1. Introduction
Transcatheter edge-to-edge mitral valve repair (Mitral TEER) has emerged as an alternative treatment option for patients who are not eligible for cardiac surgery. Although
Mitral TEER is less effective at reducing mitral regurgitation (MR) compared to surgery, the procedure demonstrated superior safety and similar clinical improvement [
1].
Prior to and after Mitral TEER, conventional echocardiography is routinely used for assessment of left ventricle and atrium volumes, along with mitral regurgitation grade [
2,
3,
4]. Speckle tracking echocardiography, a novel imaging modality for assessing myocardial mechanics such as strain, has been shown to have clinical prognostic value in patients with MR [
5,
6]. In a recent study by the COAPT group, improved left atrial (LA) strain at the 6-month follow-up was associated with subsequently lower rates of the composite endpoints of all-cause mortality or heart failure hospitalization, both after TEER and GDMT alone [
7]. Lower pre-TEER left ventricular (LV) global longitudinal strain was shown to be associated with a worse prognosis after TEER [
8]. However, LA and LV strain is not routinely assessed before and after Mitral TEER procedure
Studies have shown that after successful Mitral TEER, changes in echocardiographic parameters are associated with improved outcomes, including left ventricular reverse remodeling (LVRR), defined as a 10% reduction in left ventricular end-diastolic volume (LVEDV), which is associated with LA dimension reduction and improvement in heart failure symptoms and survival [
9,
10]. Indeed, speckle tracking echocardiography evaluating both myocardial deformation (percentage of change in myocardial length and strain) and volumes throughout the cardiac cycle [
11], may improve the assessment of the left atrial remodeling and function before and after
Mitral TEER.
Previous studies focused on LA reverse remodeling, following Mitral TEER by assessing left ventricle and atrium volumes [
2,
4,
12]. However, less is known regarding LA and LV strain changes in the early phase after the procedure. The objective of the study was to assess the effect of Mitral TEER on LA and LV strain early after TEER procedure.
2. Materials and Methods
2.1. Study Design
An observational retrospective study was conducted, using patients’ charts and imaging studies from Poriya Medical Center's (PMC) ongoing Mitral TEER registry.
The study was approved by the Ethical Review Board of Poriya Medical Center and complies with the guidelines of the Declaration of Helsinki. All patients included in this study provided informed consent.
2.2. Patient Population
In total, 109 patients had undergone Mitral TEER between March 2015 and February 2022 at PMC. The patients were assessed by a multidisciplinary heart team and were referred to Mitral TEER due to high surgery risk. All patients were diagnosed with moderate-severe MR and remained symptomatic despite optimal medical therapy. For patients who underwent a repeated Mitral TEER, we included only the first procedure in the analysis. Patients who underwent a combined procedure of mitral and tricuspid clipping, had either missing or low image quality (inability to track LV or LA segments, foreshortened LA views – in either pre procedure or discharge studies), and those with missing images were excluded, leaving 44 patients available for analysis. Patient follow up for all-cause mortality was 511days (333 to 793, 95% Confidence interval).
2.3. Procedure
Mitral TEER was performed per clinical indications after assessment by a cardiologist. All procedures were performed with the Mitraclip® device (Abbott Vascular, Santa Clara, CA, USA), according to a standard technique. [
1] The procedure was performed under general anesthesia, with the imaging guidance of fluoroscopy and 2-dimensional (2D) and 3-dimensional (3D) TEE.
2.4. Two-Dimensional and Doppler Echocardiography
2D-TTE were performed prior to the Mitral TEER and again at discharge. Studies were done by experienced sonographers on various machine systems (GE Vivid E9, GE Medical Systems, Milwaukee, WI, USA; Philips Epiq7CVx, Philips Medical System, Andover, MA, USA; Siemens Acuson SC2000 PRIME, Siemens Medical Solutions, Malvern, PA, USA). Interpreting echocardiographers were not blinded to the clinical diagnosis and Mitral TEER or post procedural course.
2.5. Tissue Strain Echocardiography
The LA and LV measurements were retrospectively analyzed, using the software eSie VVI, Siemens Medical System, Mountain View, CA, USA. Acquired 4-chamber, 3 chamber and 2-chamber cine images were uploaded to the software. By the supervision of guiding echocardiographer (SC), the myocardial mechanics analysis was reviewed and approved. The endocardial region of interest was manually marked. The cardiac cycle was determined by the onset of R wave for LA strain and volume curves. Biplane LA function was assessed, including reservoir parameters (maximal and minimal volumes, maximal strain) as half of the patients were in atrial fibrillation lacking LA booster function. Biplane LV volumes were assessed in a similar fashion using 4-chamber, and 2-chamber views (bi plane volumes). LV longitudinal strain was measured from the Acquired 4-chamber, 3 chamber and 2-chamber views.
