1. Introduction
Acute respiratory infections (ARIs) are a major cause of morbidity in children worldwide, frequently leading to hospitalizations, particularly due to conditions such as bronchiolitis and bronchopneumonia [
1,
2,
3]. Recently, there has been considerable debate regarding the role of vitamin D in immune responses to these infections and the potential need for supplementation. Numerous studies indicate that low vitamin D levels are prevalent among infants and children with respiratory tract infections (RTIs), highlighting a strong correlation between deficiency, the severity and frequency of the infections, and the requirement for critical care while hospitalized. Additionally, ongoing research continues to explore the effects of vitamin D supplementation during acute respiratory conditions [
4]. However, maintaining normal to elevated serum 25(OH)D (25-hydroxyvitamin D) levels appears to positively influence the incidence and severity of some, though not all, types of respiratory infections in children [
5,
6].
Vitamin D is a fat-soluble steroid primarily known for its essential role in regulating calcium-phosphorus metabolism, crucial for normal bone growth and development. However, its non-classical actions, mediated through vitamin D receptors (VDRs) expressed in various extrarenal tissues, are now well-documented. A key example is the immune system, where cells such as lymphoid, myeloid, dendritic cells, and macrophages increase VDR levels during acute infections. This promotes the production of active vitamin D, which, in turn, stimulates the generation of host defense proteins like cathelicidin and defensin. Additionally, vitamin D plays a role in innate immune signaling by promoting cytokine production and activating pattern recognition receptors (PRRs), which detect components of pathogenic microorganisms and trigger a rapid innate immune response [
7,
8]. These functions of vitamin D are crucial for local immunity in the lungs, as they are present not only in macrophages but also in lung epithelial cells [
9]. This evidence underscores the role of vitamin D in the pathogenesis of certain acute and chronic lung diseases [
10].
Vitamin D deficiency and insufficiency pose significant public health challenges globally, not only due to their involvement in various diseases but also because of the limited data on population-wide vitamin D status in many countries [
11,
12]. In Bulgarian children, few studies have investigated vitamin D status, and most focus on specific conditions such as idiopathic scoliosis, myopia, kidney and bowel disorders [
13,
14,
15]. To date, few studies have explored its relationship with chronic respiratory infections and acute pneumonia [
16,
17]. Through our current research, we aimed to expand the data on vitamin D status in relation to respiratory infections in the Bulgarian pediatric population, focusing on various acute respiratory diseases. To achieve this, we included children with not only acute pneumonia but also bronchiolitis, laryngotracheitis, and acute bronchitis. Additionally, we aimed to compare our findings with existing data from studies conducted in different countries and populations, which are further analyzed in this article.
Calcidiol (25-hydroxycholecalciferol) is the primary vitamin D metabolite present in circulation, with a lifespan of 2–3 weeks, making its concentration the gold standard for assessing vitamin D status [
18,
19,
20]. Measuring blood concentrations of 25-hydroxycholecalciferol and parathormone (PTH) allows for a precise evaluation of vitamin D status in children with acute respiratory infections. PTH levels serve as an indicator of vitamin D sufficiency, as normal PTH values require optimal levels of 25(OH) vitamin D [
21].
The data obtained from these measurements aim to clarify the correlation between these laboratory parameters and the clinical manifestations of acute respiratory diseases in childhood. This study specifically sought to determine if there is a relationship between vitamin D levels and the frequency of certain acute infections of the respiratory tract in children.
2. Materials and Methods
This prospective clinical research was carried out at the Pediatrics Department of Dr. Georgi Stranski University Hospital, Pleven. A total of 129 children, aged 0–17 years, who were hospitalized due to acute respiratory infections (ARIs) between July 2021 and December 2023, were included in the study. The participants were divided into five subgroups:
acute bronchopneumonia (n=42),
acute laryngotracheitis (n=7),
acute bronchiolitis (n=32),
acute bronchitis (n=18), and a
control group (n=30).
The children with acute bronchiolitis were between 2 months and 2 years of age.
Control subjects were healthy children who attended follow-up examinations at the clinic.
Exclusion criteria included: (1) children with acute non-infectious respiratory conditions (e.g., foreign body aspiration, congenital malformations); (2) those with chronic respiratory or circulatory system conditions (e.g., asthma, cystic fibrosis, primary ciliary dyskinesia, immunodeficiencies, congenital cardiovascular malformations); and (3) children with renal or endocrine diseases.
Serum 25(OH)D and parathyroid hormone (PTH) levels were measured using an electrochemiluminescent immunoassay on a Roche Cobas e411 immunological analyzer.
Vitamin D status was categorized based on the guidelines of the Bulgarian Society of Endocrinology as follows: vitamin D deficiency: <25 nmol/L, vitamin D insufficiency: 25–50 nmol/L, vitamin D sufficiency: 50–120 nmol/L, and hypervitaminosis D: >120 nmol/L. Serum PTH levels were classified as normal within the range of 15 to 65 pg/mL [
22].
