1. Introduction
The global incidence of influenza is estimated to be between 27 and 54 million people annually and between 300,000 and 650,000 hospitalizations (
https://www.cdc.gov/flu/about/burden/preliminary-in-seasonestimates.htm). As many studies have shown, vaccination is effective in reducing the risk of seasonal influenza infection by 40–60% if the epidemic viruses correspond to the vaccine viruses [
1]. For the influenza virus, the main antigens are surface glycosylated proteins, such as hemagglutinin (HA) and neuraminidase (NA). The main antigenic component of the influenza virus is HA [
2], and anti-hemagglutinating antibodies are the main criterion for assessing humoral immunity to influenza after infection or vaccination [
3]. The strain composition of influenza vaccines has to be updated almost annually due to the high variability of HA of influenza viruses. Due to the high variability of HA of influenza viruses, the strain composition of influenza vaccines must be updated almost annually. In case of genetic drift or antigenic shift in circulating strains, vaccine-induced HI antibodies might be less effective at cross-neutralization. Therefore, it is worth take into account immunity directed against the second most important antigenic component of the influenza virus, NA, when studying susceptibility after vaccination and consider NA as a critical target in future promising influenza vaccine platforms [
4,
5].Anti-NA antibodies are capable of reducing the severity of influenza infection, thereby preventing the development of secondary complications and effectively limiting the transmission of the virus [
6]. In adults, pre-existing antibodies to NA have been shown to reduce the duration of shedding of the influenza A(H1N1)pdm09 virus and the duration of illness during natural infection [
7]. The study of antibodies to NA may play a role in predicting herd immunity against newly emerging influenza viruses, as well as in assessing the protection provided by seasonal influenza vaccines in the event of a mismatch between the epidemic and vaccine strains [
8].
Despite the known importance of NA-directed antibodies in protection against influenza, the NA content of seasonal influenza vaccines is currently not regulated or assessed by manufacturers [
9] and the threshold value of NI antibodies corresponding to protection is not clearly defined. It has long been established that the HI antibody titer > 1:40 corresponds to a 50% reduction in the risk of influenza infection [
10].A study by Memoli, M. J., using intranasal challenge of healthy volunteers demonstrated that HI titers >1:40 were protective against mild to moderate influenza infection but did not by themselves reduce symptom incidence. Although the HI antibody titer correlated with some reduction in disease severity scores, the total baseline NA inhibitory antibody titer correlated more significantly with disease severity scores and had a greater impact on recovery than the baseline HI titer [
4].
Antigenic changes in influenza virus surface glycoproteins allow avoidance of pre-existing humoral immunity. Antigenic drift is a feature of not only HA but also NA.NA antigenic drift is discordant with HA antigenic drift and it does not always happen more slowly. Thus, between 2010 and 2016, influenza vaccines included the A/California/07/09(H1N1)pdm09-like virus, indicating a slowdown in the antigenic drift of the HA of A/H1N1pdm09 influenza viruses. At the same time, human monoclonal antibodies identified antigenic sites in the A/H1N1pdm09 NA that changed shortly after the emergence of the new pandemic virus [
11].
After 2019, significant antigenic drift of N1 neuraminidase was observed [
11]. It was found that the NA of the circulating 2019 strain acquired a significant number of mutations in the head domain, and therefore demonstrated a dramatic change in antigenic properties, especially after the loss of the N-linked glycosylation site at 245 position (due to S245N and S247T mutations) and P468H substitution resulted in significant antigenic drift of N2 neuraminidase [
13]. In March 2019, the World Health Organization (WHO) recommended to replace the A/H3N2 component of influenza vaccines for the northern hemisphere for the 2018–2019 epidemic season with influenza viruses similar to A/Kansas/14/2017 (H3N2) [
14]. Just like for for HA, genetic changes in NA does not always lead to antigenic changes. A single amino acid substitutions in NA of A/Brisbane/59/2007 was shown to result in reduction of inhibition by polyclonal antibodies evolved to earlier strains. These data suggest the importance of NA inhibition estimation to detect antigenic drift in the presence of sequence changes in NA [
12].
Since there are not so much data regarding the efficacy of influenza vaccines in cases of mismatch between vaccine and epidemic strains, the aim of this study was to investigate the cross-reactivity and functional properties of NI antibodies produced as a result of immunization with seasonal influenza vaccines.
2. Materials and Methods
2.1. Surveyed Contingents and Samples
Blood serum samples were obtained as part of a serological study [
15] involving healthy volunteers over 18 years of age who have no contraindications to be vaccinated and who signed written informed consent. Immunization was done as previously described in a specialized clinic of the A.A. Smorodintsev Research Institute of Influenza in an open mode. This study used blood samples obtained before vaccination and on the 21st day after vaccination. The study was approved by the Local Ethics Committee of the A.A. Smorodintsev Research Institute of Influenza, protocol No. 131 dated October 10, 2018. The study included three sеasonal trivalent inactivated influenza vaccines (IIV) with the composition of vaccine strains recommended by WHO for the northern hemisphere in the 2018–2019 flu season: A/Michigаn/45/2015(H1N1)pdm09-like virus; A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus; and B/Colorado/06/2017 (B/Victoria/2/87 linеage)-like virus [14 WHO]. All vaccines were produced in accordance with the Russian Pharmacopoeia from purified candidate viruses grown in embryonated chicken eggs. The content of HA in the vaccine formulations was estimated using the single radial immunodiffusion method; the NA content was not determined. The split vaccine “Ultrix” (“FORT”, Moscow, Russia) contained 15 μg HA of each strain in a dose of 0.5 mL [
16]. The subunit vaccine “Grippol Plus” (Petrovax Pharm, Moscow, Russia) contained 5 μg GA of each strain (antigens manufactured by Abbott Biologicals BV, Olst, the Netherlands) and 500 μg Polyoxidonium® adjuvant in a dose of 0.5 mL [
17]. Another subunit vaccine “Sovigripp” NPO “Mikrogen”, Republic of Bashkortostan, Russia) contained 5 μg GA of influenza strains A(H1N1)pdm09 and A(H3N2), 11 μg of influenza B strain and 500 μg of Sovidon® adjuvant in a dose of 0.5 mL [
18]. After receiving permission from the Local Ethics Committee at the Federal State Budgetary Scientific Institution “IEM” No. 3/23 dated September 20, 2023, the clinical samples were transferred to the researchers in anonymized form.
2.2. Influenza Viruses
Influenza viruses A/Michigan/45/2015(H1N1)pdm09; A/Singapore/INFIMH-16-0019/2016 (H3N2), A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 and A/Brisbane/34/2018 (H3N2) were used as antigens to perform HI.
We used H6Nx reassortant viruses containing NA of recent A/H1N1pdm09 and A/H3N2 viruses (
Table 1) to measure NI titers in enzyme-linked lectin assay (ELLA).
Continuation of the
Table 1. Genome composition of diagnostic reassortant viruses of subtype H6 for use in ELLA
| Name |
Origin of HA |
Origin of NA |
| H6N2/18 |
A/Herring Gull/Sarma/51c/2006 (H6N1) |
A/Brisbane/34/2018 (H3N2) |
Viruses were purified and concentrated by ultracentrifugation as previously described [
15].
2.3. The Enzyme-Linked Lectin Assay (ELLA)
For the ELLA setup as described previously [
15], 96-well flat-bottomed ELISA plates with high binding were used. 150 μl of fetal calf serum fetuin solution at a concentration of 50 μg/mL in 0.1 M carbonate/bicarbonate buffer (pH = 9.5-9.7) were added to the wells. Substrate adsorption took place at a temperature of +4°C overnight. Before immediate use, the plates were washed twice with sterile PB. The working dilution of diagnostic viruses A/H6N1 was prepared in 0.01 M PB containing BSA at a concentration of 10 mg/mL. The 65 μl of the working dilution of the virus were added to each well of the plate with diluted sera. For control, control samples were placed in each plate: negative control – 130 µl of 0.01 M FB with a BSA content of 10 mg/mL and positive control – a mixture of 65 µl of the working dilution of the virus with an equivalent volume of 0.01 M FB with a BSA content of 10 mg/mL.
The result of the enzymatic reaction was obtained in the form of optical density (OD) data determined for a series of dilutions of each blood serum. The OD set was used to plot a graph of the dependence of the residual enzymatic activity of NA in the presence of anti- NA antibodies, calculated as a function of the dilution of the immune serum sample:
The titer of serum anti- NA antibodies was determined as the reciprocal of the dilution of the sample giving 50% inhibition of NA activity. Results were expressed as binary logarithms of the reciprocal of the final dilution.
2.4. Hemagglutination Inhibition Test (HI)
HI was performed as described previously [
15] with sera treated with receptor destroying enzyme (RDE, DenkaSeikenCo., Tokyo , Japan).
