1. Introduction
Since year early 20
th century, iodine deficiency disorder (IDD) has been a major problem to the people due to the manifestation of diseases such as goiter or cretinism [
1]. However, this was not fully understood until 1819s, when chronic goiter was linked to the iodine deficiency [
2]. Initially, iodine-deficient patients were treated by taking oral iodine tablets [
3]. Nowadays, the most common intervention for IDD is by the consumption of iodine through an iodine-fortified cooking salt or table salt [
4] which eventually form the basis of the Universal Salt Iodization (USI) program [
5,
6] . The fortification of iodine in table salt involves adding iodine, typically in the form of potassium iodate and potassium iodide. However, the use of potassium iodide is less common due to its instability [
7].
Despite worldwide implementation of USI, IDD remain a significant public health challenge especially among pregnant woman and children. During pregnancy, insufficient of iodine can result in poor health outcomes including congenital hypothyroidism [
8] and increased risk of stillbirth [
9]. For children, low iodine intake can lead to mental development delay [
10], affecting their overall development and educational outcomes. Moreover, geographical factor, such as people in rural areas often hinders assessment of adequate level of iodine due to logistical and infrastructure barrier [
11,
12]. Therefore, addressing IDD requires continuous monitoring, public awareness and targeted interventions to ensure the populations receive adequate iodine intake.
Monitoring of iodine is crucial to make sure the fortified iodine is between the recommended level. Currently, the most common and affordable method for iodine level monitoring in iodized table salt is using titration technique. However, this approach suffers from a highly laborious method, requires a skilled technician and could encounter human error. Although these can be prevented with an automated titrator, a large sample size could lead to a varying degree of reproducibility and poor robustness of the method. The use of instrument such as inductively-coupled plasma – mass-spectrometry (ICP-MS) would easily defeat the problem, but the use of plasma to burn the samples, especially salt samples could lead to a formation of crystal in the capillary tubing of the system [
13,
14] and damage the measurement in the long run.
Alternatively, the use of more common and reliable instrument such as high-performance liquid chromatography (UHPLC) can be advantageous since UHPLC is more affordable and used by many laboratories. Recent wider usage of mixed mode column of non-polar with weak anion-exchange functionality [
15,
16], which allows the separation of ionic compounds such as iodide. For instance, weak anion-exchange properties contain ammonium-based functional groups that would interact with a negatively charged analytes in the mobile phase at high pH and interact with positively charged analytes at low pH [
17].
This study developed and optimized iodine detection method in table salt using UHPLC with diode array detector due to the ability of the instrument to have high accuracy, robustness and great reproducibility, as well as high throughput due to the use of automated sample injection. UHPLC is also a more common instrument to have in the laboratory compared to the other instrument. The objective of the study was to produce a protocol of detection method for total iodine in iodized table salt in sodium bisulfite medium. Sodium bisulfite was used to reduce the iodate form to its iodide form so that it can be detected by the UHPLC system. However, the effect of sodium bisulfite toward the iodide form of table salt also needs to be observed.
2. Materials and Methods
2.1. Chemicals and Reagents
Deionized water (18.2 MΩ grade, Sartorius, Göttingen, Germany) was used throughout the study. All chemicals and reagents were obtained from Sigma-Aldrich (Wisconsin, United States of America (USA)). Except for solvents, all other chemicals and reagents used was American Chemical Society (ACS)-grade. The list including sodium chloride, sodium bisulfite, sodium phosphate monohydrate, sodium pyrophosphate decahydrate, phosphoric acid, potassium iodide and potassium iodate. A gradient-grade solvents were used for methanol and acetonitrile.
2.2. Instrumentation
The study was performed using an Ultra-High Performance Liquid Chromatography (UHPLC) with a diode array detector (Ultimate 3000, Thermo Scientific, Massachusetts, USA). The system was paired with the analytical column of Thermo Scientific Acclaim® Mixed-Mode WAX-1, 2.1 mm × 150 mm, with 5 μm particle size. All chromatographic data were recorded and processed using Chromeleon software with version 7.2.
2.3. Procedures
2.3.1. Chromatographic Conditions
The iodine in iodized salt was determined by the detection of iodide in the samples. The chromatographic condition for iodide detection was done according to the previously reported studies with minor modification [
18,
19]. The isocratic conditions of mobile phase A (120 mM sodium phosphate, monobasic pH 3.0) and mobile phase B (methanol) enabled the separation at 1:1 (v/v) ratio with the flow rate of 0.2 mL/min. The chromatograms were observed at 223 nm wavelength after 50 μL of sample injection at 30 °C of column temperature. The total run time was 15 minutes with observed symmetrical peak of iodide which can be noted at 10.11 minutes.
2.3.2. Column Flushing
To prolong the lifespan of the column, it was stored in 1:1 (v/v) ratio of acetonitrile and 150 mM phosphate buffer (pH3.0) by flushing the system each day, for 30 minutes at 0.50 mL/min.
2.3.3. Standard Preparation
The standard solutions were prepared by dissolving 0.5 g sodium chloride in a total volume of 10 mL which consist of 2.5 mL of 2 M sodium bisulfite, with certain volume of 100 mg/L iodide stock and certain volume of deionized water. For instance, iodide concentration of 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/L, required a volume of 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mL of 10 mg/L iodide stock respectively, and finally diluted with deionized water to the final volume of 10.00 mL.
