1. Introduction
Currently, grapevine cultivation worldwide is facing significant challenges due to reduced winter rainfall and higher summer temperatures, as noted by the IPCC (2018). This situation underscores the pressing need for efficient water management to sustain adequate wine grape production.
One approach to mitigate water scarcity is the adoption of rootstocks. Initially introduced in response to the devastating phylloxera (Daktulosphaira vitifoliae) infestation in the late 19th century, which nearly wiped out European vineyards, rootstocks offer more than just phylloxera resistance. They also bring benefits such as regulating cultivar vigor (Gambetta et al. 2012), enhancing nutrient uptake (Cochetel et al. 2017), and increasing drought tolerance (Bascuñan-Godoy et al. 2017). Combining scion and rootstock varieties that can endure prolonged drought periods without compromising growth or wine quality represents a promising strategy under investigation (Lovisolo et al., 2016; Serra et al., 2014). However, additional research is needed to comprehensively understand the distinct physiological changes triggered by different rootstock types and to assess their diverse responses compared to grapevines grown on their own roots (own-rooted grapevines).
Various studies have investigated the stomatal behavior of non-grafted cultivars concerning water use efficiency (Davies et al., 2002; Schulz, 2003; Medrano et al., 2015). According to these studies and in simplified form, cultivars are categorized into two types based on their stomatal response to water stress. Isohydric cultivars react to xylem tension induced by high water demand and water scarcity in the soil by closing stomata to prevent water loss (Schultz, 2003; Brodribb & Holbrook, 2003). However, this conservation mechanism, while reducing transpiration, also lowers CO2 assimilation, thereby impacting plant growth and productivity. Conversely, anisohydric cultivars exhibit less responsiveness to water stress, keeping stomata open, which minimally affects their growth rate but elevates the risk of xylem cavitation when soil water availability fails to meet water demand (Schulz et al., 2003; Keller, M., 2015). In the latter scenario, prolonged water scarcity may lead to leaf shedding, significantly compromising production (Keller, M., 2015). However, this classification is often ambiguous and varies depending on environmental factors (Lovisolo et al., 2010), soil characteristics (Tramontini et al., 2014), duration of water stress (Tombesi et al., 2015), and the specific combinations of scion and rootstock (Kounduras et al., 2008; Tramontini et al., 2013a). This ambiguity is highlighted in studies by Hochberg et al. (2013) and Tramontini et al. (2013b), where Cabernet Sauvignon is classified as isohydric, while Lovisolo et al. (2010) regard it as an anisohydric cultivar. Therefore, it should be considered a complex trait that should be studied in each genotype, since it is of great interest for vineyard management.
Another plant mechanism to cope with water stress involves the regulation and functionality of aquaporins, which are transmembrane water channel proteins facilitating water movement (Maurel et al., 2008; Vandeleur et al., 2009). These proteins are expressed in tissues where water transport from roots to leaves occurs. In Vitis vinifera L., plasma membrane intrinsic proteins (PIPs), identified as aquaporins, have been extensively studied. It has been observed that they exhibit diurnal responses to various environmental cues (Zwieniecki and Secchi, 2017; Vandeleur et al., 2009; Maurel et al., 2008) and facilitate water transport in both roots and leaves (Afzal et al., 2016). Another category of aquaporins, tonoplast intrinsic proteins (TIPs), may promote water movement from the vacuole to the cytosol under conditions of high water demand, or function as cytoplasmic osmoregulators by aiding in the movement of ions and small solutes from the vacuole to the cytosol during stress recovery in leaves and roots (Zarrouk et al., 2016; Maurel and Prado, 2017; Afzal et al., 2016).
The expression levels of aquaporins in leaves vary according to water stress conditions, with their response influenced by stomatal conductance depending on the expression site (plasma membrane or tonoplast) (Vandeleur et al., 2009). Therefore, if the expression levels of TIP aquaporins are maintained or increased under high water demand conditions, they may lead to a near anisohydric behavior by sustaining water flow into tissues (Sade and Moshelion, 2017; Zwieniecki and Secchi, 2017).
The impact of rootstock on stomatal behavior and the involvement of aquaporins in how grafted grapevines react to water stress have been overlooked in prior studies. Previous research indicates that the physiological responses of grafted cultivars to water scarcity could be shaped by the characteristics of the rootstock (Marguerit et al., 2012; Shtein et al., 2017; Gullo et al., 2018).
Based on this understanding, our study aimed to compare stomatal responses and growth across various grapevine genotypes, including both own-rooted cultivars and graft combinations of scions and rootstocks, under conditions of water deficit. Additionally, we investigated the expression levels of PIP and TIP aquaporins in leaves of grafted plants to elucidate how rootstock influences aquaporin response to water scarcity.
2. Results
This study comprised two distinct experiments. The first experiment focused on four non-grafted genotypes, including two cultivars (Carménère and Cabernet Sauvignon, CAR and CS respectively) and two rootstocks (1103P known for its drought tolerance, and SO4 characterized by low drought tolerance). The second experiment investigated scion/rootstock combinations, totaling three combinations. In both experiments, plants underwent a progressive water deficit phase (non irrigation, Ni), followed by a recovery phase (Rec). These plants were contrasted with plants under full irrigation (Fi). Additionally, in the second experiment, the expression levels of aquaporins were analyzed after both the water stress and recovery phases. In the experiment concerning scion/rootstock combinations, a single bud of the CS cultivar was omega-grafted onto a 20 cm of rootstock stem (Hartmann et al., 2002).
The experiments took place during the summer months of January and February in central Chile, reflecting the typical growth conditions for this species. The vapor pressure deficit (VPD) recorded during measurements exceeded 3.81 kPa, indicating that plants experienced stressful environmental conditions conducive to high water demand and the onset of water stress. A detailed table outlining the environmental conditions at the evaluation site is provided as supplementary information (see Table S1).
2.1. Stem Water Potential and Stomatal Conductance
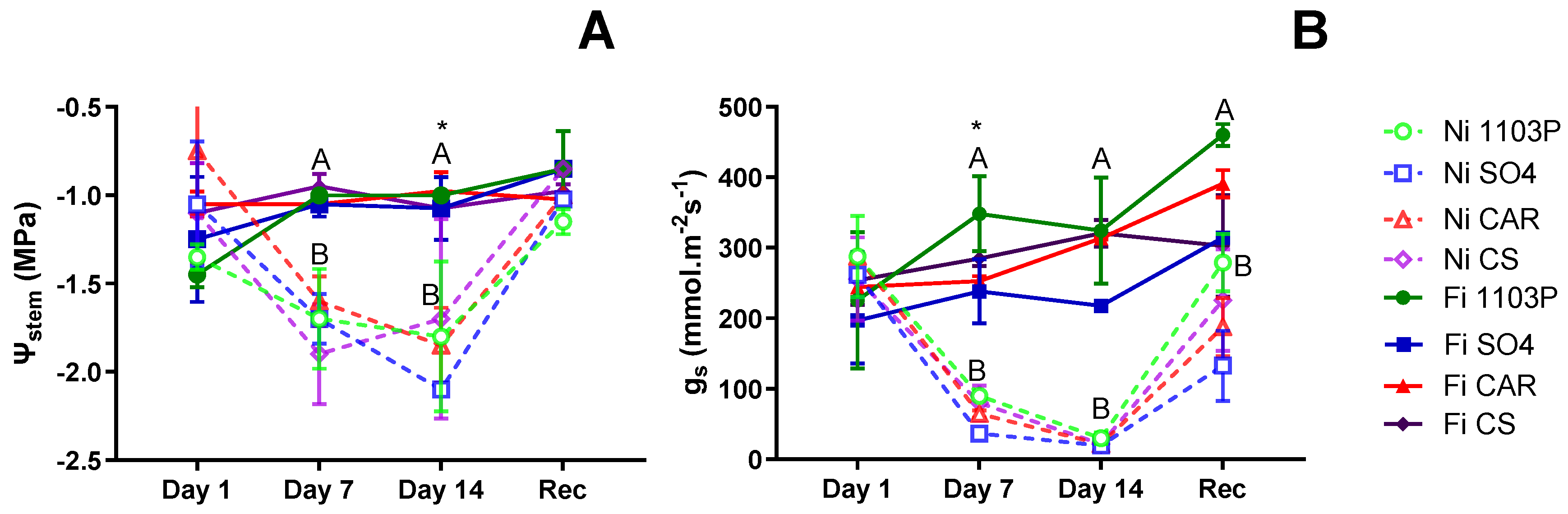
Figure 1A presents the stem water potential (Ψ
stem) values for the first experiment on own-rooted genotypes subjected to water stress treatments. On the first day of evaluation, no significant differences were observed among genotypes, and after 7 days of water stress, differences were only associated with irrigation treatment (p=0.0056). After 14 days of water stress, significant differences were detected between water treatments (p=0.01716) and among genotypes independent of water treatment (p=0.04073), but no interaction between them. Specifically, the SO4 genotype exhibited the lowest Ψ
stem value (-1.75 MPa), while the CAR genotype experienced the less negative Ψ
stem (-1.24 MPa). The CS and 1103P genotypes displayed intermediate responses. Following a week in the recovery irrigation treatment, plants exhibited Ψ
stem magnitudes similar to those observed at the beginning of the experiment.
