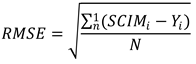1. Introduction
Spinal cord injury (SCI) is a severe and life-changing event, often resulting in psychopathological outcomes and disability even long-term [
1]. Additionally, SCI significantly impacts social and professional domains [
2].
Despite significant scientific progress in knowledge and understanding of the pathogenesis and treatment, it remains a devastating condition involving motor, sensory, and autonomic dysfunctions below the injury level, temporary or permanent [
3]. Currently, there is no effective treatment for completely repair spinal cord neural damage and the management of this injury is mainly focused to supportive measures aimed at preventing additional complications and rehabilitation [
4,
5].
SCI remains a significant global health issue, with 0.91 million new cases of SCI, 20.64 million prevalent cases, and 6.20 million years lived with disability [
6]. Furthermore, SCI has countless expenses affecting healthcare systems, community, and society, especially with invaluable lifetime healthcare costs for patients and their families according to clinical and socio-demographic characteristics [
6,
7]. Men are commonly more affected, accounting for 78% of new cases, while the onset age shifted from approximately 20 to 45 years [
8]. Traumatic causes of SCI, such as motor vehicle accidents, falls, and violence account for the majority of cases globally, with motor vehicle accidents being the leading cause in younger populations, and falls being more frequent among the elderly [
9,
10]. Non-traumatic SCI, which results from congenital or genetic disorders or acquired conditions, also accounts for a significant proportion of cases, despite the absence of epidemiological research. As healthcare systems improve and life expectancy increases, the prevalence of non-traumatic SCI is expected to rise, particularly in older adults [
11].
SCI condition represents a significant challenge in inpatient rehabilitation, requiring complex, multifaceted treatment approaches [
12]. The main goal of the rehabilitation process following SCI is to restore functional independence, but the outcomes are highly variable and depend on numerous factors. The outcomes of SCI rehabilitation are traditionally prognosticated based on clinical and demographic variables, such as lesion level and completeness of injury, age and sex, functional and clinical scale scores, complications, and comorbidities [
13]. However, patients after SCI are at risk of experiencing both physical and psychological issues [
14]. Individuals with SCI often experience significant stress, anxiety, and depression. In fact, patients with SCI tend to have higher levels of depression and stress, as well as lower self-esteem, compared to healthy individuals [
2]. Previous literature indicates that patients with SCI often face acute psychological distress, which can exacerbate emotional and cognitive responses, further affecting rehabilitation outcomes [
15]. A significant number of individuals with SCI may face mental health challenges following their injury, with mental health disorder diagnoses ranging from 10% to as much as one-third of affected individuals [
16]. Several studies suggest that psychological variables, such as anxiety and depression, may significantly influence rehabilitation outcomes and be crucial to recovery success [
17,
18], and that are also related to lower resilience [
19]. Furthermore, people with severe medical conditions, as well as those with SCI, often exhibit decreased emotional expressiveness, including traits associated with alexithymia, defined by difficulty in recognizing and describing emotions and distinguishing between feelings and their associated physical sensations. It also involves challenges in experiencing and expressing emotional states [
20]. This emotional dysregulation may adversely affect motivation for self-regulation and rehabilitation in the context of illness or disability [
21,
22,
23,
24]. In the literature, the correlation between alexithymia and SCI remains complex and not linear [
25,
26]. Conversely, protective psychological traits such as social skills, resilience, and high self-esteem are linked to better adaptation and improved recovery outcomes [
27]. Despite this, the role of psychological variables in predictive models for SCI outcomes remains quite underexplored, creating a gap in our understanding and management of these patients in the context of SCI inpatient rehabilitation.
Moreover, both traumatic and non-traumatic spinal cord injuries resulted in significant improvements across all rehabilitation domains with a standardized rehabilitation program, supporting its overall efficacy, including psychological ones [
28]. Mental health outcomes, such as depression, anxiety, and stress, were consistent across patients with in the post-acute phase. However, patients with non-traumatic spinal cord injuries are at higher risk for poor mental health outcomes, regardless of demographic or injury-related factors traumatic and non-traumatic spinal cord injuries [
29].
Rehabilitation outcomes often focus mainly on physical function, leaving the impact of psychological variables, essential for assessing the need for psychological screening measures and improving the identification of rehabilitation needs [
18]. While the literature regarding the impact of positive psychological variables on outcomes after SCI is limited, positive psychological factors significantly influence post-rehabilitation subjective well-being in individuals with SCI and are related to increased life satisfaction following SCI. This may provide a potential approach for interventions aimed at facilitating positive outcomes [
30].
Artificial Neural Networks (ANNs) have recently become a more precise alternative method for distinguishing between different classes of potential prognostic factors and predicting outcomes in patients needing neurorehabilitation, including those with spinal cord injuries, showing significant potential in diagnosis, prognostication, management, rehabilitation planning, and chronic complication prevention [
13,
31,
32].
Therefore, this study aims to deeper understand the role of psychological variables in rehabilitation outcomes for patients with SCI, to allow an improvement in rehabilitation strategies and clinical practices guidelines in the neurorehabilitation framework. We hypothesize that the percentage weight assigned to these psychological factors within the artificial neural network (ANN) model significantly influences the prediction of SCIM scores at discharge. Additionally, we performed a secondary analyses on the subgroup of patients with traumatic injuries to investigate the role of psychological factors in these people for which the spinal cord injury was a sudden and unexpected event.