2.3.4. Sample preparation
The iodized table salt samples were weighted 0.5 g in a 15-mL tube. The samples were then dissolved in 2.50 mL of 2 M sodium bisulfite solution and 7.50 mL deionized water for a final volume of 10.00 mL. The sample solution was ready for UHPLC analysis.
2.4. Method Validation
This protocol was validated according to the International Council for Harmonization (ICH) guidelines (validation of analytical procedures). Our study includes the validation of accuracy, linearity, limit of detection (LOD), limit of quantification (LOQ), specificity, precision’s repeatability and intermediate-precision, and robustness. This is to ensure the reliability of the method when done periodically, and in different laboratory setup.
2.4.1. Accuracy
Three iodine concentration at 1.0 mg/L, 1.5 mg/L and 2.0 mg/L were tested where each of the concentration was injected three times. The relative standard deviation (RSD) was obtained to check the accuracy of the method.
2.4.2. Linearity, LOD and LOQ
The absorbance response of iodide signal was studied in term of the peak area at different total iodine concentration. The concentration was from 0.50 mg/L, 1.0 mg/L, 1.5 mg/L, 2.0 mg/L and 2.5 mg/L in 5% (w/v) sodium chloride and 0.5 M sodium bisulfite medium. Each of concentration level was repeated in triplicate. Mean value of the peak area was plotted against its respective iodine concentration forming a regression line. The LOD and LOQ can be obtained by the standard deviation (SD) of y-intercept of a linear response and a slope, which is expressed as follows:
where σ is the SD of y-intercept of linear response and
S is the slope of calibration curve.
2.4.3. Specificity
The study of specificity was done by comparing the chromatograms of each component used in the standards including the injection of blank of 50% (v/v) mobile phase A and B, the injection of 5% (w/v) sodium chloride, the injection of 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite, and (4) 1.50 mg/L iodide in 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite. The chromatograms were then overlayed to see any effect of the solution composition towards the detection of iodide.
2.4.4. Precision—Repeatability and Intermediate-Precision
Seven (7) replicates of iodine at the concentration of 1.0 mg/L, 1.5 mg/L and 2.0 mg/L were determined to test the RSD of the method’s repeatability, in 5% (w/v) sodium chloride and 0.5 M sodium bisulfite medium. This procedure was also repeated on the second day using freshly prepared standard samples, to check the intermediate-precision’s RSD.
2.4.5. Robustness
Two factors of robustness were investigated including the column temperature of 40 °C against the default setting of 30 °C, and the concentration of sodium bisulfite at 0.25 M against its existing concentration at 0.5 M.
2.5. Sodium Bisulfite Stability Study
The use of sodium bisulfite was to convert the iodate form to its iodide form through reduction reaction. In a 15-mL tube, 2.5 mL of 2M sodium bisulfite solution was added to dissolve 0.5 g sodium chloride. The iodate with concentrations of 1.38 mg/L and 3.45 mg/L was prepared by adding 1.38 mL and 3.45 mL of 100 mg/L of iodate stock to the solution, respectively. Finally, deionized water was then added to each of the 15-mL tube to its final homogenous volume of 10.00 mL. The prepared solutions were repeated every day for ten (10) days prior to the UHPLC injections using the same 2 M sodium bisulfite stock that was kept refrigerated in 8 °C.
2.6. Sample Spike and Recovery
Individually, a 0.5g of iodized table salt with pre-determined iodine concentration was placed in a 15-mL tube. Then, 2.50 mL of 2M sodium bisulfite solution was added to dissolve the sample. Afterward, the samples were spiked with 0.50 mL, 1.00 mL and 1.50 mL of 10.0 mg/L iodide solution, respective to the equivalent concentration of 10.0 mg/Kg, 20.0 mg/Kg and 30.0 mg/Kg of iodine in the samples. Similarly for iodate spike, 0.69 mL, 1.38 mL and 2.07 mL of 10.0 mg/L iodate solution were added to the samples with respect to the equivalent concentration of 10.0 mg/Kg, 20.0 mg/Kg and 30.0 mg/Kg of iodine in the samples. Finally, the spiked solutions were topped up with deionized water to the final total volume of 10.00 mL. The spiked samples were then ready for UHPLC analysis.