2.5. Microneutralization Reaction (MN)
For the MN setup, monolayer of MDCK cell was grown in flat-bottomed 96-well polystyrene plates for adherent cultures (Sarstedt, Nümbrecht, Germany). After removing the maintenance medium and washing the plates twice with phosphate-buffered saline, 50 μl of falling two-fold dilutions (starting with a 1:10 dilution) of blood serum samples in DMEM containing trypsin TPCK at a concentration of 2 μg/mL were added. Then 50 μl of a standard dose of virus 200 TCID50/0.05 mL (= 100 TCID50/0.1 mL), diluted to the indicated concentration with the same medium, were added to each well. The plates were incubated in a CO2-fed thermostat at 34°C for 72 h. Inhibition of virus reproduction was determined by hemagglutination test with 0.75% suspension of chicken erythrocytes.
2.6. Statistical Processing of Results
The results were processed using the statistical package “GraphPad”. The following descriptive statistics were used to describe the data obtained: arithmetic mean (M), standard deviation (σ). When comparing samples in case of failure to meet the assumptions of normal distribution of the dependent variable within each group and homogeneity of variance, nonparametric criteria were used (Mann-Whitney, Wilcoxon signed ranks, Friedman rank analysis of variance, Kruskal-Wallis rank analysis of variance). For nominal data, Fisher’s exact test was used. The null hypotheses tested by the criteria were rejected at p < 0.05.
3. Results
3.1. Genetic Analysis of NA Subtypes N1 and N2 and Enzymatic Activity
Seasonal IIVs used in this study included vaccine strains of influenza A viruses recommended by WHO for the northern hemisphere in the 2018–2019 season: A/Michigan/45/2015 (H1N1)pdm09-like virus; A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus. To study immunity to drift variants, we used A/Guangdong-Maonan/SWL1536/2019 (reference virus recommended for use in the 2020-2021 Northern Hemisphere vaccine) and influenza virus A/Brisbane/34/2018 (A/Kansas/14/2017-like (recommended for use in the 2019-2020 Northern Hemisphere vaccine) [
19].
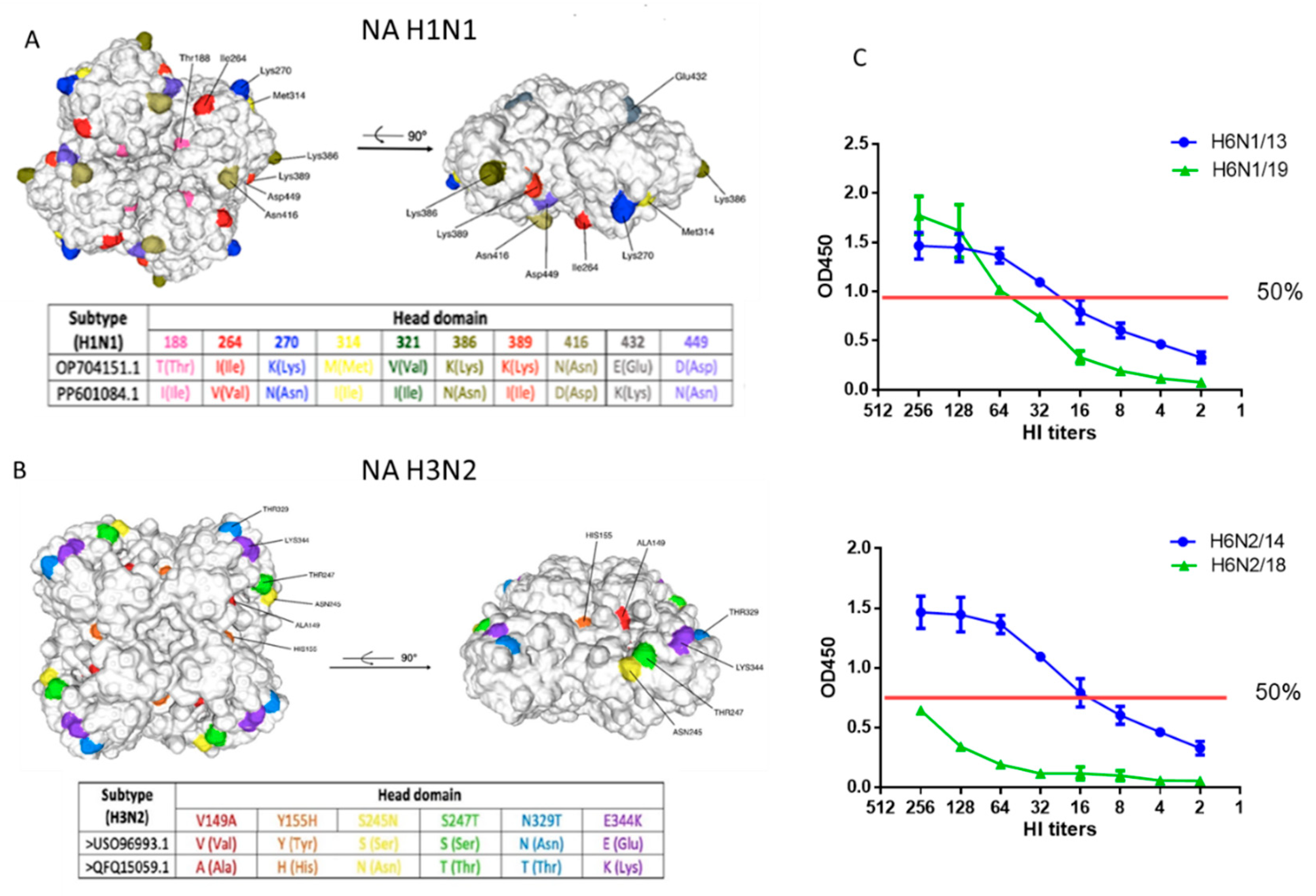
A total of 20 substitutions (4.3% differences) were identified the NA amino-acid sequence 1-469 of circulating 2019 strain A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 compared to the previously circulating strain A/South Africa/3626/2013(H1N1)pdm09.A number of mutations have been identified in the head domain (
Figure 1, А). As has been shown before, one of the main factors of the antigenic changes of NA A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 is the loss of the N-linked glycosylation site (Asn386), which can influence antigenicity of N1 [
20]. Another antigenic site includes residues 270 and 314, which are on the opposite side of the NA monomer in position that has been demonstrated to be recognized by both mouse and human NI antibodies. The two sites (386 and 270/314) may be independent NI epitopes, but it is possible that the 270/314 substitutions may have an additional effect on the distal epitope, which is buried by the glycan at position Asn386 [
11]. The T188I mutation is critical for altering the binding activity of immune antiserum [
21].
Substitutions T188I, M314Iand K389I are associated with moderate to strong resistance to neuraminidase inhibitors [
22,
23].
Structural analysis showed that the amino acid change from isoleucine to valine (V321I) distorts the hydrophobic pockets and affects residues in the active site of NA. In addition, the V321I mutation may affect the hydrophobic region, which may destabilize a nearby loop containing the oseltamivir-interacting residue R368. These residues may be new potential markers for decreased susceptibility to oseltamivir. [
24].
Mutation E432K is associated with mutant antigenic drift/escape and moderate drug resistance to oseltamivir, zanamivir and peramivir [
25]. Mutation D449N is involved in viral oligomerization, host protein binding, and small ligand binding according to FluSurver (A*STAR Bioinformatics Institute, Singapore). Thus, the above changes can lead to changes in the conformation and functional properties of NA of A(H1N1)pdm09.
Strains A /Hong Kong/4801/2014(H3N2) and A/Brisbane/34/2018(H3N2) differed by 15 amino acid substitutions (3.2% differences) in NA, 9 of the most significant ones were located in the head domain outside the active centers (
Figure 1, B).Substitutions V149A, Y155H, S247T, T392I are associated with potential resistance of the virus to NA inhibitors [
26,
27,
28].Mutation E344K has been previously described as being associated with changes in antigenic properties for binding to monoclonal antibodies [
29]. The S245N substitution potentially results in the formation of a new glycosylation site – changes in positions 245-247.Due to its proximity to the active site of the enzyme, the attached glycan at position 245 was found to sterically protect NA-inhibitory antibodies, although it also reduced viral replication in vitro in human nasal epithelial cells [
30]. Thus, the identified genetic mutations in H2 could also lead to structural and functional changes in the corresponding protein.
In the test with the high molecular weight substrate fetuin, it was shown that the H6N1/19 virus containing NA A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm 09 was slightly inferior in enzymatic activity to the H6N1/13 virus containing NA A/South Africa/3626/2013 in the linear range of the titration curve (
Figure 1, C).The H6N2/18 virus containing the NA of A/Brisbane/34/2018(H3N2) was significantly inferior in enzymatic activity to the H6N2/14 virus containing the NA of the A/HongKong/4801/2014 (H3N2) (
Figure 1, C).
Thus, the acquired mutations in NA of A/H1N1pdm09 influenza virus did not greatly affect the enzymatic activity of N1, but the activity of the new N2 was quite significantly reduced.