2.6. Statistical Analysis
The data was analyzed using SPSS software, Version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous data with normal distribution was reported as the mean and one standard deviation (SD) and compared using dependent samples t-tests. Continuous variables without a normal distribution were reported as medians and interquartile ranges and were analyzed using a Mann-Whitney U test. Categorical variables were reported as absolute numbers and percentages and were compared using the Chi-Square tests. A 2-sided p-value of ≤ 0.05 was considered statistically significant.
3. Results
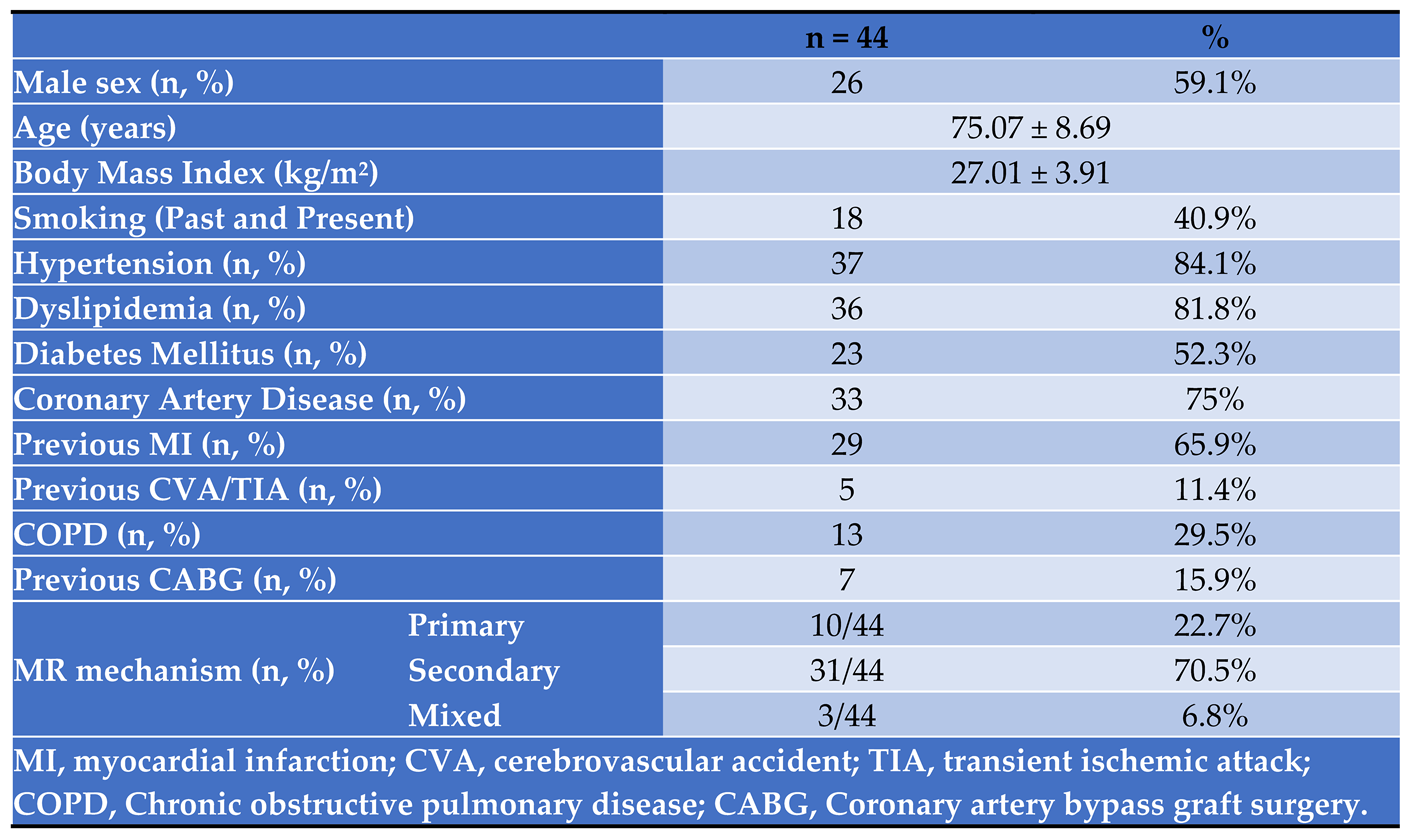
In total, 44 patients who underwent Mitral TEER procedure were included in the analysis. Their baseline characteristics are shown in
Table 1. All the patients had multiple comorbidities including coronary risk factors, 75% had coronary artery disease, and a third had chronic lung disease.