2. Materials and Methods
2.1. Patients and Data
A review of clinical records related to hospital admissions and discharges was conducted, selecting data from electronic archives. The focus was on patients aged over 18 years admitted to the Spinal Centre of an Italian rehabilitation hospital (I.R.C.C.S. Fondazione Santa Lucia of Rome) between 2017 and 2023 after their first SCI. This study involved a secondary analysis of an extensive database comprising 1.308 patients, previously utilized in several studies [
13] and enriched with additional data. Exclusion criteria were patients with cognitive impairments that compromised their ability to engage in the neurorehabilitation program fully or interfered with accurate outcome assessment, individuals who experienced a discharge or transfer lasting more than three weeks before readmission (as these cases were classified as secondary admissions), patients with a length of stay of fewer than seven days, and those who deceased during their rehabilitation period. As our hospital functions as a research institution, all patients provided informed consent at the time of admission, authorizing the utilization of their clinical data for translational research purposes.
2.2. Clinical Assessment and Neurorehabilitation
At admission, the database was populated with key variables: three continuous variables, including age, Spinal Cord Independence Measure (SCIM) score (version II or III) [
33,
34] , and Walking Index for Spinal Cord Injury (WISCI) score; two ordinal variables, which included the American Spinal Injury Association Impairment Scale (AIS) score [
35] (A, B, C, or D) and lesion level (cervical, thoracic, or lumbar); and several binary variables, such as gender, etiology (traumatic or non-traumatic), surgical intervention (yes/no), and presence of complications like pressure sores(yes/no), heterotopic ossifications (HOs) (yes/no), respiratory complications (yes/no), pulmonary embolism (yes/no), deep vein thrombosis (yes/no), urologic complications (excluding urinary infections) (yes/no), preservation of motor tracts (motor complete/incomplete) (yes/no), and other complications (yes/no).
The psychological assessment battery utilized validated instruments to measure various constructs, as detailed below. The trait version of the State-Trait Anxiety Inventory (STAI-T), a 20-item self-report measure of trait anxiety, has been commonly utilized to examine ordinary levels of worrying and general anxiety state in daily life in both general populations and individuals with SCI [
28,
36,
37,
38]. The Depression Questionnaire (DQ) of the Cognitive Behavioural Assessment 2.0 is a well-validated self-report measure of depression, consisting of 24 dichotomous items adapted from the Beck Depression Inventory and the Zung Self-Rating Depression Scale. It was used to evaluate clinical depression by asking individuals to identify their current experience of each symptom as well in patients with SCI [
37,
38]. The “cut-off” score is equivalent to one standard deviation above the mean of the norms, as used in other studies: patients were defined as “anxious” and “depressed” when their scores on the two scales were higher than the mean of uninjured subjects plus one standard deviation. The Eysenck Personality Questionnaire (EPQ) Extroversion scale (E Scale) consists of 12 dichotomous items, with each response scoring 0 or 1. This questionnaire enables the identification of personality traits that may develop into certain disorders or, conversely, constitute a protective factor against the possible onset of psychological problems [
38,
39]. The Rosenberg Self-Esteem Scale (RSES) is the standard measure for assessing global self-esteem. The scale comprises 10 items, 5 negatively worded, and employs a 4-point Likert response format, ranging from 'strongly agree' to 'strongly disagree.' Higher scores indicate greater self-esteem, reflecting individuals with enhanced self-awareness and self-regard. Since self-esteem is considered a trait-like construct, the RSES may be particularly valuable as a moderator variable in various psychological studies [
40]. The Toronto Alexithymia Scale-20 (TAS-20) is a 20-item Likert scale to measure three-factor alexithymia - difficulties identifying and describing feelings, and externally oriented thinking. It focuses on the difficulties in regulating emotions instead of the effects and consequences of these issues [
41].
Despite numerous variables were regularly documented upon discharge; however, only the SCIM score was examined as the main outcome of neurorehabilitation in this study.
2.3. ARtificial Intelligence Assistant for Neural Network Analysis (ARIANNA)
We used a Feed Forward Neural Network Multilayer Perceptron formed by the input layers from which entered seventeen variables, two hidden layers of 5 elements each one and a single output node. Hyperbolic tangent was used as activation function for all the units in the hidden and output layers. The information unidirectionally moved from input to output layers. The chosen computational procedure was based on an online training (details: initial learning=1.2; lower learning=0.001, learning epochs=10, momentum=0.9 interval center=0, interval offset=±0.5, memsize=1000, steps without error=1, error change=0.0001, error ratio=0.001). This configuration, called ARtificial Intelligence Assistant for Neural Network Analysis (ARIANNA) was developed using the dedicated tool Neural Networks module of IBM Statistical Package for the Social Sciences software (SPSS, version 23), and was validated and used in previous studies on neurorehabilitation [
42,
43,
44] Statistical Analysis.
Mean ± standard deviation were used to describe the continuous and ordinal variables, whereas percentage frequencies were used for nominal variables. The pre-post comparisons were performed using the paired Wilcoxon test. The percentage weight assigned by ANN to each variable were reported as percentage of the total. Correlation were performed using linear regression and computing the Pearson’s coefficient and the Root Mean Square Error (RMSE) that was:
Statistical significance threshold for all the analysis was set at 5%. All the statistical analyses, as well as the ANN, have been performed using SPSS (Statistical Package for the Social Sciences) software of the IBM, version 23.
