1. Introduction
Bovine laminitis causing large economic losses and major animal-health concern in the global dairy sector [
1]. This disease was first characterized as a diffuse process involving aseptic inflammation of the dermal lamellae of the claw [
2]. Subsequently, researchers offered a broader definition for bovine laminitis as a system disorder with localized signs in the claw [
3]. This disease may result in different laminitis related claw horn disruption (CHDs) including sole ulcer, sole hemorrhage and white line disease [
4]. The overall prevalence of these lesions is highly linked to breeding, management, and environmental factors [
5]. In clinical contexts, bovine laminitis is primarily ranked to secondary to many inflammatory disorders, including ruminal acidosis, metritis, and gram-negative pleuropneumonia [
3]. Experimental models of dairy cow laminitis have been developed by simulating these clinical scenarios. The OF-induced model is more legitimate and widely used than the some other types of inducement models [
6,
7]. This model has similar clinical manifestations and characteristic histopathological changes with acute laminitis cases [
8,
9]. A latest research group confirmed that excessive oligofructose (a type of non-structural carbohydrate) have been shown to cause serious clinical issue including acute laminitis and respiratory adaptations for acute metabolic acidosis [
10]. In the past, the OF-induced model was employed more commonly in equine laminitis research than in cow laminitis [
11,
12]. Thus, this investigation based on the successful induction of laminitis in dairy cows with oligofructose, for the first time to examine the apoptosis response at gene and protein level in the lamellae of dairy cow’s claw.
The investigators have come across the evidence showing that bovine laminitis affects the structural integrity of the lamellae. These tissues are relevant to provide appropriate orientation of the third phalanx (P3) and transmit primarily mechanical load to the hoof as they connect P3 to the hoof capsule [
13]. In the lamellae structure, epidermal lamellae and dermal lamellae are interconnected, separated by a contact interface known as the basement membrane (BM) [
14]. Disruption of epidermal and dermal lamellae allows P3 to sink and rotate inside the capsule, causing severe lameness and pain [
15]. The BM, as a boundary between two distinct layers, is a primary part of the extracellular matrix (ECM). BM destruction and separation are the two main histological changes that are believed to cause epidermal separation in bovine laminitis [
16,
17]. Nevertheless, the underlying biological pathway is still unknown.
Apoptosis is the most composite and necessary biological progression that, during animal development, cellular homeostasis and illness, destroys and removes aggravated cells by bacterial inhibition [
18]. Apoptosis has been suggested to play a role in causing acute laminitis. Researchers proposed that when an animal is exposed to carbohydrate and black walnut overload, apoptosis of cells takes place that bind to the lamellar layers of the hoof and cause sepsis-related laminitis [
19]. According to the toxic and metabolic models of laminitis, a rise in apoptotic basal lamina may be caused by the fact that bacterial infections can cause apoptosis in a variety of cell types [
20]. In vivo studies have shown that thermolysin and streptococcal oncogenic exotoxin B aggravate the detachment of horse lamellar sprouts [
21]. The vascular theory for laminitis proposes that any ischemia event may increase the proportion of apoptotic basal layer cells in the lamellae of laminitic horses as compared to non-laminitic horses [
20]. Apoptosis has been reported to trigger cell death following ischemia-reperfusion injuries in humans [
22], rats [
23], rabbits [
24] heart muscle tissue, and rat liver [
25]. In these species, it was noticed that apoptosis occurs during the reperfusion period. In this period, oxygen free radicals are formed that can cause apoptosis in different cells [
25]. Recently, it was reported that the death of basal layer cells takes place after 34 to 72 hours of a laminitis-inducing dose of carbohydrates [
26]. Moreover, Mungall and Pollitt (2002) revealed that MMP activity can also be initiated by ischemia-reperfusion events [
27]. Thus, the existence and activation of MMPs in the lamellar tissue of laminitic dairy cows may occur as a consequence of an ischemia event and start an apoptotic cascade [
28]. Even though hundreds of articles have been published on the role of apoptosis in both biological and pathological processes in different species, there is a scarcity of data on apoptosis in dairy cow hooves.
To the best of our understanding, no studies on the apoptotic status in the laminar tissue of OF-induced bovine laminitis have been completed. Thus, the present study focused largely on determining the apoptotic status of laminar tissue in OF dairy cows at the gene and protein levels, as well as the identification of potential treatment avenues for this sickness. We speculated that oral oligofructose challenge induces apoptosis in the laminar tissue of laminitis dairy cow.
2. Materials and Methods
2.1. Experimental Animals
In this experiment, 12 healthy non-pregnant Chinese Holstein cows, which have normal locomotion [
29], lacking a history of claw horn lesions were utilized. Cows body weight ranged from 335-403 kg (379.71±19.87 kg), age ranged from 18-26 months (20.67±3.01 mo) and BCS [
30] ranged from 2.7-3.3 (3.00±0.23). All the cows used in this experiment bought from the Qingxi dairy farm in Xiangfang District Harbin, P.R. China. The experimental cows were housed in the University animal hospital shed with a rubber floor thirty days before experimental trial. The cows were provided with adequate supply of clean drinking water and mixed forages ad libitum. The experimental cows were monitored for their hoof temperature, body temperature, and blood pressure, and walk for 5 min, daily to evaluate their health. At the end of the acclimation period, there was no discomfort in the experimental animals earlier the experiment was started.
2.2. Experimental Design and Treatment
Twelve dairy cows were assigned into two groups, comprising OF-treated group (n = 6) and Control group (n = 6). 17g/kg BW of oligofructose (Bailong Biotech, Inc., Dezhou, Shandong, China) dissolved in 20 mL/kg BW of deionized water was given to the OF-treated group, while the control group accepted 20 mL/kg BW of deionized water at 0 h via a stomach tube (length 2.2 m, diameter 25 mm) following the method represented by [
6,
7]. Oligofructose (5%) was given orally once daily for three days. Shortly before overloading the advanced oral dose of OF, cow were trained for the clinical examination.