3.1. TEER Procedure Results and 2D-Doppler Echocardiography
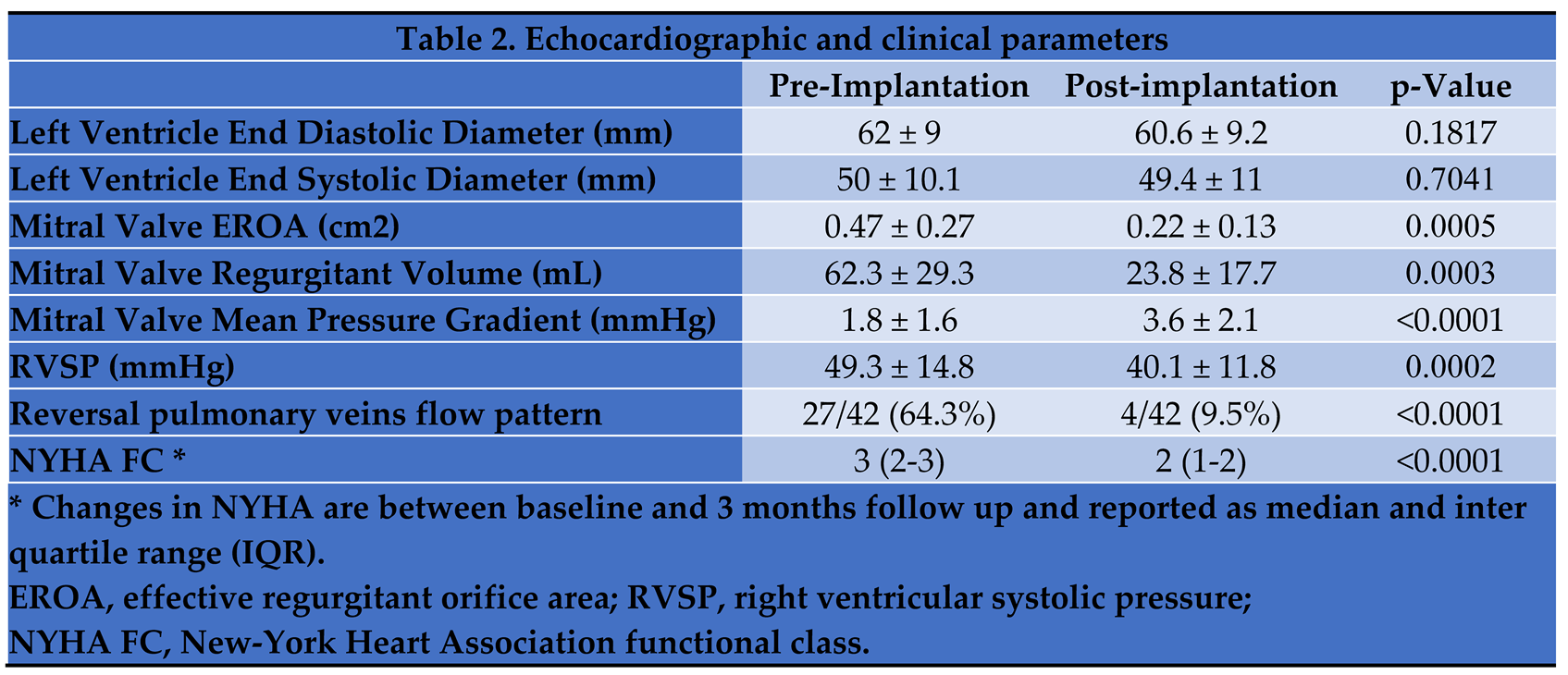
Following Mitral TEER, the effective regurgitant orifice area decreased significantly, while mitral valve mean pressure gradient was higher compared to baseline
Table 2. Intraprocedural hemodynamic measurements showed a decrease in LA peak V wave from 30 ± 13 to 21 ± 9 mmHg (p = 0.0023). At 3 months follow-up the NYHA functional class dropped by 1 point compared to assessment at baseline.
