a primary disturbance in renal sodium excretion. Depending on the stage in the development of nephrotic syndrome, the rate of progression in the development of hypoproteinemia, and the absolute levels of plasma oncotic pressures, functional hypovolemia can develop, resulting in secondary sodium retention.
Variations in plasma oncotic pressure and glomerular basement membrane permeability also influence sodium excretion [
44]. Experimental studies by Udwan showed that oxidative stress causes angiotensin-stimulated secretion of aldosterone and is required for development of edema [
45]. An increased urinary plasminogen-plasmin to creatinine ratio is an independent factor in edema formation in adults and also supports the idea that plasmin-dependent ENaC is involved in sodium retention and edema formation [
46].
Hepatic disorders: In liver disorders, there is a state of water and sodium retention. There is a maladaptive response triggered by intra-hepatic vascular resistance leading to increased nitric oxide (NO) release, splanchnic arterial vasodilation, increased vascular endothelial growth factor (VEGF), and portosystemic shunting, resulting in a low effective arterial blood volume (EABV) with a low systemic vascular resistance (SVR) and high cardiac output (CO) [
47]. The low EABV activates systemic signals that lead to water reabsorption.
The pathophysiological state is driven by intra-hepatic and portal vein hemodynamic pressures that activate angiotensin II leading to proximal tubular sodium reabsorption by sodium hydrogen exchanger (NHE) and NaPi (sodium phosphate cotransporters), which leads to concomitant water reabsorption through the apical side. In addition to sodium reabsorption in the distal convoluted tubule by sodium chloride cotransporter (NCC), there is also evidence of catecholamine amplification in cirrhotic states that activate NHE reabsorption and aldosterone-mediated sodium retention via ENAC [
48,
49]. The excess of water is mediated by AVP and aquaporin channel (AQP2) insertion. Either due to intrahepatic physical forces or changes in the composition of intrahepatic blood (oxygen tension, hormones, concentration of substances absorbed in the gut, etc.), abnormal sodium retention is initiated and edema develops [
50]. In patients with hepatorenal syndrome, there is a decrease in systemic vascular resistance, hypovolemia, and hypotension that causes high-pressure baroreceptors in the carotid body and aortic arch to activate the renin-angiotensin-aldosterone system, the sympathetic nervous system, and the release of vasopressin to maintain effective arterial blood volume and increase cardiac output and heart rate. These mechanisms have negative effects on renal function by increasing sodium and water retention and by constricting renal vessels that reduce renal blood flow [
51,
52].
These medical disorders have chronic effects on sodium and water regulation. They also periodically undergo acute decompensation which can increase fluid and electrolyte management problems. Finally, patients may have more than one disorder which complicates clinical assessment. Clinicians manage these disorders frequently in hospitalized patients, and a "simple" rule to manage sodium disorders could provide an effective initial approach to correcting fluid and electrolytes in these patients.
6. The Beginning of Sodium Management
In 1958, Edelman et al. evaluated the relationships between serum sodium concentration, serum osmolarity and total exchangeable sodium (TBNa+), total exchangeable potassium (TBK+), and total body water (TBW) [
53]. Ninety- eight chronically ill patients were studied, and the authors performed simultaneous measurements of serum sodium concentration, serum osmolarity, total exchangeable sodium, total exchangeable potassium, and total body water. The TBW was measured by dilution of deuterium oxide, rapid exchangeable TBNa
+ and TBK
+ were measured with radioisotopes, and serum Na
+ was measured with flame photometry. The relationship between TBNa
+, TBK
+, and TBW was expressed in the following formula:
Based on their data, the authors established the effect of the three major components of body composition on serum sodium concentrations. Edelman recognized the intercept resulting from the linear regression of the data could have physiological significance. The zero intercept of the regression equation for plasma sodium concentration in plasma water versus (Nae+ + Ke+)/ TBW is a negative constant (-25.6 mEq/L), and this measures the amount of osmotically inactive exchangeable sodium and potassium per unit of body water.
Based on these findings, the formulas for calculating sodium deficits were reexamined. In 1986, Rose simplified Edelman’s formula and emphasized the relevance of urinary losses of water, sodium, and potassium to determine the direction and magnitude of change in sodium concentration due to these losses. He expressed it as serum sodium concentration rather than plasma water concentration [
54].
