Submitted:
28 October 2024
Posted:
30 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
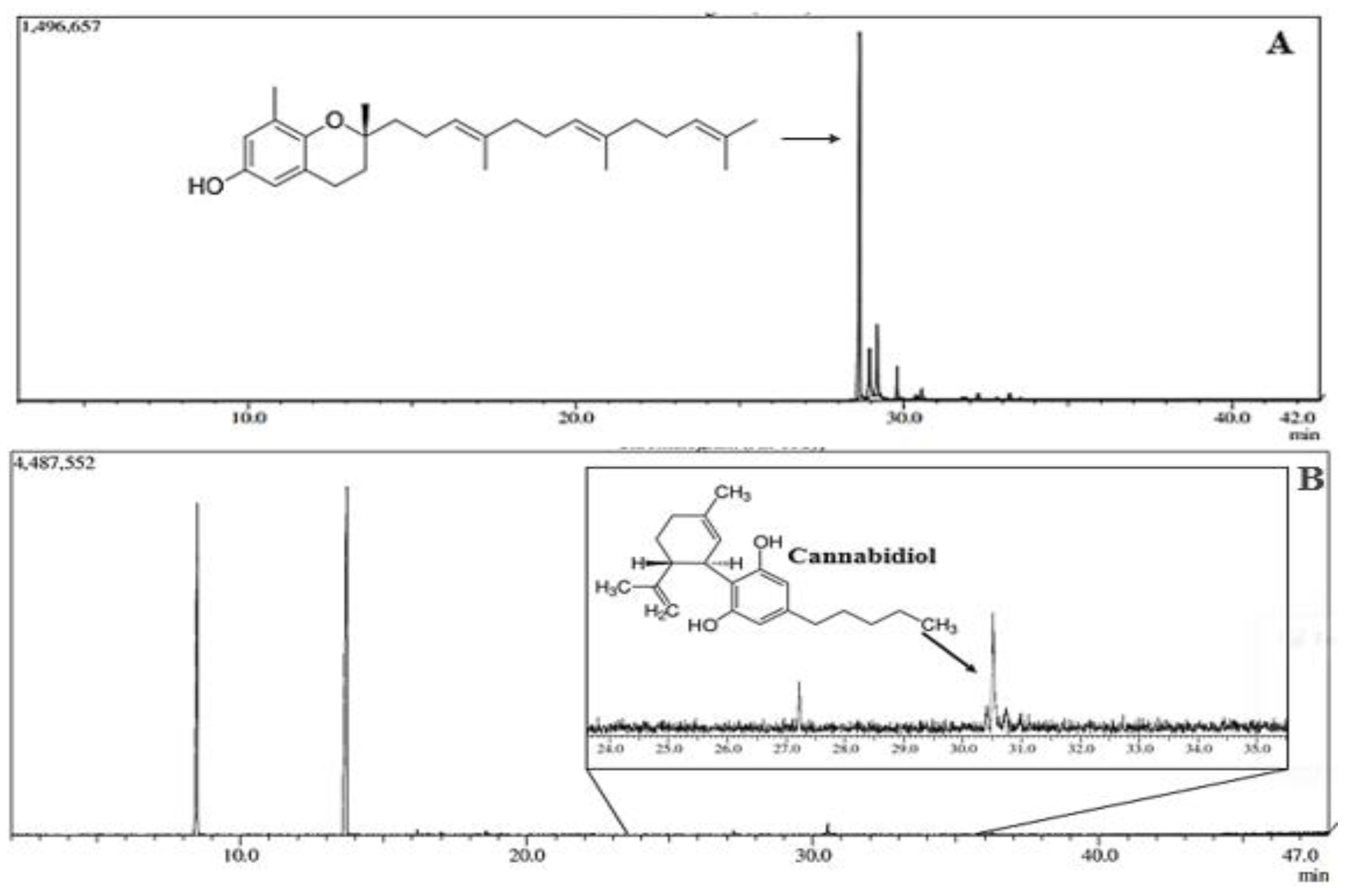
2.1. Chemical Analysis of Bixa orellana and Cannabis sativa Oils
2.2. Characterization of Injectable Nanodispersion (Chronic in®) of B. orellana