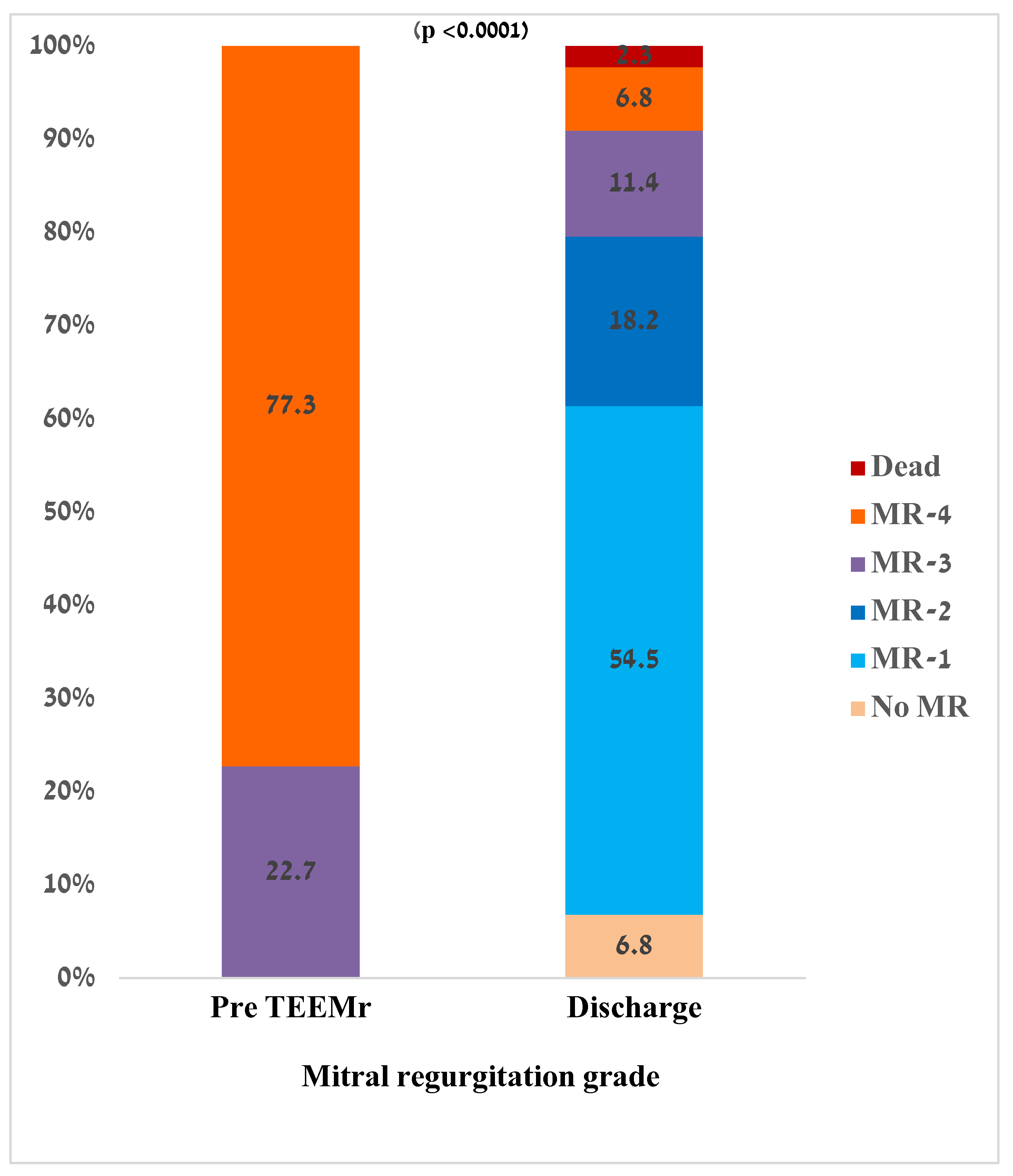
Pre procedural MR severity and residual MR severity at discharge are presented in
Figure 1. Following Mitral TEER, MR severity at discharge significantly decreased compared to pre procedure values (1 (1-2) vs 4 (4-4), p < 0.0001). While before Mitral TEER procedure all the patients had significant MR (MR-3 or MR-4), approximately 80% of patients were discharged with MR-2 or less. There was no relation between MR grade decrease and the MR mechanism (primary vs secondary, p = 0.825).