Renal and extrarenal fluid and electrolyte losses affect the responses in patients during the treatment of both hyponatremia and hypernatremia. This was considered by Barsoum and Levine, and due to the lack of available formulas to account for these aspects, a new formula was proposed to provide better estimates of changes in serum sodium concentration with infused fluid, while also considering renal losses[
55].
Nguyen and Kurtz noted that clinicians cannot usually measure Na
e+ and K
e+ and thought that the lack of these measurements required a different approach. They also considered additional factors, such as changes in plasma sodium in hyperglycemic states, transcellular shifts of sodium and potassium in hypokalemia-induced hyponatremia, and a component of the total Na
e+ and K
e+ that was osmotically inactive and could not affect the concentration of sodium in plasma water (Na
pw+) [
56]. They created new formulas to calculate the volume of the infusion needed to produce a desired change in serum sodium concentration, which accounted for gains and losses of water, sodium, and potassium during treatment, electrolyte free urinary water clearance, the effect of fluid imbalance on Na
s+ in hyperglycemia states, and volume of water required to be infused in patients with hypervolemic hypernatremia who were on loop diuretics [
57].
Due to the challenges during the management of hyponatremia, Adrogué and Madias proposed a practical approach to manage this disorder with a focus on a diagnostic evaluation directed at the pathogenesis and known causes of hyponatremia to help clinicians create individualized plans for management and on the use of formulas for fluid administration [
58].
The formula for the effect of gaining 1L of any infusion (inf) on the patient’s sodium:
The formula for the effect of losing 1L of any fluid (fl) on the patient’s sodium:
These new formulas have helped clinicians manage patients with dysnatremias. However, questions about the original Edelman data were still present. Oppelaar and colleagues in Amsterdam reanalyzed the original data from the Edelman study to determine if the association between plasma water sodium concentration and exchangeable cation concentration per TBW is affected by body weight, edema, profound hyponatremia, and normal or high sodium concentrations [
3]. To estimate the effect of body composition, the authors determined body water percentage by dividing TBW (measured by isotopes) by body weight. They found that the Edelman equation was significantly affected by total body weight and the presence of edema. In addition, with plasma sodium levels below and above 133 mmol/L, the slope of the Edelman equation changes. The limitations in this study included the fact that only one subject had severe hypernatremia (sodium >150 mmol/L), and most of the subjects had hyponatremia.
The exact formulas are available in
Table 2.
7. Clinical Scenarios
Previous investigations have improved the basic understanding of factors that affect serum sodium levels and clinicians have used them in patients with common sodium disorders.
7.1. Hyponatremia
Hyponatremia is a common electrolyte disorder in both inpatients and outpatients. It is defined as a serum sodium concentration <135 mEq/L, and it represents an imbalance in which total body water is greater than the body solutes. The first step to understanding hyponatremia is to measure serum sodium and serum osmolality to determine if the serum is hypotonic, hypertonic, or isotonic [
59]. The ability to differentiate between these conditions is key for appropriate treatment; additional variables should be taken into consideration, and these include potassium levels, glucose levels, and losses of electrolytes in urine. The pathogenesis of hypokalemia-induced hyponatremia using a three-compartment model, and the Edelman equation was studied by Nguyen. who found changes in potassium can trigger an alteration in the plasma water sodium concentrations [
60].
The applicability of the formulas initially described has been studied by several investigators. Edelman’s concept was used in experimental models by Overgaard-Steensen, and these authors concluded that it is a valid tool in managing and preventing acute hyponatremia in most cases [
61]. Mohmand evaluated the response of hypertonic saline in 62 patients with hyponatremia [
61]. They wanted to evaluate the accuracy of the Adrogué-Madias formula and found that it underestimates the increase of sodium concentration after giving hypertonic saline and concluded that additional variables, such as unaccounted hypovolemia and other transitory causes of water retention can cause overcorrection. Nagase et al. conducted a retrospective study including 221 patients in Japan hospitalized between 2014 and 2020 [
63]. The authors wanted to investigate the potential factors including the predictive correction of patients with severe hyponatremia using the Edelman equation. Appropriate correction was considered when serum sodium concentration increased 4–10 mEq/L during the first 24 h and 18 mEq/L in the first 48 h after the beginning of treatment and occurred in approximately 60% of patients (132 out of 221).