3.2. Antibodies to A/H1N1pdm09 and A/H3N2 Viruses in Human Sera Before and After Immunization with Seasonal IIVs 2018-2019 Years of Formulation
Figure 2, A shows the results of studying antibodies to vaccine and drift influenza viruses in the blood serum of 64 examined patients of different ages. Blood samples were obtained before immunization and 21 days after the administration of vaccines. It was shown that vaccination with seasonal IIVs caused increases in antibodies to homologous and drift viruses, but to drift viruses more often among older patients.
Examination of patient sera collected before vaccination showed a statistically significant reduction in the inhibition of H6N1/19 virus NA compared to the inhibition of H6N1/13 virus NA (
Figure 2, B). The results of detection of antibodies to NA of A/H3N2 viruses showed that despite a significant decrease in the enzymatic activity of NA of the A/Brisbane/34/2018(H3N2) virus compared to the A/Hong Kong/2014/8296(H3N2) virus, a significant decreased in the inhibitory activity of antibodies specific to the A/Hong Kong/2014/8296(H3N2) virus was observed against the A/Brisbane/34/2018(H3N2) virus in the same blood samples, while statistically significant differences in the levels of NI antibodies to N2 were noted between older patients and adults. Thus, a significantly reduced ‘herd’ immunity to drift influenza viruses A/H1N1pdm09 and A/H3N2 was shown, compared to previously circulating strains.
We analyzed combined seroconversions to influenza A virus antigens that were considered reliable when they were fourfold for HI antibodies and twofold for NI antibodies. As shown in
Figure 2, C, the total number of seroconversions was lower in the younger group. Interestingly, more than half of the seroconversions to new strains coincided with seroconversions to earlier viruses, but still a certain proportion of increases in antibodies to new viruses was detected independently of the presence of increases to previous antigenic variants (
Figure 2, C).
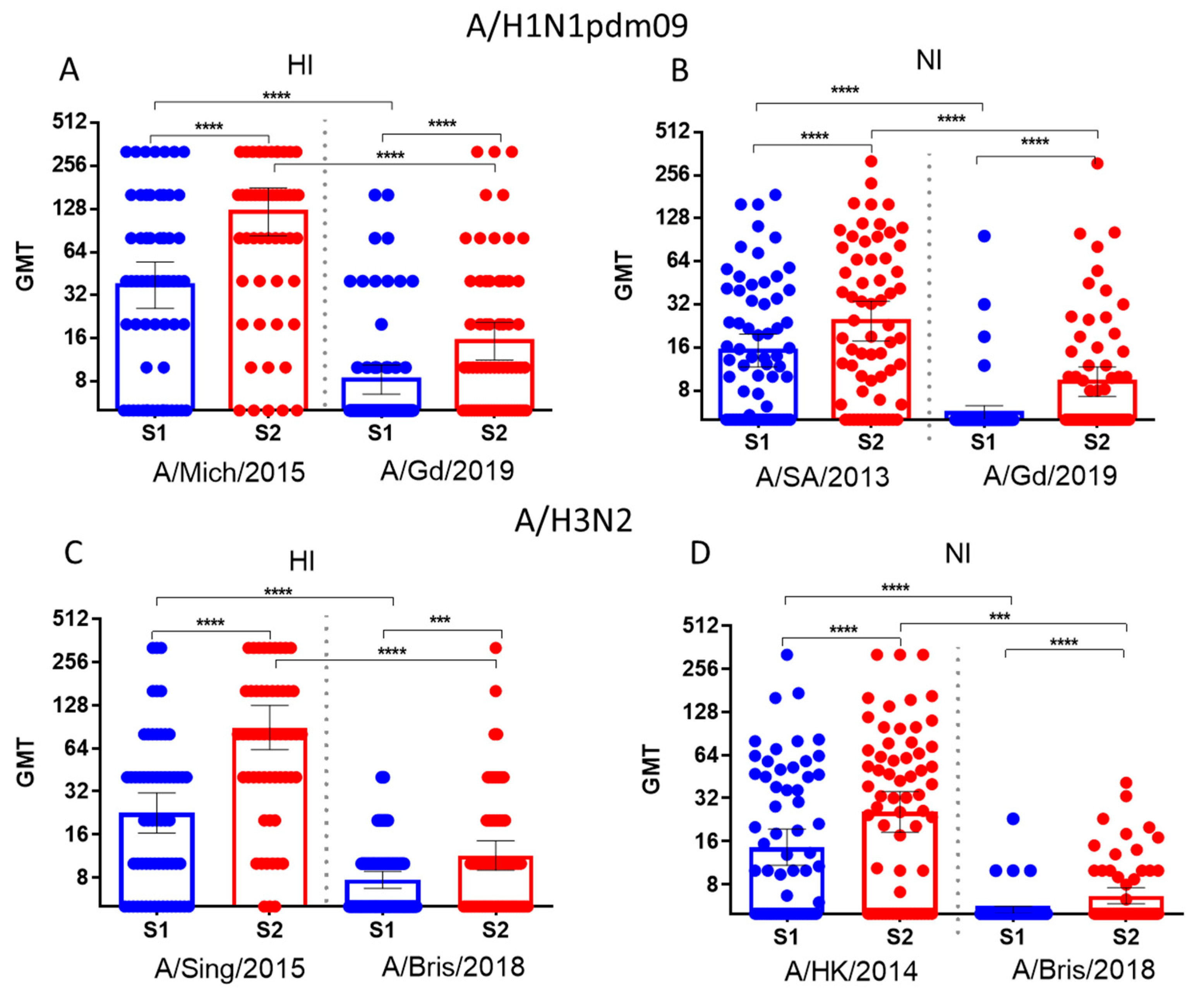
Figure 3 shows that after immunization with seasonal influenza vaccines, significant increases in antibody titers were observed. These antibodies reacted with both earlier and later variants of the A/H1N1pdm09 and A/H3N2 viruses.
The levels of immunoprotection expressed as the proportion of individuals with HI antibody titers ≥1:40 to and NI antibody titers ≥1:20 are presented in
Figure 4 and 5.
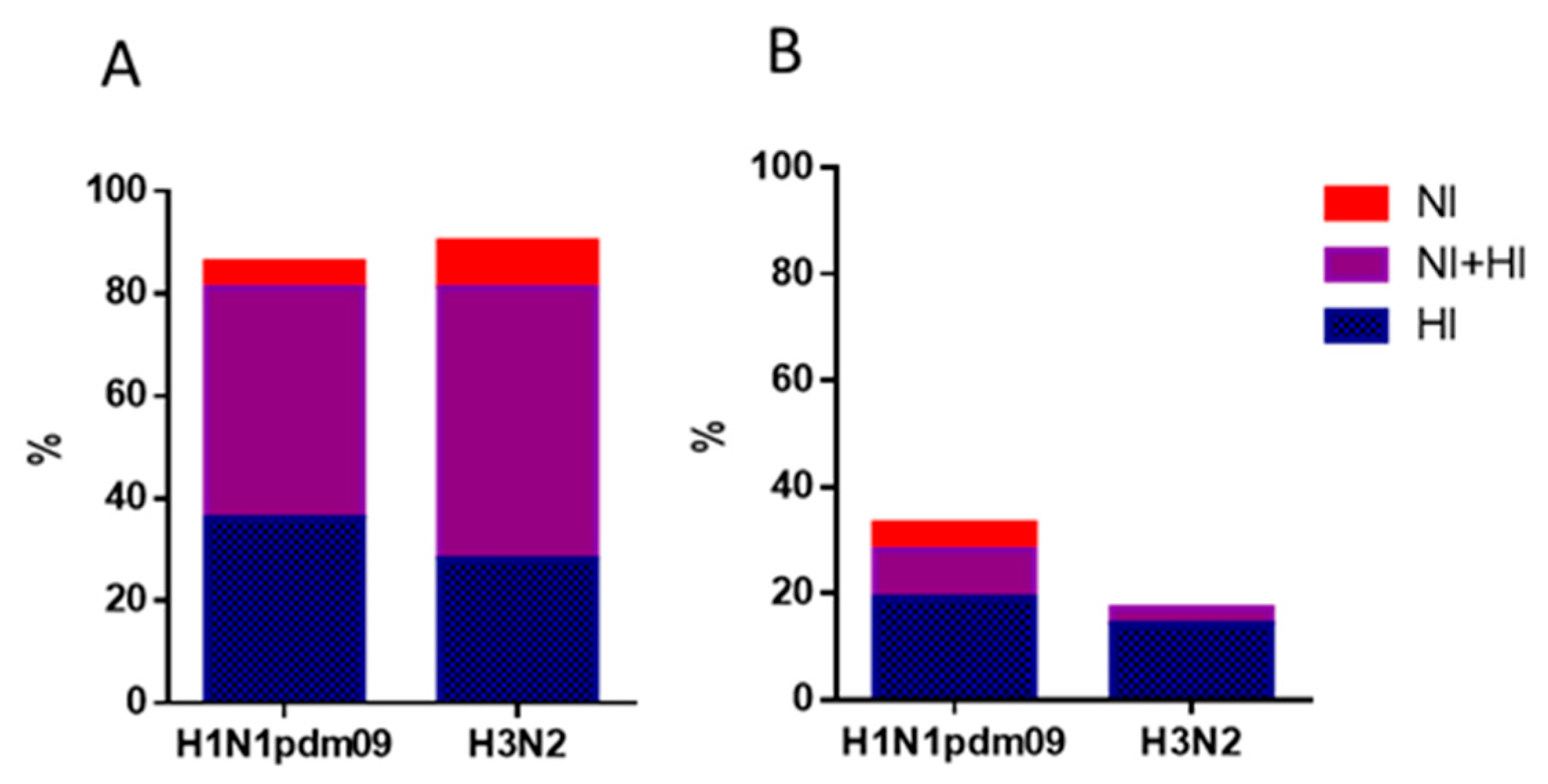
Figure 4 shows the proportions of individuals with antibody titers 21 days after vaccination to HA only (blue bars), NA only (red bars), or both HA and NA. It was shown that protective levels of antibodies to either HA or NA after seasonal IIVs of formula 2018-2019 flu season were acquired by 85.9 and 90.6% of patients to A/H1N1pdm09 and A/H3N2 viruses, respectively (
Figure 4, A). But to late viruses, it turned out that 32.8% developed protective titers to A/H1N1pdm09 virus and only 17.2% to A/H3N2 virus. Moreover, antibodies to NA alone without the formation of HI antibodies in the above titers were observed in no more than 3-6% of cases (
Figure 4, A, B), and only NI antibodies were not detected at all to the A/Brisbane/34/2018(H3N2) virus (
Figure 4, B).