3.2. Myocardial Mechanics – LA and LV Strain and Volumes
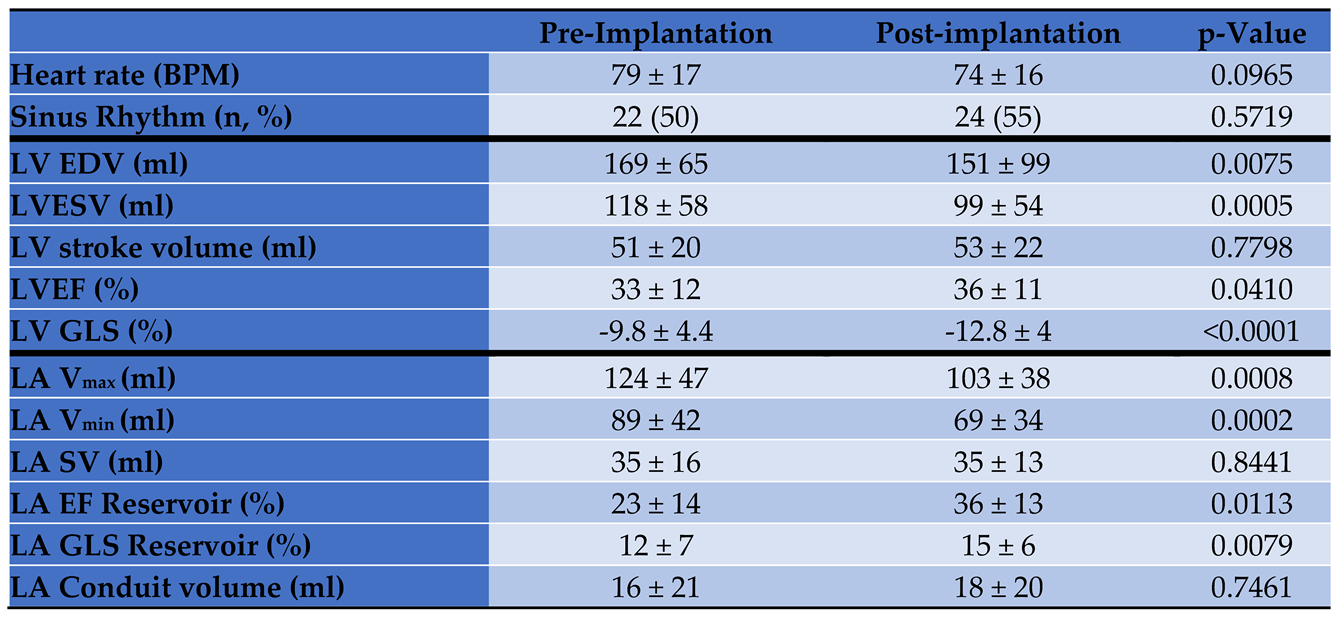
Left ventricular and left atrial myocardial strain and volume assessments, at baseline and prior to discharge 24-48 hours post Mitral TEER, are presented in
Table 3. Patients with secondary MR had similar strains and volumes at baseline. LV end diastolic and end systolic volume decreased by 11%, and 16%, respectively while LV stroke volume did not change. LV GLS showed a significant 30% improvement after Mitral TEER (p < 0.001). Similarly, LA maximal and minimal volumes were reduced by 17% and 22.5% respectively, while LA reservoir stroke volume did not significantly change (p = 0.8441). At discharge, LA GLS reservoir improved by 25% after Mitral TEER (14.7 ± 6.4 and 12.2 ± 7, p = 0.0079). The extent of changes in volume and strain did not correlate with the MR mechanism.
4. Discussion
Our study investigated the early changes in LA strain parameters immediately after Mitral TEER. The main finding of the study is that following successful Mitral TEER both left chambers decrease in size and improve their mechanics – while their respective stroke volumes remain unchanged. These changes occurred very early after TEER.
Mitral regurgitation results in LA and LV remodeling, which are associated with a poor prognosis and an increased risk of cardiac death [
13,
14]. Following successful Mitral TEER, LV reverse remodeling is associated with LV strain changes, LA dimension reduction and improvement in heart failure symptoms and survival [
15,
16,
17,
18]. While most studies focused on LV strain following Mitral TEER [
15,
16,
17,
18], only a few studies have reported the LA strain changes, and importantly the immediate changes were not reported previously.