Statistical analysis was conducted using IBM SPSS Statistics v.26.0 for Windows, along with Microsoft Office Excel 2019. To determine where statistically significant differences in 25(OH)D levels existed between patients with specific diagnoses and the control group, the non-parametric Mann-Whitney U test was applied. A statistically significant difference was defined as a p-value < 0.05.
The study was approved by the Committee on Research Ethics at the Medical University – Pleven (Approval Code: 658; Approval Date: June 29, 2021). Informed consent for participation was obtained from the parents or guardians of all enrolled patients.
3. Results
A total of 129 children were enrolled in the study, consisting of 52 boys (40.3%) and 77 girls (59.7%). The mean age of the participants, calculated in months, was 31 ± 46.26, with an age range of 0 to 14 years.
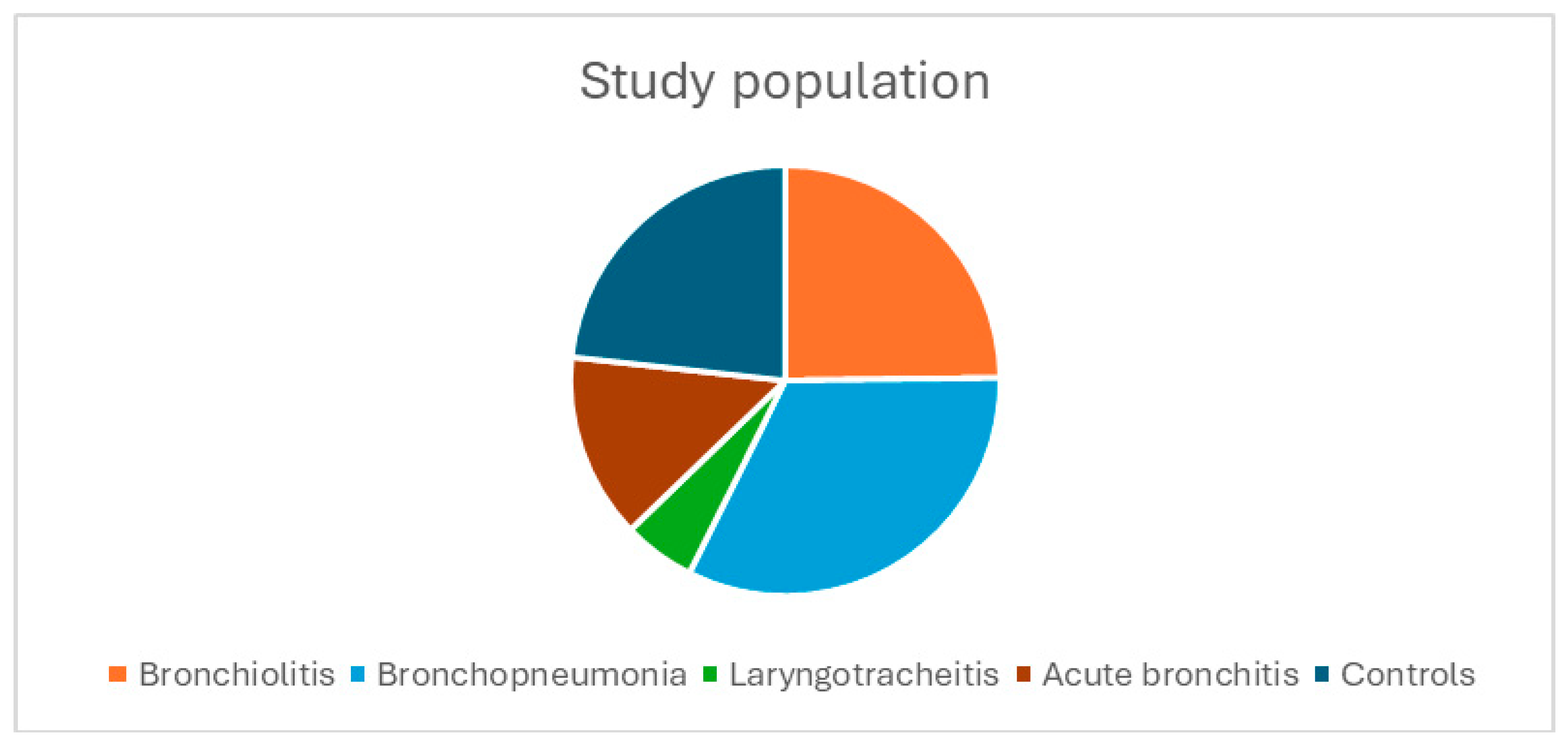
Figure 1 shows the distribution of the study population, with the bronchopneumonia group being the largest (n = 42), followed by the bronchiolitis group (n = 32), the acute bronchitis group (n = 18), the laryngotracheitis group (n = 7), and the control group (n = 30).