2.4. Statistical Analysis
Both continuous and ordinal variables were reported in terms of mean and standard deviation, whereas binary and nominal variables were pointed out as percentage frequencies. Predictive scores were selected for reporting the ANN results, raw percentage importance (RI) of each variable (the total of all RIs is 100%) and normalized importance (NI, with respect to the most important variable). All data were used for training the ANN and not splitted for testing. The hyperparameters were setted as follows. The training criteria was an online batch, the optimization performed by scaled conjugate, the initial values were lambda=0.0000005, sigma=0.00005, interval center=0, interval offset=0.5 mem size=1000, the error steps= 1 (data=auto) training timer was on with a max time=15 and automatic max epochs. Error change was =1.0e-4 and the error ratio=0.001. The mean absolute error (MAE) was used to assess the performance of ANN
A linear regression analysis was also conducted to identify, among the analyzed factors, those significantly entering into the model. The linear regression had the same input variables of ANN, and the same predicted output that was the SCIM-score at discharge. Particularly as regards SCIM score, SCIM-score assessed at admission to neurorehabilitation hospital was one of the input variable of both ANN and regression model, the predicted variable for both the models was the SCIM-score assessed at discharge. Unstandardized (B) and standardized (Beta) coefficients of linear regressions were reported together with p-values of variables entered (p≤0.05) or not (p>0.05) into the model. Pearson coefficient was used to assess correlations, and linear fitting of prediction versus actual outcomes were performed using the least squares method. Mean absolute error (MAE) was used to compare the absolute differences between computed and actual scores. For the statistical analyses, as well as the ANN, SPSS (Statistical Package for the Social Sciences) software of the IBM, version 23, was adopted
3. Results
3.1. Cohort of SCI Patients
The patients included in this analysis were 303, with a mean age of 52.3±18.2 years, 33% of them with a cervical lesion, 49% with a thoracic lesion and 18% with a lumbar lesion. These and other demographic and clinical characteristics measured at admission into the rehabilitation hospital are reported in
Table 1. The SCIM score at admission was 21.8±15.8 and at discharge it was 57.4±22.5, with a statistically significant improvement (
p<0.001). The mean length of stay in the rehabilitation hospital was 166±120 days.
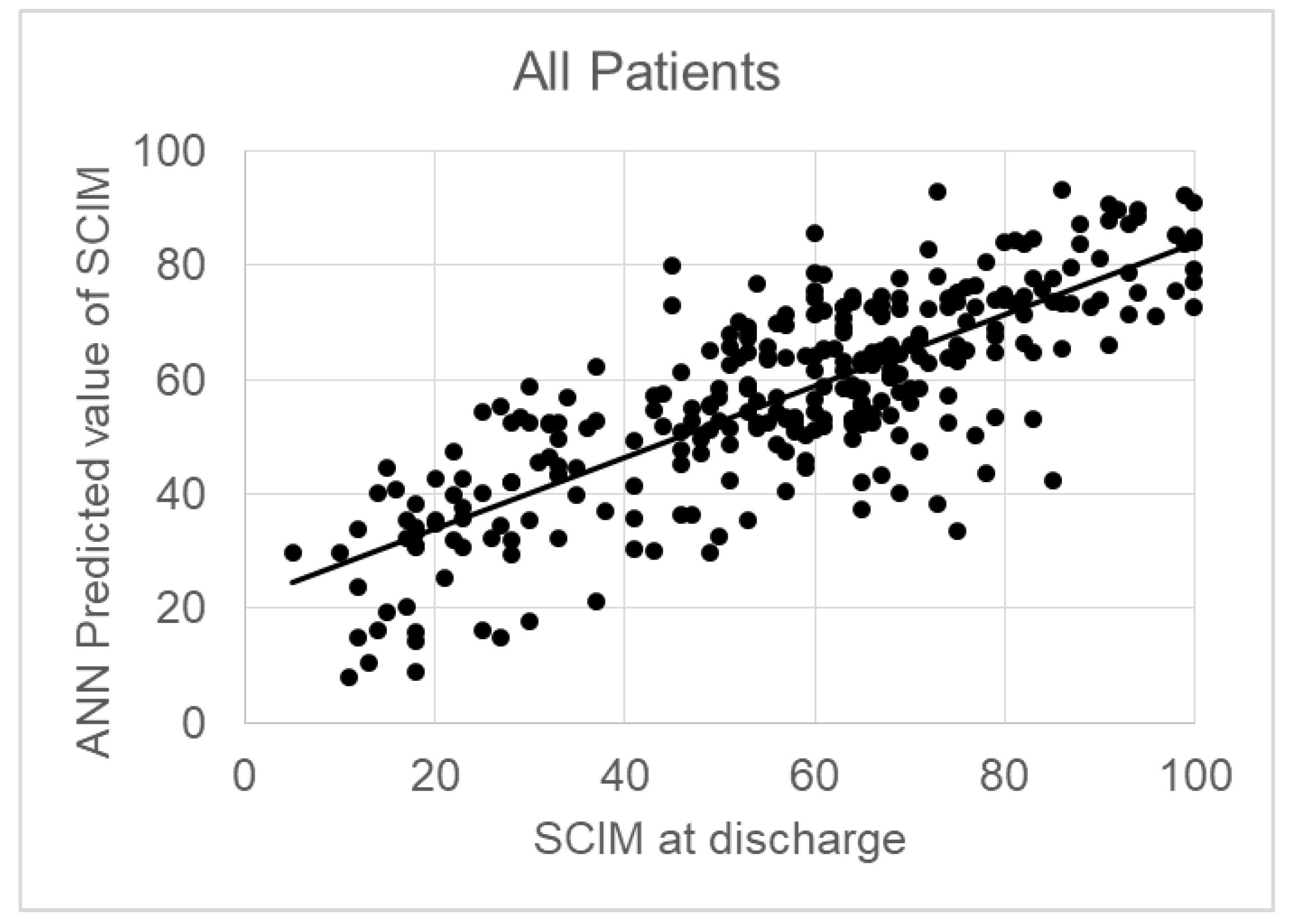
Table 1 also reports the percentage weight associated with each input variable for predicting the SCIM at discharge. The SCIM-score at admission was the most important factor for predicting the SCIM-score at discharge (weight: 10.3%). The sum of the weights associated to psychological factors covered the 36.3% of the total. The correlation between predicted values and actual ones was R=0.794 (p<0.001).
Figure 1 shows this regression, the formula of which was: SCIM-score at T1 = 0.62* Predicted SCIM + 22, with a RMSE=21.