4.1. LA Function Post Mitral TEER
Previous studies have demonstrated that after successful reduction of degenerative MR severity, LA peak positive strain decreased significantly in patients with normal/high baseline strain, when treated with either Mitral TEER or mitral valve surgical repair at 12 months follow-up [
19]. In comparison, in our study, LA GLS reservoir was higher at discharge compared to pre-Mitral TEER values. However, while all the patients, in the former study, suffered from primary MR, in our study the majority of the patients (70.5%) suffered from secondary MR with very different pathophysiology, clinical course, and prognosis [
19]. Additionally, the researchers found a significant decline of LA strain that was more marked in surgical patients than in Mitral TEER patients, while we studied only patients who underwent Mitral TEER. Surgical MVR requires cutting the LA open and is in many cases accompanied with a MAZE procedure for treatment of atrial fibrillation, both of which may hamper LA function. However, another study evaluating LA remodeling, reported that compared to baseline LA reservoir strain improved significantly after Mitral TEER, along with significant improvements in three-dimensional (3D) minimum LA volume index and maximum LA volume index [
19]. It is important to note that in that study the follow-up was performed at 12-month post procedure and 8% of the patients included in the study suffered from primary MR. In our study, we assessed LA strain indices early within post-procedural index hospitalization, and more than ¼ of the patients suffered from either primary or mixed etiology MR.
The LA reservoir phase is essential for LV filling because the energy stored by the LA during ventricular systole is released after mitral valve opening, greatly contributing to LV stroke volume, a mechanism that depends on LA compliance, which is altered in chronic MR patients [
20]. Reducing MR severity may decrease preload and LA expansion, improving LA compliance. In our study we found a significant early improvement in LA GLS reservoir, at discharge (after 24-48 hours). Additionally, compared with baseline, LA minimum and maximum volumes were reduced without a significant change in LA stroke volume, further demonstrating the early changes in the LA after TEER. As a result of the MR reduction, the backward flow into the LA decreases, subsequently decreasing LA minimal and maximal volumes. Since both minimum and maximum LA volumes are lower post-Mitral TEER, the LA stroke volume remains the same. Similar results regarding LA volumes were described by other Mitral TEER studies [
19,
21].
4.2. LV Function Post Mitral TEER
LV function was assessed after Mitral TEER implantation in a few studies. Following Mitral TEER, a previous study reported no change at all in LV GLS one year after the procedure [
22]. Furthermore, one study described a decrease in LV GLS compared to baseline [
15]. In this study LV and LA longitudinal strain worsened only in patients that had an increase in N-terminal pro brain natriuretic peptide (NT-proBNP) after Mitral TEER, suggesting an increase of myocardial wall stress. Cimino et al. concluded that MR reduction results in improvement of LV GLS, only if reverse remodeling occurred. Those patients with LV reverse remodeling and LV GLS improvement had better baseline parameters. [
16] Another study showed LV and RV strain improvement after clip implantation as well as lower post-procedural LV strain values in patients with worse preexisting RV function [
17]. Six-month 3D LV GLS was correlated significantly with improvements in NYHA functional class and 6-min walk distance. Early improvements, at discharge, were seen in 2D LV longitudinal strain and in 3D LV longitudinal strain. Our study demonstrated similar results and a very early improvement in LV GLS. Post clip implantation, the volume-overload is reduced and therefore wall stress is ameliorated, manifested by better LV function [
18]. The changes of LV may also explain the early LA remodeling, since during systole and early diastole, LA expansion and shortening on longitudinal axis are primarily influenced by movement of the valvular plane. Therefore, LA stain may reflect the longitudinal LV systolic function in addition to LA intrinsic function [
21]. Along with the strain changes, we found no significant change in the overall stroke volume of the LV, although there was significant decrease in MR degree and LA volume. This may be explained by the MR reduction, which causes an increase in the forward stroke volume with a concomitant decrease in the backward flow into the LA whereas total stroke volume remains unchanged significantly.
Chamber size, wall stress and pressures are interrelated as depicted in the LaPlace theorem from 1806 and Woods application in 1892 [
23]. Generally, wall stress increases as the chamber size (radius) increases [
24]. As previously demonstrated, myocardial wall stress increases oxygen consumption [
25,
26,
27]. Thus, improved myocardial physiology, with potentially reduced oxygen demand for the ejection of similar volumes at rest, may explain the improvement in dyspnea and effort tolerance in patients with severe MR after successful TEER.