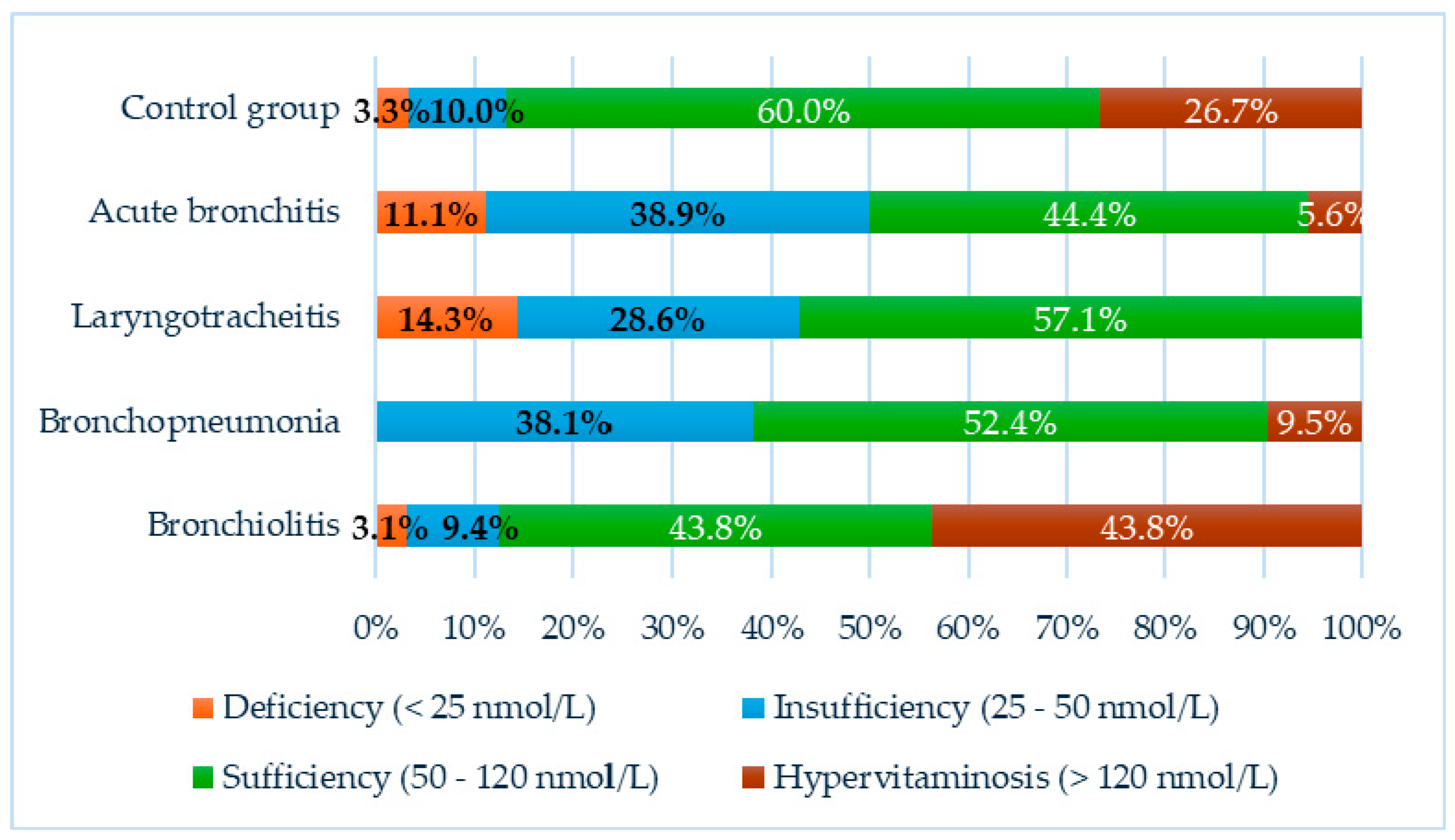
In our study, both subnormal levels of vitamin D and cases of hypervitaminosis were observed in the individual groups:
In the acute bronchitis group, 7 children (38.9%) were found to have insufficient vitamin D levels, 2 children (11.1%) were deficient, and 1 child (5.6%) had hypervitaminosis. The remaining 8 children (44.4%) were classified as sufficient.
In the group of children with laryngotracheitis, 2 children (28.6%) exhibited vitamin D insufficiency, 1 child (14.3%) had vitamin D deficiency, and the remaining 4 children (57.1%) had sufficient levels.
Among children with bronchopneumonia, 16 (38.1%) had vitamin D insufficiency, and 4 (9.5%) presented with hypervitaminosis. Vitamin D levels in the remaining 22 children (52.4%) in this group were found to be within the reference range, with no children classified as deficient.
Abnormalities in vitamin D status were observed in children with acute bronchiolitis, with 3 children (9.4%) exhibiting insufficiency, 1 child (3.1%) exhibiting deficiency, and 14 children (43.8%) having hypervitaminosis. The remaining 14 children (43.8%) were classified as sufficient.
