Submitted:
29 October 2024
Posted:
29 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Testing
2.3. Treatment Outcome
2.4. Statistical Analyses
2.5. Ethical Approval and Informed Consent
3. Results
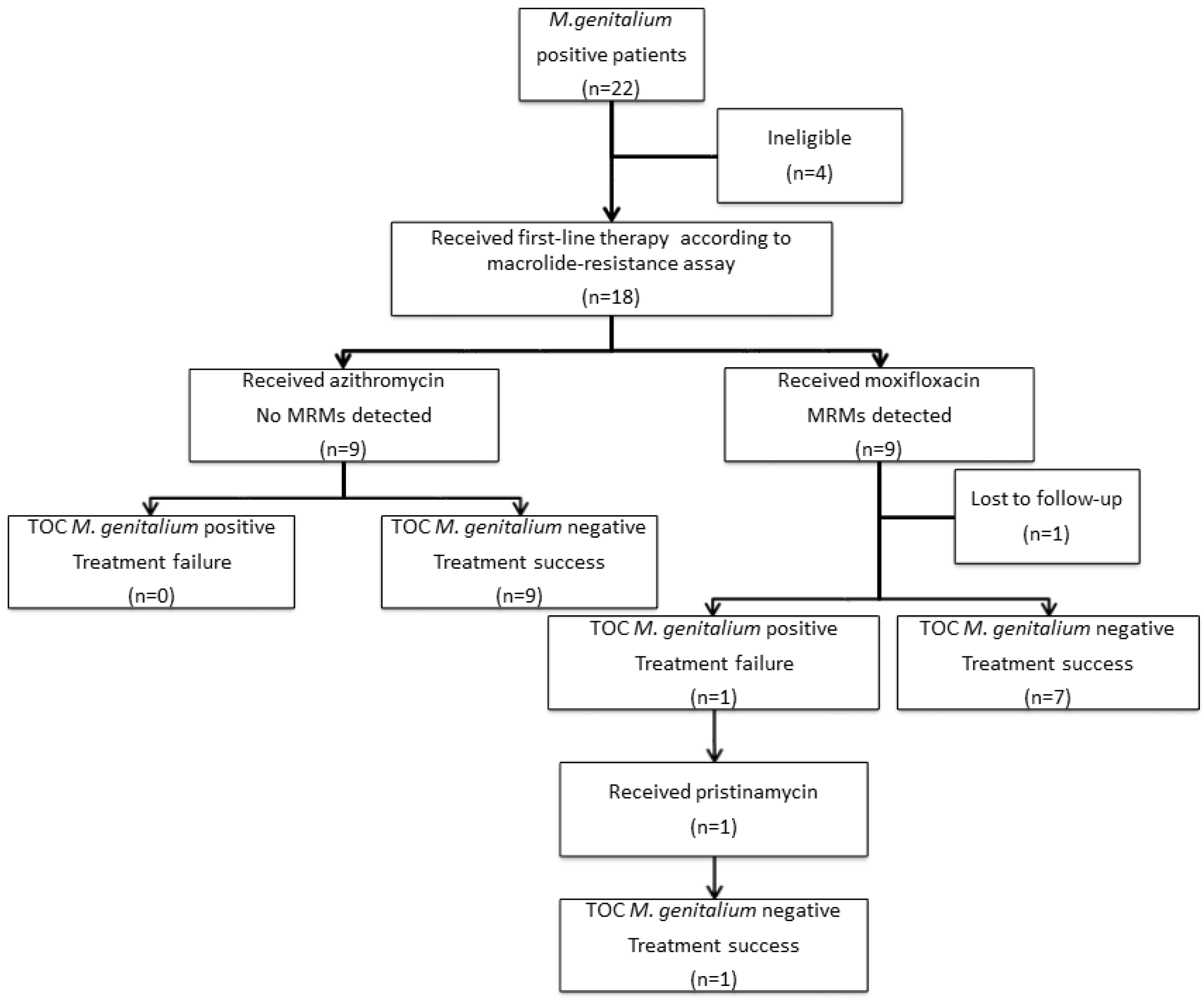
3.1. Selection of Cases, Treatment Outcomes and Demographic Characteristics
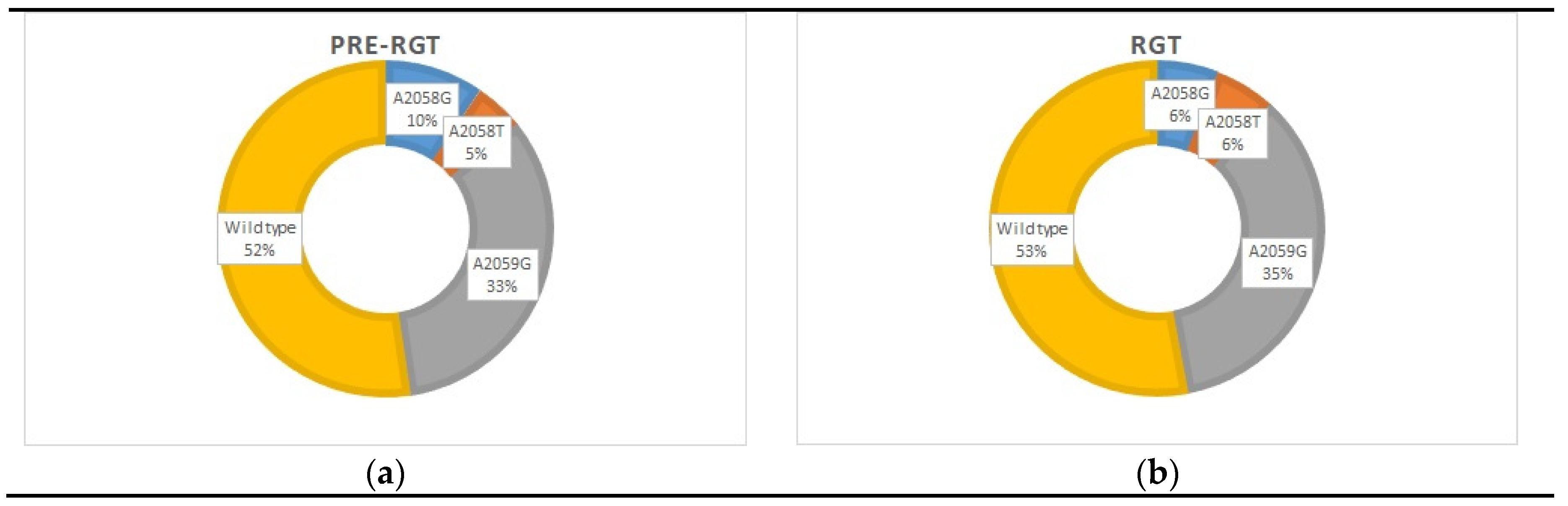
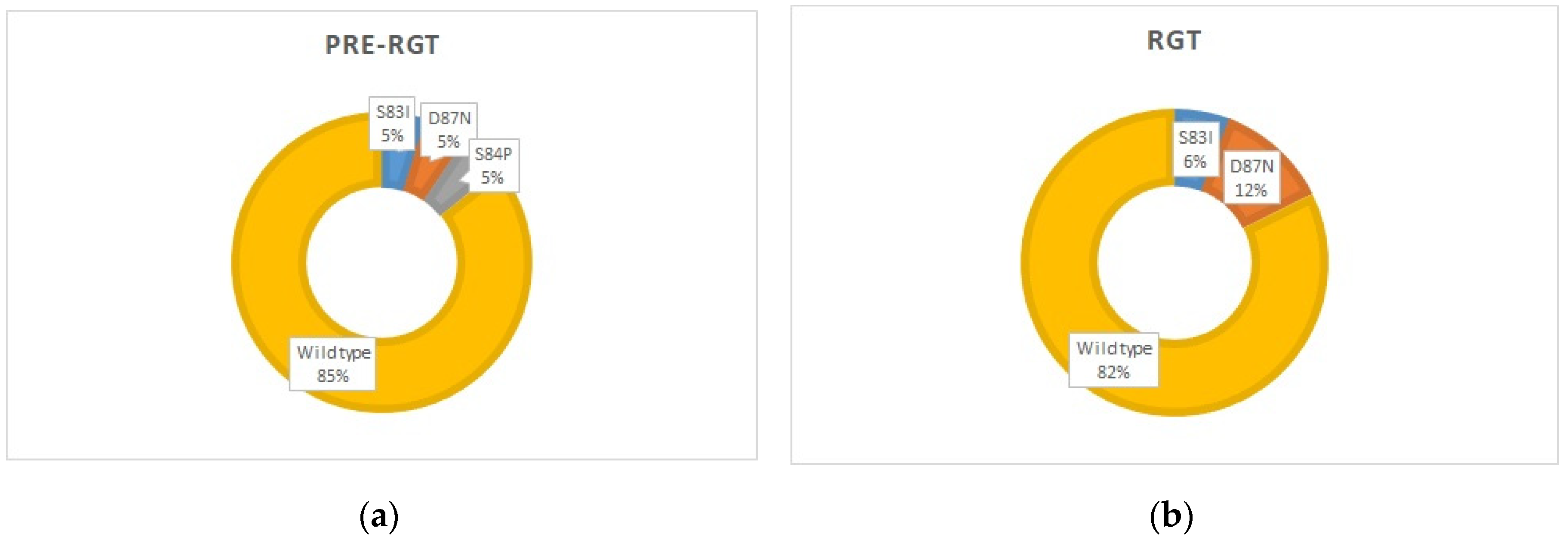
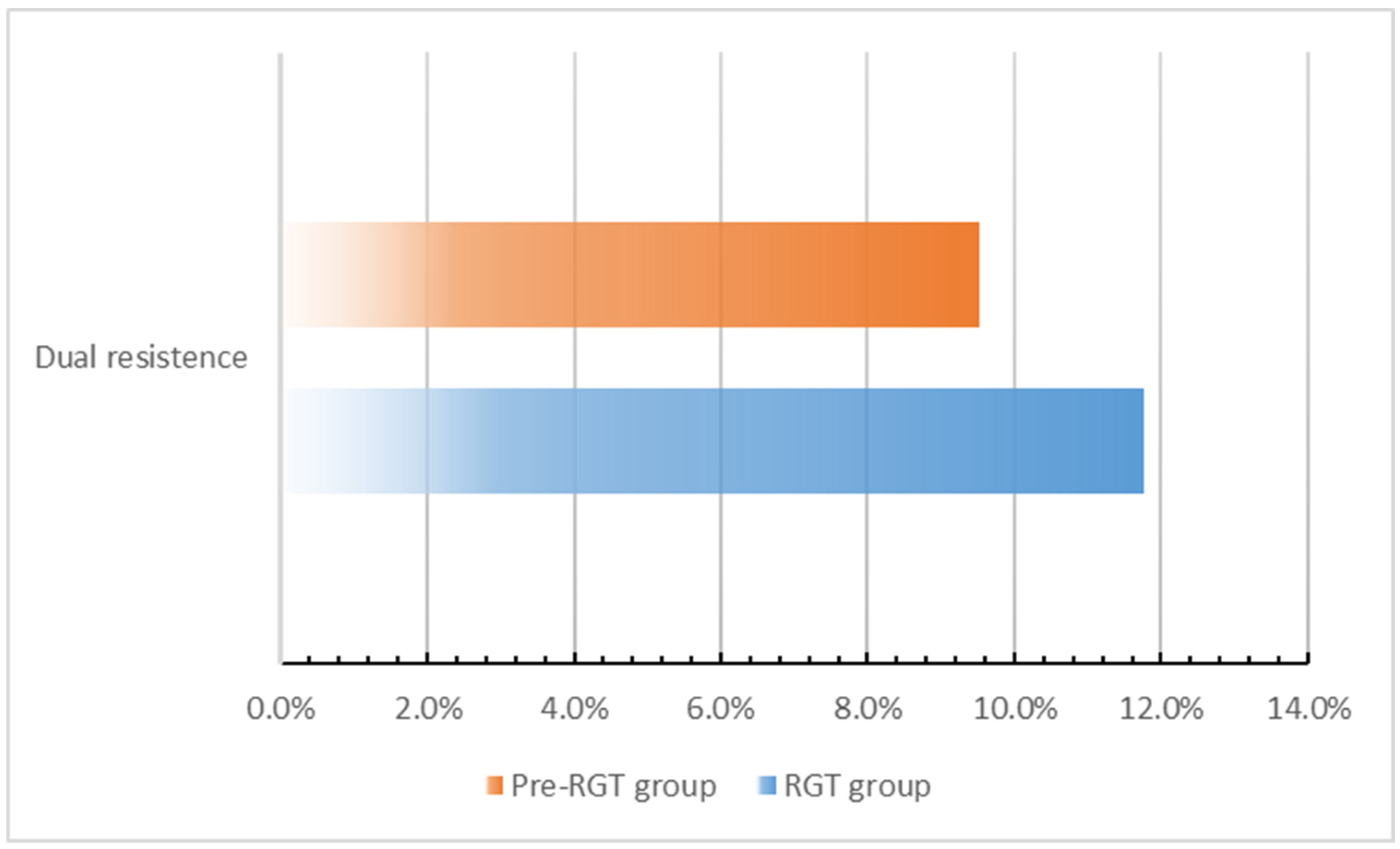
3.2. Macrolide and Quinolone Resistance Mutations (MRMs and QRAMs)
3.3. Treatment Failure Rate and Mean Time to Cure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonnenberg P, Ison CA, Clifton S, Field N, Tanton C, Soldan K, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol. 2015, 44, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Edouard S, Tissot-Dupont H, Dubourg G, Bernard A, Fournier P, Ravaux I, et al. Mycoplasma genitalium, an agent of reemerging sexually transmitted infections. Apmis. 2017, 125, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011, 24, 498–514. [Google Scholar] [CrossRef]
- Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015, 61, 418–426. [Google Scholar] [CrossRef]
- Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatology Venereol. 2022, 36, 641–650. [Google Scholar] [CrossRef]
- Jensen JS, Hansen HT, Lind K. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol. 1996, 34, 286–291. [Google Scholar] [CrossRef]