3. Results
3.1. Method Development
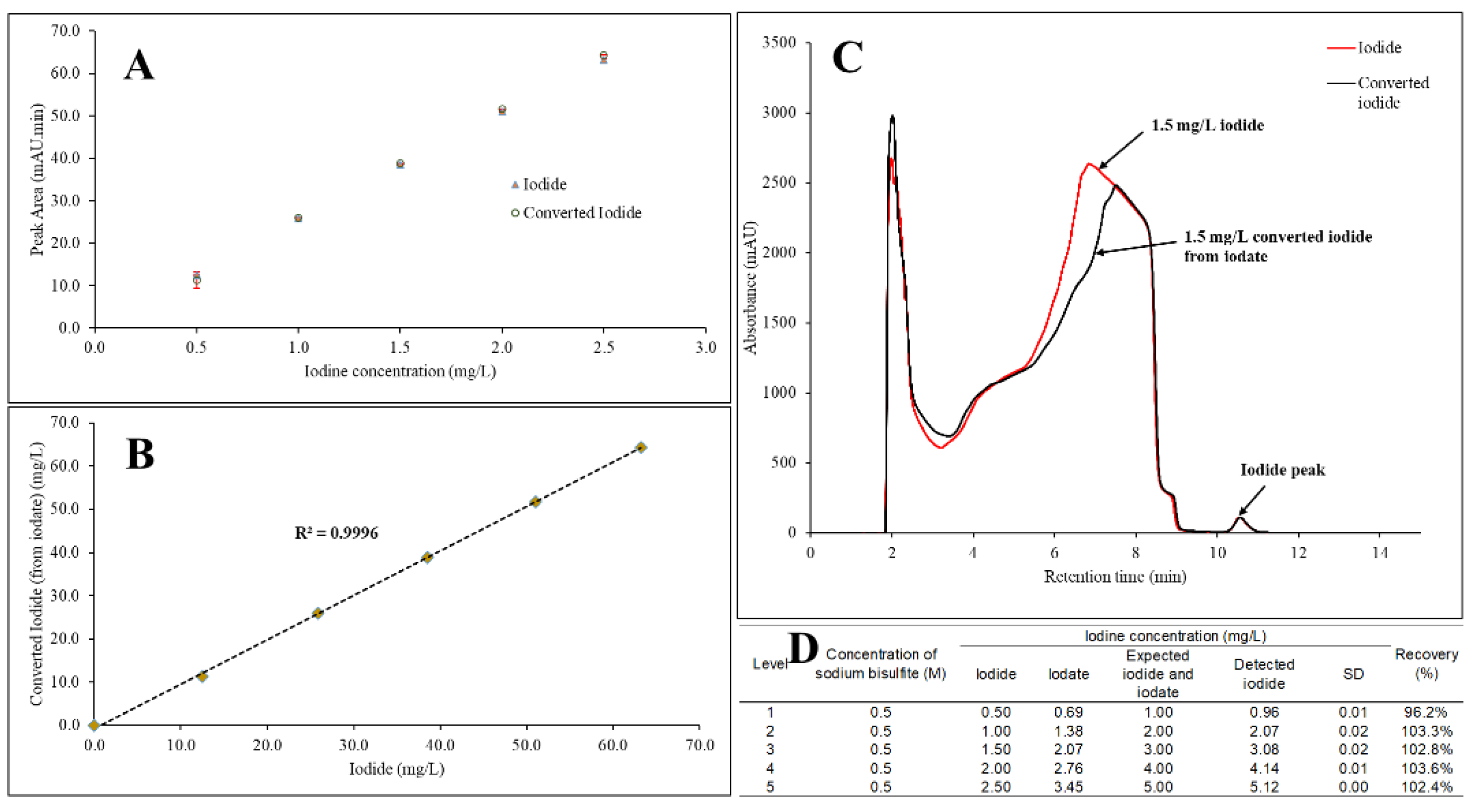
The effect of 0.5 M sodium bisulfite addition to the iodized table salt samples was investigated for the total iodine concentration of 0.5 mg/L to 2.5 mg/L, with respect to both iodide and iodate forms. From here onward, the reduced iodate after its reaction with sodium bisulfite will be denoted as “converted iodide”. It was found that the individual absorbance intensity was similar between iodide and converted iodide (
Figure 1A). A linear correlation was observed between iodide and converted iodide at R
2 of 0.9996 (
Figure 1B). Furthermore, chromatograms overlay shows similar retention time and peak intensity for both iodide and converted iodide (
Figure 1C). Additionally, both iodate and iodide were mixed at equal level of iodine concentration, in 0.5 M sodium bisulfite medium, from 0.5 mg/L to 2.5 mg/L, and it was found that an excellent recovery was calculated from 96.2% to 103.6% (
Figure 1D).
3.2. Method Validation
3.2.1. Accuracy
Three iodine concentrations were investigated for their accuracy at 1.0, 1.5 and 2.0 mg/L. The three samples were prepared in 5% (w/v) sodium chloride to mimic the matrix of real samples. It was found that the relative standard deviation (RSD) was varied from 3.3% to 4.8% (
Table 1), indicating an excellent accuracy.
3.2.2. Linearity
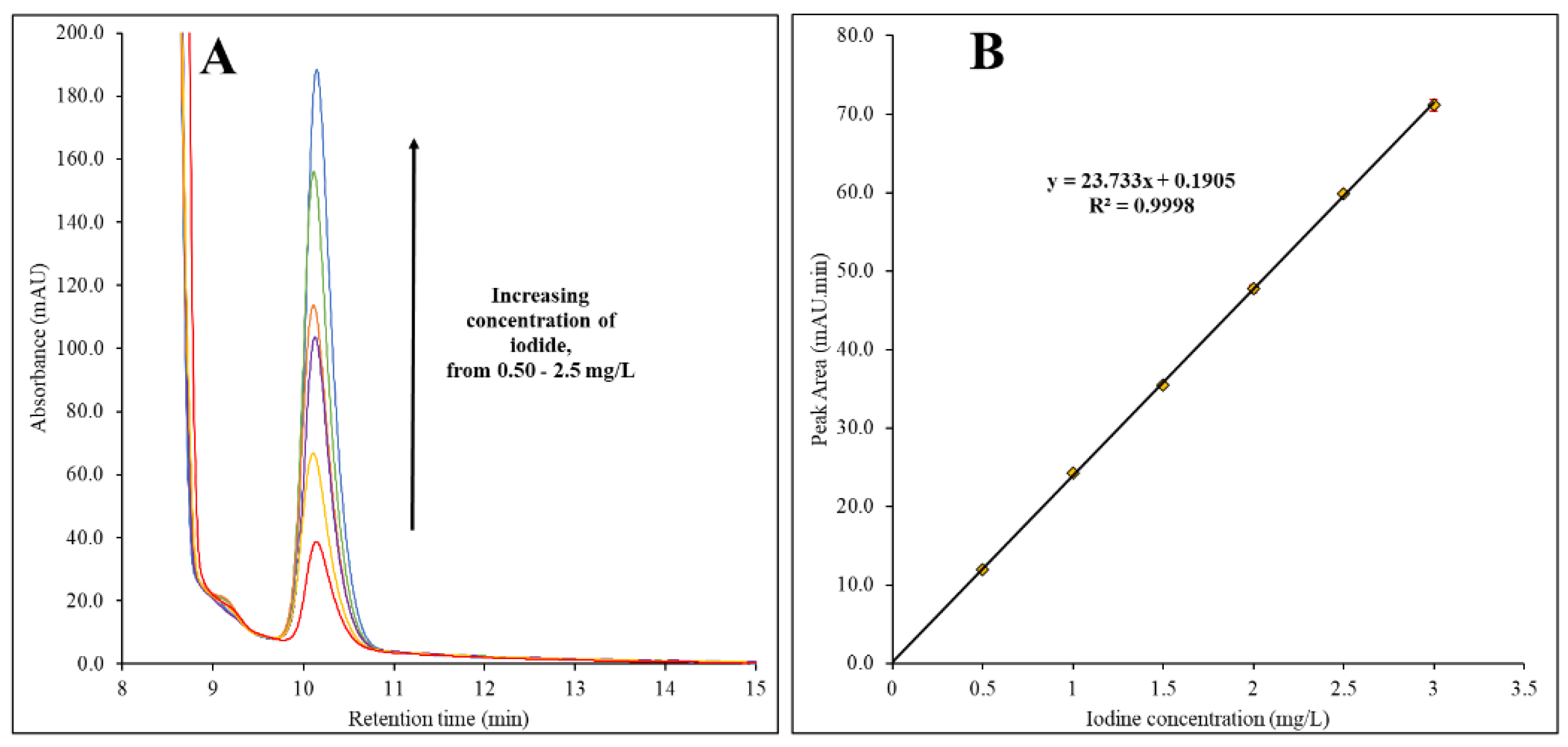
Different concentration of iodine including 0.50 mg/L, 1.0 mg/L, 1.5 mg/L, 2.0 mg/L and 2.5 mg/L were analyzed using UHPLC in 0.5 M sodium bisulfite medium (
Figure 2). Each of the concentration level were prepared in triplicate. Based on the observed chromatograms overlay (