These formulas are a valuable tool for management, but dynamic assessment remains important. Clinicians should remember that excessive correction can harm patients [
5].
7.2. Hypernatremia
Clinical conditions leading to hypernatremia usually reflect a relative deficit in total body water in relation to total body sodium content [
64]. Traditional recommendations advise restoring 50% of the calculated water deficit in the first 24 hours and the remaining deficit in the next 48-72 hours but do not consider ongoing water losses. Newer evidence in a large retrospective study by Chauhan et al. comparing two rates of correction at either less than 12 mmol/L/day or more than 12 mmol/L/day in patients with acute and chronic hypernatremia and found no differences in mortality between the two correction protocols [
65]. This result suggests that a more rapid correction rate is less of an issue in patients with hypernatremia than in patients with hyponatremia.
The initial management should identify the underlying cause of hypernatremia and determine the patient’s volume status and body weight to allow calculation of the water deficit. Placing patients into hypovolemic, euvolemic, or hypervolemic categories provides a starting point for deciding on the type and rate of fluid to be administered. Frequent clinical assessment and serial laboratory tests are essential to ensure gradual correction of hypernatremia by 0.5 mEq/L per hour and no more than 10 mEq/L in 24 hours in adult patients with chronic hypernatremia, and the goal to normalize plasma sodium in 24 hours in acute hypernatremia [
54]. Based in the Edelman equation, hypernatremia can develop through either a loss of free water or a gain of serum sodium. Studies evaluating the Adrogué-Madias formula have been done; for example, Liamis and his coinvestigators followed 204 patients with hyponatremia and 117 with hypernatremia [
66]. The formula did not accurately predict the serum sodium concentration changes in a subgroup of patients with hypernatremia who had severe extracellular volume depletion and reduced renal function. A cohort of patients with hyper- and normonatremia in the ICU was assessed by Lidner to compare different formulas (Adrogué-Madias, Barsoum-Levine, Kurtz-Nguyen and an electrolyte-free water clearance formula proposed by the authors) [
67]. All formulas correlated well with the measured change in serum sodium, but both over- and underestimations occurred. These findings are consistent in another study that showed the same four equations could not reliably predict sodium changes and inputs during a 12–30 h period [
68].
8. Discussion
The long-established concept of two-compartment model of sodium distribution has changed with new evidence of a third dynamic compartment [
5,
11]. The relevance of sodium content in the skin and other tissue sites has led to studies on its role under normal conditions and in different clinical disorders, such as hypertension, T2DM, CKD, SLE, multiple sclerosis, and others, using tools such as the
23Na-MRI. An increase or decrease in sodium concentration can affect the glycocalyx in the endothelium that works as a buffer in the body [
9]. Total body sodium is regulated by the interaction of several effector systems that prevent large variations in response to any large variations in sodium intake [
34,
35].
There are several mechanisms to preserve homeostasis of water and sodium in the normal range, and an imbalance in either water or sodium can cause both signs and symptoms, such as edema [
7,
8]. The role of sodium is important in several disorders, such as heart failure, chronic kidney disease, nephrotic syndrome, cirrhosis, and hepatorenal syndrome. Hyponatremia is a frequent clinical finding and a common electrolyte disorder worldwide [
69].