- Wihlfahrt K, Günther V, Mendling W, Westermann A, Willer D, Gitas G, et al. Sexually Transmitted Diseases—An Update and Overview of Current Research. Diagnostics. 2023, 13, 1656. [Google Scholar] [CrossRef]
- Philipova I, Hadad R, Levterova V, Kantardjiev T, Unemo M. Mycoplasma genitalium antimicrobial (azithromycin and moxifloxacin) resistance and treatment outcome in Sofia, Bulgaria, 2018-2021. J Eur Acad Dermatology Venereol. 2023 Mar 27;n/a(n/a). [CrossRef]
- Lau A, Bradshaw CS, Lewis D, Fairley CK, Chen MY, Kong FYS, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis. 2015, 61, 1389–1399. [Google Scholar] [CrossRef]
- Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020, 20, 1302–1314, Available from: https://www.sciencedirect.com/science/article/pii/S1473309920301547. [Google Scholar] [CrossRef]
- Li Y, Le W-J, Li S, Cao Y-P, Su X-H. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS. 2017, 28, 1106–1114. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. US Department of Health and Human Services, Centres for Disease Control and Prevention; 2019.
- Sakalauskienė G V, Radzevičienė A. Antimicrobial Resistance: What Lies Beneath This Complex Phenomenon? Diagnostics. 2024, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Read TRH, Fairley CK, Murray GL, Jensen JS, Danielewski J, Worthington K, et al. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis. 2019, 68, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Durukan D, Read TRH, Murray G, Doyle M, Chow EPF, Vodstrcil LA, et al. Resistance-Guided Antimicrobial Therapy using doxycycline–moxifloxacin and doxycycline–2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: Efficacy and tolerability. Clin Infect Dis. 2020, 71, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Conway RJH, Cook S, Malone C, Bone S, Hassan-Ibrahim MO, Soni S. Resistance-guided treatment of Mycoplasma genitalium infection at a UK sexual health centre. Int J STD AIDS. 2021, 32, 758–765. [Google Scholar] [CrossRef]
- Dumke R, Spornraft-Ragaller P. Antibiotic Resistance and Genotypes of Mycoplasma genitalium during a Resistance-Guided Treatment Regime in a German University Hospital. Antibiotics 2021, 10, 962. [Google Scholar] [CrossRef]
- Philipova I, Levterova V, Simeonovski I, Kantardjiev T. Azithromycin treatment failure and macrolide resistance in Mycoplasma genitalium infections in Sofia, Bulgaria. Folia medica (Plovdiv). 2022, 64, 422–429. [Google Scholar] [CrossRef]
- Tabrizi SN, Tan LY, Walker S, Twin J, Poljak M, Bradshaw CS, et al. Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology. PLoS One. 2016, 11, e0156740. [Google Scholar]
- Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008, 47, 1546–1553. [Google Scholar] [CrossRef]
- Deguchi T, Maeda S-I, Tamaki M, Yoshida T, Ishiko H, Ito M, et al. Analysis of the gyrA and parC genes of Mycoplasma genitalium detected in first-pass urine of men with non-gonococcal urethritis before and after fluoroquinolone treatment. J Antimicrob Chemother. 2001, 48, 742–744. [Google Scholar] [CrossRef]
- Twin J, Jensen JS, Bradshaw CS, Garland SM, Fairley CK, Min LY, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One. 2012, 7, e35593. [Google Scholar]
- Horner P, Ingle SM, Garrett F, Blee K, Kong F, Muir P, et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Transm Infect. 2018, 94, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Read TRH, Fairley CK, Tabrizi SN, Bissessor M, Vodstrcil L, Chow EPF, et al. Azithromycin 1.5 g over 5 days compared to 1g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis. 2017, 64, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jensen JS, Unemo M. Antimicrobial treatment and resistance in sexually transmitted bacterial infections. Nat Rev Microbiol. 2024, 1–16. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Mycoplasma genitalium guide: key information and resources. [Internet]. Government of Canada. 2023. Available from: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/mycoplasma-genitalium.html#Key_information.