Figure 2A), it was found that the peak intensity of iodide at 10.11 min, increased when the concentration increased. It was found that a linear calibration was formed when the peak area was plotted against the iodine concentration, which resulted in R
2 of 0.9998 (
Figure 2B). Based on the plotted calibration curve, the calculated limit of detection (LOD) and limit of quantification (LOQ) was 0.06 mg/L and 0.18 mg/L of iodine concentration, which are equivalent to 1.2 mg/Kg and 3.7 mg/Kg of total iodine in iodized table salt, respectively.
3.2.3. Specificity
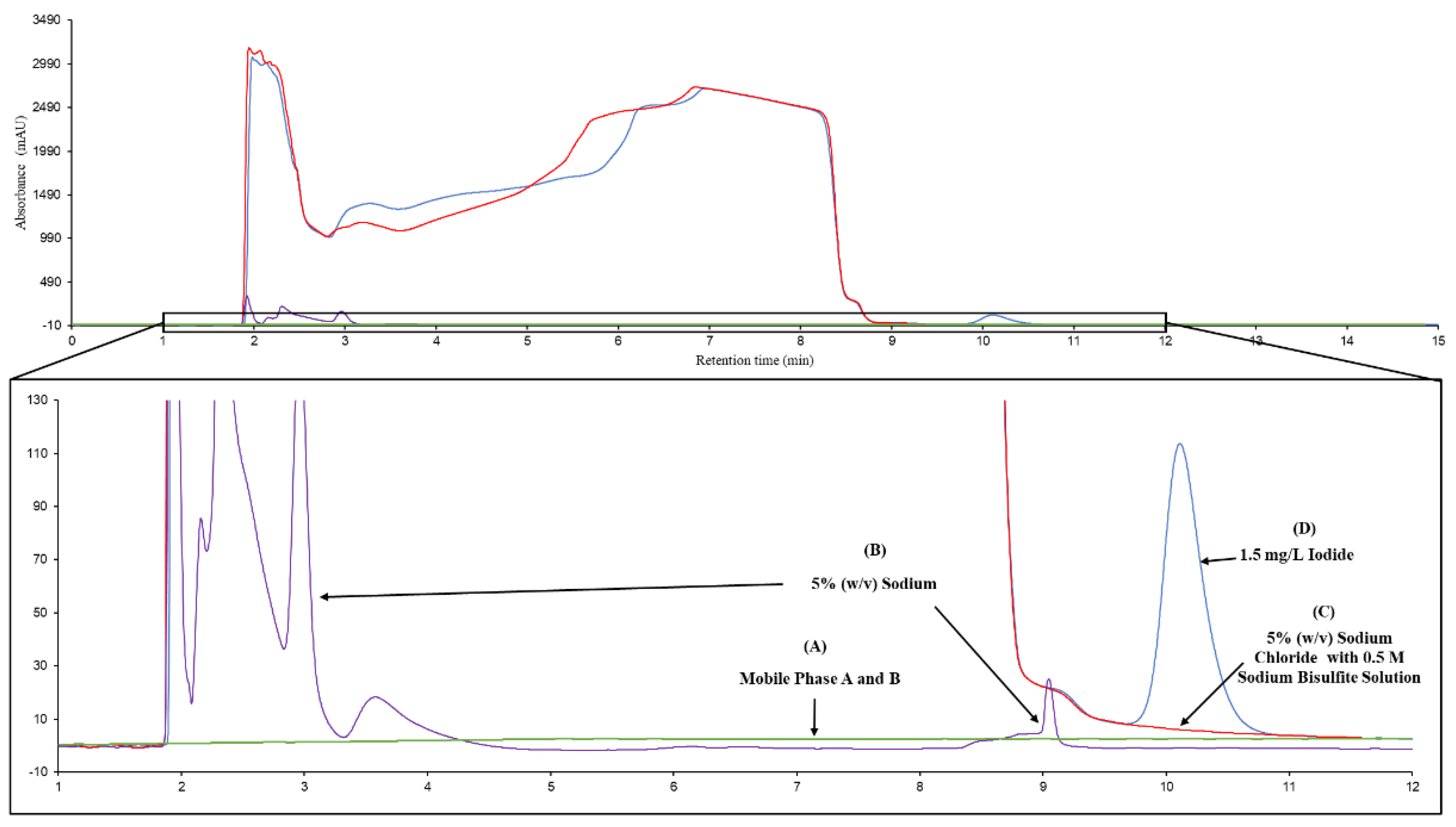
Several sample matrices were studied for the effect of iodide signals in the chromatograms (
Figure 3), including (1) blank of mobile phase A and B at 50% (v/v) (
Figure 3A); (2) 5% (w/v) sodium chloride(
Figure 3B); (3) 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite (
Figure 3C), and (4) 1.50 mg/L iodide in 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite (
Figure 3D), respectively. No obvious interference was observed when blank injection was done. On the other hand, small foreign peaks were observed in the retention time from 1.90 minutes to 4.00 minutes and 9.10 minutes when 5% (w/v) sodium chloride was injected. Also, it was observed that a very large and bulky peaks shown in the chromatograms of 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite and 1.50 mg/L iodide in 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite with the retention time between 1.90 min to 9.50 min, indicating the addition of 0.5 M sodium bisulfite in the solution affect the chromatogram.
3.2.4. Precision