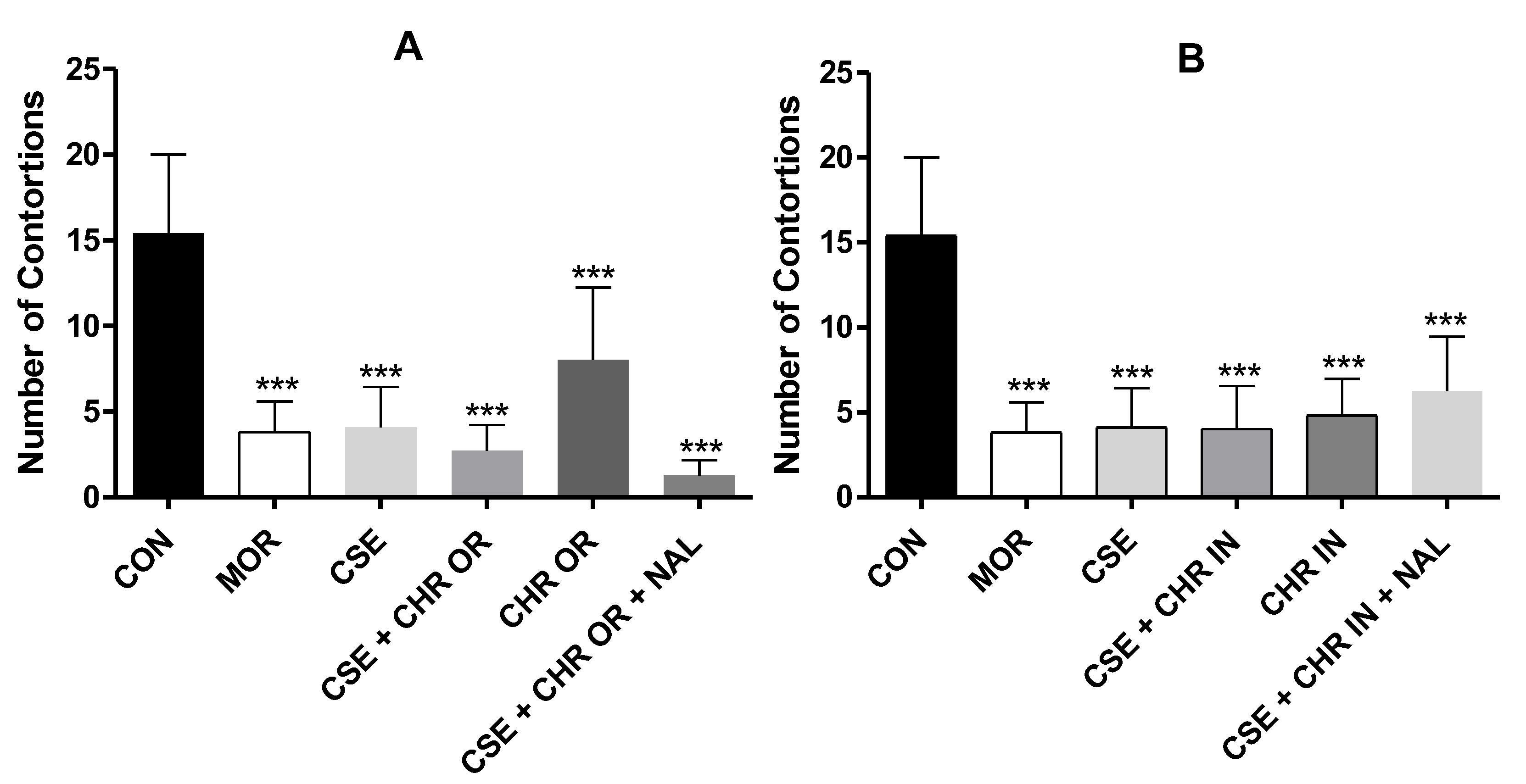
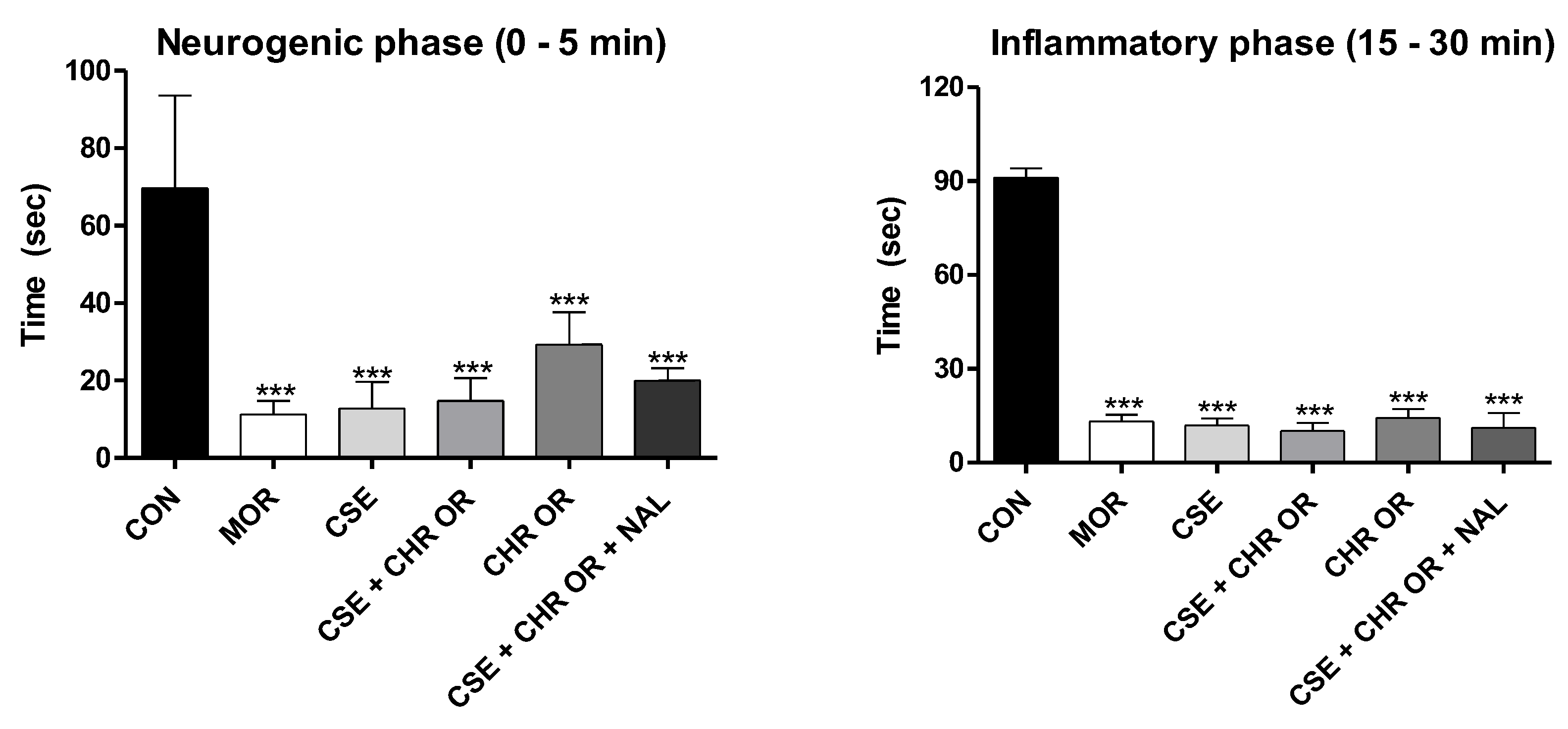
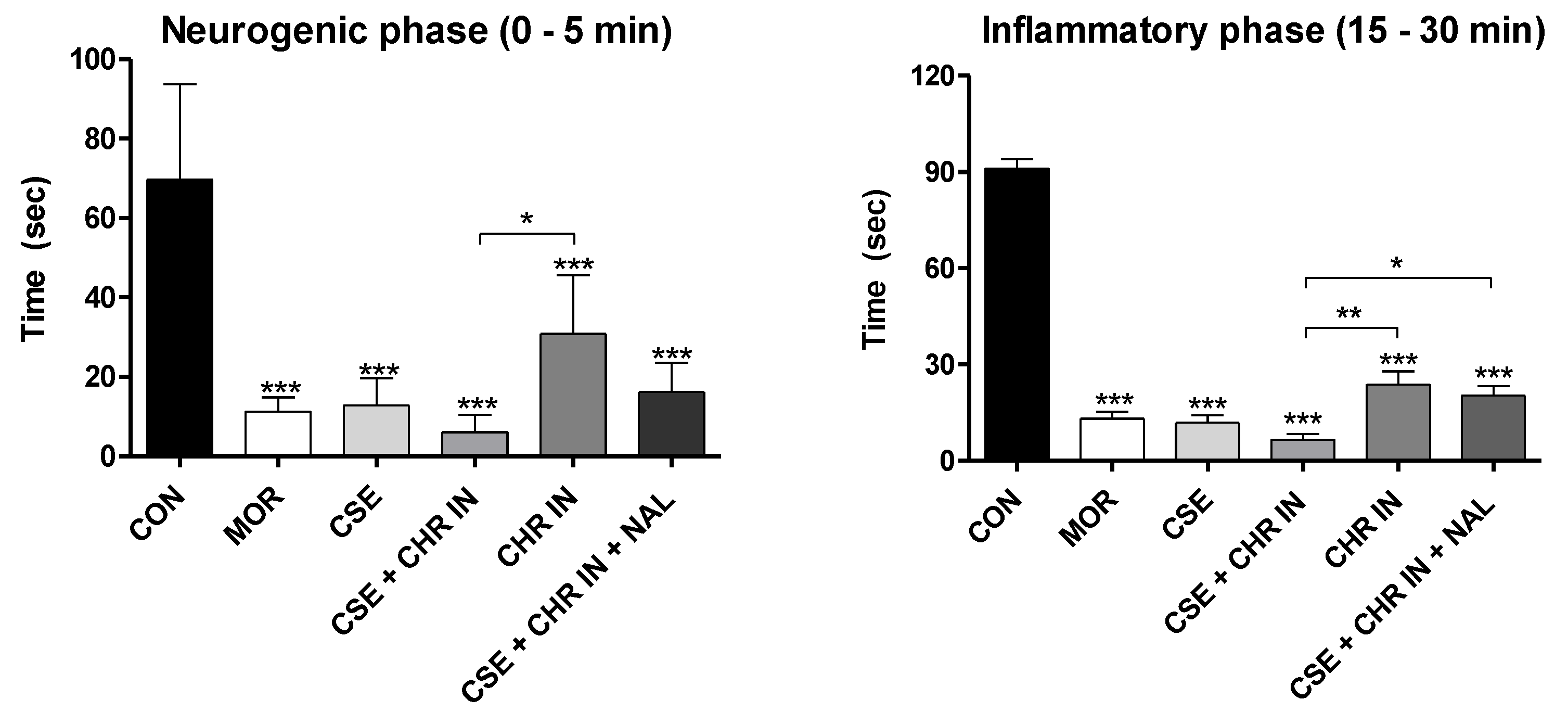
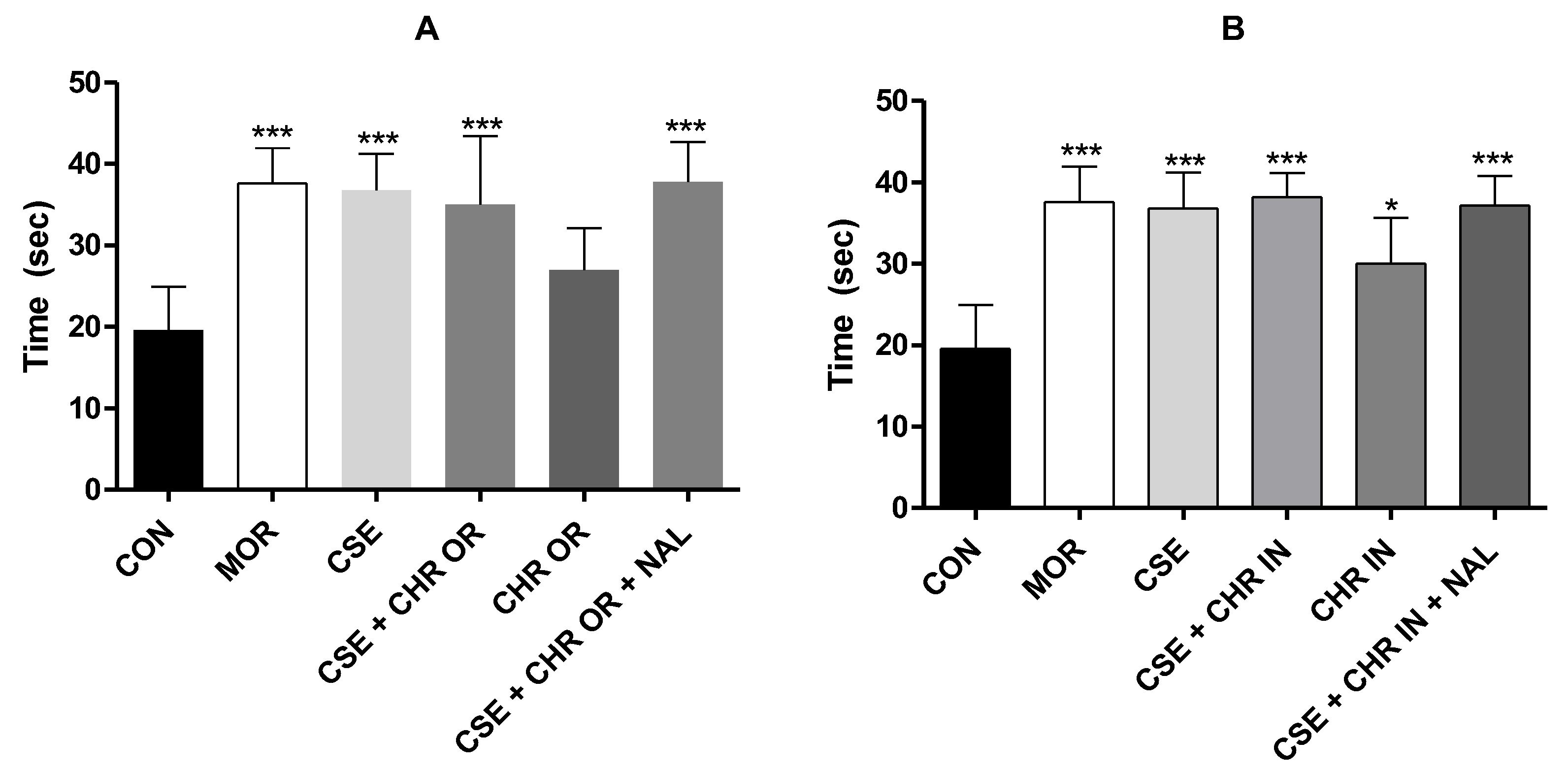
2.3. Evaluation of Treatments in Pain Models
3. Materials and Methods
3.1. Acquisition of Cannabis sativa Extract and Chemical Analysis by G.C.
3.2. Acquisition of B. orellana Oil and Chemical Analysis
3.3. Obtention of the Granules of Bixa orellana (Chronic®)
3.4. Obtention of Polymeric Nanoparticles Containing Bixa orellana oil (Chronic In®)
3.5. Characterization of B. orellana Nanosuspension (Chronic In®)
3.6. Study Design and Ethical Considerations
3.7. Animals Used
3.8. Experimental Design
3.9. Formalin Test
3.10. Hot Plate Test
3.11. Cold-Water Tail Withdrawal Test
3.12. Acetic Acid-Induced Abdominal Writhing
3.13. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Adams, J. U., Geller, E. B., & Adler, M. W. (1994). Receptor selectivity of icv morphine in the rat cold water tail-flick test.