Limping examination was evaluated at -72, 0, 6, 12, 18, 24, 36, 48, 60, and 72 h. During this period, cows were directed to walk and trot by hand in a straight line, and then to turn in a small circle on the same ground of Animal Hospital, Northeast Agricultural University Harbin, China. Each cow's limp scores were examined by five licensed veterinarians adopting the approach reported by [
29]. Once all licensed vets examined a score of ≥2, then cow was recommended lame. At every 6 h, all cows were allowed for the clinical examination including respiratory rate, heart frequency range, the rectal temperature, eating routine, and feces consistency, hoof coronary band, weight shift, diastolic blood pressure (electronic sphygmomanometer), hoof temperature, and rumen movements, hoof discomfort, rumen pH (pH meter; Benchtop; Mettler Toledo Inc., Switzerland). After 72 h of OF-overloading, a dose of 20 mg/kg of pentobarbital sodium and phenytoin sodium (Fatal-plus; 20 mg/kg IV) were overdosed to each cow by intravenous injection to be euthanized [
6,
7]. Considering cow welfare ethics, supportive therapy, including Ringer lactate (15 mL/kg of BW; Heping Animal Medicine Co., Ltd. Harbin, China) at 18 and 24 h and calcium borate (14 mg of Ga/mL; 1.4 mL/kg of BW; Heping Animal Medicine Co., Ltd. Harbin, China) at 18 h were presented following OF overload.
2.3. Laminar Tissue Sampling
After 72 h of OF overload, when the clinical signs of the cows in the OF group mimicked acute laminitis in dairy cows [
6,
7], the cows in the control and OF groups were slaughtered and hoof laminar tissue biopsies were obtained. Following the procedure outlined by [
8], the deceased animals' left hind claws were detached in 5-10 min, put in an iced pack, and instantly taken to the laboratory. Laminar tissue was exposed by cutting the hoof wall, and hoof capsule was isolated. Then, a laminar tissue was separated into small tissue sections (1-2 mm2). It was then instantly refrigerated in a liquid nitrogen and transported to storage at -80˚C. The rest hoof tissue was placed in a 10% formalin-phosphate buffer and fixed overnight (room temperature). The entire work was performed in ice. Proper gloves and sterile mask were utilized to prevent the tissue exposure. Remarkably, the tissue of the laminar wall is present inside hoof capsule and is tightly bound to it. The lamella wall specifically covers the entire axial, abaxial, and dorsal side of the claw, reaching 3–7 cm from the coronet to the sole corium. The unconnected lamella wall derived 2cm beneath the coronet includes the axial and abaxial aspects of the laminar wall tissue. Laminar wall tissue dimension commonly measures (6 x 3 cm) in length and width .
2.4. RNA Isolation and cDNA Synthesis
The total RNA was extracted from laminar tissue of 12 cows using the RNA Miniprep-Kit (Invitrogen, Carlsbad, USA) following the manufacturer's instructions. The tissue samples (100 mg) were homogenized with 1 mL of TRIzol reagent (Invitrogen, Carlsbad, USA). Then, it was subjected to a non-DNAse/RNAse centrifuge tube, and the total RNA without DNA, protein and isopropanol precipitates was taken out with chloroform and washed with 75% ethanol. The quality and quantity of the isolated total RNA were determined utilizing an ultranucleic acid protein testing kit. (NO-ONE, Gene Company, USA). Quality of each RNA samples was assessed by 1% agarose gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA). Then the extracted RNA samples were reduced to 1ug/uL by measuring optical density. 1ug of total RNA was extracted from each sample using the manufacturer's protocols for Prime-Script™ RT Kit (Takara, Dalian, Liaoning, China). Reverse transcription was done to get Complementary DNA (cDNA). For Realtime RT-qPCR analysis, the cDNA thus acquired was diluted 1:3 with DEPC water and preserved in -20˚C until further utilized. DEPC water was prepared from dH2O with DEPC and then autoclaved to take out DEPC.
2.5. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
In the present work, the primers used for identifying the genes which includes glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Bcl2, Bax, and P53, were designed from the Shanghai Sheng Gong Biotechnology, Co., Ltd. (BBI Life Sciences, Shanghai, China) (
Table 1). The efficiency of all primer sequences were determined by the Blast Computer Program from the (NCBI) National Center for Biotechnology Information database (Bethesda, Maryland, USA).
RT-qPCR was performed utilizing the Green chimeric florescence detection method of SYBR Premix Ex Taq TM ‖ Kit (Takara, Dalian, Liaoning, China) through with the Light Cycler 480 RT-PCR system (Roche, Germany). The PCR reaction mixture (20 μL final volume) comprises of 2 μL cDNA template and 18 µL of principal blend for the PCR. Then, PCR master mix composed of 6.4 uL DEPC water, 10 uL SYBR green florescence dye , and 1.6 uL miscellaneous primer solution (10 uM for each forward and reverse primer). The ultimate primer dosage volume consisted of 0.4 uM/uL. PCR conditions are as follows: pre-denaturation, 1 cycle, 95°C, 1 min; quantitative valuation, 40 cycles of 95°C 5s and 60°C 1 min; melting curve analysis, 95°C 5s, 60°C 1min, 95°C 1 cycle; cooling, 1 cycle at 50°C for 30s. The Ct values for the different genes were calculated by Light Cycler 480 software 2.0 (Roche, Germany) and the Abs Quant/Fit points approach. Using the △△Ct technique, GAPDH as the internal reference gene, the PCR efficiency of each gene was evaluated.