The precision study was done including repeatability and intermediate-precision (
Table 2). The study of repeatability was done by injecting triplicate of each sample at 1.0 mg/L, 1.5 mg/L and 2.0 mg/L of iodine concentration in 5% (w/v) sodium chloride. Each of the triplicate samples were injected seven (7) times and it was found that the average RSD for 1.0 mg/L, 1.5 mg/L and 2.0 mg/L of iodine concentration was at 0.3%, 0.5% and 0.4%, respectively. On the other hand, the study of intermediate-precision was performed for two (2) different days using 1.0 mg/L, 1.5 mg/L and 2.0 mg/L of iodine concentration in 5% (w/v) sodium chloride, which was done in triplicate. It was found that two (2) days average RSD for 1.0 mg/L at 1.0%, 1.5 mg/L at 2.0% and 2.0 mg/L at 1.7%. Overall, the RSD for repeatability and intermediate-precision was at 0.4% and 1.6%, respectively.
3.2.5. Robustness
Several different conditions were applied to the method setup including changing the column temperature to 40 °C and decrease the concentration of sodium bisulfite by half to 0.25 M (
Table 3). The robustness study was done to 1.0 mg/L, 1.5 mg/L and 2.0 mg/L of iodine concentration in 5% (w/v) sodium chloride. It was found that the retention time of iodide peak was affected to 9.28 ± 0.11 min when the column temperature was increased to 40 °C while retention time of 10.02 ± 0.01 min was the shifted peak of iodide when 0.25 M sodium bisulfite was used. Nevertheless, the average RSD for the three level of iodine was at 1.2% and 0.8% respective to 40 °C of column temperature and 0.25 M sodium bisulfite. It was shown that the overall RSD averaged at 0.8% showing a highly robust method.