- Aggarwal, V., Kashyap, D., Sak, K., Tuli, H. S., Jain, A., Chaudhary, A., Garg, V. K., Sethi, G., & Yerer, M. B. (2019). Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. In International Journal of Molecular Sciences (Vol. 20, Número 3). MDPI AG. [CrossRef]
- Aktary, N., Sultana, S., & Hossain, M. L. (2019). Assessment of analgesic and neuropharmacological activity of leaves of Bixa orellana (Family: Bixaceae). International Journal of Scientific Reports, 6(1), 13. [CrossRef]
- Atalay, S., Jarocka-karpowicz, I., & Skrzydlewskas, E. (2020). Antioxidative and anti-inflammatory properties of cannabidiol. Em Antioxidants (Vol. 9, Número 1). MDPI. [CrossRef]
- Bannon, A. W., & Malmberg, A. B. (2007). Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley ... [et al.], Chapter 8. [CrossRef]
- Batista, E. K. F., Trindade, H. I., Lira, S. R. S., Muller, J. B. B. S., Silva, L. L. B., & Batista, M. C. S. (2016). Atividades antinociceptiva e antiinflamatória do extrato etanólico de Luehea divaricata. Revista Brasileira de Plantas Medicinais, 18(2), 433–441. [CrossRef]
- Batista, M. A., Dos Santos, A. V. T. de L. T., Nascimento, A. L. Do, Moreira, L. F., Souza, I. R. S., Silva, H. R. da, Pereira, A. C. M., Hage-Melim, L. I. da S., & Carvalho, J. C. T. (2022). Potential of the Compounds from Bixa orellana Purified Annatto Oil and Its Granules (Chronic®) against Dyslipidemia and Inflammatory Diseases: In Silico Studies with Geranylgeraniol and Tocotrienols. Molecules, 27(5). [CrossRef]
- Borges, R. S., Lima, E. S., Keita, H., Ferreira, I. M., Fernandes, C. P., Cruz, R. A. S., Duarte, J. L., Velázquez-Moyado, J., Ortiz, B. L. S., Castro, A. N., Ferreira, J. V., da Silva Hage-Melim, L. I., & Carvalho, J. C. T. (2018). Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology, 26(1), 183–195. [CrossRef]
- Bunman, S., Muengtaweepongsa, S., Piyayotai, D., Charlermroj, R., Phuengwas, S., & Makornwattana, M. (2022). Study the Effect of Cannabidiol Topical on Antinociceptive and Anti-inflammatory Activities in Animal Model. [CrossRef]
- Collier, J., Dinneen, L. C., Johnson, C. A., & Schneider, C. (1968). THE ABDOMINAL CONSTRICTION RESPONSE AND ITS SUPPRESSION BY ANALGESIC DRUGS IN THE MOUSE. Em Br. J. Pharmac. Chemother (Vol. 32).
- Costa, B., Colleoni, M., Conti, S., Parolaro, D., Franke, C., Trovato, A. E., & Giagnoni, G. (2004). Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn-Schmiedeberg’s Archives of Pharmacology, 369(3), 294–299. [CrossRef]
- de Oliveira Carvalho, H., Gonçalves, D. E. S., Picanço, K. R. T., de Lima Teixeira dos Santos, A. V. T., Lucia, M., Hu, X., Fernandes, C. P., Ferreira, I. M., & Carvalho, J. C. T. (2021). Actions of Cannabis sativa L. fixed oil and nanoemulsion on venom-induced inflammation of Bothrops moojeni snake in rats. Inflammopharmacology, 29(1), 123–135. [CrossRef]
- de Oliveira Carvalho, H., Laura, A., Sauma, R., Emanuelle, D., Gonçalves, S., Gomes, L., Ribeiro Da Silva, H., César, A., Pereira, M., Carlos, J., & Carvalho, T. (2022). Intramuscular compatibility of an injectable nanodispersion anti-inflammatory (Chronic®) from a standardized Bixa orellana oil: a toxicological study in Wistar rats. [CrossRef]
- Dubuisson, D., & Dennis, S. G. (1977). THE FORMALIN TEST: A QUANTITATIVE STUDY OF THE ANALGESIC EFFECTS OF MORPHINE, MEPERIDINE, AND BRAIN STEM STIMULATION IN RATS AND CATS—Em Pain (Vol. 4). ElsevirrlNorth-Holland Biomedical Press.