At the beginning (day 1), stomatal conductance (g
s) values were not different between genotypes., but after 7 days of water stress (
Figure 1B) they exhibited differences attributed to water treatment (p=0.00032) and genotypes (p=0.0072), but no interaction observed. Notably, the 1103P rootstock consistently demonstrated higher g
s levels regardless of irrigation treatment, while the SO4 genotype experienced the most substantial decrease in g
s due to water shortage. Meanwhile, the CAR and CS genotypes displayed intermediate responses. In contrast to observations regarding
stem, no genotype-related differences were observed after 14 days without irrigation, with all plants reaching minimum g
s values. Following one week of recovery, plants showed increased g
s levels, although they did not reach the levels observed in control plants, and no distinctions among genotypes were evident.
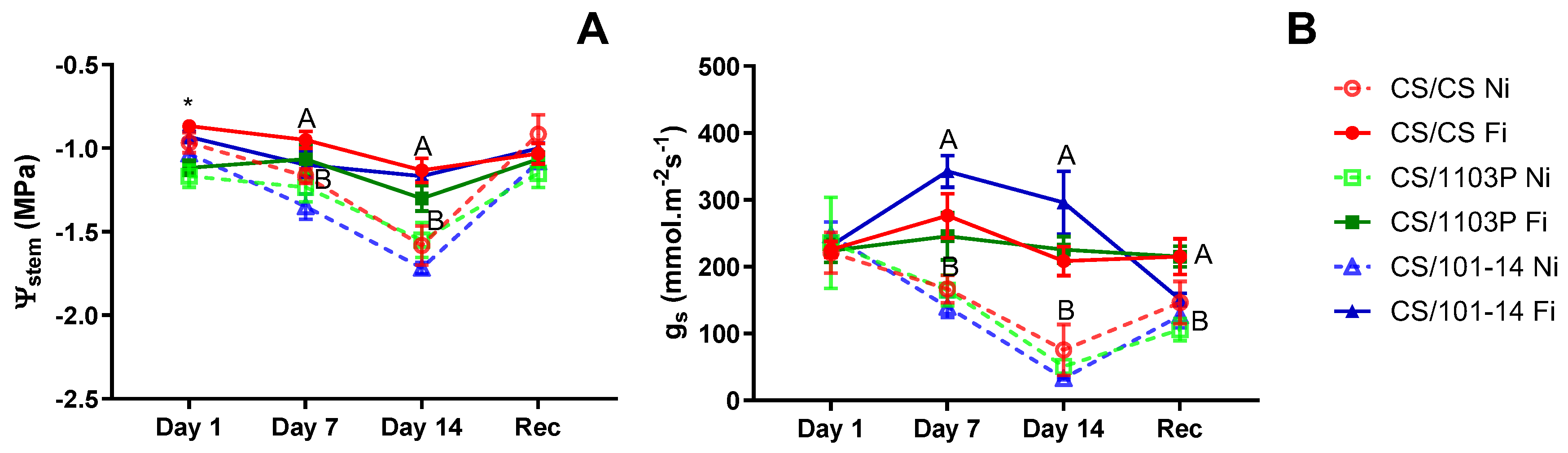
Figure 2A depicts the stem water potential (Ψ
stem) for the examined scion/rootstock combinations in the second essay. Initially, the CS/1103P combination exhibited a significantly lower Ψ
stem compared to other combinations (p=0.0063). However, after 7 and 14 days of non-irrigated treatment, no differences were observed among combinations, with distinctions arising solely due to irrigation treatment (p=0.034 and p=0.0049, respectively). Throughout the trial, the CS/101-14 combination consistently displayed the lowest Ψ
stem values under the Ni treatment. Stomatal conductance (g
s) did not exhibit significant differences among combinations on all evaluation dates (
Figure 2B). All significant differences observed were attributed to irrigation treatment, starting on day 7 (p=0.0012), a trend that persisted until the conclusion of treatment (p=0.0003). Following one week of recovery, all combinations showed signs of recovery, although they were unable to reach initial levels, thereby maintaining differences between irrigated and non-irrigated combinations (p=0.0339).
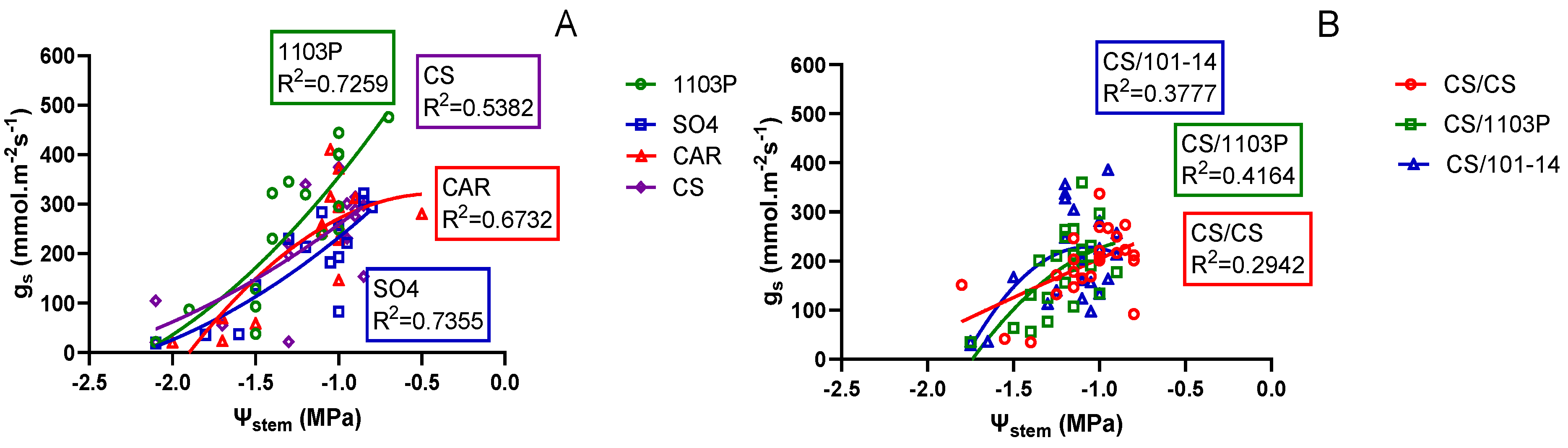
When examining the relationship between Ψ
stem and g
s (
Figure 3A), differences among genotypes can be observed. These differences are visible only when the Ψ
stem is greater than -1.5 MP. In this Ψ
stem range, genotype 1103 showed a smaller decrease in g
s, which could suggest an anisohydric response. In the case of the CS, CAR and SO4 genotypes, for the same water potential range, lower g
s values were observed, suggesting a greater sensitivity to water stress. That, could be associated to a near isohydric behavior. Below -1.5 MPa, all genotypes demonstrated a similar response, with stomata practically closing due to the extreme conditions of the Ni treatment, as observed in both g
s and
stem on day 14 (
Figure 1).