4.3. Study Limitations
Since the study was conducted in a single center, we had a relatively small number of patients. Therefore, a larger sample size is necessary for validating our results. Another limitation of our study is that LA strain measurement requires a good echocardiographic imaging, and we had to exclude from our study patients with low echocardiographic quality and it may affect the significance of our results. Both sinus rhythm and atrial fibrillation patients were included in the study and further investigation regarding LA strain differences between those two groups is needed, after clip implantation.
5. Conclusions
A successful Mitral TEER leads to early LV and LA reverse remodeling, manifesting as a functional improvement, which can be detected very early after the procedure by the LV and LA strain study. Those immediate changes may further contribute to our understanding of the conditions associated with improved functional and clinical course following Mitral TEER.
References
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; Loghin, C.; Trento, A.; Skipper, E.R.; Fudge, T.; Letsou, G.V.; Massaro, J.M.; Mauri, L. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Maslow, A.D.; Poppas, A. Primary mitral valve regurgitation: Update and review. Global Cardiology Science & Practice 2017, 2017, e201703. [Google Scholar] [CrossRef]
- Izumi, S.; Miyatake, K.; Beppu, S.; Park, Y.D.; Nagata, S.; Kinoshita, N.; Sakakibara, H.; Nimura, Y. Mechanism of mitral regurgitation in patients with myocardial infarction: a study using real-time two-dimensional Doppler flow imaging and echocardiography. Circulation 1987, 76, 777–785. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; O'Gara, P.T.; Rigolin, V.H.; Sundt, T.M., 3rd; Thompson, A.; Toly, C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Namazi, F.; van der Bijl, P.; Hirasawa, K.; Kamperidis, V.; van Wijngaarden, S.E.; Mertens, B.; Leon, M.B.; Hahn, R.T.; Stone, G.W.; Narula, J.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic Value of Left Ventricular Global Longitudinal Strain in Patients With Secondary Mitral Regurgitation. J Am Coll Cardiol 2020, 75, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Barbieri, A.; Grigioni, F.; Reggianini, L.; Zanasi, V.; Leuzzi, C.; Ricci, C.; Piovaccari, G.; Branzi, A.; Modena, M.G. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail 2010, 12, 382–388. [Google Scholar] [CrossRef]
- Pio, S.M.; Medvedofsky, D.; Delgado, V.; Stassen, J.; Weissman, N.J.; Grayburn, P.A.; Kar, S.; Lim, D.S.; Redfors, B.; Snyder, C.; Zhou, Z.; Alu, M.C.; Kapadia, S.R.; Lindenfeld, J.; Abraham, W.T.; Mack, M.J.; Asch, F.M.; Stone, G.W.; Bax, J.J. Left Atrial Improvement in Patients With Secondary Mitral Regurgitation and Heart Failure: The COAPT Trial. JACC Cardiovascular Imaging 2024. [Google Scholar] [CrossRef]
- Shechter, A.; Hong, G.J.; Kaewkes, D.; Patel, V.; Visrodia, P.; Tacon, P.R.; Koren, O.; Koseki, K.; Nagasaka, T.; Skaf, S.; Makar, M.; Chakravarty, T.; Makkar, R.R.; Siegel, R.J. Prognostic Value of Left Ventricular Global Longitudinal Strain in Transcatheter Edge-to-Edge Repair for Chronic Primary Mitral Regurgitation. European Heart Journal Cardiovascular Imaging 2024. [Google Scholar] [CrossRef]
- Nita, N.; Scharnbeck, D.; Schneider, L.M.; Seeger, J.; Wöhrle, J.; Rottbauer, W.; Keßler, M.; Markovic, S. Predictors of left ventricular reverse remodeling after percutaneous therapy for mitral regurgitation with the MitraClip system. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions 2020, 96, 687–697. [Google Scholar] [CrossRef]
- Rudolph, V.; Knap, M.; Franzen, O.; Schlüter, M.; de Vries, T.; Conradi, L.; Schirmer, J.; Treede, H.; Wegscheider, K.; Costard-Jäckle, A.; Meinertz, T.; Reichenspurner, H.; Baldus, S. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 2011, 58, 2190–2195. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. European Heart Journal Cardiovascular Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O'Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; Sundt, T.M., 3rd; Thomas, J.D. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Bellamy, M.; Avierinos, J.F.; Breen, J.; Eusemann, C.; Rossi, A.; Behrenbeck, T.; Scott, C.; Tajik, J.A.; Enriquez-Sarano, M. Left atrial remodelling in mitral regurgitation--methodologic approach, physiological determinants, and outcome implications: a prospective quantitative Doppler-echocardiographic and electron beam-computed tomographic study. Eur Heart J 2007, 28, 1773–1781. [Google Scholar] [CrossRef]