2.6. Western Blot
The 100 mg of preserved laminar tissue was intermingled to RIPA lysis buffer (Beyond Biotech, Shanghai, China). The main ingredient of RIPA buffer included 1% deoxycholate, 1% Triton X-100, and sodium lauryl sulfate (0.1%). After adding 10 μL of phenylmethylsulfonyl fluoride (protease inhibitor, Beyond Biotech, Shanghai, China), the tissue underwent grinding in a grinder (4°C, 4 min), before spinning (12,000 rcf/min, 15 min). BCA method was utilized to find out the concentration of protein. By utilizing SDS- PAGE, the concentration of protein was ascertained in the tissue sample. 10 μL of protein sample was poured into each well. The total concentration of protein was measured as 25 μg. After separation on 10% polyacrylamide gel, the desirable proteins were moved to nitrocellulose (NC) filter membranes applying the semi-dry technique (300 mA, 1.5 h). The membrane was blocked by shaking it in 5% nonfat milk in TBS (Tris-buffer saline) containing Tween-20 (0.1% Tween-20 TBS; TBST) for 2 h at ambient temperature. After blocking, the membranes were exposed to primary antibodies such as Bcl2 (1:750), Bax (1:1000), Bif-1 (1:1000), and caspase-3 (1:500), caspase-8 (1:1000), caspase-9/9p (1:1000), and β-actin at 1:2000 dilution, from (BIOSS Antibodies Beijing Biosynthesis Biotechnology Co., LTD. P.R. China), followed by incubation (4°C, 12 h), and thrice washing with TBST (15 min/washing). The membrane was subsequently diluted with secondary antibody (HRP-labeled goat anti-rabbit IgG) at a ratio of 1:5,000 in 1 x TBST (BBL Life Sciences, Shanghai, China) and incubated at room temperature for 2 hours, followed by shaking (2 hours, room temperature). NC membrane was thrice washed with TBST (15 min) using rocking. Finally, the strip was settled on the plate of the TANON 5200 exposure device, and the liquid was absorbed using filter paper. The ECL solution (Meilun Biotechnology Co., Ltd., Dalian, China) was subsequently diluted to evenly coat the protein bands after the drops. A photograph of the western blot with the proper exposure time was acquired. ImageJ software was utilized to find out the gray value of the protein in each gel, and the gray ratio of the desired protein band to the internal reference protein band was calculated to find out the relative expression of the desirable protein.
2.7. Immunohistochemistry
Immunohistochemistry was conducted to measure the expression level of Bax, and P53 in laminar tissue. Tissue samples were chopped into an appropriate size, followed by fixation (4% paraformaldehyde, 24 h), and sectioned and embedded. The dewaxing of sections was carried out by an overnight incubation in oven (80 °C), followed by immersion in H2O2 solution (3%) in the dark (10 min) for blockade of endogenous peroxidase, and repaired antigen in a pressure pan with sodium citrate solution. The segment were covered with BSA (20 min, room temperature), then overnight primed (4℃) with primary antibody (1:200, Bax; 1:200, P53 Novus Biologicals, USA), and treated with streptavidin- labeled streptavidin-labeled horseradish peroxidase (HRP) (30 min, room temperature). The segment were then glued with neutral glue, followed by staining with hematoxylin and 3,3′-Diaminobenzidine (DAB), and preserved in an oven. Finally, the stained tissue segment from each group were evaluated through a microscope, and calculated with Image-Pro Plus 6.0 software (Media Cybernetics, USA).
2.8. Statistical Analysis
Data analysis was completed utilizing GraphPad Prism software (Version 7.04, GraphPad software Inc., San Diego, CA, USA). The experimental data were analyzed with normal distribution. When assessing results from local histology investigations, differences across groups were analyzed by the independent student-t test and Bonferroni’s multiple comparison tests. Differences were assumed when P<0.05. All data were presented as mean ± SD.
3. Results
3.1. Clinical Manifestation of Dairy Cow Laminitis
All dairy cows of OF group displayed clinical signs of distinctive acute ruminal and systemic acidosis manifestations, including persistent profuse diarrhea, absence of dietary intake, in-appetence, depression, anorexia, swelled of carpal (tarsal) joints, inflamed hoof coronary band, altered weight displacement, raised heart rate, raised diastolic blood pressure, raised hoof temperature, raised body temperature, reduced respiration, and inattention of digital (toe) arteries, decreased rumen contraction, hoof pain, reduced rumen pH, intermittent fever, and lameness [
28,
31,
32,
33]. The cows of control group showed no symptoms of systemic illness. All these symptoms were the identical as those described by [
6,
7,
8]. During limping examination, clinical signs of laminitis were initially ascertained at 24 hours after OF overload and continued to change until a maximal limp score of 3–5 was observed at 60 h to 72 h [
33], which were in line with Danscher et al [
7], who confirmed acute laminitis at 60–120 h of OF-overload.
3.2. Apoptosis-Related Genes Expression in Laminar Tissue of Laminitis Dairy Cow
The expressions of apoptosis-related genes such as Bcl2, Bax, and P53 in control and OF groups are shown in
Figure 1. We observed that the expression level of Bcl-2 (
P<0.001) highly significantly reduced in the OF cows laminar tissue compared with the control cows. Similarly, the expression level of Bax (
P=0.0037) highly significantly enhanced (
P<0.01), while P53 (
P=0.011) significantly enhanced (
P<0.05) in the laminar tissue of OF group compared with the control group. These research results confirmed that enhanced or reduced expression of genes may be associated to severe apoptosis in damaged laminar tissue suggesting that apoptosis was induced in laminar tissue of OF cows.