Numbers of persons and percentage rates of seroprotection in age groups are given in
Supplementary Table S1. The proportions of individuals with protective levels of antibodies to antigens of new viruses were generally higher in patients from the older group both before and after vaccination, although the differences were not statistically significant (
Figure 5). Only to the vaccine virus A/H1N1pdm09, HI antibody titers were slightly higher in younger patients before vaccination, but after vaccination, the proportion of seroprotection became the same in both age groups (
Figure 5). At the same time, the level of seroprotection to NA of the later A/H1N1pdm09 virus after IIVs vaccination was statistically significantly lower among the group of patients under 60 years of age.
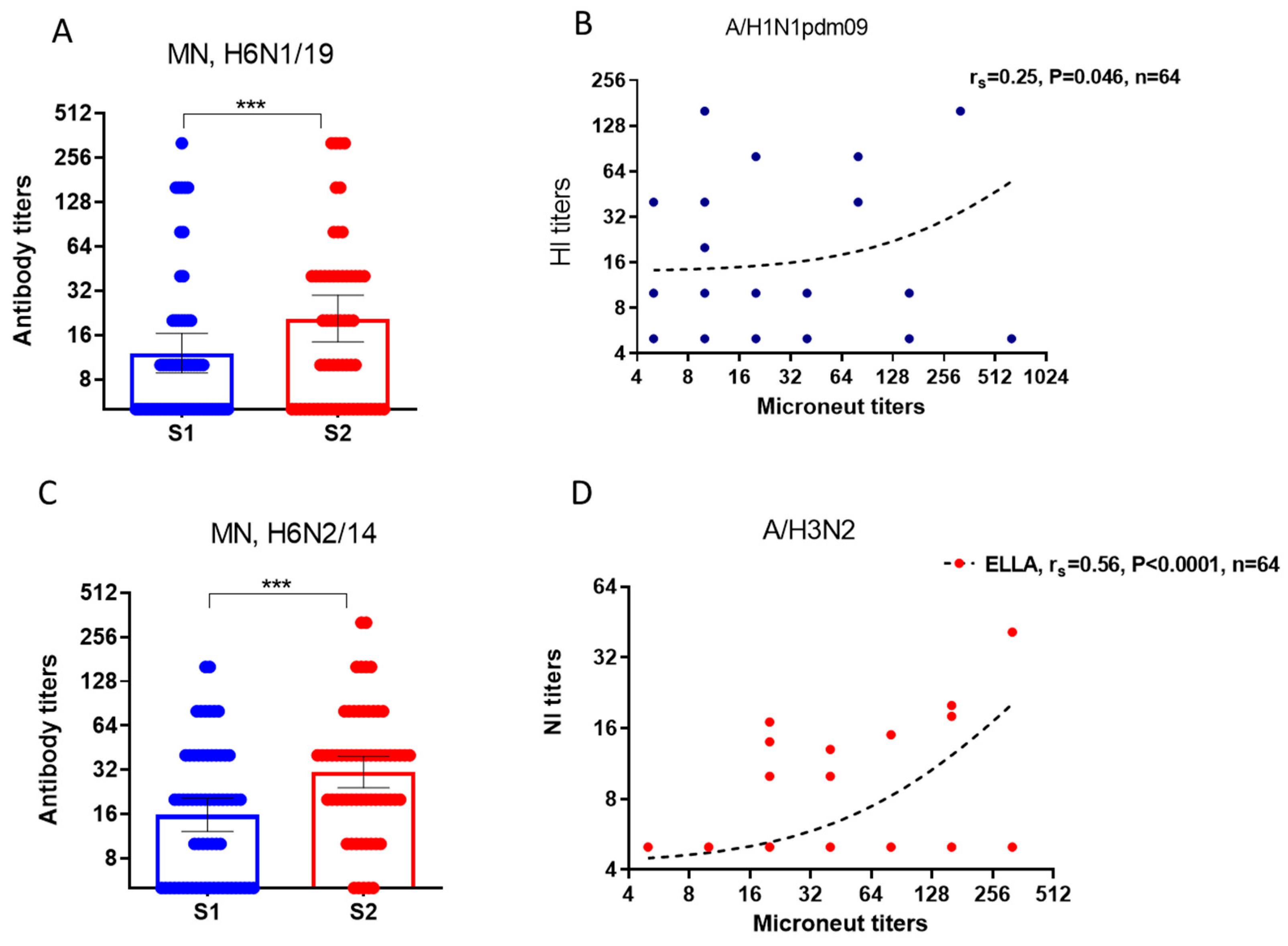
We studied neutralizing antibodies to H6Nx viruses before and after vaccination, as well as the degree of association of neutralizing antibodies with HI and NI antibodies in the same serum samples. Assuming that pre-vaccination antibodies could potentially be acquired by the subjects after past infections, in contrast to post-vaccination antibodies, we performed a correlation analysis of pre-vaccination and post-vaccination antibodies separately. It was shown that neutralizing antibodies to viruses with irrelevant HA subtype H6 increased statistically significantly in response to vaccination with seasonal IIVs (
Figure 6, A, C). MN antibodies to reassortant strains H6N1/19 virus containing NA from A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 weakly positive correlated with HI antibodies before vaccination (
Figure 6, B). At the same time, neutralizing antibodies to reassortant strains H6N2/18 virus containing NA of A/Brisbane/34/2018(H3N2) virus after vaccination correlated notably with antibodies detected in ELLA (
Figure 6, D). Thus, antisera containing HI and NI antibodies exhibit neutralizing properties
in vitro against viruses with unrelated HA of H6 subtype.