In the control group, 1 child (3.3%) exhibited vitamin D deficiency, 3 children (10%) showed insufficiency, 8 children (26.7%) had hypervitaminosis, and 18 children (60%) were classified as sufficient.
The results are illustrated in
Figure 2.
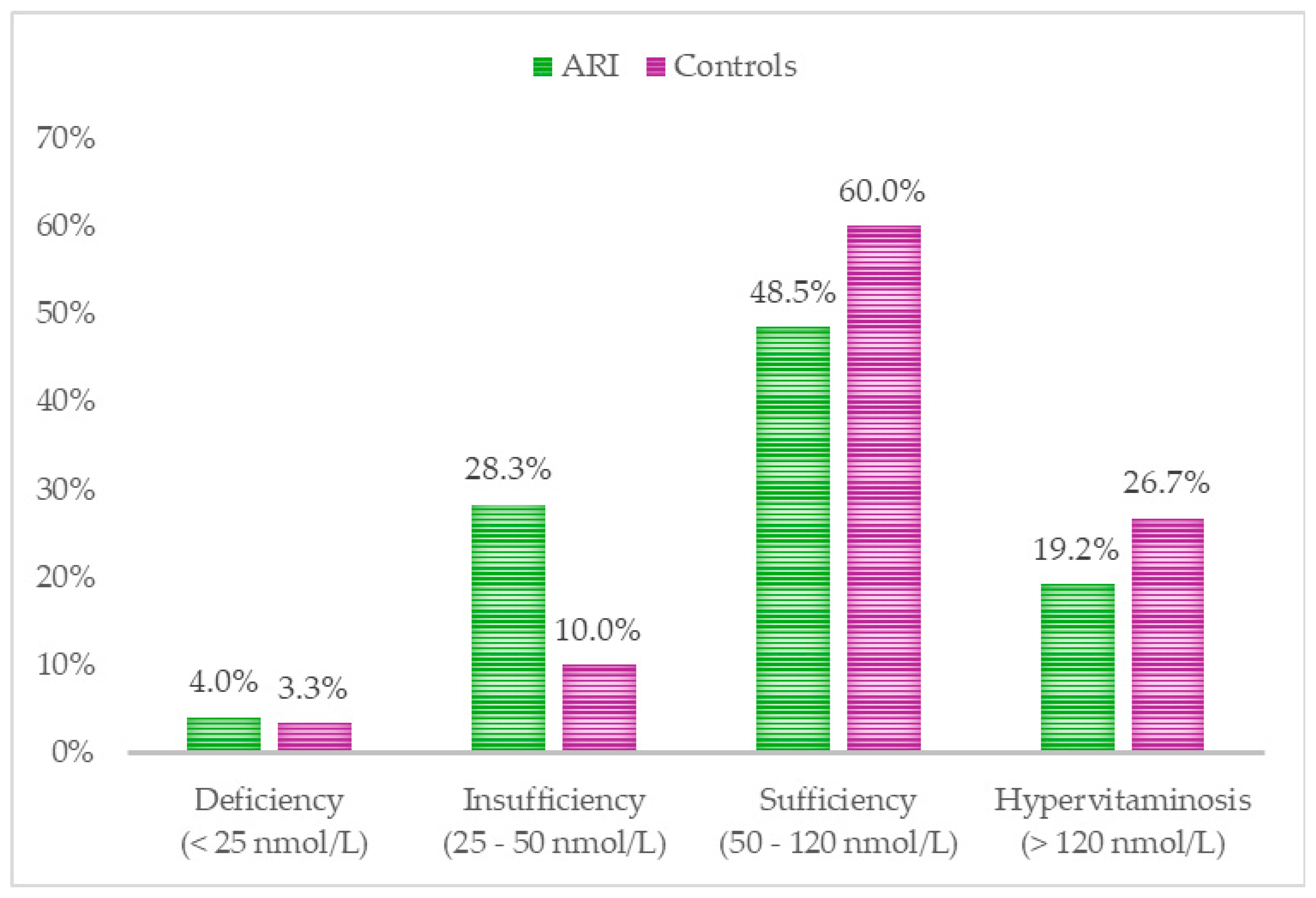
Comparison of serum 25 (OH) D levels between children with ARIs and healthy controls revealed the following: 28.3% of ARI patients exhibited vitamin D insufficiency, compared to 10% of the healthy group. 4% of children with ARIs were found to have vitamin D deficiency, compared to 3.3% in the control group. Interestingly, a higher percentage of hypervitaminosis (26.7%) was observed among the healthy children. Regarding sufficient levels of vitamin D, 60% of healthy children were found to have adequate levels, in contrast to 48.5% of those with ARIs. (
Figure 3).
Statistical analysis using the non-parametric Mann-Whitney test (U) indicated the following differences in 25(OH)D levels between children with acute respiratory infections and the control group: Bronchiolitis (U=370.000, z=-1.550, p=0.121), Bronchopneumonia (U=366.000, z=-3.016, p=0.003), Laryngotracheitis (U=41.000, z=-2.081, p=0.037), and Acute Bronchitis (U=144.000, z=-2.684, p=0.003).