The concepts created by Edelman have guided the approach to dysnatremias for over half a century [
1]. Edelman’s work continues to be the major foundation that guides the expected changes in plasma sodium with changes in sodium, potassium, and water balance [
70]. However, his study has limitations, and sodium concentration was not corrected for the degree of glycemia, it doesn’t provide information about the time to equilibrium during dynamic fluctuations in fluid or sodium balance, and it does not include factors other than TBNa
+, TBK
+, or TBW measured in the steady state that could influence rapidly changing sodium concentrations during the development or treatment of sodium disorders. His equation has become a starting point for developing other predictive formulas, and some of these have included and some have omitted the regression’s intercept [
71]. The Adrogué -Madias formula, for example, evaluates the increase of sodium after infusion of 1 L of hypertonic saline, which has been valuable for hyponatremia management; however, it does not consider additional factors, such as loss of sodium, potassium, and water, exchanges of sodium between osmotically active and osmotically inactive sites during correction of hyponatremia, and changes in water binding to hydrophilic biopolymers during correction of hyponatremia [
72]. The Nguyen–Kurtz equation is the most developed variation and includes the primary slope of the Edelman formula (1.03) and the original x-intercept of −23.8, which are conceptually important [
56,
60,
73].
The use of formulas can help establish the initial infusion rates for the correction of both hypo-and hypernatremia, but they can underestimate the change in serum sodium achieved by infusion [
74]. Studies have compared the accuracy of all formulas, and while they have correlated well with the measured change in serum sodium, no formula is clearly superior [
67,
68]. Although several formulas have been available and some have more complexity than others, no formula seems superior [
1]. It is important to remember that predictive formulas that disregard urinary electrolyte and water losses are most likely to fail [
75].
Effective management of dysnatremias demands rigorous monitoring and regular treatment reassessment [
70]. It is critical to clarify the etiology of the disorder before treatment is started. A slow and controlled correction must be closely monitored to avoid neurological complications [
76]. Clinicians and students should continuously reinforce their knowledge to have a stronger foundation of concepts that will be needed in practice. Rondon-Berrios recently introduced the use of Edelman’s equation and incorporated it to bar diagrams named “Gamblegram”, and called it “Edelman Gamblegram”, which provides a teaching method for dysnatremias [
77]. This method could be used as a learning tool.
9. Conclusions
To understand sodium and all its clinical implications is a skill clinicians should develop and use during patient care; this will improve the management and outcomes of patients with dysnatremias. The initial formula created by Edelman more than 50 years ago has helped clinicians over time and encouraged others to create variations that can be used in a more practical way to predict serum sodium concentrations. However, recent studies have reported both overestimation and underestimation of changes in sodium that can lead to significant uncertainty about the management of patients. These formulas can still be considered as tools in early stages of management, but clinicians should depend on frequent measurements to provide reliable treatment.
Author Contributions
JF: CP, and KN participated in the literature review, manuscript drafting, manuscript editing, and manuscript revisions. Each author approved the submitted version of the manuscript. Each author agrees to be personally accountable for his or her contributions.
Funding
There were no funding sources available for this project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All information in this review was recovered from the references used for the review.
Conflicts of Interest
None.
References
- Rohrscheib M: Sam R, Raj DS, Argyropoulos CP, Unruh ML, Lew SQ, et al. Edelman Revisited: Concepts, Achievements, and Challenges. Front Med [Internet]. 2022 Jan 10 [cited 2024 Jun 25];8. Available from: https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2021.808765/full.
- Lindner G, Schwarz C. Electrolyte-Free Water Clearance versus Modified Electrolyte-Free Water Clearance: Do the Results Justify the Effort. Nephron Physiol. 2012 Mar 6;120(1):p1–5.
- Oppelaar JJ, Vuurboom MD, Wenstedt EFE, van Ittersum FJ, Vogt L, Olde Engberink RHG. Reconsidering the Edelman equation: impact of plasma sodium concentration, edema and body weight. Eur J Intern Med. 2022 Jun 1;100:94–101.
- Sam R, Feizi I. Understanding Hypernatremia. Am J Nephrol. 2012 Jun 27;36(1):97–104.
- Sterns RH. Disorders of Plasma Sodium — Causes, Consequences, and Correction. N Engl J Med. 2015 Jan 1;372(1):55–65.
- Walker WF, Johnston IDA. 2 - Water and Electrolyte Metabolism. In: Walker WF, Johnston IDA, editors. The Metabolic Basis of Surgical Care [Internet]. Butterworth-Heinemann; 1971 [cited 2024 Jun 25]. p. 13–47. Available from: https://www.sciencedirect.com/science/article/pii/B9780433345800500070.
- Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. 12th ed. Philadelphia, Pa: Saunders/Elsevier; 2011. 1091 p.