3.4. Stability Study for Sodium Bisulfite as Reducing Agent
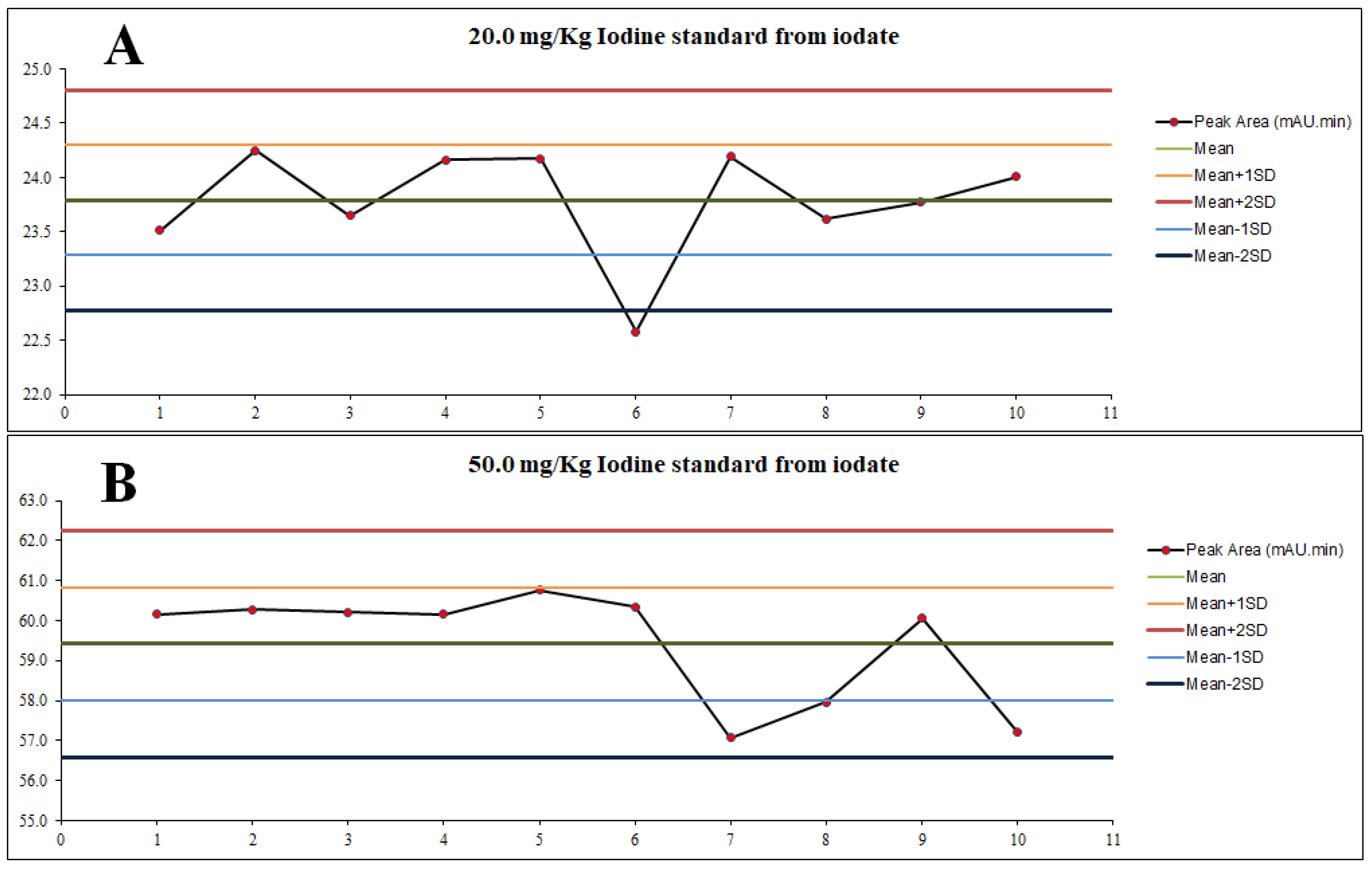
An equivalent of sample concentration was prepared using iodate standard in 5% (w/v) sodium chloride and 0.5 M sodium bisulfite medium at 20.0 mg/Kg and 50.0 mg/Kg of total iodine concentration, respectively. The stability of 0.5 M sodium bisulfite was observed based on the peak area produced by the converted iodide (from iodate) at the concentration of 20.0 mg/Kg and 50.0 mg/Kg of total iodine, respectively, for ten (10) different days. It was found that the peak area remain stable within mean ± 2SD in both concentration, 23.79 ± 1.02 min.mAU for 20.0 mg/Kg and 59.42 ± 2.84 min.mAU, except for day-6 for 20.0 mg/Kg, which was slightly below the allowable limit (22.58 min.mAU), calculated at only 5.1% of relative difference (
Figure 4).
3.5. Sample Spike and Recovery
Six iodized table salt samples were analyzed and individually spiked with both iodide and iodate as shown in the tabulated data (
Table 4). It was shown that the method was able to detect the iodine concentration which ranging from 7.4 mg/Kg to 24.2 mg/Kg of total iodine concentration. The spiked samples show a very good recovery from 89.8% to 101.8% for iodide spike and from 87.2% to 106.9% for iodate spike.
4. Discussion
Typically, ionic compounds such as iodide is analyzed by ion chromatography due to the separation principle is based on ion-exchange properties of the compounds [
20,
21]. Nowadays, new column technology has emerged and thus the introduction of weak anion-exchange column for UHPLC separation that is meant for ionic compounds separation [
16]. The use of UHPLC is more preferable due to its versatility compared to ion chromatography [
22]. Due to this, experts in handling UHPLC are generally common making it suitable for mass adoption. Our study developed and optimized a UHPLC technique of detecting total iodine concentration in the form of iodide. However, most of iodized table salt is fortified with potassium iodate. So, a conversion of iodate to its iodide form was required, and this was done by reducing all the iodate form in the samples to its iodide form by the reaction with sodium bisulfite.
During the method development, evidently, iodate was completely converted to its iodide form when 0.5 M sodium bisulfite was added from 0.5 mg/L to 2.5 mg/L of iodine concentration due to observed similar intensity of iodide and the converted iodide at 10.11 min. The complete conversion of iodate was further supported by the data of linear correlation between iodide and converted iodide at R2 of 0.9996. The addition of 0.5 M sodium bisulfite (in excess) shows no effect to the peak of iodide suggesting a suitable standardization method of sodium bisulfite in the standard and iodized table salt samples. Therefore, the standardization of 0.5 M sodium bisulfite addition was introduced in the sample preparation steps despite not knowing the form of iodine in the iodized table salt.