3.2. Patients with Traumatic Lesion
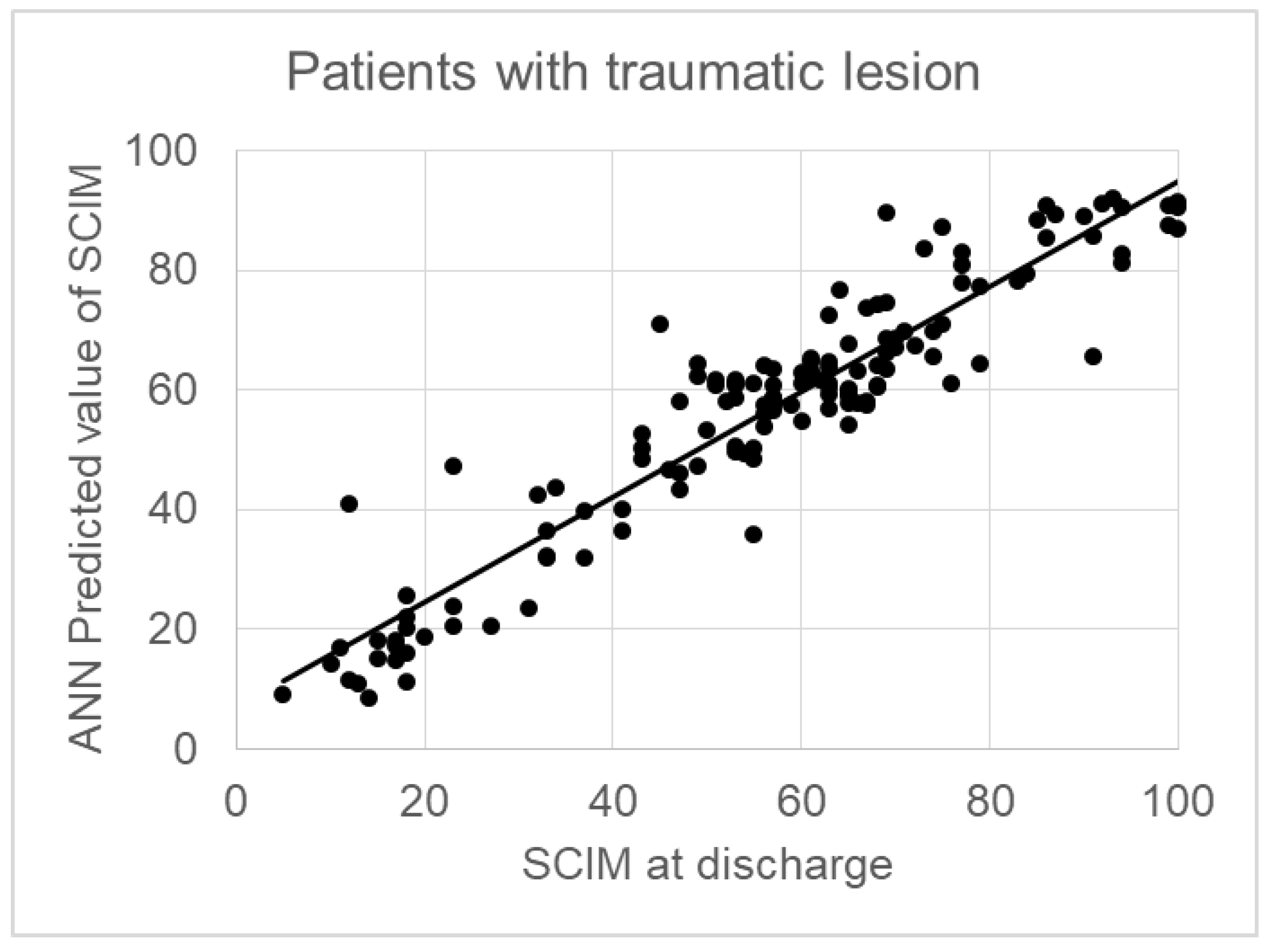
The sum of psychological factors was 36.3% on the whole sample, 40.9% in patients with traumatic lesions. In 140 patients the cause of the lesion was traumatic.
Table 1 reports the demographical, clinical and psychological variables in these patients, together with the weights assigned by the ANN to each one of these variables for predicting the SCIM at discharge. The weight of psychological factors increased to 40.9%, and also the correlation between predicted and actual values resulted very high (R=0.940, p<0.001) with a regression line close to y=x (SCIM-score at T1 = 0.88* Predicted SCIM + 6.8) and a RMSE=5. The sum of psychological factors was 36.3% on the whole sample, 40.9% in patients with traumatic lesions The sum of psychological factors was 36.3% on the whole sample, 40.9% in patients with traumatic lesions. The correlation between predicted and actual outcome (SCIM) was R=0.794 (y=0.62*SCIM+22, p<0.001) for the whole sample (see
Figure 1) and R=0.940 for subjects with a traumatic lesion (y=0.88*SCIM+6.8, p<0.001) as shown in
Figures 2.