- Ellison DH, Schrier RW. Chapter 7 - Disorders of Extracellular Volume. In: Feehally J, Floege J, Tonelli M, Johnson RJ, editors. Comprehensive Clinical Nephrology [Internet]. Sixth. ELSEVIER; 2019 [cited 2024 Jun 25]. p. 80–92. Available from: https://drive.google.com/file/d/1nkhqmVouEuvgwZLLCAy4Nn158vDOXPtH/view?usp=drive_open&usp=embed_facebook.
- Oberleithner H. Two barriers for sodium in vascular endothelium? Ann Med. 2012 Jun;44(sup1):S143–8.
- Wenstedt EFE, Olde Engberink RHG, Vogt L. Sodium Handling by the Blood Vessel Wall: Critical for Hypertension Development. Hypertens Dallas Tex 1979. 2018 Jun;71(6):990–6.
- Olde Engberink RHG, Selvarajah V, Vogt L. Clinical impact of tissue sodium storage. Pediatr Nephrol. 2020 Aug 1;35(8):1373–80.
- Hofmeister LH, Perisic S, Titze J. Tissue sodium storage: evidence for kidney-like extrarenal countercurrent systems? Pflüg Arch - Eur J Physiol. 2015 Mar;467(3):551–8.
- Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012 Feb;463(2):269–78.
- Warner RR, Myers MC, Taylor DA. Electron Probe Analysis of Human Skin: Element Concentration Profiles. J Invest Dermatol. 1988 Jan 1;90(1):78–85.
- Giovannetti G, Flori A, Martini N, Francischello R, Aquaro GD, Pingitore A, et al. Sodium Radiofrequency Coils for Magnetic Resonance: From Design to Applications. Electronics. 2021 Jul 26;10(15):1788.
- Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, et al. 23Na Magnetic Resonance Imaging-Determined Tissue Sodium in Healthy Subjects and Hypertensive Patients. Hypertension. 2013 Mar;61(3):635–40.
- Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na + storage in the skin. Am J Physiol-Heart Circ Physiol. 2004 Jul;287(1):H203–8.
- Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, et al. Cutaneous Na+ Storage Strengthens the Antimicrobial Barrier Function of the Skin and Boosts Macrophage-Driven Host Defense. Cell Metab. 2015 Mar 3;21(3):493–501.
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013 Apr 25;496(7446):518–22.
- Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, et al. Novel Mechanism for Buffering Dietary Salt in Humans. Hypertension. 2017 Nov;70(5):930–7.
- Kannenkeril D, Karg MV, Bosch A, Ott C, Linz P, Nagel AM, et al. Tissue sodium content in patients with type 2 diabetes mellitus. J Diabetes Complications. 2019 Jul;33(7):485–9.
- Kannenkeril D, Jung S, Harazny J, Striepe K, Ott C, Dahlmann A, et al. Tissue sodium content correlates with hypertrophic vascular remodeling in type 2 diabetes. J Diabetes Complications. 2021 Dec 1;35(12):108055.
- Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J Am Soc Nephrol JASN. 2017 Jun;28(6):1867–76.
- Matthias J, Maul J, Noster R, Meinl H, Chao YY, Gerstenberg H, et al. Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci Transl Med. 2019 Feb 20;11(480):eaau0683.
- Huhn K, Linz P, Pemsel F, Michalke B, Seyferth S, Kopp C, et al. Skin sodium is increased in male patients with multiple sclerosis and related animal models | PNAS [Internet]. 2021 [cited 2024 Jul 4]. Available from: https://www.pnas.org/doi/full/10.1073/pnas.2102549118.
- Kopp C, Beyer C, Linz P, Dahlmann A, Hammon M, Jantsch J, et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatol Oxf Engl. 2017 Apr;56(4):556–60.
- Carranza-León DA, Oeser A, Marton A, Wang P, Gore JC, Titze J, et al. Tissue sodium content in patients with systemic lupus erythematosus: association with disease activity and markers of inflammation. Lupus. 2020 Apr;29(5):455–62.