The method validation of shows an excellent accuracy due to the RSD that was in the range of 3.3% to 4.8 %. Different concentration of iodine from 0.5 mg/L – 2.5 mg/L in 0.5 M sodium bisulfite medium was found to be linearly correlated (R2 of 0.9998) with LOD and LOQ of 0.06 mg/L and 0.18 mg/L, respectively. It is worth mentioned that the concentration of iodine for 0.50 mg/L, 1.0 mg/L, 1.5 mg/L, 2.0 mg/L and 2.5 mg/L are equivalent to 10 mg/Kg, 20 mg/Kg, 30 mg/Kg, 40 mg/Kg and 50 mg/Kg, in the iodized table salt samples, respectively, after it was dissolved in the solution for sample preparation prior to the injection in UHPLC.
The comparison of chromatograms of blank of mobile phase, 5% (w/v) sodium chloride, 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite and 1.50 mg/L iodide in 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite shows that all of the solutions does not affect the peak intensity of iodide except the addition of 5% (w/v) sodium chloride mixed with 0.5 M sodium bisulfite which affect the chromatogram from 1.90 – 9.50 min. Nevertheless, the peak of iodide was clearly distinguished at 10.11 min suggest the separation method has good specificity of iodide detection.
The method was also found to be very precise due to the resulted RSD of 0.3% - 0.5% for repeatability and RSD of 1.0% - 2.0% for intermediate-precision.
The robustness of the method was tested by changing the column temperature to 40 °C and decrease the concentration of sodium bisulfite to 0.25 M, and changing the column causing the retention time of iodine shifted to 9.28 ± 0.11 min whereas decreasing the sodium bisulfite concentration shifted the retention time to 10.02 ± 0.01 min. These effects were expected due to the properties of weak-anion exchange which can be affected by different temperature and different pH where the pH would slightly change due to the change of concentration of sodium bisulfite [
23,
24]. Even so, the RSD still suggest an excellent robustness which ranging from 0.8% - 1.2%.
The addition of excess sodium bisulfite, i.e., at 0.5 M might be degraded overtime due to the its instability when expose to the air because of its nature as a very good antioxidant [
25]. Thus, the stability test was crucial to determine the availability of the reagent to convert any possible iodate form exist in the iodized table salts. It was shown that the peak area of iodide in the chromatograms remain stable within the mean ± 2SD at 20.0 mg/Kg and 50.0 mg/Kg of iodine concentration when observed for ten (10) days, except for day-6 of 20.0 mg/Kg observed 5.1% below allowable limit. The fact that only one day shows an outlier suggest the cause of the problem was due to external factors such as the instrumentation error or the laboratory environment [
26]. The reason was further supported by the peak area result of 50.0 mg/Kg monitoring at day-6, which observed to be remained stable within the allowable limit. The aim was to study the stability of sodium bisulfite if not prepared fresh daily. Thus, the results indicate that the use of 0.5 M sodium bisulfite remain stable for at least ten (10) consecutive days for the concentration of 20.0 mg/Kg and 50.0 mg/Kg of total iodine.
Finally, the method successfully analyzed six iodized table salt samples with all the samples were individually spiked with both iodide and iodate standards. Good recovery was concluded due to the results of 89.8% - 101.8% recovered when spiked with iodide and 87.2% - 106.9% when spiked with iodate, indicating that the sample matrix does not affect the detection of iodide.
5. Conclusions
We have successfully determined and validated the total iodine in iodized table salt. The form of iodine for the UHPLC detection is iodide in which the addition of sodium bisulfite enabled the reduction of iodate into its iodide forms. This method was able to detects total iodine from 0.5 – 3.0 mg/L, which is equivalent to 10 – 60 mg/Kg of total iodine in iodized table salt. The LOD and LOQ were 0.06 mg/L (1.2 mg/Kg) and 0.18 mg/L (3.7 mg/Kg). The method was validated with great specificity, accuracy at 4.2% RSD, precision at 0.4% RSD for repeatability and 1.6% RSD for intermediate-precision and robustness at 0.8% RSD. Six (6) samples were analyzed in triplicate and it was found that the samples were recovered greatly from 87.2% - 106.9% when spiked with individual iodate and iodate standard at equivalent concentration of 10, 20 and 30 mg/Kg, respectively. The use of sodium bisulfite for the conversion of iodate to iodide form, observed to be remain stable for at least 10 days. The study concluded that this method was suitable to be used as a laboratory protocol for the determination of total iodine in iodized table salt.
Author Contributions
Conceptualization, M.A.J.; methodology, M.A.J.; software, M.A.J.; validation, M.A.J., A.A.R. and M.F.M.N.; formal analysis, M.A.J.; investigation, M.A.J.; resources, A.A.R.; data curation, M.A.J.; writing—original draft preparation, M.A.J.; writing—review and editing, A.A.R. and M.F.M.N.; visualization, M.A.J.; supervision, M.F.M.N.; project administration, A.A.R.; funding acquisition, M.F.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Ministry of Health Malaysia, grant number NMRR-14-502-21091.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. J. Nutr. 2008, 138, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; Wierckx, K.; Delanghe, J.R.; Kaufman, J.-M. Value and Pitfalls in Iodine Fortification and Supplementation in the 21st Century. Br. J. Nutr. 2011, 106, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Mannar, M.G.V.; Dunn, J.T. Salt Iodization for the Elimination of Iodine Deficiency; International Council for Control of Iodine Deficiency Disorders: Netherlands, 1995; ISBN 9070785137. [Google Scholar]
- Bégin, F.; Unicef, K.C.; Asia, E.; Regional, P. Iodized Salt Legislation in South and East Asia and the Pacific : An Overview. 2013, 16–17.