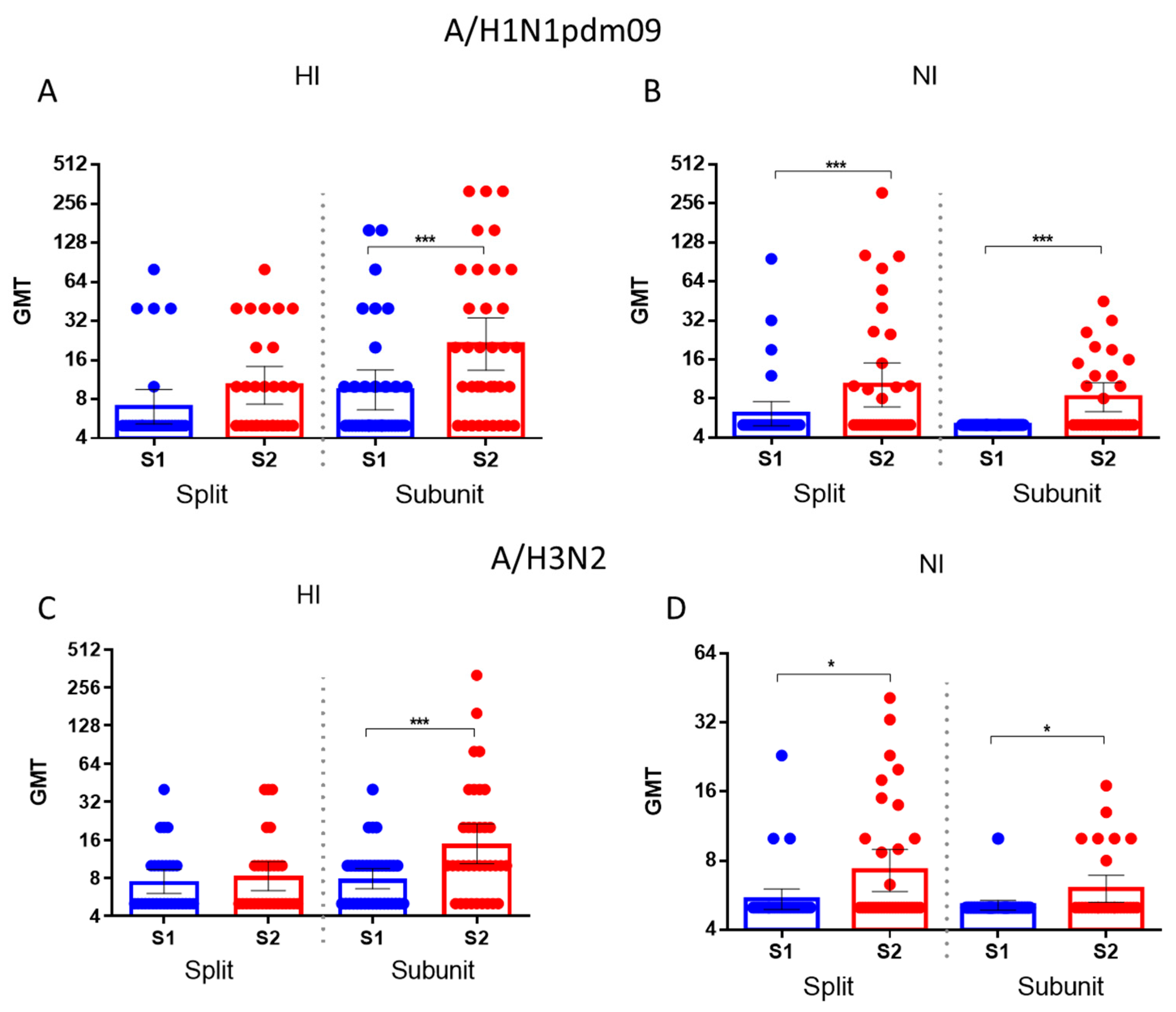
3.3. Antibodies to A/H1N1pdm09 and A/H3N2 Viruses in Human Sera Before and After Immunization with Either a Split or Subunit IIV
It was shown that the increase in HA antibodies to the drift influenza A/H1N1pdm09 and A/H3N2 influenza virus after immunization with the split vaccine was insignificant, while the subunit vaccine caused a significant increase in antibodies to HA and NA of the drift virus (
Figure 7, A, С). At the same time, the increases in antibodies to HA of drift viruses were statistically significant in response to both the split and subunit vaccines (
Figure 7B,D).
Thus, acquired mutations in NA resulted in decreased binding to immune antisera obtained through immunization with earlier strains. It was shown that the titers of HI antibodies to the A/Michigan/45/2015(H1N1)pdm09 virus and NI antibodies to the A/South Africa/3626/2013 (H1N1)pdm09 strain in patient’s sera before vaccination were significantly higher compared to the titers of antibodies to HA and NA of the A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09 virus. Significant increases in average antibody titers were observed to the A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09 strain, and not only to earlier viruses. Thus, seasonal IIVs based on past strains induce cross-reactive antibodies against later strains. Neutralizing antibodies increased significantly to reassortant strains H6N1/19 and H6N2/18, including NA of the A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 and to A/Brisbane/34/2018(H3N2) viruses respectively in the sera of patients vaccinated with influenza IIVs based on earlier strains.
4. Discussion
Antibodies to influenza surface antigens are one of the main factors determining human susceptibility to influenza infection. Antigenic changes in the HA protein may reduce the efficacy of vaccines and also can prevent recognition by pre-existing antibodies. Analysis of influenza viruses, including the 2019-2020 season, shows continuous evolution with the emergence in each season of genetic variants of influenza viruses A(H1N1)pdm09, A(H3N2) that have changes in the HA gene compared to vaccine strains [
31].Changes in NA, on the one side, can help viruses evade neutralizing antibodies developed from previous infections or vaccinations [
32]. However, the impact of NA drift on vaccine effectiveness tends to be less pronounced than that of HA drift. And besides а major advantage of NA is its separate from HA evolution and ability to induce cross-protection. NA antibodies often display broad cross-reactivity within a subtype [
33,
34] which suggests that these antibodies can have meaning in predicting ‘herd’ immunity against newly emerging influenza viruses, as well as in assessing of possible protection against seasonal influenza viruses in case of when HA is mismatched [
8].
Health authorities like the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend annual influenza vaccination as the best way to achieve high seroprotection rates within a community to minimize influenza’s impact. For influenza vaccines, a common threshold for protection is often defined as a HI titer of 1:40 or greater. In general, studies have shown seroprotection rates in adults after vaccination to be approximately 70% to 90% [
35], but may vary depending on different factors like age, health status, and history of previous vaccinations and the match between the vaccine strains and circulating strains. In our study it was shown the rate of seroprotection after seasonal IIVs as determined by HI antibodies was 80%to recent strains, and to drift strains up to 33%.
Anti-NA titers in humans have been shown to correlate independently with protection regardless of HI antibodies [
36]. As HI is a signal peptide, the neutralizing antibodies to it act in the early stages of infection by preventing attachment to cellular receptors [
37].NA plays a critical role in the virus’s life cycle by facilitating the release of progeny viruses from infected host cells. There is evidence that аnti-NA immunity reduces virus shedding/infectivity in humans [
7,
38]. Оn the household transmission model recently it was shown that possessing higher preexisting antibody levels to the HA head, HA stalk, and NA was associated with lower susceptibility to infection although only anti-NA antibodies were associated with reduced the possibility of infection. These results suggest that influenza vaccines created to causeNA immunity in addition to HA immunity may not only contribute to protection against infection but reduce infectivity of vaccinated individuals upon infection [
38].
It is often believed that сurrently availiable IIV do not induce strong anti-NA responses [
39], and that infection but not vaccination induces anti-NA antibodies in humans [40].However, a number of studies, including our recent study, demonstrated that seasonal IIVs cause an increase not only in antibodies to HA, but also to measurable response to NA, which lasted throughout the year after vaccination [
15].The present study also found that seasonal IVIs induced an immune response to HA and NA of drift strains of influenza A/H1N1pdm09 and A/H3N2 (
Figure 2), although with a lower level of seroprotection (
Figure 4). In our study, when measuring the level of seroprotection we used titers of NI antibodies 1:20. Although NI antibodies have been intensively studied due to their broad cross-reactivity, NI antibody seroprotection levels have not yet been determined. It has recently been shown that pre-existing neuraminidase antibody titers 1:40 determined using an enzyme-linked immunosorbent assay (ELISA) significantly reduced the duration of seasonal influenza A virus shedding in adults [
7]. But in regard to NA-inhibitory antibodies, several studies suggest that the different NI antibody levels obtained in several laboratories (1:8- ≥ 1:20) may be protective against natural influenza infection [
41,
42,
43]. It was revealed that what that NA antibodies at the level of seroprotection, independent of antibodies to HA, were formed less frequently than only antibodies to HA, and independent NI antibodies at the level of seroprotection were not formed at all to the A/H3N2 drift virus (
Figure 5).
The current study also shows that patients under 60 years of age have a lower response to drift viruses as a result of vaccination with the than those over 60 years of age (
Figure 2, C;
Figure 5).In this case, statistically significant differences were only in the level of seroprotection to the NA of the A/H1N1pdm09 drift virus. This can be explained by the fact that vaccination can activate memory B cells and T cells, but the breadth and quality may vary based the individual’s pre-existing immunity. A number of studies have shown that middle-aged patients have a reduced immune response to N1 of A/H1N1pdm09 viruses [
44]. But it’s still unclear, whether early-life exposure to influenza viruses is important for the subsequent development of broadly cross-reactive NI antibodies, as has been shown for HA.
A reduced immune response to N1 of drifted A/H1N1pdm09 virus among subjects under 60 years old and a very low content of protective antibodies to N2 in all those were examined, regardless of age can be hypothesized that in the case of a mismatched HA, it will most likely be necessary to vaccinate against the N1-containing influenza virus those who under 60 years of age, and the entire population against virus possessing N2.
5. Conclusions
Seasonal vaccines caused an increase in antibodies not only to HA but also to NA of viruses close in vaccination date and those that arose later. Drift A/H1N1pdm09 and A/H3N2 viruses demonstrated significantly lower reactivity with NI and NI antibodies to early influenza viruses. In response to seasonal IIVs, the level of seroprotection increased, including to drift viruses A, but to a greater extent to A/H1N1pdm09. A reduced immune response to N1 of drifted A/H1N1pdm09 virus vas obtained among subjects under 60 years old. Based on the data obtained, it can be hypothesized that in the case of a mismatched HA, it will most likely be necessary to vaccinate against the N1-containing influenza virus those who under 60 years of age, and the entire population against virus possessing N2.
The limitations of this study are related to the relatively small number of surveys. The study was conducted before the COVID-19 pandemic, when quarantine measures and personal protective equipment were widely used and the influenza situation changed, in particular, population immunity to influenza decreased.