- Maifeld A, Wild J, Karlsen TV, Rakova N, Wistorf E, Linz P, et al. Skin Sodium Accumulates in Psoriasis and Reflects Disease Severity. J Invest Dermatol. 2022 Jan;142(1):166-178.e8.
- Chattopadhyay A, Tully J, Shan J, Sheikh S, Ohliger M, Gordon JW, et al. Sodium in the skin: a summary of the physiology and a scoping review of disease associations. Clin Exp Dermatol. 2023 Jul 7;48(7):733–43.
- Gagnon KB, Delpire E. Sodium Transporters in Human Health and Disease. Front Physiol [Internet]. 2021 Feb 25 [cited 2024 Jun 25];11. Available from: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2020.588664/full.
- Pohl HR, Wheeler JS, Murray HE. Sodium and Potassium in Health and Disease. In: Sigel A, Sigel H, Sigel RKO, editors. Interrelations between Essential Metal Ions and Human Diseases [Internet]. Dordrecht: Springer Netherlands; 2013 [cited 2024 Jun 25]. p. 29–47. (Metal Ions in Life Sciences; vol. 13). Available from: https://link.springer.com/10.1007/978-94-007-7500-8_2.
- Titze J, Maillet A, Lang R, Gunga HC, Johannes B, Gauquelin-Koch G, et al. Long-term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002 Sep 1;40(3):508–16.
- Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, et al. Long-Term Space Flight Simulation Reveals Infradian Rhythmicity in Human Na+ Balance. Cell Metab. 2013 Jan;17(1):125–31.
- Bonventre JV, Leaf A. Sodium homeostasis: steady states without a set point. Kidney Int. 1982;21(6):880–3.
- Greger R. Physiology of Renal Sodium Transport. Am J Med Sci. 2000 Jan 1;319(1):51–62.
- Gizowski C, Bourque CW. The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrol. 2018 Jan;14(1):11–25.
- Borst JG, Borst-de Geus A. Hypertension explained by Starling’s theory of circulatory homoeostasis. The Lancet. 1963 Mar 30;281(7283):677–82.
- Bie P. Mechanisms of sodium balance: total body sodium, surrogate variables, and renal sodium excretion. Am J Physiol-Regul Integr Comp Physiol. 2018 Nov;315(5):R945–62.
- Chen J, Zeng R. Edema. In: Wan XH, Zeng R, editors. Handbook of Clinical Diagnostics [Internet]. Singapore: Springer Singapore; 2020 [cited 2024 Jun 26]. p. 11–2. Available from: http://link.springer.com/10.1007/978-981-13-7677-1_3.
- Andreoli T. Pathogenesis of Renal Sodium Retention in Congestive Heart Failure. Miner Electrolyte Metab. 1999 Apr 17;25(1–2):11–20.
- SALAJOVA KB, MALIK J, VALERIANOVA A. Cardiorenal Syndromes and Their Role in Water and Sodium Homeostasis. Physiol Res. 2024 Apr 30;73(2):173–88.
- Pedersen EB, Thomsen IM, Lauridsen TG. Abnormal function of the vasopressin-cyclic-AMP-aquaporin2 axis during urine concentrating and diluting in patients with reduced renal function. A case control study. BMC Nephrol. 2010 Dec;11(1):26.
- Usberti M, Gazzotti RM, Poiesi C, D’Avanzo L, Ghielmi S. Considerations on the Sodium Retention in Nephrotic Syndrome. Am J Nephrol. 1995;15(1):38–47.
- Vande Walle JGJ, Donckerwolcke RA. Pathogenesis of edema formation in the nephrotic syndrome. Pediatr Nephrol. 2001 Mar 8;16(3):283–93.
- Udwan K. Role of oxidative stress in primary sodium retention and edema formation in nephrotic syndrome [Internet] [phdthesis]. Université Pierre et Marie Curie - Paris VI; 2015 [cited 2024 Jun 26]. Available from: https://theses.hal.science/tel-01542593.
- Chen JL, Wang L, Yao XM, Zang YJ, Wang Y, Li ZJ, et al. Association of Urinary Plasminogen-Plasmin with Edema and Epithelial Sodium Channel Activation in Patients with Nephrotic Syndrome. Am J Nephrol. 2019 Jul 3;50(2):92–104.
- Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol WJG. 2014 Mar 14;20(10):2555–63.
- Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006 Oct;55(Suppl 6):vi1–12.
- Cárdenas A, Ginès P. 16 Sodium Balance in Cirrhosis. In: Burnier M, editor. Sodium in Health and Disease. First. CRC Press; 2007. p. 317–32. (978).
- Oliver JA, Verna EC. Afferent mechanisms of sodium retention in cirrhosis and hepatorenal syndrome. Kidney Int. 2010 Apr 2;77(8):669–80.
- Chmielewski J, Lewandowski R, Maddur H. Hepatorenal Syndrome: Physiology, Diagnosis and Management. Semin Interv Radiol. 2018 Aug;35(03):194–7.
- Badura K, Frąk W, Hajdys J, Majchrowicz G, Młynarska E, Rysz J, et al. Hepatorenal Syndrome—Novel Insights into Diagnostics and Treatment. Int J Mol Sci. 2023 Dec 14;24(24):17469.
- Edelman IS, Leibman J, O’meara MP, Birkenfeld LW. Interrelations Between Serum Sodium Concentration, Serum Osmolarity and Total Exchangeable Sodium, Total Exchangeable Potassium and Total Body Water1. J Clin Invest. 1958 Sep 1;37(9):1236–56.
- Rose BD. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986 Dec 1;81(6):1033–40.
- Barsoum NR, Levine BS. Current prescriptions for the correction of hyponatraemia and hypernatraemia: are they too simple? Nephrol Dial Transplant. 2002 Jul 1;17(7):1176–80.
- Kurtz I, Nguyen MK. Evolving concepts in the quantitative analysis of the determinants of the plasma water sodium concentration and the pathophysiology and treatment of the dysnatremias. Kidney Int. 2005 Nov;68(5):1982–93.
- Nguyen MK, Kurtz I. Derivation of a new formula for calculating urinary electrolyte-free water clearance based on the Edelman equation. Am J Physiol-Ren Physiol. 2005 Jan;288(1):F1–7.
- Adrogué HJ, Madias NE. The Challenge of Hyponatremia. J Am Soc Nephrol. 2012 Jul;23(7):1140.
- Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol. 2014 Nov;46(11):2153–65.
- Nguyen MK, Kurtz I. Role of potassium in hypokalemia-induced hyponatremia: lessons learned from the Edelman equation. Clin Exp Nephrol. 2004 Jun;8(2):98–102.
- Overgaard-Steensen C, Larsson A, Bluhme H, Tønnesen E, Frøkiær J, Ring T. Edelman’s equation is valid in acute hyponatremia in a porcine model: plasma sodium concentration is determined by external balances of water and cations. Am J Physiol-Regul Integr Comp Physiol. 2010 Jan;298(1):R120–9.
- Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol CJASN. 2007 Nov;2(6):1110–7.
- Nagase K, Watanabe T, Nomura A, Nagase FN, Iwasaki K, Nakamura Y, et al. Predictive correction of serum sodium concentration with formulas derived from the Edelman equation in patients with severe hyponatremia. Sci Rep. 2023 Jan 31;13(1):1783.
- Al-Absi A, Gosmanova EO, Wall BM. A Clinical Approach to the Treatment of Chronic Hypernatremia. Am J Kidney Dis. 2012 Dec 1;60(6):1032–8.
- Chauhan K, Pattharanitima P, Patel N, Duffy A, Saha A, Chaudhary K, et al. Rate of Correction of Hypernatremia and Health Outcomes in Critically Ill Patients. Clin J Am Soc Nephrol CJASN. 2019 May 7;14(5):656–63.
- Liamis G, Kalogirou M, Saugos V, Elisaf M. Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant. 2006 Jun 1;21(6):1564–9.
- Lindner G, Schwarz C, Kneidinger N, Kramer L, Oberbauer R, Druml W. Can we really predict the change in serum sodium levels? An analysis of currently proposed formulae in hypernatraemic patients. Nephrol Dial Transplant. 2008 Nov 1;23(11):3501–8.