3.3. Apoptosis-Related Proteins Expression in Laminar Tissue of Laminitis Dairy Cow
Apoptosis-related proteins expression such as Bcl2, Bax, Bif1, Caspase3, Caspase8, and Caspase9/9p in OF and control groups are shown in
Figure 2. The Bcl2 protein expression (
P=0.0008) was highly significantly decreased (
P<0.01), while the Bax (
P=0.0017) and Bif1 (
P=0.0092) proteins expression were highly significantly enhanced in the laminar tissue of OF group as compared with the the control group. However, the caspase3 (P=0.0141), caspase8 (
P=0.0276), and caspase9/9p (
P=0.0238) proteins expression significantly increased (
P<0.05) in the OF group's laminar tissue compared with the control group. These results confirmed that enhanced or reduced expression of proteins may be associated to apoptosis in afflicted cows.
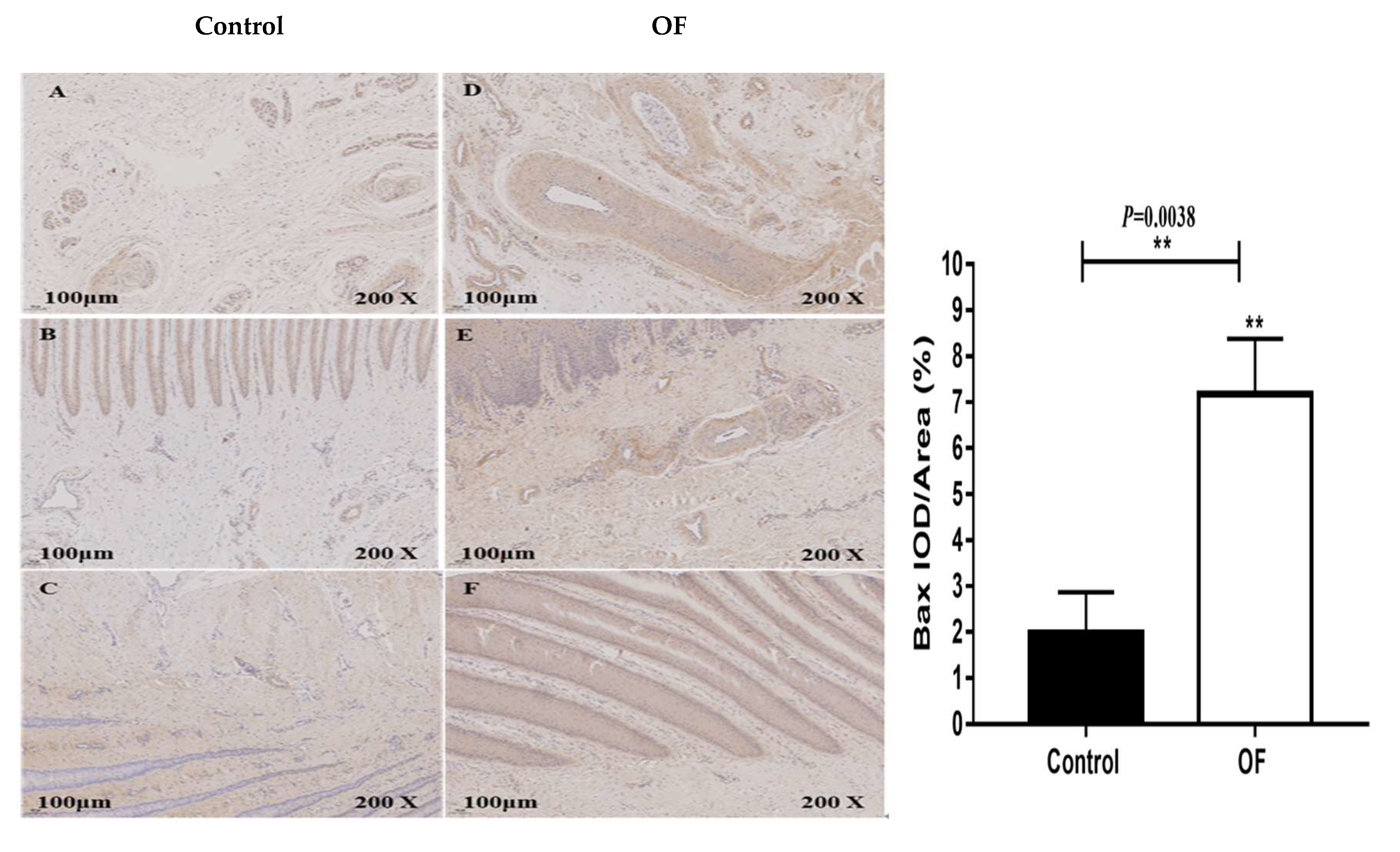
3.4. Immuno-Expression of Bax and P53 Proteins in Laminar Tissue of Laminitis Dairy Cow
The immunohistochemical results of Bax protein expression in the cows laminar tissue showed that the mean Bax staining in the cytoplasm of the OF group laminar tissue was 7.18 %, whereas it was 1.98% in the control tissue. Statistically, the expression of Bax protein was highly significantly increased in the cytoplasm of laminar tissue in the OF group than in the control group (
P=0.0038), as shown in
Figure 3. This may be due to severe apoptosis in the laminar tissue confirming that increase in Bax expression play important role in the laminar tissue damage of OF cows.