4. Discussion
This study aimed to assess the role of psychological factors in predicting rehabilitation outcomes following SCI using an ANN approach with data from a single Italian hospital. The potential of predicting the SCIM score at discharge from rehabilitation based on demographic, clinical, and psychological variables obtained at admission was evaluated in patients with both traumatic and non-traumatic SCI admitted. The ARIANNA model, based on ANN, allowed for precise identification of the relative weight of each variable in predicting rehabilitation outcomes. The analysis indicated that the level of independence, as measured by the SCIM score at discharge, was primarily determined by clinical variables such as the SCIM score at admission, lesion level (cervical, thoracic, lumbar), and motor completeness of the injury (complete vs. incomplete injury). The ANN further highlighted an influence of age. These findings are consistent with previous studies that have highlighted that SCIM baseline is a robust predictor of long-term functional outcomes [
42] and the importance of lesion characteristics in determining rehabilitation potential, such as level and severity of SCI and age [
13,
45,
46,
47]. Psychological factors collectively accounted for 36.3% of the total predictive weight for functional outcomes, with even higher contributions (40.9%) observed in patients with traumatic injuries. Among the traumatic injury group the most influential variables were anxiety and depression levels, with weights of 10.3% and 10.6% respectively. These two variables contributed significantly to the model's predictions. This aligns with previous research indicating that psychological health is integral to functional recovery in patients with SCI [
18]. High levels of anxiety and depression have been associated with lower rehabilitation outcomes, as they can impede motivation and engagement in therapy [
16,
17]. Patients with SCI who exhibit depressive symptoms, including psychomotor disturbance, changes in appetite, and sleep disturbances, are likely to experience longer lengths of hospitalization. This extended hospitalization is associated with decreased mobility and functional independence at discharge compared to patients without these symptoms [
47]. Moreover, depressive symptoms contribute to a slower rate of functional improvement and are linked to increased morbidity and mortality [
46]. Anxiety can lead to extreme preventive behaviours, leading individuals to avoid situations they perceive as potentially anxiety-inducing or threatening. The prolonged persistence of such behaviours may result in disuse and increased disability, further diminishing rehabilitation opportunities [
48]. On the contrary, protective psychological traits, such as resilience and high self-esteem, positively correlate with better adaptation and recovery outcomes, enabling individuals to effectively navigate the challenges associated with their injuries and encouraging active participation in rehabilitation for patients with SCI [
27,
49]. Similar results have been demonstrated in stroke and cardiological rehabilitation. In both cases, higher levels of depression at the onset of rehabilitation were significantly correlated with lower rehabilitation outcomes, specifically reflecting a decrease in exercise capacity and poorer emotional, physical, and social quality of life; conversely, anxiety levels showed a positive association with improvements in exercise capacity and emotional and physical quality of life [
50,
51]. However, also in stroke and cardiac rehabilitation, several psychological variables and emotional regulation significantly affect patient engagement and overall recovery outcomes. Specifically, increased self-efficacy is related to better functional outcomes, while coping styles and personality traits influence rehabilitation success. Problem-focused coping helps manage controllable stressors, and emotion-focused approaches support chronic condition management. Traits like extraversion, conscientiousness, and openness are linked to lower systemic inflammation and adherence to health guidelines [
50,
52]. Although these findings specifically address cardiac events, they may also apply to conditions like SCI. Both vascular events and SCI occur suddenly, and the episode can be traumatic. As a result, patients may develop a sedentary lifestyle, experience reduced psychological and physical motivation, and be inclined not to respect the therapeutic regimen [
50].
Furthermore, individuals with severe medical conditions often exhibit reduced emotional expressiveness, such as alexithymia, which may impact their motivation for self-regulation and rehabilitation when dealing with illness or disability [
21,
23]. Specifically, patients with SCI may develop secondary alexithymic features due to the unconscious suppression or denial of their emotional experiences as a psychological response to their injury [
24]. However, other research has found no significant increase in alexithymia scores in this population [
25] and that alexithymia does not appear to be associated with the outcomes of inpatient therapy [
26]. This suggests that the relationship between alexithymia and SCI may be more complex than initially assumed, requiring further investigation to determine how alexithymia influences rehabilitation outcomes in this population. This data highlights the dual role psychological factors can play in either hindering or facilitating recovery, suggesting that interventions targeting mental health could improve overall rehabilitation outcomes.
The implications of our findings are related to clinical practice. It has already been examined how psychological resources significantly impact the lives of individuals with SCI [
53]. The challenges faced by individuals with SCI extend beyond the period of hospitalization. Upon returning home after institutional rehabilitation, they may experience heightened levels of depression due to increased dependency, limited social skills, insufficient social support beyond their family, and restricted employment opportunities [
17]. These findings underscore important considerations for the psychological management of individuals with SCI, highlighting the necessity of targeted psychological interventions during inpatient rehabilitation. Therefore, aligning rehabilitation strategies with individual psychological profiles can increase recovery efficacy. The integration of psychological assessments into routine rehabilitation protocols can lead to a more holistic approach to patient care. Addressing the emotional and psychological dimensions of recovery is essential for optimizing long-term outcomes. Mental health screening should become a standard component of rehabilitation assessments, allowing for the identification of patients at risk of lower outcomes due to psychological distress [
54].
Clinically, this study underlines the necessity for a multidisciplinary approach to SCI rehabilitation, where mental health professionals work closely with physical therapists, neurologists, and rehabilitation specialists. Collaboration across disciplines would ensure that the psychological dimension is addressed concurrently with physical rehabilitation. It also highlights the need for long-term psychological support, as the effects of depression, anxiety, and other psychological factors may extend beyond the acute rehabilitation phase, impacting the long-term quality of life and functional independence of patients with SCI. Training rehabilitation staff in recognizing and addressing psychological factors can create a more supportive environment for patients, facilitating their engagement in the rehabilitation process.
The study presents some limitations. Firstly, the sample was recruited from a single specialized neurorehabilitation center, which may limit the generalizability of the results. The characteristics of patients in different contexts may vary significantly, affecting the applicability of findings to other patient populations or less specialized settings. Therefore, future studies should be conducted in more diverse contexts to validate the findings and ensure that clinical recommendations can be effectively applied across a broader range of rehabilitation settings. Secondly, the complexity of the relationship between these factors and other aspects of the patient's life may not have been fully explored. For instance, factors such as cognitive dimensions, social support, coping resources, and environmental variables can significantly impact the recovery process and may influence rehabilitation outcomes [
55]. Integrating such variables into future predictive models could enhance the understanding of the complexities of rehabilitation experiences and optimize intervention strategies. Future studies should aim to integrate these variables into predictive models, potentially increasing their accuracy and applicability across diverse populations. Moreover, the study's cross-sectional design limits our ability to conclude changes over time and the causal relationships between psychological factors and rehabilitation outcomes. Longitudinal studies could provide deeper insights into how psychological factors evolve and impact recovery trajectories, thereby informing more targeted and effective rehabilitation approaches. Finally, the reliance on self-reported measures of psychological factors introduces potential biases related to response styles and social desirability. Future research should consider utilizing a combination of self-report measures and objective assessments to mitigate these biases and provide a more comprehensive understanding of the psychological dimensions of recovery after SCI, addressing these limitations could enhance the clinical utility of this research. However, the predictive capacity of ANNs comes with challenges. As noted, ANNs often function as "black boxes," making it difficult to discern the relationships between variables [
13,
32,
56]. This limitation necessitates further exploration of alternative modeling techniques that might enhance interpretability while maintaining predictive power. Incorporating methods that provide clearer insights into the interactions among variables could inform better clinical decision-making and improve individualized patient management.