While no overall statistically significant difference in 25(OH)D levels was observed between the ARI population (mean: 81.06 ± 47.018) and the control group (p = 0.073, U = 1163.000), significant differences were noted in comparisons of the control group with children suffering from bronchopneumonia, acute bronchitis, and laryngotracheitis (see
Table 1).
Additionally, the Mann-Whitney U test was utilized to assess differences in parathyroid hormone (PTH) levels across diagnostic groups and control group. The results showed the following: Bronchiolitis (U = 155.000, z = -4.579, p = 0.000); Bronchopneumonia (U = 628.000, z = -0.023, p = 0.982); Laryngotracheitis (U = 97.000, z = -0.310, p = 0.756); and Acute Bronchitis (U = 257.000, z = -0.277, p = 0.782).
A statistically significant difference in PTH levels was observed between control group and children with acute bronchiolitis, likely attributable to a high prevalence of hypervitaminosis in this group (see
Table 2).
4. Discussion
Vitamin D deficiency and its consequences have long been a focus of scientific research. In recent years, the immunomodulatory effects of active vitamin D, driven by its local synthesis in the lungs, immune system cells, and other extrarenal tissues, have been increasingly understood [
23]. There is evidence of widespread expression of the enzyme 1α-hydroxylase throughout the body, which catalyzes the final step in the synthesis of the active form 1,25-dihydroxyvitamin D3 (1,25 D), along with the expression of vitamin D receptors (VDRs) that mediate its localized actions [
24].
Regarding the association between low vitamin D serum levels and pediatric pulmonary pathology, systematic reviews up to 2019 reported that studies were limited and inconclusive [
25]. However, in the following years, research on this topic has increased significantly. A recent meta-analysis from 2022 concluded that children with low vitamin D levels are more susceptible to developing respiratory infections [
26]. Our study aims to contribute to the existing data on the relationship between specific acute respiratory infections in childhood and low vitamin D levels.
A 2017 systematic review examining vitamin D status in Southern European countries found that, despite the higher availability of UVB radiation compared to Northern Europe, vitamin D deficiency is still prevalent. The review also indicated that neonates, infants, and adolescents tend to have higher rates of vitamin D deficiency or insufficiency compared to adults and the elderly [
27]. However, data on vitamin D status in Bulgarian children, particularly its connection to the incidence of acute respiratory infections, remain scarce.
One key challenge in our study was the lack of universal consensus and limited guidelines for defining vitamin D status in both children and adults [
28,
29,
30]. The Institute of Medicine and the European Calcified Tissue Society define vitamin D deficiency as serum 25-hydroxyvitamin D (25(OH)D) levels below 50 nmol/L, severe deficiency as levels below 30 nmol/L, and vitamin D insufficiency as levels between 52.5 and 72.5 nmol/L [
31,
32]. In our study, we followed the criteria recommended by the Bulgarian Society of Endocrinology for adults, which defines vitamin D deficiency as <25 nmol/L and insufficiency as 25–50 nmol/L. However, many of the studies we referenced used criteria from the aforementioned organizations or different ones, which may lead to potential confusion and varying interpretations of the data.
In our study, 28.3% of children with acute respiratory infections and 10% of the control group had vitamin D levels below 50 nmol/L, classified as insufficiency by the Bulgarian Society of Endocrinology. Although the difference in deficiency (<25 nmol/L) percentages was not as large, it was still more prevalent in the case group (4%) compared to the control group (3.3%). These results are similar to those in a study of Nigerian children with ARIs, which reported serum vitamin D levels <50 nmol/L in 10.8% of cases compared to 3.2% in controls [
33]. Comparable findings were reported in a study conducted in China, which investigated hospitalized children with various acute respiratory infections, as well as another study on children with community-acquired pneumonia, both finding vitamin D levels below 50 nmol/L. However, in these studies, the results were interpreted as vitamin D deficiency, while in our study, they were classified as insufficiency [
34,
35]. An Indian study reported that three-fourths of children with recurrent acute respiratory infections were vitamin D deficient, using a cutoff value of <50 nmol/L for deficiency [
36].
Surprisingly, 26.7% of healthy children had vitamin D levels above 120 nmol/L, which we classified as hypervitaminosis in this study. Fortunately, no clinical signs of intoxication were observed in these patients. Moreover, according to the aforementioned studies, these levels could be interpreted as sufficient, as vitamin D toxicity typically occurs when serum 25(OH)D levels exceed 375 nmol/L (150 ng/mL) [
37].