In the scion/rootstock combination experiment, the relationship between
stem and g
s (
Figure 3B) showed more subdued differences for generally lower gs magnitudes. In this case it is not possible to distinguish a stress response pattern..
2.2. Above-Ground Growth Rate
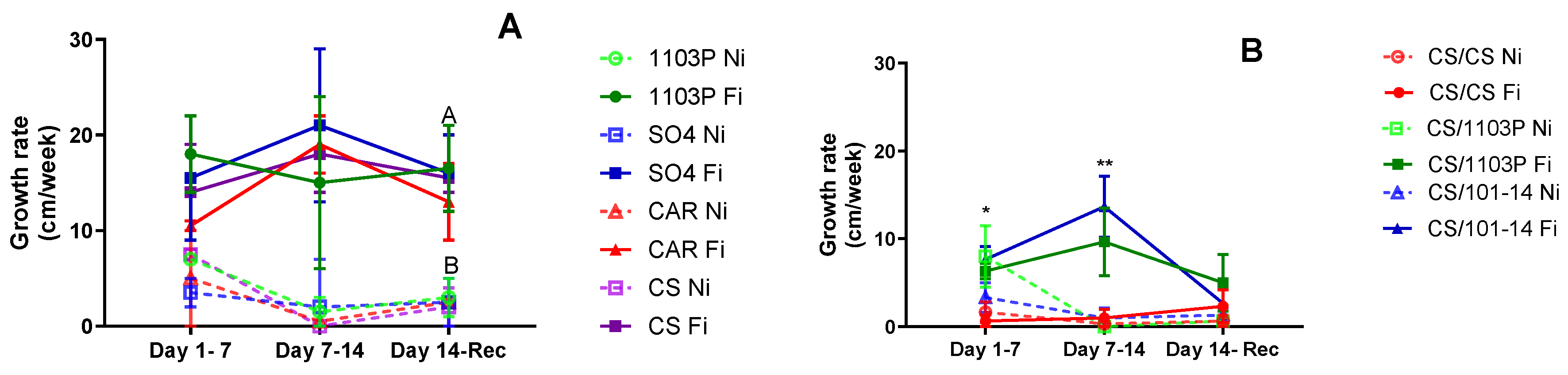
Figure 4A displays the shoot growth rate for the four own-rooted genotypes studied. While no significant differences were observed among genotypes initially, Ni plants exhibited decreased growth rates or ceased growth altogether as water stress progressed, resulting in significant differences due to water treatment (p=0.01305).
In
Figure 4B, the shoot growth rate for scion/rootstock combinations is depicted. During the first week of evaluations (days 1 to 7), no differences were noted between irrigated and non-irrigated plants. However, the CS/CS combination consistently displayed the slowest growth rate, regardless of irrigation treatment (p=0.0158). By the final week of water stress treatment (days 7-14), an interaction between irrigation treatment and combinations emerged (p=0.003). The CS/101-14 Fi combination maintained the highest growth rate, while CS/CS Fi and all non-irrigated combinations exhibited the lowest growth rates. Following one week of recovery, all combinations demonstrated similar growth rates, with no discernible differences between treatments.
2.3. Aquaporin Activity
In experiment two, scion/rootstock combinations, the expression of PIP and TIP aquaporins was measured in leaves at two time points; at 14 days Ni and after one week of recovery.
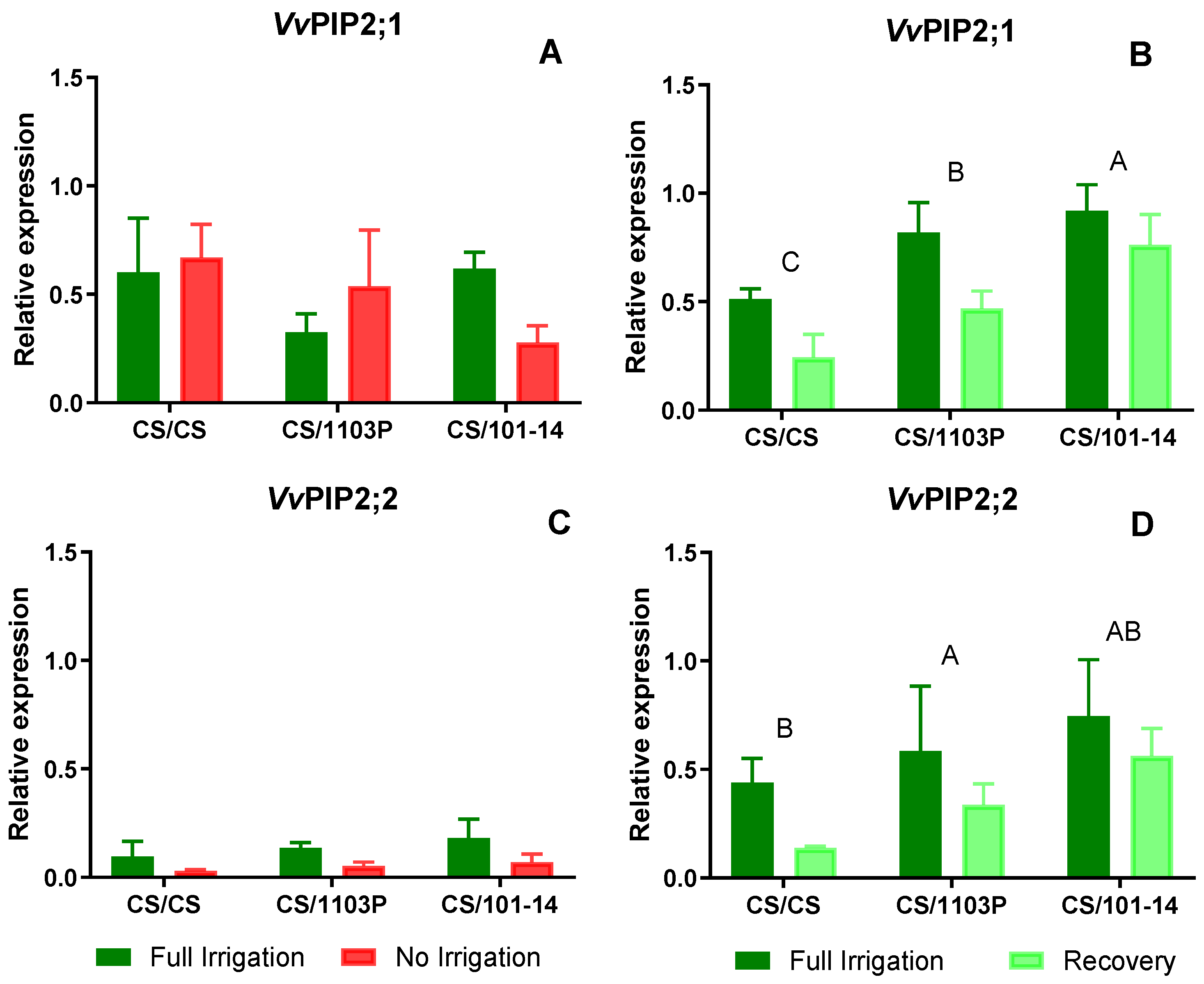
Figure 5 depicts the expression levels of PIP aquaporins. Regarding
VvPIP2;1 (
Figure 5A), no differences were observed between combinations or water treatments after 14 days of treatment. For
VvPIP2;2 (
Figure 5C), expression levels were low in both water treatments, with slightly higher levels in Fi plants, although these differences were not statistically significant (p=0.061).