5. Conclusions
These results highlight the impact of psychological factors on rehabilitation outcomes following SCI, through the application of ANNs. Our results suggest that in addition to established clinical predictors—such as lesion level, baseline SCIM score, and motor completeness—psychological factors significantly contribute to the prediction of the SCIM at discharge. Psychological variables accounted for 36.3% of the model's predictive weight, with an enhanced contribution (40.9%) observed in traumatic SCI cases. Among psychological variables, anxiety, and depression emerged as dominant negative predictors, consistent with literature associating these factors with diminished recovery potential. Conversely, traits like self-esteem and emotional regulation functioned as protective factors, increasing functional outcomes and underlining the importance of psychological well-being in the neurorehabilitation treatment. These findings advocate for the integration of psychological assessment tools into routine SCI rehabilitation protocols, aiming to tailor individualized therapeutic interventions that address both physical and mental health dimensions.
The high correlation coefficient between predicted and actual SCIM outcomes, particularly in traumatic SCI cases, underscores the importance of psychological variables in refining prognostic accuracy. The model’s overall predictive strength highlights its utility in clinical practice, suggesting that psychological screening could enhance patient classification and optimize rehabilitation strategies. Future investigations should focus on expanding the model’s application to broader patient populations, validating these findings across different rehabilitation settings, and refining its predictive algorithms. These results underscore the relevance of a multidimensional approach to SCI management, integrating psychological parameters to enhance the accuracy of outcome prediction and the efficacy of rehabilitation interventions including psychological treatment.
Author Contributions
Conceptualization, M.I., G.S.; methodology, M.I., G.S..; software, M.I.; formal analysis, M.I..; investigation, M.I., G.S., F.T.; resources, G.S.; data curation, G.S., M.I., F.T.; writing—original draft preparation, G.S., M.I., F.T.; writing—review and editing, G.S., M.I., F.T., E.L., M.M..; visualization, G.S., M.I., F.T.; supervision, G.S., M.I.; project administration, G.S.; funding acquisition, M.I., G.S. All authors have read and agreed to the published version of the manuscript.
Funding
this research was supported by two grants of Italian Ministry of Health: i)“NEURO-METAVERSE: Application in Neurorehabilitation and Neuroscience of Metaverse Technologies as Virtual Reality and Artificial Intelligence”; ii) DISCLOSER RF-2019-12369396.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
Authors would like to thank the coordinator of the neurorehabilitation gym and all the individuals with SCI who allowed us to collect data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- H.-T. Jenkins and T. D. Cosco, “Spinal cord injury and aging: an exploration of the interrelatedness between key psychosocial factors contributing to the process of resilience,” Health Psychol. Behav. Med., vol. 9, no. 1, pp. 315–321, Apr. 2021. [CrossRef]
- Serpanou, et al., “The Association Between Spirituality, Self-Esteem, Anxiety and Depression: A Comparative Exploratory Study Between People with a Spinal Cord Injury and Healthy Subjects in Greece,” J. Relig. Health, Jul. 2024. [CrossRef]
- Anjum, et al., “Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms,” Int. J. Mol. Sci., vol. 21, no. 20, p. 7533, Oct. 2020. [CrossRef]
- T. Y. Wang et al., “Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature,” Front. Surg., vol. 8, p. 698736, 2021. [CrossRef]
- D. Yari et al., “Recent Advances in the Treatment of Spinal Cord Injury,” Arch. Bone Jt. Surg., vol. 12, no. 6, pp. 380–399, 2024. [CrossRef]
- M. Diop and D. Epstein, “A Systematic Review of the Impact of Spinal Cord Injury on Costs and Health-Related Quality of Life,” PharmacoEconomics - Open, Aug. 2024. [CrossRef]
- J. Donovan and S. Kirshblum, “Clinical Trials in Traumatic Spinal Cord Injury,” Neurother. J. Am. Soc. Exp. Neurother., vol. 15, no. 3, pp. 654–668, Jul. 2018. [CrossRef]
- Eli, D. P. Lerner, and Z. Ghogawala, “Acute Traumatic Spinal Cord Injury,” Neurol. Clin., vol. 39, no. 2, pp. 471–488, May 2021. [CrossRef]
- M. Yadollahi, M. Karajizadeh, N. Bordbar, and Z. Ghahramani, “Incidence and pattern of traumatic spine injury in a single level I trauma center of southern Iran,” Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi, vol. 26, no. 4, pp. 199–203, Jul. 2023. [CrossRef]
- H. S. Kim et al., “Epidemiology of Spinal Cord Injury: Changes to Its Cause Amid Aging Population, a Single Center Study,” Ann. Rehabil. Med., vol. 45, no. 1, pp. 7–15, Feb. 2021. [CrossRef]
- D. M. Molinares, D. R. Gater, S. Daniel, and N. L. Pontee, “Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management,” J. Pers. Med., vol. 12, no. 11, p. 1872, Nov. 2022. [CrossRef]
- T. Tian, S. Zhang, and M. Yang, “Recent progress and challenges in the treatment of spinal cord injury,” Protein Cell, vol. 14, no. 9, pp. 635–652, Sep. 2023. [CrossRef]
- F. Tamburella, E. Lena, M. Mascanzoni, M. Iosa, and G. Scivoletto, “Harnessing Artificial Neural Networks for Spinal Cord Injury Prognosis,” J. Clin. Med., vol. 13, no. 15, p. 4503, Aug. 2024. [CrossRef]