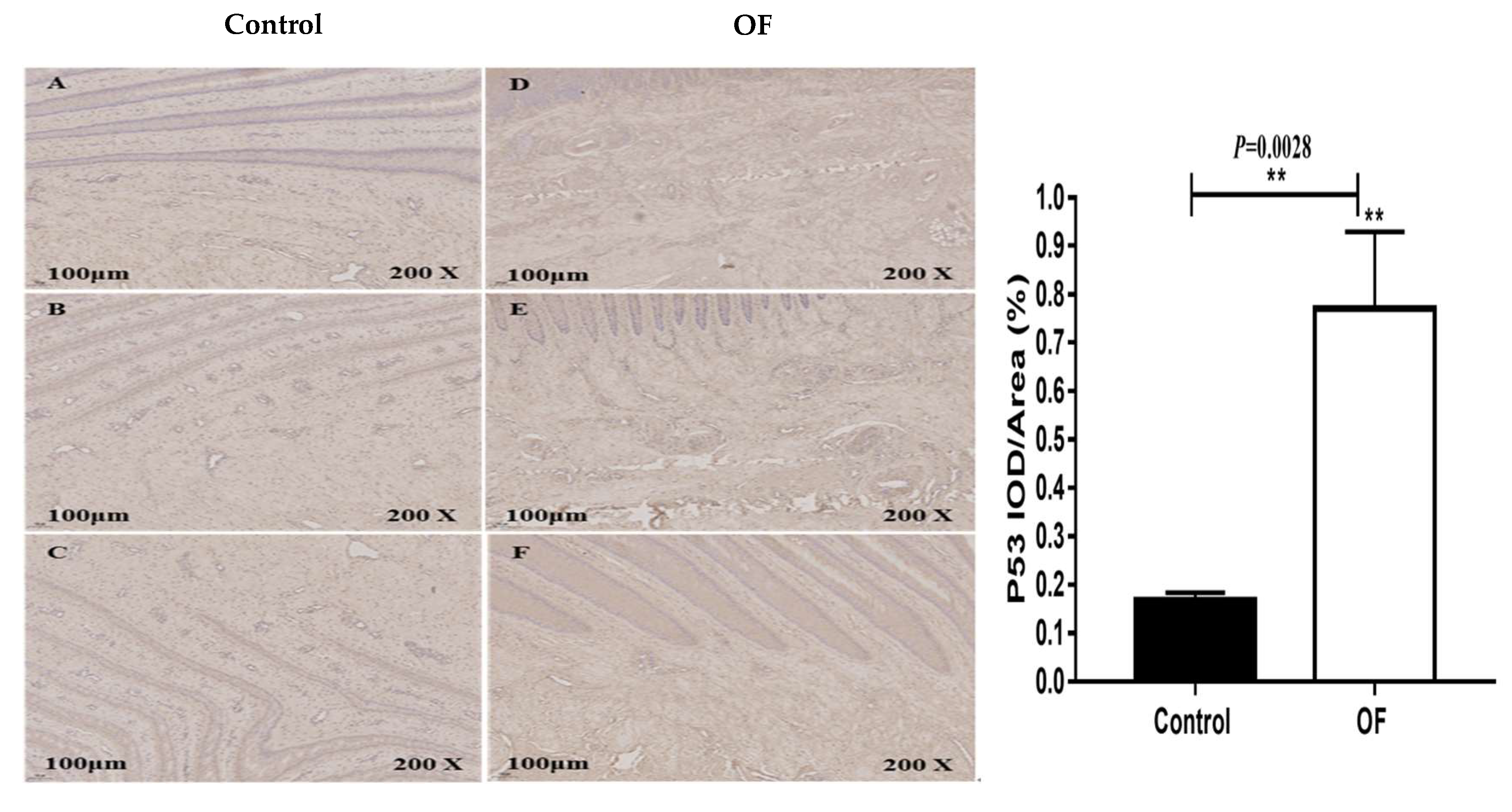
The immunohistochemical findings for the expression of P53 protein in the laminar tissue of cows showed that the mean P53 staining in the cytoplasm of the OF group laminar tissue was 0.77 %, whereas it was 0.17 % in the control tissue. Statistically, the expression of P53 protein was highly significantly increased in the cytoplasm of laminar tissue in the OF group than in the control group (P=0.0028), as shown in
Figure 4. This may be due to severe apoptosis in the laminar tissue of OF cows.
4. Discussion
The damaging effects of oligofructose (OF) overload in dairy cows has been widely investigated in acute rumen acidosis (ARA), laminitis and synovitis [
7,
34,
35]. Overconsumption of digestible carbohydrates leads to the alterations in the microbial balance and fermentation process. During acute rumen acidosis, lactate levels in the rumen juice was elevated and dissolved into systemic blood stream [
36]. In tandem with lactate, lipopolysaccharide (LPS) is believed to play an important role in the progression of rumen acidosis. LPS is expected to move into the circulation when experimentally elicited subacute ruminal acidosis (SARA) [
37,
38,
39]. These compounds trigger systemic inflammatory process as well as many organ damage such as laminitis, ruminitis, and synovitis, however the role of apoptosis in the dairy cow laminar tissue during the induction of OF-induced laminitis remains unknown. In our published data, research proved that bovine laminitis was successfully constituted using the OF-overload method, and OF cows attained clinical hallmarks of typical acute ruminal and systemic acidosis manifestation and obvious histological evidence in the laminar tissue, which includes BM injury and detachment, sinking of the epidermal lamellae, and changes to the basal cell configuration [
28,
31,
32]. We obtained an same portion of laminar tissue from our previous work and examined the apoptotic indicators at the gene and protein levels.
In the present work, the expression of apoptosis-associated genes, involving Bcl2, Bax, and P53; Bax, Bcl2, caspase 3, caspase 8, and caspase 9/9p proteins expressions; and the distribution of of Bax and P53 proteins positive cells in the laminar wall tissue were assessed to examine alterations in apoptosis activity in the cow's hoof with OF overload-induced laminitis. The present work found that OF overload-induced bovine laminitis caused an increase in Bax and P53 gene expression, a decline in Bcl2 gene expression, and an raise in Bax, Bif1, caspase 3, caspase 8, and caspase 9/9p protein expressions inside the laminar tissues. Nevertheless, the immunohistochemical examination of P53 and Bax discovered that the number of positive cells of P53 and Bax enhanced in the laminitic cows laminar tissue. This demonstrated that OF dairy cow caused an increase in apoptosis in the laminar tissue.