We observed no statistically significant difference in 25(OH)D levels between the general population of children with ARIs and the control group, primarily due to the considerable proportion (43.8%) of children with hypervitaminosis in the acute bronchiolitis group. Additionally, only 9.4% were found to be insufficient, and 3.1% presented with vitamin D deficiency. These findings differ from recent studies, which reported vitamin D insufficiency rates of 47.8%, 62.5%, and 73% in different cohorts of children with acute bronchiolitis [
38,
39,
40]. The acute bronchitis, laryngotracheitis, and pneumonia groups exhibited the highest percentages of vitamin D insufficiency (<50 nmol/L), with a statistically significant difference in 25(OH)D levels compared to the control group.
In our investigation of parathyroid hormone (PTH) serum levels, we did not observe the expected elevated values in children with vitamin D deficiency (<25 nmol/L), likely due to ongoing 25(OH)D supplementation during childhood. This may be also linked to the lack of a universal consensus on the lower levels of vitamin D at which parathyroid hormone should rise, as studies investigating the relationship between vitamin D and parathyroid hormone levels in children have produced conflicting results [
41]. Interestingly, we found subnormal PTH levels in the group of children with acute bronchiolitis, which we attribute to the high prevalence of hypervitaminosis in this group. Unfortunately, we were unable to compare these findings with similar studies, as we could not locate relevant research in the context of acute respiratory infections in childhood.
Lastly, this study has several limitations. The distribution of patients across the study groups was uneven, as enrollment was based on the respiratory conditions presented at the clinic during the study period. Furthermore, the number of patients was constrained by the available institutional funding, which limited the sample size. Due to these financial constraints, we were unable to monitor the markers over time and could only measure them once, during the initial phase of infection. Furthermore, we were unable to obtain reliable information from the parents regarding current or previous vitamin D supplementation, particularly as some families were from lower socioeconomic backgrounds. We also acknowledge that ongoing supplementation during infection may have contributed to the optimal or elevated vitamin D levels observed in some patients with acute respiratory infections.
We believe that to better understand the role of vitamin D status in ARI during childhood, its relationship with inflammatory markers (CRP, pro-inflammatory cytokines, and WBC count) should be further explored, which we plan to address in a future study.
5. Conclusions
Our study found that low serum 25(OH)D levels are associated with specific acute respiratory diseases in children, including bronchopneumonia, acute bronchitis, and laryngotracheitis. Therefore, we believe that the immunomodulatory effects of vitamin D warrant close attention. Ongoing evaluation of serum 25(OH)D levels in children with acute respiratory infections is necessary to better understand the true correlation between vitamin D status and these infections. This knowledge will be essential for developing effective therapeutic strategies to manage and prevent acute respiratory infections in the future.
Maintaining sufficient vitamin D levels is crucial for the health of pediatric patients, and supplementation ought to be provided when there is insufficiency or deficiency.
Author Contributions
Conceptualization, G.P. and V.B.; methodology, G.P.; software, E.M.; validation, V.B., G.P. and E.M.; formal analysis, E.M.; investigation, G.P.; resources, G.P.; data curation, E.M.; Writing the initial version of the manuscript, G.P.; writing—review and editing, V.B.; visualization, G.P.; supervision, V.B.; project administration, V.B.; funding acquisition, G.P. All authors have reviewed and consented to the final version of the manuscript.
Funding
This study received financial support from Medical University Pleven, under project number 8/2021.
Institutional Review Board Statement
Adhering to the principles set forth in the Declaration of Helsinki, this study received ethical approval from the Ethics Committee of Scientific Research at the Medical University – Pleven (Phone: +359 64 884 197; Address: 1 Kliment Ohridski St., Pleven, Bulgaria). The approval was granted on June 29, 2021, with the code 658-CENID/29.06.2021.
Informed Consent Statement
Consent after being fully informed was acquired from all study subjects.