- W. Ding et al., “Spinal Cord Injury: The Global Incidence, Prevalence, and Disability From the Global Burden of Disease Study 2019,” Spine, vol. 47, no. 21, pp. 1532–1540, Nov. 2022. [CrossRef]
- Calderone, D. Cardile, R. De Luca, A. Quartarone, F. Corallo, and R. S. Calabrò, “Cognitive, behavioral and psychiatric symptoms in patients with spinal cord injury: a scoping review,” Front. Psychiatry, vol. 15, p. 1369714, 2024. [CrossRef]
- H. Bombardier, C. B. Azuero, J. R. Fann, D. D. Kautz, J. S. Richards, and S. Sabharwal, “Management of Mental Health Disorders, Substance Use Disorders, and Suicide in Adults with Spinal Cord Injury: Clinical Practice Guideline for Healthcare Providers,” Top. Spinal Cord Inj. Rehabil., vol. 27, no. 2, pp. 152–224, 2021. [CrossRef]
- M. Qasheesh, M. A. Shaphe, A. Iqbal, and A. H. Alghadir, “Association of psychological variants with functional outcomes among people with spinal cord injury,” Sci. Rep., vol. 11, no. 1, p. 20325, Oct. 2021. [CrossRef]
- M. Wallace, J. Duff, and L. C. Grant, “The influence of psychological need on rehabilitation outcomes for people with spinal cord injury,” Spinal Cord, vol. 61, no. 1, pp. 83–92, Jan. 2023. [CrossRef]
- M. Bhattarai, S. M. Smedema, and K. Maneewat, “An integrative review of factors associated with resilience post-spinal cord injury,” Rehabil. Couns. Bull., vol. 64, no. 2, pp. 118–127, 2021. [CrossRef]
- G. J. Taylor, R. M. Bagby, and J. D. A. Parker, Disorders of affect regulation: Alexithymia in medical and psychiatric illness. in Disorders of affect regulation: Alexithymia in medical and psychiatric illness. New York, NY, US: Cambridge University Press, 1997, pp. xxii, 359. [CrossRef]
- Fukunishi, “Psychosomatic aspects of patients on hemodialysis. 2. Alexithymic trait of hemodialysis patients with diabetic nephropathy,” Psychother. Psychosom., vol. 52, no. 1–3, pp. 58–65, 1989. [CrossRef]
- Fukunishi, “Anxiety associated with kidney transplantation,” Psychopathology, vol. 26, no. 1, pp. 24–28, 1993. [CrossRef]
- Fukunishi, Y. Chishima, and M. Anze, “Posttraumatic stress disorder and alexithymia in burn patients,” Psychol. Rep., vol. 75, no. 3 Pt 1, pp. 1371–1376, Dec. 1994.
- Fukunishi, I. Koyama, and H. Tobimatsu, “Psychological acceptance and alexithymia in spinal cord injury patients,” Psychol. Rep., vol. 76, no. 2, pp. 475–481, Apr. 1995. [CrossRef]
- R. E. O’Carroll, R. Ayling, S. M. O’Reilly, and N. T. North, “Alexithymia and sense of coherence in patients with total spinal cord transection,” Psychosom. Med., vol. 65, no. 1, pp. 151–155, 2003. [CrossRef]
- C. Spitzer, U. Siebel-Jurges, S. Barnow, H. J. Grabe, and H. J. Freyberger, “Alexithymia and interpersonal problems,” Psychother. Psychosom., vol. 74, no. 4, pp. 240–246, 2005. [CrossRef]
- R. Kornhaber, L. Mclean, V. Betihavas, and M. Cleary, “Resilience and the rehabilitation of adult spinal cord injury survivors: A qualitative systematic review,” J. Adv. Nurs., vol. 74, no. 1, pp. 23–33, Jan. 2018. [CrossRef]
- P. Kennedy and B. A. Rogers, “Anxiety and depression after spinal cord injury: a longitudinal analysis,” Arch. Phys. Med. Rehabil., vol. 81, no. 7, pp. 932–937, Jul. 2000. [CrossRef]
- C. E. Migliorini, P. W. New, and B. J. Tonge, “Comparison of depression, anxiety and stress in persons with traumatic and non-traumatic post-acute spinal cord injury,” Spinal Cord, vol. 47, no. 11, pp. 783–788, Nov. 2009. [CrossRef]
- K. B. Kortte, M. Gilbert, P. Gorman, and S. T. Wegener, “Positive psychological variables in the prediction of life satisfaction after spinal cord injury,” Rehabil. Psychol., vol. 55, no. 1, pp. 40–47, Feb. 2010. [CrossRef]
- Cerasa, et al., “Predicting Outcome in Patients with Brain Injury: Differences between Machine Learning versus Conventional Statistics,” Biomedicines, vol. 10, no. 9, p. 2267, Sep. 2022. [CrossRef]
- N. Dietz, null Vaitheesh Jaganathan, V. Alkin, J. Mettille, M. Boakye, and D. Drazin, “Machine learning in clinical diagnosis, prognostication, and management of acute traumatic spinal cord injury (SCI): A systematic review,” J. Clin. Orthop. Trauma, vol. 35, p. 102046, Dec. 2022. [CrossRef]
- M. I. Catz Flavia Steinberg, Ora Philo, Haim Ring, Jacob Ronen, Raluca Spasser, Reuven Gepstein, Ada Tamir,Amiram, “The Catz-Itzkovich SCIM: a revised version of the Spinal Cord Independence Measure,” Disabil. Rehabil., vol. 23, no. 6, pp. 263–268, Jan. 2001. [CrossRef]
- M. Invernizzi et al., “Development and validation of the Italian version of the Spinal Cord Independence Measure III,” Disabil. Rehabil., vol. 32, no. 14, pp. 1194–1203, Jan. 2010. [CrossRef]
- P. L. Dittuno and J. F. Ditunno, “Walking index for spinal cord injury (WISCI II): scale revision,” Spinal Cord, vol. 39, no. 12, pp. 654–656, Dec. 2001. [CrossRef]
- C. Spielberger, R. Gorsuch, R. Lushene, P. Vagg, and G. Jacobs, State–Trait Anxiety Inventory Self Evaluation Questionnaire, Form Y (STAI). 2003. [CrossRef]
- G. Scivoletto, A. Petrelli, L. Di Lucente, and V. Castellano, “Psychological investigation of spinal cord injury patients,” Spinal Cord, vol. 35, no. 8, pp. 516–520, Aug. 1997. [CrossRef]
- G. Scivoletto et al., “One year follow up of spinal cord injury patients using a reciprocating gait orthosis: preliminary report,” Spinal Cord, vol. 38, no. 9, pp. 555–558, Sep. 2000. [CrossRef]
- H. J. Eysenck and S. B. G. Eysenck, Manual of the Eysenck Personality Questionnaire (junior and Adult). Hodder and Stoughton Educational, 1975. [Online]. Available: https://books.google.it/books?