Apoptosis is programmed cell death that shows a main role in development, ageing, and homeostasis by regulating cell numbers in tissue [
40]. Tissue damage and dysfunction, however, are related to abnormal apoptosis. B-cell lymphoma 2 (Bcl2), an essential anti-apoptotic protein, has been documented to have a major impact on sustained cell survival by inhibiting apoptosis [
41], which has been highly significantly decreased in the OF-treated cows than the control cows. In a dynamic cell, Bcl2 generally stays linked to one of the pro-apoptotic associates, such as Bax, until stimulation of the stimulus of apoptosis occurs, and Bax is the protein that aids homo di/oligomerization generates pores in the mitochondrial membrane within the cell [
42]. Bax-interacting factor 1 (Bif-1) is another protein that connects mitochondrial morphological alterations to Bcl2-regulated programmed cell death [
43]. In response to particular apoptotic signals, a substantial proportion of Bif-1 binds Bax at the mitochondrial outer membrane despite the close linkage with Bax reimposition and cytochrome c discharge [
44]. Furthermore, Bif-1 overexpression enhances apoptosis, whereas Bif-1 deficiency abolishes the ubiquitination of Bax and inhibits the apoptotic process via numerous intrinsic death signals. As a result, it has been proposed that Bif-1 is an essential Bax activator [
44,
45]. Moreover, Bax and Bif-1 expression were noticeably increased in the OF-treated cows than in the control cows. This is in line with previous work showing a significant decrease in Bcl2 in SARA cows [
46], and milk cows with ketosis [
47]. Harris and Levine (2005) reported that P53 activation results in an organized cell cycle arrest, apoptosis, or cellular senescence [
48]. In the present work, the P53 mRNA and protein expression enhanced significantly in the OF-treated cows relative to the control cows. This is similar with recent research that indicated a rise in the expression of P53 in cows of thermal stress [
49] and in cows with milk that have ketosis [
47].
The cysteine-aspartic acid protease family includes caspases. When specific signals trigger caspases, the inactive zymogen state generates activating enzymes that initiate a cascade of events that degrades a series of proteins that enhance apoptosis. Factors that initiate apoptosis in caspase8 and caspase9 stimulate the downstream cascade of caspases which induce apoptosis. Furthermore, caspase3 is the primary apoptotic executor, and activation of this enzyme indicates irreversible apoptosis [
50,
51]. Current research has indicated that high-concentration feeding promotes the activation of caspase3, caspase8, and caspase9 in milk cows with subacute rumen acidosis in the rumen epithelium and mammary epithelial cells [
46,
52]. In a previous study, major differences in caspase3 and caspase9 expressions were also found in milk cows with ketosis [
47]. In this study, OF-treated cows showed significantly higher caspase3, caspase8, and caspase9/9p protein expressions in the laminar tissue, highlighting that dairy cow laminitis induces apoptosis.
5. Conclusions
In conclusion, the impaired activation of Bcl2 in laminar wall may lead to laminar tissue damage. Thus, our findings indicate that the imbalanced state of apoptosis at the gene and protein level may be a typical cause for epidermal attachment failure which serves a key role in the pathogenesis of dairy cow laminitis, which directly or indirectly results in the damage of laminar tissue in dairy cows. Therefore, the obtained results in this study may offer theoretical clues and a base of knowledge at core for better prevention as well as management strategies related to laminitis among dairy cows. Nonetheless, further large-scale gene and protein-level studies are required to fully explain the pathogenesis of laminitis in dairy cows.
Author Contributions
M.A.H., H.B.W. conceived and conducted the experiments, analyzed the data, and wrote the manuscripts. J.F.D., L.T. and Z.X.H. performed some part of experiment. M.A.H. and J.F.D. evaluated the part of data. H.B.W. and Z.J.T. designed the experiments and participate in the conception and revised the manuscript. H.B.W. and Z.J.T. review and editing the manuscript. All authors read and approved the final this manuscript.
Institutional Review Board Statement
This study and all the procedures were approved in accordance with the rules and regulations of Animal Ethical Review Committee (Approval No: SRM-13) at the Department of Veterinary Surgery, College of Veterinary Medicine, Northeast Agricultural University Harbin, P.R. China.
Informed Consent Statement
All animals in this study were purchased from the Qingxi dairy farm in Xiangfang District Harbin, P.R. China. Owner of dairy farm were agreed for animal to participate in research study. He was agreed for animals to collect tissue samples and any further educational purposes. All the procedures were followed by Animal Ethical rules of College of Veterinary Medicine, Northeast Agricultural University Harbin, China.
Data Availability Statement
All data generated or analyzed during the study are included in this published article.