- Modaragamage Dona, A.C.; Afoke, J.; Punjabi, P.P.; Kanaganayagam, G.S. Global longitudinal strain to determine optimal timing for surgery in primary mitral regurgitation: A systematic review. J Card Surg 2021, 36, 2458–2466. [Google Scholar] [CrossRef]
- van Wijngaarden, S.E.; Kamperidis, V.; Al-Amri, I.; van der Kley, F.; Schalij, M.J.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Effects of Transcatheter Mitral Valve Repair With MitraClip on Left Ventricular and Atrial Hemodynamic Load and Myocardial Wall Stress. J Card Fail 2018, 24, 137–145. [Google Scholar] [CrossRef]
- Cimino, S.; Maestrini, V.; Cantisani, D.; Petronilli, V.; Filomena, D.; Mancone, M.; Sardella, G.; Fedele, F.; Lancellotti, P.; Agati, L. 2D/3D echocardiographic determinants of left ventricular reverse remodelling after MitraClip implantation. European Heart Journal Cardiovascular Imaging 2019, 20, 558–564. [Google Scholar] [CrossRef]
- Vitarelli, A.; Mangieri, E.; Capotosto, L.; Tanzilli, G.; D'Angeli, I.; Viceconte, N.; Placanica, A.; Placanica, G.; Cocco, N.; Ashurov, R.; Al-Kindy, S. Assessment of Biventricular Function by Three-Dimensional Speckle-Tracking Echocardiography in Secondary Mitral Regurgitation after Repair with the MitraClip System. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 2015, 28, 1070–1082. [Google Scholar] [CrossRef]
- Scandura, S.; Ussia, G.P.; Capranzano, P.; Caggegi, A.; Sarkar, K.; Cammalleri, V.; Mangiafico, S.; Chiarandà, M.; Immè, S.; Di Pasqua, F.; Pistritto, A.M.; Millan, G.; Tamburino, C. Left cardiac chambers reverse remodeling after percutaneous mitral valve repair with the MitraClip system. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography 2012, 25, 1099–1105. [Google Scholar] [CrossRef]
- Toprak, C.; Kahveci, G.; Kilicgedik, A.; Pala, S.; Kirma, C.; Tabakci, M.M.; Inanir, M.; Esen, A.M. Left atrial remodeling in patients undergoing percutaneous mitral valve repair with the MitraClip system: an advanced echocardiography study. Echocardiography (Mount Kisco, NY). 2016, 33, 1504–1511. [Google Scholar] [CrossRef]
- Todaro, M.C.; Choudhuri, I.; Belohlavek, M.; Jahangir, A.; Carerj, S.; Oreto, L.; Khandheria, B.K. New echocardiographic techniques for evaluation of left atrial mechanics. European Heart Journal Cardiovascular Imaging 2012, 13, 973–984. [Google Scholar] [CrossRef]
- Gucuk Ipek, E.; Singh, S.; Viloria, E.; Feldman, T.; Grayburn, P.; Foster, E.; Qasim, A. Impact of the MitraClip Procedure on Left Atrial Strain and Strain Rate. Circulation Cardiovascular imaging 2018, 11, e006553. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; Ikonomidis, I.; Chrissoheris, M.; Chalapas, A.; Kourkoveli, P.; Parissis, J.; Spargias, K. MitraClip and left ventricular reverse remodelling: a strain imaging study. ESC Heart Failure 2020, 7, 1409–1418. [Google Scholar] [CrossRef]
- Woods, R.H. A Few Applications of a Physical Theorem to Membranes in the Human Body in a State of Tension. Journal of Anatomy and Physiology 1892, 26, 362. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.C. Ventricular wall stress. Circ Res 1981, 49, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Beyar, R.; Sideman, S. Left ventricular mechanics related to the local distribution of oxygen demand throughout the wall. Circ Res 1986, 58, 664–677. [Google Scholar] [CrossRef]
- Suga, H. Total mechanical energy of a ventricle model and cardiac oxygen consumption. Am J Physiol 1979, 236, H498–H505. [Google Scholar] [CrossRef]
- Carasso, S.; Beyar, R.; Rooke, A.G.; Sideman, S. Combining transmural left ventricular mechanics and energetics to predict oxygen demand. Annals of Biomedical Engineering 1988, 16, 495–513. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).