Data Availability Statement
The data supporting the findings of this study are available within the manuscript and its supplementary information files. Additional patient-level data are not publicly available due to privacy concerns but can be requested from the corresponding author, subject to approval by the ethics committee.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Nair, H.; Simões, E. A.; Rudan, I.; Gessner, B. D.; Azziz-Baumgartner, E.; Zhang, J. S. F.; Feikin, D. R.; Mackenzie, G. A.; Moiïsi, J. C.; Roca, A.; et al. Severe Acute Lower Respiratory Infections Working Group. Global and Regional Burden of Hospital Admissions for Severe Acute Lower Respiratory Infections in Young Children in 2010: A Systematic Analysis. Lancet 2013, 381(9875), 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countries, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. The Lancet. Infectious diseases 2018, 18(11). [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; Gao, Q.; Yang, X.; Yang, N.; Hao, L. Association of Vitamin D Supplementation with Respiratory Tract Infection in Infants. Maternal & Child Nutrition 2020, 16(3), e12987. [Google Scholar] [CrossRef]
- Nicolae, M.; Mihai, C. M.; Chisnoiu, T.; Balasa, A. L.; Frecus, C. E.; Mihai, L.; Lupu, V. V.; Ion, I.; Pantazi, A. C.; Nelson Twakor, A.; Andrusca, A.; Cambrea, C. S.; Arghir, I. A.; Lupu, A.; Arghir, O. C. Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients 2023, 15(15), 3430. [Google Scholar] [CrossRef] [PubMed]
- Zisi, D.; Challa, A.; Makis, A. The Association between Vitamin D Status and Infectious Diseases of the Respiratory System in Infancy and Childhood. Hormones 2019, 18(4), 353–363. [Google Scholar] [CrossRef]
- Esposito, S.; Lelii, M. Vitamin D and Respiratory Tract Infections in Childhood. BMC Infectious Diseases 2015, 15(1), 487. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J. H. Vitamin D, Infections and Immunity. Rev Endocr Metab Disord 2022, 23(2), 265–277. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F. P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J. W.; Mader, S.; White, J. H. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J Immunol 2004, 173(5), 2909–2912. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A.; Bręborowicz, A. Review Paper. Vitamin D Impact on Immune Functions: Implications for Preventive Strategy of Allergic Disease? Adv Dermatol Allergol 2012, 29(3), 176–181. [Google Scholar]
- Hansdottir, S.; Monick, M. M.; Hinde, S. L.; Lovan, N.; Look, D. C.; Hunninghake, G. W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense1. The Journal of Immunology 2008, 181(10), 7090–7099. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health Problem? The Journal of steroid biochemistry and molecular biology 2013, 144PA, 138. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. J. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15(17), 3842. [Google Scholar] [CrossRef] [PubMed]
- Veleva, N.; Dimtrova, G.; Oscar, A.; Kemilev, P.; Mladenov, O.; Dimitrov, G.; Haikin, V.; Hristova, R.; Persenska, E.; Yankova, E.; Svinarov, D. Vitamin D status in children with myopia. Bulgarian Review of Ophthalmology 2020, 64(1), 33–37. [Google Scholar] [CrossRef]
- Mileva, S.; Galunska, B.; Gospodinova, M.; Gerova, D.; Svinarov, D. Vitamin D3 Status in Children with Acute Diarrhea. Integr Food Nutr Metab 2014, 1(2), 98–99. [Google Scholar]
- Marinov, D. B.; Dimitrova, T. T.; Department of Hygiene and Epidemiology, Faculty of Public Health, Medical University of Varna, Bulgaria. VITAMIN D STATUS AND DIETARY HABITS OF CHILDREN WITH ADOLESCENT IDIOPATHIC SCOLIOSIS IN VARNA. JofIMAB 2021, 27 (1), 3589–3592. [CrossRef]
- Velikova, T.; Lazova, S.; Perenovska, P.; Miteva, D.; Velikov, P.; Petrova, G. Vitamin D Status in Children with Chronic Lung Disease. 2018, 62, 10–17.
- Rimpova, N.; Valcheva, V.; Tsakova, A.; Shivachev, H.; Iliev, D. Association of Low Vitamin D Status with Childhood Pneumonia Severity in Hospitalized Bulgarian Patients. Russian Journal of Infection and Immunity 2021, 12. [Google Scholar] [CrossRef]
- Burgazliev, G.; Georgiev, T. What is Vitamin D – the vitamin-hormone? Science Dietetics 2010, 2, 232–234. [Google Scholar]
- Holick, M. F. VITAMIN D STATUS: MEASUREMENT, INTERPRETATION AND CLINICAL APPLICATION. Annals of epidemiology 2009, 19(2), 73. [Google Scholar] [CrossRef]
- Hollis, B. W. Assessment of Vitamin D Status and Definition of a Normal Circulating Range of 25-Hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes 2008, 15(6), 489–494. [Google Scholar] [CrossRef]
- Lips, P.; Duong, T.; Oleksik, A.; Black, D.; Cummings, S.; Cox, D.; Nickelsen, T. A Global Study of Vitamin D Status and Parathyroid Function in Postmenopausal Women with Osteoporosis: Baseline Data from the Multiple Outcomes of Raloxifene Evaluation Clinical Trial. J Clin Endocrinol Metab 2001, 86(3), 1212–1221. [Google Scholar] [CrossRef]
- Borisova, A.-M.; Boyanov, M.; Kolarov, Z.; Popivanov, P.; Stoilov, R.; Svinarov, D.; Shinkov, A.; Pilosof, V. Recommendation for Diagnosis, Prevention and Treatment of Vitamin D Deficiency.; Bulgarian Society of Endocrinology: Bulgaria, 2019.