Acknowledgments
This work was supported by the National Key R&D Program of China (Project No.2017YFD0502200) and Heilongjiang Provincial Funding for National Subjects (GX18B023). The funders had no role in the study’s design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zhang, X.; Ding, J.; Li, Y.; Song, Q.; Li, S.; Hayat, M.A.; Zhang, J.; Wang, H. The changes of inflammatory mediators and vasoactive substances in dairy cows’ plasma with pasture-associated laminitis. BMC Vet. Res. 2020, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.A. Clinical, morphological and experimental studies of laminitis in cattle. Acta Vet. Scand. 1963, 4, 1–304. [Google Scholar]
- Greenough, P.R. Bovine laminitis and lameness: a hands on approach. Els. Health Sci. 2007. [Google Scholar]
- Randall, L.V.; Green, M.J.; Huxley, J.N. Use of statistical modelling to investigate the pathogenesis of claw horn disruption lesions in dairy cattle. Vet. J. 2018, 238, 41–48. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, D.; van Arendonk, J.A.M.; Vallee, A.A.A.; Bovenhuis, H. Genetic parameters for claw disorders and the effect of preselecting cows for trimming. J. Dairy Sci. 2013, 96, 6070–6078. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Pollitt, C.C.; Van-Eps, A.W.; Milinovich, G.J.; Trott, D.J.; Wattle, O.; Andersen, P.H. Acute bovine laminitis: A new induction model using alimentary oligofructose overload. J. Dairy Sci. 2004, 87, 2932–2940. [Google Scholar] [CrossRef]
- Danscher, A.M.; Enemark, J.M.D.; Telezhenko, E.; Capion, N.; Ekstrom, C.T.; Thoefner, M.B. Oligofructose overload induces lameness in cattle. J. Dairy Sci. 2009, 92, 607–616. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Wattle, O.; Pollitt, C.C.; French, K.R.; Nielsen, S.S. Histopathology of oligofructose-induced acute laminitis in heifers. J. Dairy Sci. 2005, 88, 2774–2782. [Google Scholar] [CrossRef]
- Mendes, H.M.; Casagrande, F.P.; Lima, I.R.; Souza, C.H.; Gontijo, L.D.; Alves, G.E.; Vasconcelos, A.C.; Faleiros, R.R. Histopathology of dairy cows' hooves with signs of naturally acquired laminitis. Pesquisa. Vet. Brasil. 2013, 33, 613–619. [Google Scholar] [CrossRef]
- Sousa, R.D.S.; Oliveira, F.L.C.; Dias, M.R.B.; Minami, N.S.; Amaral, L.; Santos, J.A.A.D.; Barreto, J.R.A.; Minervino, A.H.H.; Ortolani, E.L. Characterization of oligofructose-induced acute rumen lactic acidosis and the appearance of laminitis in zebu cattle. Animals. 2020, 10, 429. [Google Scholar] [CrossRef]
- Leise, B.S.; Faleiros, R.R.; Watts, M.; Johnson, P.J.; Black, S.J.; Belknap, J.K. Laminar inflammatory gene expression in the carbohydrate overload model of equine laminitis. Equine. Vet. J. 2011, 43, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Dern, K.; van-Eps, A.; Wittum, T.; Watts, M.; Pollitt, C.; Belknap, J. Effect of continuous digital hypothermia on lamellar inflammatory signaling when applied at a clinically-relevant timepoint in the oligofructose laminitis model. J. Vet. Int. Med. 2018, 32, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Raber, M.; Lischer, C.J.; Geyer, H.; Ossent, P. The bovine digital cushion – a descriptive anatomical study. Vet. J. 2004, 167, 167,258–264. [Google Scholar] [CrossRef] [PubMed]
- Boosman, R.; Nemeth, F.; Gruys, E. Bovine laminitis: clinical aspects, pathology and pathogenesis with reference to acute equine laminitis. Vet. Q. 1991, 13, 163–171. [Google Scholar] [CrossRef]
- Vermunt, J. “Subclinical” laminitis in dairy cattle. N. Z. Vet. J. 1992, 40, 133–138. [Google Scholar] [CrossRef]
- Alvergnas, M.; Strabel, T.; Rzewuska, K.; Sell-Kubiak, E. Claw disorders in dairy cattle: Effects on production, welfare and farm economics with possible prevention methods. Livest. Sci. 2019, 222, 54–64. [Google Scholar] [CrossRef]
- Potterton, S.L.; Bell, N.J.; Whay, H.R.; Berry, E.A.; Atkinson, O.C.; Dean, R.S.; Main, D.C.; Huxley, J.N. A descriptive review of the peer and non-peer reviewed literature on the treatment and prevention of foot lameness in cattle published between 2000 and 2011. Vet. J. 2012, 193, 612–616. [Google Scholar] [CrossRef]
- Nagata, S.; Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017, 17, 333–340. [Google Scholar] [CrossRef]
- Catunda, A.P.; Alves, G.E.; Leme, F.O.; Carvalho, A.M.; Leise, B.S.; Johnson, P.J.; Faleiros, R.R. Apoptosis in epithelial cells and its correlation with leukocyte accumulation in lamellar tissue from horses subjected to experimental sepsis-associated laminitis. Res. Vet. Sci. 2021, 136, 318–323. [Google Scholar] [CrossRef]
- Faleiros, R.R.; Stokes, A.M.; Eades, S.C.; Kim, D.Y.; Paulsen, D.B.; Moore, R.M. Assessment of apoptosis in epidermal lamellar cells in clinically normal horses and those with laminitis. Am. J. Vet. Res. 2004, 65, 578–585. [Google Scholar] [CrossRef]
- Mungall, B.A.; Kyaw-Tanner, M.; Pollitt, C.C. In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet. Microbiol. 2001, 79, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Abbi, R.; Quaini, F.; Kajstura, J.; Cheng, W.; Nitahara, J.A.; Quaini, E.; Di Loreto, C.; Beltrami, C.A.; Krajewski, S.; Reed, J.C. Apoptosis in the failing human heart. New England J. Med. 1997, 336, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Fliss, H.; Gattinger, D. Apoptosis in ischemic and reperfused rat myocardium. Circ. Res. 1996, 79, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Burleson, K.O.; Kloner, R.A.; Babior, B.M.; Engler, R.L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Invest. 1994, 94, 1621–1628. [Google Scholar] [CrossRef]