- Foss, J. D., Farkas, D. J., Huynh, L. M., Kinney, W. A., Brenneman, D. E., & Ward, S. J. (2021a). Behavioural and pharmacological effects of cannabidiol (CBD) and the cannabidiol analogue KLS-13019 in mouse models of pain and reinforcement. British Journal of Pharmacology, 178(15), 3067–3078. [CrossRef]
- Foss, J. D., Farkas, D. J., Huynh, L. M., Kinney, W. A., Brenneman, D. E., & Ward, S. J. (2021b). Behavioural and pharmacological effects of cannabidiol (CBD) and the cannabidiol analogue KLS-13019 in mouse models of pain and reinforcement. British Journal of Pharmacology, 178(15), 3067–3078. [CrossRef]
- Hill, K. P., Palastro, M. D., Johnson, B., & Ditre, J. W. (2017). Cannabis and Pain: A Clinical Review. Em Cannabis and cannabinoid research (Vol. 2, Número 1, p. 96–104). NLM (Medline). [CrossRef]
- İnaltekin, A., & Kivrak, Y. (2021). Evaluation of the effect of vortioxetine on pain threshold by hot-plate test in mice. Noropsikiyatri Arsivi, 58(4), 274–277. [CrossRef]
- Menezes, P. M. N., Araújo, T. C. de L., Pereira, E. C. V., Neto, J. A., Silva, D. S., Brito, M. C., Lima, K. S. B., Monte, A. P. O. do, Matos, M. H. T. de, Lima, R. S. de, Ribeiro, L. A. de A., Silva, F. S., & Rolim, L. A. (2021). Investigation of antinociceptive, antipyretic, antiasthmatic, and spasmolytic activities of Brazilian Cannabis sativa L. roots in rodents. Journal of Ethnopharmacology, 278, 114259. [CrossRef]
- Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., & Langer, R. (2021). Engineering precision nanoparticles for drug delivery. Em Nature Reviews Drug Discovery (Vol. 20, Número 2, p. 101–124). Nature Research. [CrossRef]
- Mlost, J., Bryk, M., & Starowicz, K. (2020a). Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Em International Journal of Molecular Sciences (Vol. 21, Número 22, p. 1–22). MDPI AG. [CrossRef]
- Mlost, J., Bryk, M., & Starowicz, K. (2020b). Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Em International Journal of Molecular Sciences (Vol. 21, Número 22, p. 1–22). MDPI AG. [CrossRef]
- Moore, C. F., & Weerts, E. M. (2022). Cannabinoid tetrad effects of oral Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in male and female rats: sex, dose-effects and time course evaluations. Psychopharmacology, 239(5), 1397–1408. [CrossRef]
- Nascimento, G., Ferrari, D. P., Guimaraes, F. S., Del Bel, E. A., Bortolanza, M., & Ferreira- Junior, N. C. (2020). Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology, 163. [CrossRef]
- Neelakantan, H., Tallarida, R. J., Reichenbach, Z. W., Tuma, R. F., Ward, S. J., & Walker, E. A. (2015). Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behavioural Pharmacology, 26(3), 304–314. [CrossRef]
- P1zziketti, R. J., Pressman, N. S., Geller, E. B., Cowan, A., Adler, M. W., & Pizziketti, R. J. (1985). 23 Elsevier RAT COLD WATER TAlL-FLICK: A NOVEL ANALGESIC TEST THAT DISTINGUISHES OPIOID AGONISTS FROM MIXED AGONIST-ANTAGONISTS. in European Journal of Pharmacology (Vol. 119).
- Raja, S. N., Carr, D. B., Cohen, M., Finnerup, N. B., Flor, H., Gibson, S., Keefe, F. J., Mogil, J. S., Ringkamp, M., Sluka, K. A., Song, X. J., Stevens, B., Sullivan, M. D., Tutelman, P. R., Ushida, T., & Vader, K. (2020). The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Em Pain (Vol. 161, Número 9, p. 1976–1982). Lippincott Williams and Wilkins. [CrossRef]
- Ranasinghe, R., Mathai, M., & Zulli, A. (2022). Revisiting the therapeutic potential of tocotrienol. Em BioFactors (Vol. 48, Número 4, p. 813–856). John Wiley and Sons Inc. [CrossRef]
- Razavi, Y., Rashvand, M., Sharifi, A., Haghparast, A., Keyhanfar, F., & Haghparast, A. (2021). Cannabidiol microinjection into the nucleus accumbens attenuated nociceptive behaviors in an animal model of tonic pain. Neuroscience Letters, 762, 136141. [CrossRef]