After one week of recovery, no differences in expression levels due to water treatment were observed for the studied PIP aquaporin genes (
Figure 5 B and D). However, significant differences were noted in the expression levels of
VvPIP2;1 and
VvPIP2;2 (
Figure 5 B and D, respectively) due to the grafting combinations (p=0.005 and p=0.014, respectively), with CS/101-14 exhibiting higher expression levels of both aquaporins.
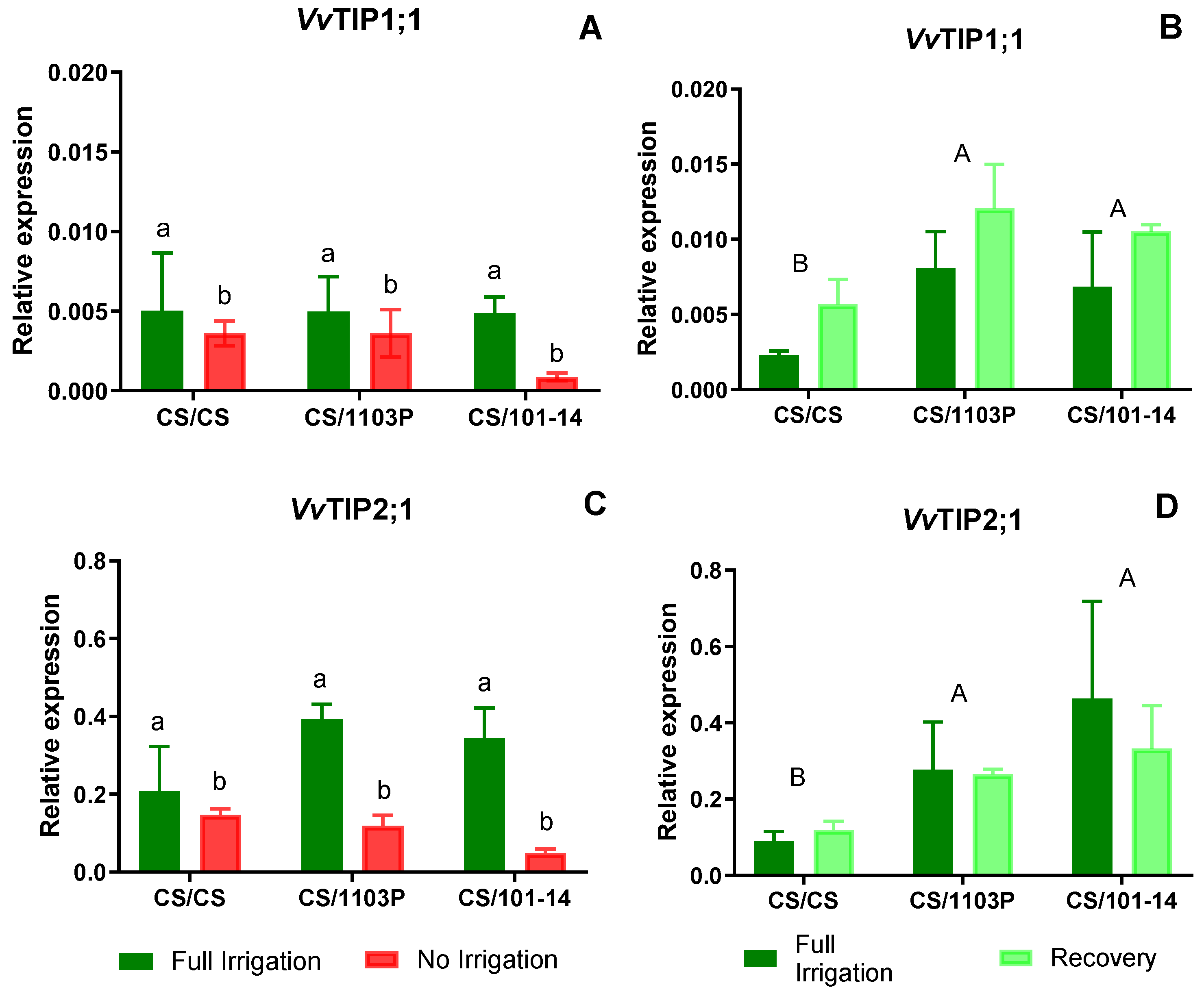
Figure 6 (A and C) shows the expression levels of VvTIP1;1 and VvTIP2;1 aquaporin genes. In both cases, differences are observed between Ni and Fi treatments, with higher expression in Fi (p=0.0067 and p=0.0280, respectively).
In plants undergoing recovery, expression levels of the
VvTIP1;1 aquaporin gene (
Figure 6B) increased notably. Iinterestingly, differences were attributed to the genotype combinations (p=0.014), particularly with CS/CS displaying lower expression levels than other combinations, which exhibited uniformly high expression levels without notable differences among them. Regarding
VvTIP2;1 (
Figure 6D) aquaporin expression levels, differences were also observed due to combinations (p=0.0004), with CS/CS exhibiting the lowest expression levels. No significant differences were noted between control (Fi) and recovered plants.
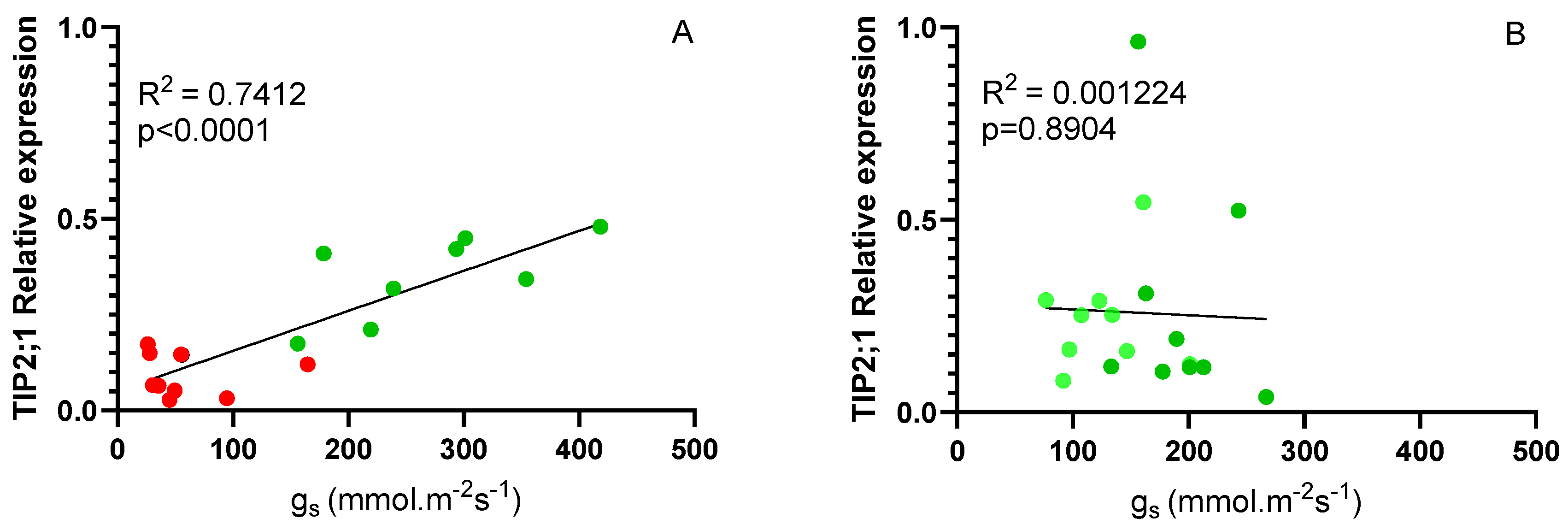
Considering the differences in the expression of VvTIP 2;1 aquaporins between genotypes (
Figure 6 B), a correlation analysis was performed between these values and stomatal conductance. As shown in
Figure 7 A, considering Fi and Ni plants at 14 days, a high correlation was obtained (0.7412) clearly indicating that the expression of this gene is related to stomatal behavior. For the different scion/rootstock combinations in Fi and Rec conditions the correlation was not significant, since in both cases the plants had an adequate water supply. It has been suggested that TIP aquaporins may regulate leaf water potential, contributing to differences between isohydric and anisohydric plants (Sade and Moshelion, 2017).