- Hanna RM, Yang WT, Lopez EA, Riad JN, Wilson J. The utility and accuracy of four equations in predicting sodium levels in dysnatremic patients. Clin Kidney J. 2016 Aug;9(4):530–9.
- Burst V. Etiology and Epidemiology of Hyponatremia. In: Peri A, Thompson CJ, Verbalis JG, editors. Frontiers of Hormone Research [Internet]. S. Karger AG; 2019 [cited 2024 Jun 29]. p. 24–35. Available from: https://karger.com/books/book/123/chapter/5061998.
- Adrogué HJ, Madias NE. Osmotically Inactivated Sodium in Acute Hyponatremia: Stay With Edelman. Am J Kidney Dis. 2019 Sep 1;74(3):297–9.
- Portales-Castillo I, Sterns RH, Bress J, Proano RA. Where Do the Salt and Water Go? A Case of Profound Hyponatremia. Am J Kidney Dis Off J Natl Kidney Found. 2018 Dec;72(6):885–9.
- Wagner B, Malhotra D, Schmidt D, Raj DS, Khitan ZJ, Shapiro JI, et al. Hypertonic Saline Infusion for Hyponatremia: Limitations of the Adrogué-Madias and Other Formulas. Kidney360. 2023 Apr;4(4): e555.
- Nguyen MK, Kurtz I. Determinants of plasma water sodium concentration as reflected in the Edelman equation: role of osmotic and Gibbs-Donnan equilibrium. Am J Physiol-Ren Physiol. 2004 May;286(5): F828–37.
- Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013 Apr;28(2): 216.e11-216.e20.
- Sterns RH. Formulas for fixing serum sodium: curb your enthusiasm. Clin Kidney J. 2016 Aug;9(4):527–9.
- Förch A, Deetjen P, Heller AR. [Dysnatremia]. Anaesthesiol. 2023 Apr;72(4):293–306.
- Rondon-Berrios H. Edelman Gamblegrams: a tool to teach and learn disorders of water/plasma tonicity homeostasis. Adv Physiol Educ. 2024 Jun;48(2):200–4.
Table 1.
Homeostatic mechanisms for extracellular fluid volume.
Table 1.
Homeostatic mechanisms for extracellular fluid volume.
| Afferent (sensing) |
Efferent (effector) |
| Cardiopulmonary receptors (atrial, ventricular, pulmonary) |
Renin angiotensin aldosterone system (RAAS) |
| High-pressure baroreceptors (carotid, aortic, renal) |
Prostaglandins
Arginine vasopressin (AVP) |
| Central nervous system receptors |
Natriuretic peptides (ANP, BNP, CNP) |
| Hepatic receptors |
Other hormones (NO, Endothelin, Kallikrein-kinin system) |
Table 2.
Formulas for sodium management.
Table 2.
Formulas for sodium management.
| Year of publication |
Authors |
Formula |
| 1958 |
Edelman et al |
(Na+) pw = 1.11 x [(Nae+ + Ke+) / TBW] - 25.6 |
| 1986 |
Rose |
[Na]Serum = (Nae + Ke)/TBW |
| 1997 |
Adrogué-Madias |
Δ (Na+) s = (Na+ inf – Na+ s) / TBW 1 |
| 2002 |
Barsoum-Levine |
Δ Serum [Na+] = {(Vi x [Na+] i) - (Vu x [Na+] u) – (ΔV) [Na+] s)} / (TBW + Δ V) |
| 2003 |
Nguyen-Kurtz equation |
Δ Na+ plasma = [[[(Na1 + 23.8) × TBW1] – [[Na2] + 23.8) x (TBW1 + VNET) + 1.03 × ([E]i x Vi) − ([E]o x Vo)]] / [ [Na+]2 + 23.8 – 1.03 [E]IVF] |
| 2005 |
Modified Electrolyte Free Water Clearance equation |
MEFWC = V (1 – [1.03 [Na+ + K+] urine / [Na+] p + 23.8)]) |
| 2005 |
Nguyen-Kurtz equation hyperglycemia correction |
MEFWC = V (1 – [1.03 [Na+ + K+] urine / [Na+] p + 23.8) + (1.6/100 × ([glucose]p − 120)]) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