- Aranow, C. Vitamin D and the Immune System. J Investig Med 2011, 59(6), 881–886. [Google Scholar] [CrossRef]
- Balan, K. V.; Babu, U. S.; Godar, D. E.; Calvo, M. S. Vitamin D and Respiratory Infections in Infants and Toddlers: A Nutri-Shine Perspective. Handbook of vitamin D in human health 2013, 4, 276. [Google Scholar]
- Cepeda S, J.; Zenteno A, D.; Fuentes S, C.; Bustos B, R. [Vitamin D and pediatrics respiratory diseases]. Rev Chil Pediatr 2019, 90(1), 94–101. [Google Scholar] [CrossRef]
- Raju, A.; Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F. D.; Zafar, S.; Panthangi, V.; Kurupp, A. R. C.; Khan, S. Role of Vitamin D Deficiency in Increased Susceptibility to Respiratory Infections Among Children: A Systematic Review. Cureus 2022, 14(9), e29205. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Lambrinou, C.-P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K. D. A Systematic Review of Vitamin D Status in Southern European Countries. Eur J Nutr 2018, 57(6), 2001–2036. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J. P.; Adler, R. A.; Banfi, G.; Bikle, D. D.; Binkley, N. C.; Bollerslev, J.; Bouillon, R.; Brandi, M. L.; Casanueva, F. F.; di Filippo, L.; Donini, L. M.; Ebeling, P. R.; Fuleihan, G. E.-H.; Fassio, A.; Frara, S.; Jones, G.; Marcocci, C.; Martineau, A. R.; Minisola, S.; Napoli, N.; Procopio, M.; Rizzoli, R.; Schafer, A. L.; Sempos, C. T.; Ulivieri, F. M.; Virtanen, J. K. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocrine Reviews 2024, 45(5), 625–654. [Google Scholar] [CrossRef] [PubMed]
- Cariolou, M.; Cupp, M. A.; Evangelou, E.; Tzoulaki, I.; Berlanga-Taylor, A. J. Importance of Vitamin D in Acute and Critically Ill Children with Subgroup Analyses of Sepsis and Respiratory Tract Infections: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9(5), e027666. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Spolidoro, G. C. I.; Milani, G. P.; Agostoni, C. Vitamin D in Pediatric Age: Current Evidence, Recommendations, and Misunderstandings. Front Med (Lausanne) 2023, 10, 1107855. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K. D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H. A.; Obermayer-Pietsch, B.; Bianchi, M. L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur J Endocrinol 2019, 180(4), P23–P54. [Google Scholar] [CrossRef]
- Holick, M. F.; Binkley, N. C.; Bischoff-Ferrari, H. A.; Gordon, C. M.; Hanley, D. A.; Heaney, R. P.; Murad, M. H.; Weaver, C. M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 2011, 96(7), 1911–1930. [Google Scholar] [CrossRef]
- Umeadi, E.; Echendu, S.; Ufoaroh, C.; Igwe, W.; Ezeudu, C.; Anyabolu, E. N.; Chinelo, J.; Egbuonu, I. Vitamin D and Acute Respiratory Infections in Children. Nigerian medical journal : journal of the Nigeria Medical Association 2022, 63, 204–212. [Google Scholar] [CrossRef]
- Kuang, L.; Liang, Z.; Wang, C.; Lin, T.; Zhang, Y.; Zhu, B. Serum 25-Hydroxy Vitamin D Levels in Children with Acute Respiratory Infections Caused by Respiratory Virus or Atypical Pathogen Infection. Nutrients 2023, 15(6), 1486. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, X.; Guo, L.; Li, H.; Sun, C.; Cui, X.; Zhang, Q.; Song, G. Association between Serum 25-Hydroxyvitamin D Concentration and Pulmonary Infection in Children. Medicine 2018, 97(1), e9060. [Google Scholar] [CrossRef] [PubMed]
- Jaybhaye, A. P.; Sangle, A. L.; Ugra, D.; Chittal, R. Y. A Hospital-Based Study of Vitamin D Levels in Children With Recurrent Respiratory Infections. Cureus 2022, 14(8), e27864. [Google Scholar] [CrossRef] [PubMed]
- Levita, J.; Wilar, G.; Wahyuni, I.; Bawono, L. C.; Ramadaini, T.; Rohani, R.; Diantini, A. Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports. Toxics 2023, 11(7), 642. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Rajvanshi, N.; Suvarna, K.; Kumar, P.; Goyal, J. P. Association of Vitamin D with Disease Severity in Infants with Bronchiolitis. Eur J Pediatr 2024, 183(6), 2717–2723. [Google Scholar] [CrossRef]
- Golan-Tripto, I.; Loewenthal, N.; Tal, A.; Dizitzer, Y.; Baumfeld, Y.; Goldbart, A. Vitamin D Deficiency in Children with Acute Bronchiolitis: A Prospective Cross-Sectional Case- Control Study. BMC Pediatr 2021, 21(1), 211. [Google Scholar] [CrossRef]
- Alakaş, Y.; Celiloğlu, C.; Tolunay, O.; Matyar, S. The Relationship between Bronchiolitis Severity and Vitamin D Status. J Trop Pediatr 2021, 67(4), fmab081. [Google Scholar] [CrossRef]
- Taylor, S. N. Vitamin D in Toddlers, Preschool Children, and Adolescents. Annals of Nutrition and Metabolism 2020, 76 (Suppl. 2), 30–41. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).