3. Discussion
In the initial experiment, the stem water potential and stomatal conductance values allowed us to observe distinct behaviors among the genotypes studied under water stress conditions. As described in the literature (Schultz, 2003; Brodribb & Holbrook, 2003), certain genotypes tend to maintain stem water potential through a rapid stomatal closure response, resulting in reduced gs values and prevention of dehydration. Conversely, other genotypes keep their stomata open for longer periods, leading to a decrease in Ψstem to very negative values, potentially causing cavitation and plant mortality.
In this study, plants were subjected to severe progressive water stress conditions. After 14 days without irrigation, all genotypes showed a decrease in gs to approximately 20 mmol m−2 s−1, indicating extreme stomatal closure induced by water stress, consistent with previous research (Tombesi et al., 2015; Pou et al., 2008; Brodribb & Holbrook, 2003). Additionally, the Ψstem after 14 days reached values of -2.2 MPa. However, after 7 days without irrigation, the 1103P rootstock exhibited the highest gs, along with a moderate reduction in Ψstem. This indicates that despite the stress conditions, it kept its stomata open and showed rapid recovery, aligning with previous descriptions of its drought resistance. In contrast, the SO4 rootstock showed the lowest gs values and slower recovery compared to CS, consistent with results obtained by Bondada and Shutthanandan (2012).
Furthermore, irrigation treatments significantly influenced growth rates, with 1103P and SO4 being the least affected during water scarcity periods. Despite lower Ψstem and gs values, the growth rate remained stable in the 1103P rootstock. However, this was not the case for CS and CAR, as both experienced a decline in growth rate.
Although definitive conclusions cannot be drawn from this experiment, it appears that the 1103P rootstock shows a greater ability to keep stomata open under progressive stress conditions without significantly reducing its water potential. This behavior aligns with an anisohydric response. Conversely, the other three genotypes studied seem to react with more pronounced stomatal closure, approaching a near-isohydric behavior, although CS has been previously classified as anisohydric (Jara-Rojas et al., 2015; Hochberg, 2013; Tramontini et al., 2013b). It should be noted that the differences between isohydric and anisohydric behaviors are more evident under moderate water stress conditions (Tramontini et al., 2014), so the differences in this experiment were only noticeable after 7 days without irrigation.
In the second experiment, no significant differences were observed in Ψstem and gs between scion/rootstock combinations throughout the evaluation dates (
Figure 2). These parameters behaved similarly to those analyzed in the first trial, although the lowest levels reached after 14 days without irrigation were less extreme. This is likely due to more moderate environmental conditions during the trial period (Table.S1). The differences found are attributed solely to irrigation treatments. The CS/CS and CS/1103P combinations gradually decreased their Ψstem to approximately -1.5 MPa, indicating moderate water stress. In contrast, the CS/101-14 combination decreased to approximately -1.7 MPa (
Figure 2a). After a week of recovery, these parameters improved in all combinations, with water potential reaching values similar to fully irrigated (Fi) plants.
Irrigation treatments significantly influenced growth rates, with the CS/1103 and CS/101-14 combinations being the least affected. In contrast, CS/CS had a very low growth rate even in the fully irrigated treatment. This supports previous research suggesting that rootstocks can mitigate the scion’s response to water stress by affecting soil water extraction and regulating stomatal sensitivity (Toro et al., 2023; Warchefsky et al., 2016; Tramontini et al., 2013a; Marguerit et al., 2012; Gambetta et al., 2012). Although this aspect could not be verified in this study, the growth rate results could reinforce this hypothesis.
Another mechanism involved in the stress response is related to aquaporin expression. TIP aquaporins facilitate the movement of water and solutes during cellular homeostasis, particularly affecting water flow from the vacuole to the cytoplasm during rehydration (Maurel & Prado, 2017; Afzal et al., 2016). On the other hand, PIP aquaporins have been associated with improved hydraulic conductance in leaves, which is crucial for maintaining water transport efficiency under water deficit conditions (Pou et al., 2013). Previous studies have shown that in response to water stress, AQP expression decreases, while it rises again during recovery (Dayer et al., 2020; Maurel & Prado, 2017; Sade & Moshelion, 2017; Zarrouk et al., 2016; Surbanovski et al., 2013; Vandeleur et al., 2009).
In this study, the expression of two PIP aquaporins (VvPIP2;1 and VvPIP2;2) and two TIP aquaporins (VvTIP1;1 and VvTIP2;1) was analyzed. It should be noted that the expression levels of these aquaporins were only obtained in the second scion/rootstock combination trial. Expression levels were obtained from leaves of plants subjected to fully irrigated (Fi) and non-irrigated (Ni) treatments on day 14, and then at the end of the recovery period.
The results were unclear for VvPIP2;1 and VvPIP2;2 as no reduction in their expression was observed in all cases, showing a statistically non-significant trend. Generally, expression levels were lower in stressed plants from the Ni and Rec treatments, except for the CS/1103P combination, where VvPIP2;1 expression was higher in the Ni treatment after 14 days.
On the other hand, VvTIP1;1 and VvTIP2;1 aquaporins showed a significant reduction in their expression levels in Ni plants compared to Fi plants. After the recovery period, no statistical differences were observed between water treatments. However, VvTIP2;1 expression remained at similar or lower levels in the recovery (Rec) treatment compared to Fi plants.
After the recovery period, our results showed higher expression levels for the four AQP genes analyzed. Significant differences were observed between scion/rootstock combinations (Figs. 5 and 6). In particular, CS plants grafted onto 1103P and 101-14 rootstocks showed higher expression levels compared to homografts (CS/CS). Although the relative expression levels of VvTIP1;1 were lower than those of the other AQP genes, Rec plants showed higher mean expression of VvTIP1;1, suggesting a potential role for this AQP in facilitating faster leaf hydration recovery.
Previous research on the Chasselas cultivar revealed that reduced expression levels of AQPs VvTIP1;1 and VvPIP2;1 were associated with decreased petiole hydraulic conductivity under water stress, suggesting their role in regulating water transport to the leaves (Dayer et al., 2017). Another study on Chardonnay showed that during stress acclimation, the foliar expression of AQPs VvPIP2;1, VvTIP1;1, and VvTIP2;1 decreased, with VvPIP2;2 being undetectable in the leaves at this specific time (Pou et al., 2013). These results closely reflect our findings in non-irrigated combinations, emphasizing the importance of AQPs in regulating water movement under variable environmental conditions.