Supplementary Materials
The following supporting information can be downloaded at: preprints.org, Table S1: Seroprotection levels to HA and NA of influenza viruses A/H1N1pdm09 and A/H3N2 before and after vaccination with seasonal IIVs in patients of different age groups (%).
Author Contributions
YD: Conceptualization, Methodology, Writing-original draft, Writing-review & Editing; MS - Investigation, Data curation, Writing-review & editing; PK – Methodology, Investigation; ER -Methodology, Investigation; VK -Methodology, Investigation; KK - Methodology, Investigation; EB - Investigation, Validation; EK - Formal analysis; MK - Formal analysis; DL - Supervision; MSt – Methodology, Validation, Writing-review &editing, IK – Supervision, Writing-review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded within the framework of applied scientific research, topic FGWG -2023-0005.
Institutional Review Board Statement
The study was approved by the Local Ethics Committee of the A.A. Smorodintsev Research Institute of Influenza, protocol No. 131 dated October 10, 2018.After receiving permission from the Local Ethics Committee at the Federal State Budgetary Scientific Institution “IEM” No. 3/23 dated September 20, 2023, the clinical samples were transferred to the researchers in anonymized form.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martins, J.P.; Santos, M.; Martins, A.; Felgueiras, M. & Santos, R. Seasonal influenza vaccine effectiveness in persons aged 15–64 years: A systematic review and meta-analysis. Vaccines, 2023, 11(8), p. 1322.
- Kilbourne, E.D.; Johansson, B.E. & Grajower, B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proceedings of the National Academy of Sciences, 1990, 87(2), pp. 786-790.
- Xu, Q.; Wei, H.; Wen, S.; Chen, J.; Lei, Y.; Cheng, Y.; ... & Shu, Y. Factors affecting the immunogenicity of influenza vaccines in humans, BMC Infectious Diseases, 2023, 23(1), p. 211.
- Memoli, M.J.; Shaw, P.A.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; ... & Taubenberger, J.K. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio, 2016, 7(2), pp. 10-1128.
- de Vries, R.D.; Altenburg, A.F. & Rimmelzwaan, G.F. Universal influenza vaccines: science fiction or soon a reality? Expert Review of Vaccines, 2015, 14(10), pp. 1299-1301.
- Giurgea, L.T.; et al. Influenza neuraminidase: a neglected protein and its potential for a better influenza vaccine. Vaccines, 2020, 8(3), p. 409.
- Maier, H.E.; Nachbagauer, R.; Kuan, G.; Ng, S.; Lopez, R.; Sanchez, N.; ... & Gordon, A. Pre-existing antineuraminidase antibodies are associated with shortened duration of influenza A (H1N1) pdm virus shedding and illness in naturally infected adults. Clinical Infectious Diseases, 2020, 70(11), pp. 2290-2297. [CrossRef]
- Eichelberger, M.C. & Monto, A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. The Journal of Infectious Diseases, 2019, 219(1), pp. S75-S80.
- Krammer, F. & Palese, P. Advances in the development of influenza virus vaccines, Nature Reviews Drug Discovery, 2015, 14(3), pp. 167-182.
- Cheung, J.T.L.; Tsang, T.K.; Yen, H.L.; Perera, R.A.P.M.; Mok, C.K.P.; Lin, Y.P.; et al. Determining existing human population immunity as part of assessing influenza pandemic risk. Emerging Infectious Diseases, 2022, 28, pp. 977-985. [CrossRef]
- Gao, J.; Couzens, L.; Burke, D.F.; Wan, H.; Wilson, P.; Memoli, M.J.; ... & Eichelberger, M.C. Antigenic drift of the influenza A (H1N1) pdm09 virus neuraminidase results in reduced effectiveness of A/California/7/2009 (H1N1pdm09)-specific antibodies. MBio, 2019, 10(2), pp. 10-1128.
- Sandbulte, M.R.; Westgeest, K.B.; Gao, J.; Xu, X.; Klimov, A.I.; Russell, C.A.; ... & Eichelberger, M.C. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proceedings of the National Academy of Sciences, 2011, 108(51), pp. 20748-20753.
- Wan, H.; et al. The neuraminidase of A (H3N2) influenza viruses circulating since 2016 is antigenically distinct from the A/Hong Kong/4801/2014 vaccine strain. Nature Microbiology, 2019, 4(12), pp. 2216-2225.
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2018–2019 Northern Hemisphere Influenza Season. Available online:: https://www.who.int/publications/m/item/recommended-compositionof-influenza-virus-vaccines-for-use-in-the-2018-2019-northern-hemisphere-influenza-season (accessed: 6 November 2023).
- Sergeeva, M.V.; Romanovskaya-Romanko, E.A.; Krivitskaya, V.Z.; Kudar, P.A.; Petkova, N.N.; Kudria, K.S.; ... & Desheva, Y.A. Longitudinal Analysis of Neuraminidase and Hemagglutinin Antibodies to Influenza A Viruses after Immunization with Seasonal Inactivated Influenza Vaccines. Vaccines, 2023, 11(11), 1731.
- Erofeeva, M.K.; Nickonorov, I.J.; Maksakova, V.L.; Stukova, M.A.; Konshina, O.S.; Okhapkina, E.A.; Voicehovskaya, E.M.; Korovkin, S.A.; Melnikhov, S.J. & Kiselev, O.I. Protective properties of inactivated virosomal influenza vaccine. Procedia Vaccinol, 2014, 8, pp. 24-33.
- Talayev, V.; Zaichenko, I.; Svetlova, M.; Matveichev, A.; Babaykina, O.; Voronina, E. & Mironov, A. Low-dose influenza vaccine Grippol Quadrivalent with adjuvant Polyoxidonium induces a T helper-2 mediated humoral immune response and increases NK cell activity. Vaccine, 2020, 38, pp. 6645-6655.
- Erofeeva, M.K.; Stukova, M.A.; Shakhlanskaya, E.V.; Buzitskaya, Z.V.; Maksakova, V.L.; Krainova, T.I.; Pisareva, M.M.; Popov, A.B.; Pozdnjakova, M.G. & Lioznov, D.A. Evaluation of the Preventive Effectiveness of Influenza Vaccines in the Epidemic Season 2019–2020 in St. Petersburg. Epidemiol Vaccinal Prev, 2021, 20, pp. 52-60.
- World Health Organization & Organisation mondiale de la Santé. Recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season. Weekly Epidemiological Record, 2019, 94(12), pp. 141-150.
- Östbye, H.; Gao, J.; Martinez, M.R.; Wang, H.; de Gier, J.W. & Daniels, R. N-linked glycan sites on the influenza A virus neuraminidase head domain are required for efficient viral incorporation and replication. Journal of Virology, 2020, 94(19), pp. 10-1128.
- Xing, L.; Chen, Y.; Chen, B.; Bu, L.; Liu, Y.; Zeng, Z.; ... & Song, W. Antigenic drift of the hemagglutinin from an influenza A (H1N1) pdm09 clinical isolate increases its pathogenicity in vitro. Virologica Sinica, 2021, 36(5), pp. 1220-1227.
- Jagadesh, A.; Salam, A.A.A.; Zadeh, V.R. & Arunkumar, G. Genetic analysis of neuraminidase gene of influenza A(H1N1)pdm09 virus circulating in Southwest India from 2009 to 2012. Journal of Medical Virology, 2017, 89(2), pp. 202-212. [CrossRef]
- Hurt, A.C.; Selleck, P.; Komadina, N.; Shaw, R.; Brown, L. & Barr, I.G. Susceptibility of highly pathogenic A (H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Research, 2007, 73(3), pp. 228-231. [CrossRef]
- Stoner, T.D.; Krauss, S.; DuBois, R.M.; Negovetich, N.J.; Stallknecht, D.E.; Senne, D.A.; ... & Webster, R.G. Antiviral susceptibility of avian and swine influenza virus of the N1 neuraminidase subtype. Journal of Virology, 2010, 84(19), pp. 9800-9809.
- Nuss, J.M.; Whitaker, P.B. & Air, G.M. Identification of critical contact residues in the NC41 epitope of a subtype N9 influenza virus neuraminidase. Proteins: Structure, Function, and Bioinformatics, 1993, 15(2), pp. 121-132. [CrossRef]
- Monto, A.S.; McKimm-Breschkin, J.L.; Macken, C.; Hampson, A.W.; Hay, A.; Klimov, A.; ... & Zambon, M. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrobial Agents and Chemotherapy, 2006, 50(7), pp. 2395-2402.
- Sleeman, K.; Mishin, V.P.; Guo, Z.; Garten, R.J.; Balish, A.; Fry, A.M.; ... & Gubareva, L.V. Antiviral susceptibility of variant influenza A (H3N2) v viruses isolated in the United States from 2011 to 2013. Antimicrobial Agents and Chemotherapy, 2014, 58(4), pp. 2045-2051.
- Leang, S.K.; Kwok, S.; Sullivan, S.G.; Maurer-Stroh, S.; Kelso, A.; Barr, I.G. & Hurt, A.C. Peramivir and laninamivir susceptibility of circulating influenza A and B viruses. Influenza and Other Respiratory Viruses, 2014, 8(2), pp. 135-139.