- M. Rosenberg, “Rosenberg Self-Esteem Scale.” Sep. 12, 2011. [CrossRef]
- R. M. Bagby, G. J. Taylor, and J. D. Parker, “The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity,” J. Psychosom. Res., vol. 38, no. 1, pp. 33–40, Jan. 1994. [CrossRef]
- M. Iosa, G. Morone, G. Antonucci, and S. Paolucci, “Prognostic Factors in Neurorehabilitation of Stroke: A Comparison among Regression, Neural Network, and Cluster Analyses,” Brain Sci., vol. 11, no. 9, p. 1147, Aug. 2021. [CrossRef]
- M. Iosa et al., “Artificial Neural Network Analyzing Wearable Device Gait Data for Identifying Patients With Stroke Unable to Return to Work,” Front. Neurol., vol. 12, p. 650542, 2021. [CrossRef]
- Ciancarelli, et al., “Identification of Determinants of Biofeedback Treatment’s Efficacy in Treating Migraine and Oxidative Stress by ARIANNA (ARtificial Intelligent Assistant for Neural Network Analysis),” Healthc. Basel Switz., vol. 10, no. 5, p. 941, May 2022. [CrossRef]
- S. C. Kirshblum et al., “International standards for neurological classification of spinal cord injury (revised 2011),” J. Spinal Cord Med., vol. 34, no. 6, pp. 535–546, Nov. 2011. [CrossRef]
- E. M. J. R. Brouwers et al., “Recovery after traumatic thoracic- and lumbar spinal cord injury: the neurological level of injury matters,” Spinal Cord, vol. 58, no. 9, pp. 980–987, Sep. 2020. [CrossRef]
- Z. Khazaeipour, S.-M. Taheri-Otaghsara, and M. Naghdi, “Depression Following Spinal Cord Injury: Its Relationship to Demographic and Socioeconomic Indicators,” Top. Spinal Cord Inj. Rehabil., vol. 21, no. 2, pp. 149–155, 2015. [CrossRef]
- J. N. Perusini and M. S. Fanselow, “Neurobehavioral perspectives on the distinction between fear and anxiety,” Learn. Mem. Cold Spring Harb. N, vol. 22, no. 9, pp. 417–425, Sep. 2015. [CrossRef]
- R. Müller, C. Peter, A. Cieza, and S. Geyh, “The role of social support and social skills in people with spinal cord injury--a systematic review of the literature,” Spinal Cord, vol. 50, no. 2, pp. 94–106, Feb. 2012. [CrossRef]
- J.-C. Chauvet-Gelinier and B. Bonin, “Stress, anxiety and depression in heart disease patients: A major challenge for cardiac rehabilitation,” Ann. Phys. Rehabil. Med., vol. 60, no. 1, pp. 6–12, Jan. 2017. [CrossRef]
- M. Griffiths, E. Kontou, and C. Ford, “Psychological support after stroke: unmet needs and workforce requirements of clinical neuropsychological provision for optimal rehabilitation outcomes,” Br. J. Hosp. Med. Lond. Engl. 2005, vol. 84, no. 11, pp. 1–8, Nov. 2023. [CrossRef]
- Y. Wang et al., “Influencing Factors of Psychological Resilience in Stroke Patients: A Systematic Review and Meta-Analysis,” Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol., vol. 39, no. 5, pp. 644–654, Jul. 2024. [CrossRef]
- C. Peter et al., “Modeling life satisfaction in spinal cord injury: the role of psychological resources,” Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil., vol. 23, no. 10, pp. 2693–2705, Dec. 2014. [CrossRef]
- Craig, Y. Tran, and J. Middleton, “Psychological morbidity and spinal cord injury: a systematic review,” Spinal Cord, vol. 47, no. 2, pp. 108–114, Feb. 2009. [CrossRef]
- M. B. Kendall, D. Amsters, S. Schuurs, D. N. Borg, K. Pershouse, and P. Kuipers, “Longitudinal effects of time since injury and age at injury on outcomes of people with spinal cord injury in Queensland, Australia,” Spinal Cord, vol. 60, no. 12, pp. 1087–1093, Dec. 2022. [CrossRef]
- J. V. Tu, “Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes,” J. Clin. Epidemiol., vol. 49, no. 11, pp. 1225–1231, Nov. 1996. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).