At the end of the 14-day irrigation experiment, a positive correlation was observed between the expression levels of VvTIP2;1 and stomatal conductance (gs) in grafted plants. This relationship was less evident for VvTIP1;1 (data not shown). This finding reinforces the role of VvTIP2;1 in regulating leaf water content under stress conditions, as supported by previous studies (Sabir et al., 2021; Dayer et al., 2017; Sade & Moshelion, 2017; Pou et al., 2013). Specifically, the positive relationship indicates that aquaporin expression decreases under water stress conditions, which aligns with the low growth rates and gs values observed in Ni plants. Thus, VvTIP2;1 expression may be more closely related to the initial response to water deficit and the drastic reduction in gs to the minimum levels observed in all combinations on day 14, at the end of the stress period. During the recovery period, no clear relationship was observed between VvTIP2;1 expression and gs, possibly due to the faster recovery of VvTIP2;1 expression compared to gs.
Previous experiments in grapevine and bean (Phaseolus vulgaris L.) confirm that TIP aquaporin expression decreases within 48 hours under water stress, with slower recovery once water supply is restored (Zarrouk et al., 2016; Pou et al., 2013; Zupin et al., 2017). In plants subjected to prolonged water stress, VvTIP1;1 reached lower levels but showed higher expression after 7 days of recovery (Zarrouk et al., 2016; Sade & Moshelion, 2017), similar to the results observed in this study in CS/1103P.
TIP aquaporins regulate cellular osmoregulation, influencing the differences between isohydric and anisohydric plants (Zarrouk et al., 2016; Sade & Moshelion, 2017). The decrease in aquaporin expression at sites of water loss, such as leaves, involves complex trafficking systems to export large amounts of TIPs and reduce tonoplast permeability. In this study, the CS/CS combination exhibited near-isohydric behavior, correlated with lower expression of PIP and TIP aquaporins during recovery, while Cabernet Sauvignon grafted onto 1103P and 101-14 showed higher expression linked to anisohydric behavior. This differential response of CS when grafted onto different rootstocks supports the argument that the binary classification of plant water use strategies into isohydric and anisohydric is an oversimplification. Lavoie-Lamoureux et al. (2017) presented a range of stomatal sensitivities in grapevines, influenced by the interaction between genotypes and soil environment. Herrera et al. (2022) further showed that grapevine stomatal responses to low water potentials increase as the growing season progresses, indicating a dynamic water use strategy. Hochberg et al. (2018) argued that it is a plant-environment interaction, with the same plant capable of exhibiting both isohydric and anisohydric behaviors depending on environmental conditions. Therefore, the plant’s water use strategy is not a fixed trait but a dynamic interaction with the environment, requiring more refined methods for precise characterization. In this context, the differential expression and activity of TIP aquaporins may be one of the mechanisms explaining the dynamic spectrum of water use exhibited by plants.
4. Materials and Methods
4.1. Plant Material and Growing Conditions
In the first experiment, four ungrafted genotypes were evaluated. The rootstocks 1103P (V. berlandieri x V. rupestris) and SO4 (V. berlandieri x V. riparia) were described as drought-tolerant and low drought-tolerant, respectively (Keller. M, 2015). Additionally, the cultivars Carménère and Cabernet Sauvignon were included. Plant material was collected during the winter (July) from the cultivar collection at the experimental station of the Pontificia Universidad Católica de Chile, located in Pirque (33°40′12.35” S, 70°35′06.96” W), and rooted in a heated propagation bed in a greenhouse.
In the experiment involving scion/rootstock combinations, a single bud of the Cabernet Sauvignon cultivar was omega-grafted onto a 20 cm rootstock stem (Hartmann et al., 2002). The scion/rootstock combinations were as follows: CS/CS, CS/1103P, and CS/101-14.
All plants were grown in 3-liter pots filled with a substrate composed of sand, peat, and perlite in a 2:1:1 volume ratio. Once established, each plant received 10 mg of slow-release fertilizer (Basacote®, Compo Expert). When the plants reached a height of 20 cm, they were watered weekly with 50 mL of a 25% Hoagland solution. To promote uniform growth, only one vertical shoot was allowed to develop, which was supported with a stake. Lateral shoots or clusters were systematically removed. The plants were initially grown under greenhouse conditions until December. Subsequently, they were moved outdoors and placed under a white mesh. Watering was done every two days, ensuring that the pots were watered to their maximum capacity (-0.033 MPa), maintaining adequate soil moisture levels until the start of the experiments.
In the initial experiment, conducted between January 23 and February 13, 2017 (DOY 23-44), 32 self-rooted plants were used. The second experiment, consisting of 36 plants, focused on scion/rootstock combinations and took place between February 20 and March 13, 2017 (DOY 51-72).
In both experiments, two water treatments were employed. The first treatment, called full irrigation (Fi), involved watering half of the plants daily to replenish 100% of the water transpired during the day, according to gravimetric estimation. The second treatment, called non-irrigated (Ni), subjected the plants to a 14-day period without watering.
Once the 14-day non-irrigation phase was completed, the plants underwent a 7-day recovery treatment (Rec), during which they were watered to saturation and subsequently replenished daily with the transpired water, reflecting the conditions of the Fi treatment. Throughout these experiments, the pots were protected with aluminum foil to prevent evaporation from the substrate surface.
4.2. Stem Water Potential, Stomatal Conductance, and Environmental Conditions
In both experiments, water relations measurements were taken at four specific time points: day 1 (the first day of treatment, following saturation watering of all plants the previous day), day 7, day 14, and day 21 (during the recovery period). These measurements were taken during the period of the day with the highest water demand, typically between 11:00 and 15:00 hours. Soil water content was determined using a capacitance probe (FDR Sensor GS-1® from Decagon Devices Inc, Pullman, WA, USA) inserted into the pot in the root zone. Sure, here’s a clearer and improved version of the sentence:
Stem water potential (ψstem) was measured on a fully expanded leaf located between nodes 7 and 10 from the base of the shoot. The leaf was enclosed in an aluminized plastic bag for at least one hour prior to measurement, following the methodology described by Scholander et al. (1965), using a pressure chamber (Pump-Up Chamber, PMS Instrument Co., Albany, OR, USA). Additionally, stomatal conductance (gs) was evaluated using a portable porometer (model SC1 from Decagon Devices) on fully expanded mature leaves. Temperature and relative humidity were recorded throughout the experiments using a hygrothermograph integrated into a data logger (HOBO® Pro v2, Onset Computer Corporation, Bourne, MA, USA). These measurements served as the basis for estimating the vapor pressure deficit (VPD) both in the greenhouse and outdoors.
4.3. Plant Growth and Biomass Measurements
Each week, the height of each plant was measured with a tape measure. Half of the plants were harvested after completing the 14-day treatment period (day 14), and the second half was harvested at the end of the recovery period (Rec). Fresh weight of roots, stems, and leaves was then determined, followed by dry weight measurement after 48 hours in an oven at 70°C.
4.4. RNA Extraction
In the scion/rootstock combination experiment, leaf samples were collected at the end of the stress period (day 14) and the subsequent recovery period (Rec) to evaluate the expression of foliar aquaporins. Leaf samples were collected from nodes 7 to 10 from the base of the shoot, always at the same time, between 09:00 and 11:00 hours, to mitigate the circadian effect on aquaporin expression. Immediately after collection, the leaves were frozen in liquid nitrogen and stored at -80°C until analysis.