- Gorstein, J.L.; Bagriansky, J.; Pearce, E.N.; Kupka, R.; Zimmermann, M.B. Estimating the Health and Economic Benefits of Universal Salt Iodization Programs to Correct Iodine Deficiency Disorders. Thyroid 2020, 30, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Chavasit, V.; Malaivongse, P.; Judprasong, K. Study on Stability of Iodine in Iodated Salt by Use of Different Cooking Model Conditions. J. Food Compos. Anal. 2002, 15, 265–276. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The Role of Iodine in Human Growth and Development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chaouki, M.L.; Benmiloud, M. Prevention of Iodine Deficiency Disorders by Oral Administration of Lipiodol during Pregnancy. Eur. J. Endocrinol. 1994, 130, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Andersen, S.; Ovesen, L.; Laurberg, P. Iodine Intake and Food Choice. In Comprehensive handbook of iodine; Academic Press: Burlington, Massachusetts, 2009; pp. 332–337. ISBN 9780123741356. [Google Scholar]
- Yun, A.J.; Doux, J.D. Iodine in the Ecosystem. In Comprehensive Handbook of Iodine; Elsevier, 2009; pp. 119–123 ISBN 9780123741356.
- Falk, H.; Geerling, R.; Hattendorf, B.; Krengel-Rothensee, K.; Schmidt, K.P. Capabilities and Limits of ICP-MS for Direct Determination of Element Traces in Saline Solutions. Fresenius. J. Anal. Chem. 1997, 359, 352–356. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Mello, P.A.; Krzyzaniak, S.R.; Cauduro, V.H.; Picoloto, R.S. Challenges and Trends for Halogen Determination by Inductively Coupled Plasma Mass Spectrometry: A Review. Rapid Commun. Mass Spectrom. 2020, 34, 0–3. [Google Scholar] [CrossRef]
- Jennings, W.G.; Majors, R.E.; Kirkland, J.J.; Unger, K.K.; Engelhardt, H.; Schomburg, G.; Pirkle, W.H.; Welch, C.J.; Armstrong, D.W.; Porath, J.O.; et al. History and Developments in Chromatographic Column Technology and Validation to 2001. In Chromatography: A Science of Discovery; Wixom, R.L., Gehrke, C.W., Eds.; John Wiley & sons, inc.: New Jersey, 2010; pp. 199–267. ISBN 978-0-470-28345-5. [Google Scholar]
- Lämmerhofer, M.; Nogueira, R.; Lindner, W. Multi-Modal Applicability of a Reversed-Phase/Weak-Anion Exchange Material in Reversed-Phase, Anion-Exchange, Ion-Exclusion, Hydrophilic Interaction and Hydrophobic Interaction Chromatography Modes. Anal. Bioanal. Chem. 2011, 400, 2517–2530. [Google Scholar] [CrossRef]
- Nogueira, R.; Lämmerhofer, M.; Lindner, W. Alternative High-Performance Liquid Chromatographic Peptide Separation and Purification Concept Using a New Mixed-Mode Reversed-Phase/Weak Anion-Exchange Type Stationary Phase. J. Chromatogr. A 2005, 1089, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Dionex Determination of Iodide and Iodate in Seawater and Iodized Table Salt by HPLC with UV Detection. Appl. Note 236 2009, 1–7.
- Rebary, B.; Paul, P.; Ghosh, P.K. Determination of Iodide and Iodate in Edible Salt by Ion Chromatography with Integrated Amperometric Detection. Food Chem. 2010, 123, 529–534. [Google Scholar] [CrossRef]
- Buchberger, W.; Czizsek, B.; Hann, S.; Stingeder, G. Preliminary Comparison of Inductively Coupled Plasma Mass Spectrometry and Electrospray Mass Spectrometry Hyphenated with Ion Chromatography for Trace Analysis of Iodide. J. Anal. At. Spectrom. 2003, 18, 512–514. [Google Scholar] [CrossRef]
- Stärk, H.-J.; Mattusch, J.; Wennrich, R.; Mroczek, A. Investigation of the IC-ICP-MS Determination of Iodine Species with Reference to Sample Digestion Procedures. Fresenius. J. Anal. Chem. 1997, 359, 371–374. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Meloan, C.E. High-Performance Liquid Chromatography and Ion Chromatography. In Food Analysis; Springer US: Boston, MA, 1994; Volume 6, pp. 324–351. ISBN 978-1-4615-7000-4. [Google Scholar]
- Staby, A.; Jensen, R.H.; Bensch, M.; Hubbuch, J.; Dünweber, D.L.; Krarup, J.; Nielsen, J.; Lund, M.; Kidal, S.; Hansen, T.B.; et al. Comparison of Chromatographic Ion-Exchange Resins. J. Chromatogr. A 2007, 1164, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.B.; Martin, D.F.; Martin, B.B.; Feliciano, D.; Acıkara, Ö.B.; Citoglu, G.S.; Özbilgin, S.; Ergene, B.; Amaral, A.C.F. Column Chromatography; 2013; ISBN 9789535110743.
- Shi, Y.; Zhan, X.; Ma, L.; Li, L.; Li, C. Evaluation of Antioxidants Using Oxidation Reaction Rate Constants. Front. Chem. China 2007, 2, 140–145. [Google Scholar] [CrossRef]
- Reckling, M.; Ahrends, H.; Chen, T.-W.; Eugster, W.; Hadasch, S.; Knapp, S.; Laidig, F.; Linstädter, A.; Macholdt, J.; Piepho, H.-P.; et al. Methods of Yield Stability Analysis in Long-Term Field Experiments. A Review. Agron. Sustain. Dev. 2021, 41, 27. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).