- Kohli, V.; Selzner, M.; Madden, J.F.; Bentley, R.C.; Clavien, P.A. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver1, 2. Transplant. 1999, 67, 1099–1105. [Google Scholar] [CrossRef]
- Morgan, S.J.; Hood, D.M.; Wagner, I.P.; Postl, S.P. Submural histopathologic changes attributable to peracute laminitis in horses. Ame. J. Vet. Res. 2003, 64, 829–834. [Google Scholar] [CrossRef]
- Mungall, B.A.; Pollitt, C.C. Thermolysin activates equine lamellar hoof matrix metalloproteinases. J. Comp. Pathol. 2002, 126, 9–12. [Google Scholar] [CrossRef]
- Ding, J.; Shi, M.; Wang, L.; Qi, D.; Tao, Z.; Hayat, M.A.; Liu, T.; Zhang, J.T.; Wang, H. Gene Expression of Metalloproteinases and Endogenous Inhibitors in the Lamellae of Dairy Heifers With Oligofructose-Induced Laminitis. Front. Vet. Sci. 2020, 7, 597827. [Google Scholar] [CrossRef]
- Sprecher, D.E.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenol. 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Ding, J.; Li, S.; Jiang, L.; Li, Y.; Zhang, X.; Song, Q.; Hayat, M.A.; Zhang, J.T.; Wang, H. Laminar inflammation responses in the oligofructose overload induced model of bovine laminitis. Front. Vet. Sci. 2020, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.A.; Ding, J.; Li, Y.U.; Zhang, X.; Zhang, J.I.; Li, S.; Wang, H.B. Determination of the activity of selected antioxidant enzymes during bovine laminitis, induced by oligofructose overload. Med. Weter. 2020, 76, 289–295. [Google Scholar] [CrossRef]
- Hayat, M.A.; Ding, J.; Zhang, X.; Liu, T.; Zhang, J.; Bokhari, S.G.; Akbar, H.; Wang, H. Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows. Animals. 2023, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.; Carretta, M.D.; Alarcón, P.; Conejeros, I.; Gallardo, D.; Hidalgo, A.I.; Tadich, N.; Cáceres, D.D.; Hidalgo, M.A.; Burgos, R.A. Oxidative response of neutrophils to platelet-activating factor is altered during acute ruminal acidosis induced by oligofructose in heifers. J. Vet. Sci. 2014, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Hidalgo, A.I.; Manosalva, C.; Cristi, R.; Teuber, S.; Hidalgo, M.A.; Burgos, R.A. Metabolic disturbances in synovial fluid are involved in the onset of synovitis in heifers with acute ruminal acidosis. Sci. Reports. 2019, 9, 5452. [Google Scholar] [CrossRef]
- Snyder, E.; Credille, B. Diagnosis and treatment of clinical rumen acidosis. Vet. Clin. N. Am. Food A. 2017, 33, 451–461. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.; Plaizier, J. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef]
- Abaker, J.A.; Xu, T.L.; Jin, D. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Li, X.; Guan, Y.; Wang, Y.; Yuan, X.; Sun, G.; Wang, Z.; Li, X. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, J.; Zhou, H.; Tan, W.; Lin, L.; Yang, J. Programmed cell death in sepsis associated acute kidney injury. Front. Med. 2022, 9, 883028. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, L.; Hou, L.; Deng, H.; Luan, S.; Liu, D.; Huang, M.; Zhao, L. Trends in targeting Bcl-2 anti-apoptotic proteins for cancer treatment. Europ. J. Med. Chem. 2022, 232, 114184. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 59,1693s–1700s. [Google Scholar] [CrossRef] [PubMed]
- Suen, D.F.; Norris, K.L.; Youle, R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Karbowski, M.; Yamaguchi, H.; Kazi, A.; Wu, J.; Sebti, S.M.; Youle, R.J.; Wang, H.G. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol. Cell. Biol. 2005, 25, 9369–9382. [Google Scholar] [CrossRef]
- Cuddeback, S.M.; Yamaguchi, H.; Komatsu, K.; Miyashita, T.; Yamada, M.; Wu, C.; Singh, S.; Wang, H.G. Molecular cloning and characterization of Bif-1: a novel Src homology 3 domain-containing protein that associates with Bax. J. Biol. Chem. 2001, 276, 20559–20565. [Google Scholar] [CrossRef]
- Dai, H.; Ma, N.; Chang, G.; Aabdin, Z.U.; Shen, X. Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Animal Biotechnol. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Du, X.; Chen, L.; Huang, D.; Peng, Z.; Zhao, C.; Zhang, Y.; Zhu, Y.; Wang, Z.; Li, X.; Liu, G. Elevated apoptosis in the liver of dairy cows with ketosis. Cellular Physiol. Biochem. 2017, 43, 568–578. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The p53 pathway: positive and negative feedback loops. Oncogene. 2005, 24, 2899–2908. [Google Scholar] [CrossRef]
- Somal, A.; Aggarwal, A.; Upadhyay, R.C. Effect of thermal stress on expression profile of apoptosis related genes in peripheral blood mononuclear cells of transition Sahiwal cow. Iranian J. Vet. Res. 2015, 16, 16,137–141. [Google Scholar]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Cur. Opi. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Deng, C.; Li, J.; Li, L.; Sun, F.; Xie, J. Effects of hypoxia ischemia on caspase-3 expression and neuronal apoptosis in the brain of neonatal mice. Exp. Therap. Med. 2019, 17, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- ul Aabdin, Z.; Cheng, X.; Dai, H.; Wang, Y.; Sahito, B.; Roy, A.C.; Memon, M.A.; Shen, X. High-concentrate feeding to dairy cows induces apoptosis via the NOD1/Caspase-8 pathway in mammary epithelial cells. Genes. 2020, 11, 107. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).