For each experimental unit, 100 mg of tissue was ground with liquid nitrogen. Total RNA was extracted from three biological samples using the 3% cetyltrimethylammonium bromide (CTAB) protocol (Yu et al., 2012). RNA concentration was measured using a NanoDrop 1000 spectrophotometer (Thermo Scientific), and its integrity was verified by electrophoresis. RNA was treated with RQ1 RNase-Free DNase (Promega) and used as a template to synthesize cDNA using Moloney murine leukemia virus reverse transcriptase (RT-MMLV; Promega). Each reaction mixture contained RNA template (2 µg), Oligo(dT)15 primers (2 µl), MMLV reverse transcriptase buffer 5x (5 µl), dNTPs (5 µl), recombinant ribonuclease inhibitor RNasin (0.63 µl), MMLV reverse transcriptase (1 µl), and DEPC-treated water (1.37 µl). The cDNA was diluted 1:4 (v/v) and a no-reverse transcriptase control was included.
Primer sequences for PIP and TIP aquaporins were selected based on previous studies by Gambetta et al. (2013) and Zarrouk et al. (2016). To confirm the identity of the products, the primers were tested with RT-PCR, and the amplified products were sequenced. Primer sequences are provided in
Table 1.
4.5. Quantitative PCR Analysis
Expression analysis was performed using real-time quantitative PCR (Stratagene Mx3000P). For transcriptional analysis, 1 μL of cDNA was used in the SYBR Green RT-PCR, along with 20 μL of Brilliant® II SYBR® Green qPCR Master Mix (Stratagene, Agilent Technologies) and 5 M of each primer in the thermocycler. The thermal profile for amplification included an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Additionally, a melting curve analysis was performed from 55°C to 95°C with 0.5°C increments. Each reaction was run in duplicate and negative controls, including a water sample and a negative RT control, were included to ensure the absence of genomic DNA contamination. The specificity of the amplification products was confirmed by the presence of a single peak in the melting curve. Before analysis, the amplification efficiency of each primer pair was determined. The threshold cycle (Ct) values obtained were averaged across the two technical replicates for each sample and gene. Subsequently, the Ct values were normalized using the Ct values of the VvUBQ and VvAct genes, ensuring consistent gene expression variation following treatment application. The normalized Ct values were then used to evaluate changes
4.6. Statistical Analysis
In both trials, a split-plot experimental design was used, with the main plot dedicated to the irrigation treatment and the subplot focused on the genotype or graft combination. The main plot (comprising two irrigation treatments) was replicated four times for the genotype experiment, while the subplot (comprising four genotypes) was replicated twice. In the combinations experiment, the main plot was the irrigation treatment (with two levels), and the subplot presented the graft combinations (with three levels), each repeated three times. In both cases, the experimental unit consisted of two plants controlled identically throughout the experiment. Data analysis was performed using linear mixed models with the REML method (Corbeil and Searle et al., 1976) in JMP pro software (v.13). Differences between the means of irrigation treatments, genotype, or combinations were determined using Student’s t-test (p<0.05) or Tukey-Kramer test (p<0.05). Regression coefficients were calculated using GraphPad Prism 9.3.1 (San Diego, California, USA).
Supplementary Material Table S1. Environmental conditions during the genotype and cultivar/rootstock experiments.
5. Conclusions
In conclusion, this study highlights the intricate and dynamic nature of plant responses to water stress, revealing significant variations among genotypes. In the first experiment, the 1103P rootstock exhibited superior drought resistance by maintaining higher stomatal conductance and moderate stem water potential, indicative of an anisohydric response. Conversely, other genotypes showed more pronounced stomatal closure, consistent with near-isohydric behavior. In the second experiment, irrigation treatments significantly influenced the growth rates of rootstock/scion combinations, with CS/1103 and CS/101-14 being the least affected. The role of aquaporins, particularly TIP AQPs, was crucial in facilitating water movement and recovery post-stress, with the expression patterns of VvTIP2;1 closely linked to stomatal conductance. These findings underscore the importance of selecting appropriate genotypes and understanding their physiological mechanisms to enhance drought resilience in plants.
References
- Afzal, Z. , Howton, T. C., Sun, Y., & Mukhtar, M. S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar]
- Bascuñan-Godoy, L. , Franck, N., Zamorano, D., Sanhueza, C., Carvajal, D, Ibacache, A. The rootstock effect on irrigated grapevine yield under arid climate conditions is explained by changes in traits related to light absorption of the scion. Sci. Hortic. 2017, 218, 284–292. [Google Scholar] [CrossRef]
- Bondada, B. , and Shutthanandan, J. Understanding differential responses of grapevine (Vitis vinifera L.) leaf and fruit to water stress and recovery following re-watering. American Journal of Plant Sciences 2012, 3, 1232–1240. [Google Scholar] [CrossRef]
- Brodribb, T. J. , & Holbrook, N. M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 2003, 132, 2166–2173. [Google Scholar]
- Cochetel, N. , Escudié, F., Cookson, S.J., Dai, Z., Vivin, P., Bert, P-F., Muñoz, M. S., Delrot, S., Klopp, C., Ollat, N., Lauvergeat. Root transcriptomic responses of grafted grapevines to heterogeneous nitrogen availability depend on rootstock genotype. Journal of Experimental Botany 2017, 68, 4339–4355. [Google Scholar] [CrossRef]
- 2481.
- Corbeil, R. R. , & Searle, S. R. Restricted maximum likelihood (REML) estimation of variance components in the mixed model. Technometrics 1976, 18, 31–38. [Google Scholar]
- Davies, W. J. , Wilkinson, S., Loveys, B., Stomatal control by chemical signaling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytologist 2002, 153, 449–44. [Google Scholar] [CrossRef]
- Dayer, S. , Perez Peña, J., Gindro K., Torregosa L., Voinesco F., Martínez, L., Prieto J., Zufferey V. Changes in leaf stomatal conductance, petiole hydraulics, and vessel morphology in grapevine (Vitis vinifera cv. Chasselas) under different light and irrigation regimes. Functional Plant Biology 2017, 44, 679–693. [Google Scholar]
- Dayer, S. , Scharwies, J. D., Ramesh, S. A., Sullivan, W., Doerflinger, F. C., Pagay, V., & Tyerman, S. D. Comparing hydraulics between two grapevine cultivars reveals differences in stomatal regulation under water stress and exogenous ABA applications. Frontiers in plant science 2020, 11, 705. [Google Scholar]
- Fujita, A. Fujita, A., Soma, N., Goto-Yamamoto, N., Mizuno, A., Kiso, K., and Hashizume, K. Effect of shading on proanthocyanidin biosynthesis in the grape berry. J. Japan. Soc. Hort. Sci. 2007, 76, 112–119. [Google Scholar] [CrossRef]
- Gambetta, G.a. , Fei, J., Rost, T.L., Knipfer, T., Mathews, M.A., Shackel, K.A., Walker, M.A., McElrone, A.J. Water uptake along the length of grapevine fine roots: developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiology 2013, 163, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A. , Manuck, C.M., Drucker, S.T., Shaghasi, T., Fort, K., Mathews, M.A., Walker, M.A. and McElrone, A.J., 2012. The relationship between root hydraulics and scion vigor across Vitis rootstocks: what role do root aquaporins play? Journal of Experimental Botany 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G. , Dattola, A., Vonella, V., Zappia, R. Evaluation of water relation parameters in Vitis rootstocks with different drought tolerance and their effects on growth of a grafted cultivar. Journal of Plant Physiology 2018, 226, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H., Kester, D., Davies., Jr, F., Geneve, R. 2002. Plant propagation, principles, and practices. Prentice-Hall. Upper Saddle River., New Jersey. EEUU. 880pp.