- Rodrigues, A. P. S., da Silva Barbosa, R., Pereira, A. C. M., Batista, M. A., Sales, P. F., Ferreira, A. M., Colares, N. N. D., da Silva, H. R., Soares, M. O. dos S., da Silva Hage-Melim, L. I., & Carvalho, J. C. T. (2022). Ormona® SI and Ormona® RC—New Nutraceuticals with Geranylgeraniol, Tocotrienols, Anthocyanins, and Isoflavones—Decrease High-Fat Diet-Induced Dyslipidemia in Wistar Rats. Nutraceuticals, 2(4), 311–322. [CrossRef]
- Sadaka, A. H., Canuel, J., Febo, M., Johnson, C. T., Bradshaw, H. B., Ortiz, R., Ciumo, F., Kulkarni, P., Gitcho, M. A., & Ferris, C. F. (2023). Effects of inhaled cannabis high in Δ9-THC or CBD on the aging brain: A translational MRI and behavioral study. Frontiers in Aging Neuroscience, 15. [CrossRef]
- Sales, P. F., Nascimento, A. L. do, Pinheiro, F. C., Alberto, A. K. M., Teixeira dos Santos, A. V. T. de L., Carvalho, H. de O., de Souza, G. C., & Carvalho, J. C. T. (2023). Effect of the Association of Fixed Oils from Abelmoschus esculentus (L.) Moench, Euterpe oleracea Martius, Bixa orellana Linné and Chronic SM® on Atherogenic Dyslipidemia in Wistar Rats. Molecules, 28(18). [CrossRef]
- Sharma, S. S., Kumar, A., & Kaundal, R. K. (2008). Protective effects of 4-amino1,8-napthalimide, a poly (ADP-ribose) polymerase inhibitor in experimental diabetic neuropathy. Life Sciences, 82(11–12), 570–576. [CrossRef]
- Shilpi, J. A., Taufiq-Ur-Rahman, M., Uddin, S. J., Alam, M. S., Sadhu, S. K., & Seidel, V. (2006). Preliminary pharmacological screening of Bixa orellana L. leaves. Journal of Ethnopharmacology, 108(2), 264–271. [CrossRef]
- Silva, J. C., Oliveira-Júnior, R. G., Silva, M. G., Lavor, E. M., Soares, J. M. D., Lima-Saraiva, S. R. G., Diniz, T. C., Medeiros, M. A. M. B., Lima, L. M., Barreiro, E. J., & Almeida, J. R. G. S. (2021). In Mice Evaluation of Antinociceptive and Anti-inflammatory Activity of N-acylhydrazone Derivative LASSBio-1587. Revista Virtual de Quimica, 13(6), 1467–1472. [CrossRef]
- Sofia, R. D., Vassar, H. B., & Knobloch, L. C. (1975). Comparative Analgesic Activity of Various Naturally Occurring Cannabinoids in Mice and Rats. In Psychopharmacology (Berl.) (Vol. 40). Springer-Verlag.
- Tejpal Singh, H. S., Aminuddin, A. A., Pang, K. L., Ekeuku, S. O., & Chin, K. Y. (2023). The Role of Tocotrienol in Arthritis Management—A Scoping Review of Literature. Em Pharmaceuticals (Vol. 16, Número 3). MDPI. [CrossRef]
- Tj, A., Berge, O.-G., Hunskaar, S., Henrik Rosland, J., & Hole, K. (1992). The formalin test: an evaluation of the method. Em Puin (Vol. 51).
- Urits, I., Gress, K., Charipova, K., Habib, K., Lee, D., Lee, C., Jung, J. W., Kassem, H., Cornett, E., Paladini, A., Varrassi, G., Kaye, A. D., & Viswanath, O. (2020). Use of cannabidiol (CBD) for the treatment of chronic pain. In Best Practice and Research: Clinical Anaesthesiology (Vol. 34, Número 3, p. 463–477). Bailliere Tindall Ltd. [CrossRef]
- Williams, A. C. D. C., & Craig, K. D. (2016). Updating the definition of pain. Em Pain (Vol. 157, Número 11, p. 2420–2423). Lippincott Williams and Wilkins. [CrossRef]
- Yong, S. T., Wong, H., & Mohammad, M. (2014). Tocotrienol and tocopherol contents of annatto seed accessions. https://www.researchgate.net/publication/318601954.
- Zielinska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Nagasamy Venkatesh, D., Durazzo, A., Lucarini, M., Eder, P., Silva, A. M., Santini, A., & Souto, E. B. (2020). Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Em Molecules (Vol. 25, Número 16). MDPI AG. [CrossRef]

| P. Zeta (mV) | Size (nm) | PDI | pH | |
|---|---|---|---|---|
| Day 1 | 19,86±0,60 | 53,15±0,64 | 0,533±0,008 | 5,8±0,01 |
| Day 30 | 19,66±1,45 | 59,90±3,63 | 0,594±0,014 | 5,8±0,01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