- Air, G.M.; Els, M.C.; Brown, L.E.; Laver, W.G. & Webster, R.G. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology, 1985, 145(2), pp. 237-248. [CrossRef]
- Powell, H. & Pekosz, A. Neuraminidase antigenic drift of H3N2 clade 3c. 2a viruses alters virus replication, enzymatic activity and inhibitory antibody binding. PLoS Pathogens, 2020, 16(6), e1008411.
- Yatsyshina, S.B.; Artamonova, A.A.; Elkina, M.A.; Valdokhina, A.V.; Bulanenko, V.P.; Berseneva, A.A. & Akimkin, V.G. Genetic characteristics of influenza A and B viruses circulating in Russia in 2019-2023. Journal of Microbiology, Epidemiology and Immunobiology, 2024.
- Altman, M.O.; Angeletti, D. & Yewdell, J.W. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunology, 2018, 31(2), pp. 142-149.
- Hansen, L.; et al. Human anti-N1 monoclonal antibodies elicited by pandemic H1N1 virus infection broadly inhibit HxN1 viruses in vitro and in vivo. Immunity, 2023, 56(8), pp. 1927-1938.
- Lei, R.; Kim, W.; Lv, H.; Mou, Z.; Scherm, M.J.; Schmitz, A.J.; ... & Wu, N.C. Leveraging vaccination-induced protective antibodies to define conserved epitopes on influenza N2 neuraminidase. Immunity, 2023, 56(11), pp. 2621-2634.
- Carlock, M.A.; Allen, J.D.; Hanley, H.B. & Ross, T.M. Longitudinal assessment of human antibody binding to hemagglutinin elicited by split-inactivated influenza vaccination over six consecutive seasons. PLOS One, 2024, 19(6), e0301157.
- Monto, A.S.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Liu, M.; Zhong, W.; ... & Ohmit, S.E. Antibody to influenza virus neuraminidase: an independent correlate of protection. The Journal of Infectious Diseases, 2015, 212(8), pp. 1191-1199.
- Vogel, M. & Bachmann, M.F. Immunogenicity and immunodominance in antibody responses. Vaccination Strategies Against Highly Variable Pathogens, 2020, pp. 89-102.
- Hoy, G.; Cortier, T.; Maier, H.E.; Kuan, G.; Lopez, R.; Sanchez, N.; ... & Gordon, A. Anti-Neuraminidase Antibodies Reduce the Susceptibility to and Infectivity of Influenza A/H3N2 Virus. medRxiv, 2024.
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; Brokstad, K.A.; ... & Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio, 2015, 6(2).
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; ... & Wilson, P.C. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell, 2018, 173(2), pp. 417-429.
- Monto, A.S. & Kendal, A.P. Effect of neuraminidase antibody on Hong Kong influenza. The Lancet, 1973, 1(7804), pp. 623-625. [CrossRef]
- Smith, A.J. & Davies, J.R. Natural infection with influenza A (H3N2). The development, persistence and effect of antibodies to the surface antigens. Journal of Hygiene (Lond), 1976, 77(2), pp. 271-282. [CrossRef]
- Murphy, B.R.; Kasel, J.A. & Chanock, R.M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. New England Journal of Medicine, 1972, 286(25), pp. 1329-1332. [CrossRef]
- Broberg, E.; Nicoll, A. & Amato-Gauci, A. Seroprevalence to influenza A (H1N1) 2009 virus—where are we? Clinical and Vaccine Immunology, 2011, 18(8), pp. 1205-1212.
Figure 1.
Molecular analysis and enzymatic activity of NA of A/H1N1pdm09 and A/H3N2 viruses. (A) Cartoon style molecular model of NA A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 from amino acid 82 to 469 (numbering as in the case of the H1N1 2009 pandemic virus)with major amino acid substitutions in the structure indicated; (B) Molecular model of NA A/Brisbane/34/2018 (H3N2) from amino acid 82 to 469 (classical H3N2 strain numbering); (C) Enzymatic activity of NA of viruses A/H6N1 and A/H6N2 was studied in the test of desialization of high-molecular substrate (fetuin), sorbed on a polymer carrier, using peroxidase-labeled lectin. The OD450 was measured depending on the hemagglutinating activity of viruses (HA).
Figure 1.
Molecular analysis and enzymatic activity of NA of A/H1N1pdm09 and A/H3N2 viruses. (A) Cartoon style molecular model of NA A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 from amino acid 82 to 469 (numbering as in the case of the H1N1 2009 pandemic virus)with major amino acid substitutions in the structure indicated; (B) Molecular model of NA A/Brisbane/34/2018 (H3N2) from amino acid 82 to 469 (classical H3N2 strain numbering); (C) Enzymatic activity of NA of viruses A/H6N1 and A/H6N2 was studied in the test of desialization of high-molecular substrate (fetuin), sorbed on a polymer carrier, using peroxidase-labeled lectin. The OD450 was measured depending on the hemagglutinating activity of viruses (HA).
Figure 2.
Antibodies to A/H1N1pdm09 and A/H3N2 viruses in sera of patients vaccinated with inactivated influenza vaccines (IIVs) corresponded to the WHO recommendations for the northern hemisphere in the 2018–2019 flu season. The total number of participants was 64 of these, 30 people were 60 years old and older and 34 were under 60 years old. (A) Antibodies to HA and NA of influenza viruses A/South Africa/3626/13 (H1N1)pdm09, A/Hong Kong/4801/2014 (H3N2), A/Guangdong-Maonan/SWL 1536/2019(H1N1)pdm09, A/Brisbane/34/2018(H3N2)before and after vaccination; (B) The HI and NI antibodies to vaccine and drift viruses A/H1N1pdm09 and A/H3N2 in sera of patients of different ages collected before vaccination. Each point represents an individual patient serum, here and below: * - P<0.05, ** - P<0.01, *** - P<0.001, **** - P<0.0001; (C) Combined seroconversions to vaccine and drifted influenza viruses on day 21 after vaccination with IIVs regardless of the vaccine type presented by Venn’s diagrams. Numbers in circles present absolute number or responders to each virus. The total number of participants was 64 of these, 30 people were 60 years old and older and 34 were under 60 years old. The number of non-responders to both antigens is not shown on Venn’s diagram. A four-fold increase in HI antibody titer after vaccination and a two-fold increase in NI antibody titer after vaccination was considered reliable antibody seroconversion.
Figure 2.
Antibodies to A/H1N1pdm09 and A/H3N2 viruses in sera of patients vaccinated with inactivated influenza vaccines (IIVs) corresponded to the WHO recommendations for the northern hemisphere in the 2018–2019 flu season. The total number of participants was 64 of these, 30 people were 60 years old and older and 34 were under 60 years old. (A) Antibodies to HA and NA of influenza viruses A/South Africa/3626/13 (H1N1)pdm09, A/Hong Kong/4801/2014 (H3N2), A/Guangdong-Maonan/SWL 1536/2019(H1N1)pdm09, A/Brisbane/34/2018(H3N2)before and after vaccination; (B) The HI and NI antibodies to vaccine and drift viruses A/H1N1pdm09 and A/H3N2 in sera of patients of different ages collected before vaccination. Each point represents an individual patient serum, here and below: * - P<0.05, ** - P<0.01, *** - P<0.001, **** - P<0.0001; (C) Combined seroconversions to vaccine and drifted influenza viruses on day 21 after vaccination with IIVs regardless of the vaccine type presented by Venn’s diagrams. Numbers in circles present absolute number or responders to each virus. The total number of participants was 64 of these, 30 people were 60 years old and older and 34 were under 60 years old. The number of non-responders to both antigens is not shown on Venn’s diagram. A four-fold increase in HI antibody titer after vaccination and a two-fold increase in NI antibody titer after vaccination was considered reliable antibody seroconversion.