- Herrera, J. C. , Calderan, A., Gambetta, G. A., Peterlunger, E., Forneck, A., Sivilotti, P.,... & Hochberg, U. (2022). Stomatal responses in grapevine become increasingly more tolerant to low water potentials throughout the growing season. The Plant Journal 2022, 109, 804–815. [Google Scholar]
- Hochberg, U. , Degu, A., Toubiana, D., Gendler, T., Zoran, N., Rachmilevitch, S., Fait, A. Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biology 2013, 13, 184–200. [Google Scholar] [CrossRef]
- Hochberg, U. , Rockwell, F. E., Holbrook, N. M., & Cochard, H. Iso/anisohydry: a plant–environment interaction rather than a simple hydraulic trait. Trends in plant science 2018, 23, 112–120. [Google Scholar]
- IPCC., & Payne, T. 2018. Summary for Policymakers. In V. Masson-Delmotte, P. Zhai, H. O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, & T. Waterfield (Eds.), Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change (p. 32). World Meteorological Organization Technical Document.
- Jara-Rojas, F. , Ortega-Farías, S., Valdés-Gómez, H., & Acevedo-Opazo, C. Gas exchange relations of ungrafted grapevines (cv. Carménère) growing under irrigated field conditions. South African Journal of Enology and Viticulture 2015, 36, 231–242. [Google Scholar]
- Keller, M. 2015. Environmental constraints and stress physiology. The science of grapevine: anatomy and physiology, 2nd ed. Elsevier/Academic Press: London, 267-341.
- Kounduras, S. , Tsialtas, I.T., Zioziou, E., Nikolaou, N. Rootstock effects on adaptive strategies of grapevine (Vitis Vinifera L. cv. Cabernet-Sauvignon) under contrasting water status: leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Lavoie-Lamoreux, A. , Sacco, D., Risse, P.A., and Lovisolo, C. Factors influencing stomatal conductance in response to water availability in grapevine: a meta-analysis. Physiologia Plantarum 2017, 159, 468–482. [Google Scholar] [CrossRef]
- Lovisolo, C. , Lavoie-Lamoureux, A., Tramontini, S., & Ferrandino, A. Grapevine adaptations to water stress: new perspectives about soil/plant interactions. Theoretical and Experimental Plant Physiology 2016, 28, 53–66. [Google Scholar]
- Lovisolo, C. , Perrone, I., Carra, A., Ferrandino, A., Flexas, J., Medrano, H., Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and their hydraulic and non-hydraulic interaction at the whole-plant level: a physiological and molecular update. Functional Plant Biology 2010, 37, 98–116. [Google Scholar]
- Marguerit, E. , Brendel, O., Lebon, E., Van Leeuwen, C., Ollat, N., Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C. , & Prado, K. 2017. Aquaporins and leaf water relations. In Plant aquaporins (pp. 155-165). Springer, Cham.
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant Aquaporins: Membrane Channels with Multiple Integrated Functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H. , Tomás, M., Martorell, S., Escalona, J.M., Pou, A., Fuentes, S., Flexas, J., Bota, J., Improving water use efficiency of vineyards in semi-arid regions. A Review. Agron. Sustain. Dev 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Pou, A. , Medrano H., Flexas J., Tyerman, SD. (2013) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant, Cell, and Environment 2013, 36, 828–843. [Google Scholar] [CrossRef]
- Reid, KE. , Olsson, N., Schlosser, J., Peng, F., and Lund, ST. (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology 2006, 6, 27. [Google Scholar] [CrossRef]
- Sabir, F. , Zarrouk, O., Noronha, H., Loureiro-Dias, M. C., Soveral, G., Geros, H., & Prista, C. Grapevine aquaporins: Diversity, cellular functions, and ecophysiological perspectives. Biochimie 2021, 188, 61–76. [Google Scholar]
- Sade, N. , & Moshelion, M. 2017. Plant aquaporins and abiotic stress. In-Plant Aquaporins (pp. 185-206). Springer, Cham.
- Scholander, P.F. , Hammel, H.T., Bradstreet, E.D., and Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Schulz, H.R. , 2003. Differences in hydraulic architecture account for near-isohydric and anisohydric behavior of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell and Environment 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Serra, I. , Strever, A., Myburgh, P.A and Deliore, A. Review: The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Australian Journal of Grape and Wine Research 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Shtein, I. , Hayat, Y., Munitz, S., Harcavi, E., Akerman, M., Drori, Scwartz, A. & Netzer, Y. From structural constraints to hydraulic function in three Vitis rootstocks. Trees 2017, 31, 851–861. [Google Scholar]
- Surbanovski N, Sargent DJ, Else MA, Simpson DW, Zhang H, et al. 2013. Expression of Fragaria vesca PIP Aquaporins in Response to Drought Stress: PIP Down-Regulation Correlates with the Decline in Substrate Moisture Content. PLoS ONE 2013, 8, e74945. [Google Scholar]
- Tombesi, S. , Nardini, A., Frioni, T., Soccolini, M., Zadra, C., Farinelli, D., Poni, S., Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevines. Nature 2015, Scientific Reports.
- Toro, G. , Pastenes, C., Salvatierra, A., Pimentel, P. 2023. Trade- off between hydraulic sensitivity, root hydraulic conductivity and water use effiency in grafted Prunus under water deficit. Agricultural water management. 282. [CrossRef]
- Tramontini, S. , Doring, J., Vitali, M., Ferrandino, A., Stoll, M., Lovisolo, C. Soil water-holding capacity mediates hydraulic and hormonal signals of near-isohydric and anisohydric Vitis cultivars in potted grapevines. Functional Plant Biology 2014, 41, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Tramontini, S. , Vitali, M., Centioni, L., Schubert, A., Lovisolo, C., 2013a. Rootstock control of scion response to water stress in grapevine. Environmental and Experimental Botany 2013, 93, 20–26. [Google Scholar] [CrossRef]
- Tramontini, S. , van Leeuwen, C., Domec, J-C., Destrac, A., Basteau, C., Vitali, M., Mosbach- Schulz, O., Lovisolo, C., 2013b. Impact of soil texture and water availability on the hydraulic control of plant and grape-berry development. Plant Soil 2013, 368, 215–230. [Google Scholar] [CrossRef]
- Vandeleur, R. , Mayo, G., Shelden, M.C., Gilliham, M., Kaise, B.N., Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology 2009, 149, 445–460. [Google Scholar]
- Warschefsky, E. J. , Klein, L. L., Frank, M. H., Chitwood, D. H., Londo, J. P., von Wettberg, E. J., & Miller, A. J. Rootstocks: diversity, domestication, and impacts on shoot phenotypes. Trends in plant science 2016, 21, 418–437. [Google Scholar]
- Yu, D.; et al. Comparison and Improvement of Different Methods of RNA Isolation from Strawberry (Fragria × ananassa). J. Agric. Sci. 2012, 4, 51–56. [Google Scholar] [CrossRef]
- Zarrouk O, Garcia-Tejero I, Pinto C, Genebra T, Sahir F, Prista C, Soares David T, Loureiro-Dias MC, Chave MM. 2016. Aquaporins isoforms in cv. Touriga Nacional grapevine under water stress and recovery- Regulation of expression in leaves and roots. Agricultural water management 2016, 164, 167–175. [Google Scholar] [CrossRef]
- Zupin, M. , Sedlar, A., Kidrič, M., & Meglič, V. Drought-induced expression of aquaporin genes in leaves of two common bean cultivars differing in tolerance to drought stress. Journal of plant research 2017, 130, 735–745. [Google Scholar]
- Zwieniecki, M. A. , & Secchi, F. 2017. Role of aquaporins in the maintenance of xylem hydraulic capacity. In-Plant Aquaporins (pp. 237-254). Springer, Cham.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).