- Wada K, Hamasuna R, Sadahira T, Araki M, Yamamoto S. UAA-AAUS guideline for M. genitalium and non-chlamydial non-gonococcal urethritis. J Infect Chemother. 2021, 27, 1384–1388. [Google Scholar] [CrossRef]
- Workowski KA. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Reports. 2021, 70. [CrossRef]
- Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae (2019). London: British Association for Sexual Health and HIV; 2019.
- The Australasian STI management guidelines. Mycoplasma genitalium [Internet]. The Australasian Sexual and Reproductive Health Alliance (ASHA). 2021. Available from: https://sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium/.
- Murray GL, Bodiyabadu K, Vodstrcil LA, Machalek DA, Danielewski J, Plummer EL, et al. parC variants in Mycoplasma genitalium: trends over time and association with moxifloxacin failure. Antimicrob Agents Chemother. 2022, 66, e00278–22. [Google Scholar]
- Sweeney EL, Bradshaw CS, Murray GL, Whiley DM. Individualised treatment of Mycoplasma genitalium infection—incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect Dis. 2022, 22, e267–70. [Google Scholar] [CrossRef]
- Manhart LE, Jensen JS. Quinolone Resistance–Associated Mutations in Mycoplasma genitalium: Not Ready for Prime Time. Sex Transm Dis. 2020, 47, 199. [Google Scholar] [CrossRef]
- Tabrizi SN, Su J, Bradshaw CS, Fairley CK, Walker S, Tan LY, et al. Prospective evaluation of ResistancePlus MG, a new multiplex quantitative PCR assay for detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol. 2017, 55, 1915–1919. [Google Scholar] [CrossRef]
- Le Roy C, Pereyre S, Hénin N, Bébéar C. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD assay and macrolide resistance detection using three distinct assays. J Clin Microbiol. 2017, 55, 3194–3200. [Google Scholar] [CrossRef]
- Adawiyah R Al, Bradshaw CS, Vodstrcil LA, Fairley CK, Zhang L, Ong JJ. Cost-effectiveness of resistance-guided therapy for Mycoplasma genitalium in Australia. Sci Rep. 2024, 14, 12856. [Google Scholar] [CrossRef]
| pre-RGT group [18], n (%) |
RGT, n (%) | |||
| Male (n = 18) | Female (n = 3) | Male (n = 14) | Female (n = 3) | |
| Median age (range) | 32 (22-49) | 28 (23-33) | 29 (18-47) | 29 (23-34) |
| Presentation | ||||
| Symptomatic | 15 (83.3) | 1 (33.3) | 12 (85.7) | 2 (66.7) |
| Asymptomatic contact | 3 (16.7) | 2 (66.7) | 2 (14.3) | 1 (33.3) |
| Specimen | ||||
| First-void urine | 14 (77.8) | 0 (0) | 7 (50) | 0 (0) |
| Urogenital swab | 4 (22.2) | 3 (100) | 5 (35.7) | 3 (100) |
| Extra-genital swab | 0 (0) | 0 (0) | 2 (14.3) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