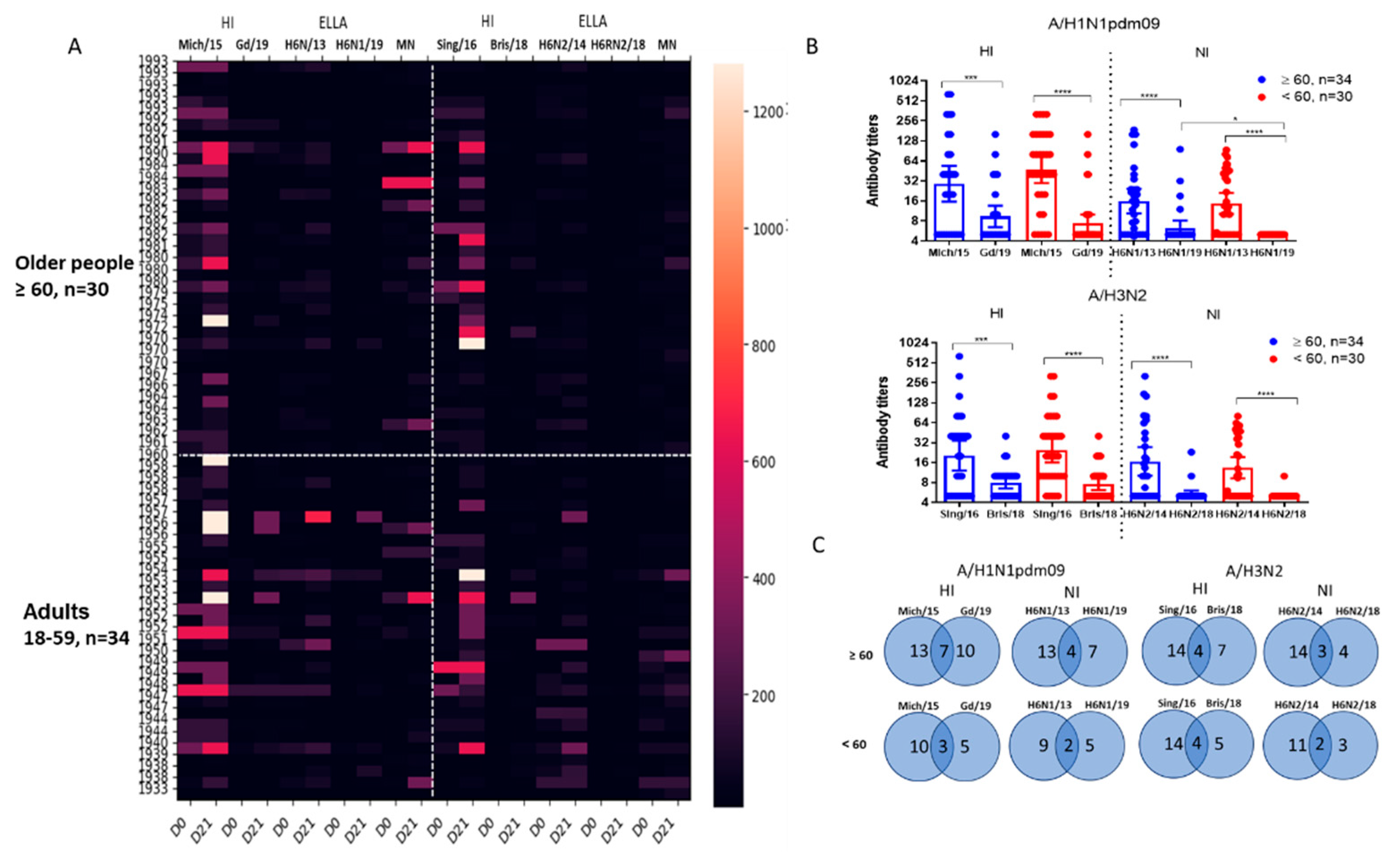
Figure 3.
Antibody titers to HA and NA of influenza viruses in paired blood sera of patients vaccinated with seasonal IIVs 2018-2019 years of formulation before and 21 days after vaccination (n=64). Population in the analyses included all participants, regardless of the age or vaccine type. Each dot represents an individual serum. (A) HI antibodies to A/Michigan/45/2015(H1N1)pdm09 and A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 viruses; (B) NI antibodies to H6N1/13 and H6N1/19 influenza viruses; (C) HI antibodies to A/Singapore/INFIMH-16-0019/2016 (H3N2) and A/Brisbane/34/2018(H3N2) viruses; (D) NI antibodies to H6N2/14 and H6N2/18 influenza viruses.* - P<0.05, ** - P<0.01, *** - P<0.001, **** - P<0.0001.
Figure 3.
Antibody titers to HA and NA of influenza viruses in paired blood sera of patients vaccinated with seasonal IIVs 2018-2019 years of formulation before and 21 days after vaccination (n=64). Population in the analyses included all participants, regardless of the age or vaccine type. Each dot represents an individual serum. (A) HI antibodies to A/Michigan/45/2015(H1N1)pdm09 and A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 viruses; (B) NI antibodies to H6N1/13 and H6N1/19 influenza viruses; (C) HI antibodies to A/Singapore/INFIMH-16-0019/2016 (H3N2) and A/Brisbane/34/2018(H3N2) viruses; (D) NI antibodies to H6N2/14 and H6N2/18 influenza viruses.* - P<0.05, ** - P<0.01, *** - P<0.001, **** - P<0.0001.
Figure 4.
Proportions of individuals with antibody titers ≥ 1:40 for HI antibodies and ≥ 1:20 for NI antibodies to HA and NA of influenza viruses A/H1N1pdm09 and A/H3N2 after vaccination with seasonal IIVs (n=64). Population in the analyses included all participants, regardless of the age or vaccine type. (A) The HI and NI antibodies to A/South Africa/3626/13 (H1N1)pdm09 and A/Hong Kong/4801/2014 (H3N2); (B) The HI and NI antibodies to A/Guangdong-Maonan/SWL 1536/2019(H1N1)pdm09 and A/Brisbane/34/2018 (H3N2).
Figure 4.
Proportions of individuals with antibody titers ≥ 1:40 for HI antibodies and ≥ 1:20 for NI antibodies to HA and NA of influenza viruses A/H1N1pdm09 and A/H3N2 after vaccination with seasonal IIVs (n=64). Population in the analyses included all participants, regardless of the age or vaccine type. (A) The HI and NI antibodies to A/South Africa/3626/13 (H1N1)pdm09 and A/Hong Kong/4801/2014 (H3N2); (B) The HI and NI antibodies to A/Guangdong-Maonan/SWL 1536/2019(H1N1)pdm09 and A/Brisbane/34/2018 (H3N2).
Figure 5.
Seroprotection levels to HA and NA of influenza viruses A/H1N1pdm09 and A/H3N2 before and after vaccination with seasonal IIVs in patients of different age groups. For HI antibodies, the seroprotection level was determined as 1:40, for NI antibodies - as 1:20. * - P<0.05, Fisher’s exact test.
Figure 5.
Seroprotection levels to HA and NA of influenza viruses A/H1N1pdm09 and A/H3N2 before and after vaccination with seasonal IIVs in patients of different age groups. For HI antibodies, the seroprotection level was determined as 1:40, for NI antibodies - as 1:20. * - P<0.05, Fisher’s exact test.
Figure 6.
Results of the study of neutralizing antibodies using the MN test in MDCK cell line. (A) The NI antibodies to the H6N1/19 virus. Population in the analyses included all participants, regardless of the age or vaccine type; (B) Correlation analysis of neutralizing antibodies and antibodies to HA and NA of the A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 virus before vaccination; (C) The NI antibodies to the H6N2/18 virus; (D) Correlation analysis of neutralizing antibodies and antibodies to HA and NA of the A/Brisbane/34/2018(H3N2) virus after vaccination.*** - P<0.001.
Figure 6.
Results of the study of neutralizing antibodies using the MN test in MDCK cell line. (A) The NI antibodies to the H6N1/19 virus. Population in the analyses included all participants, regardless of the age or vaccine type; (B) Correlation analysis of neutralizing antibodies and antibodies to HA and NA of the A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 virus before vaccination; (C) The NI antibodies to the H6N2/18 virus; (D) Correlation analysis of neutralizing antibodies and antibodies to HA and NA of the A/Brisbane/34/2018(H3N2) virus after vaccination.*** - P<0.001.
Figure 7.
Antibody titers to drifted A/H1N1pdm09 and A/H3N2 HA and NA among patients vaccinated with split influenza vaccines (n=29) and subunit influenza vaccines (n=35). Each dot represents an individual serum. (A) HI antibodies to A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 virus; (B) NI antibodies H6N1/19 influenza virus; (C) HI antibodies to A/Brisbane/34/2018(H3N2) virus; (D) NI antibodies to H6N2/18 influenza virus.* - P<0.05, *** - P<0.001.
Figure 7.
Antibody titers to drifted A/H1N1pdm09 and A/H3N2 HA and NA among patients vaccinated with split influenza vaccines (n=29) and subunit influenza vaccines (n=35). Each dot represents an individual serum. (A) HI antibodies to A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09 virus; (B) NI antibodies H6N1/19 influenza virus; (C) HI antibodies to A/Brisbane/34/2018(H3N2) virus; (D) NI antibodies to H6N2/18 influenza virus.* - P<0.05, *** - P<0.001.
Table 1.
Genome composition of diagnostic reassortant viruses of subtype H6 for use in ELLA.
Table 1.
Genome composition of diagnostic reassortant viruses of subtype H6 for use in ELLA.
| Name |
Origin of HA |
Origin of NA |
| H6N1/13 |
A/Herring Gull/Sarma/51c/2006 (H6N1) |
A/South Africa/3626/2013(H1N1)pdm09 |
| H6N2/14 |
A/Herring Gull/Sarma/51c/2006 (H6N1) |
A/HongKong/4801/2014 (H3N2) |
| H6N1/19 |
A/Herring Gull/Sarma/51c/2006 (H6N1) |
A/Guangdong-Maonan/SWL 1536/2019(H1N1)pdm09 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).