Submitted:
29 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
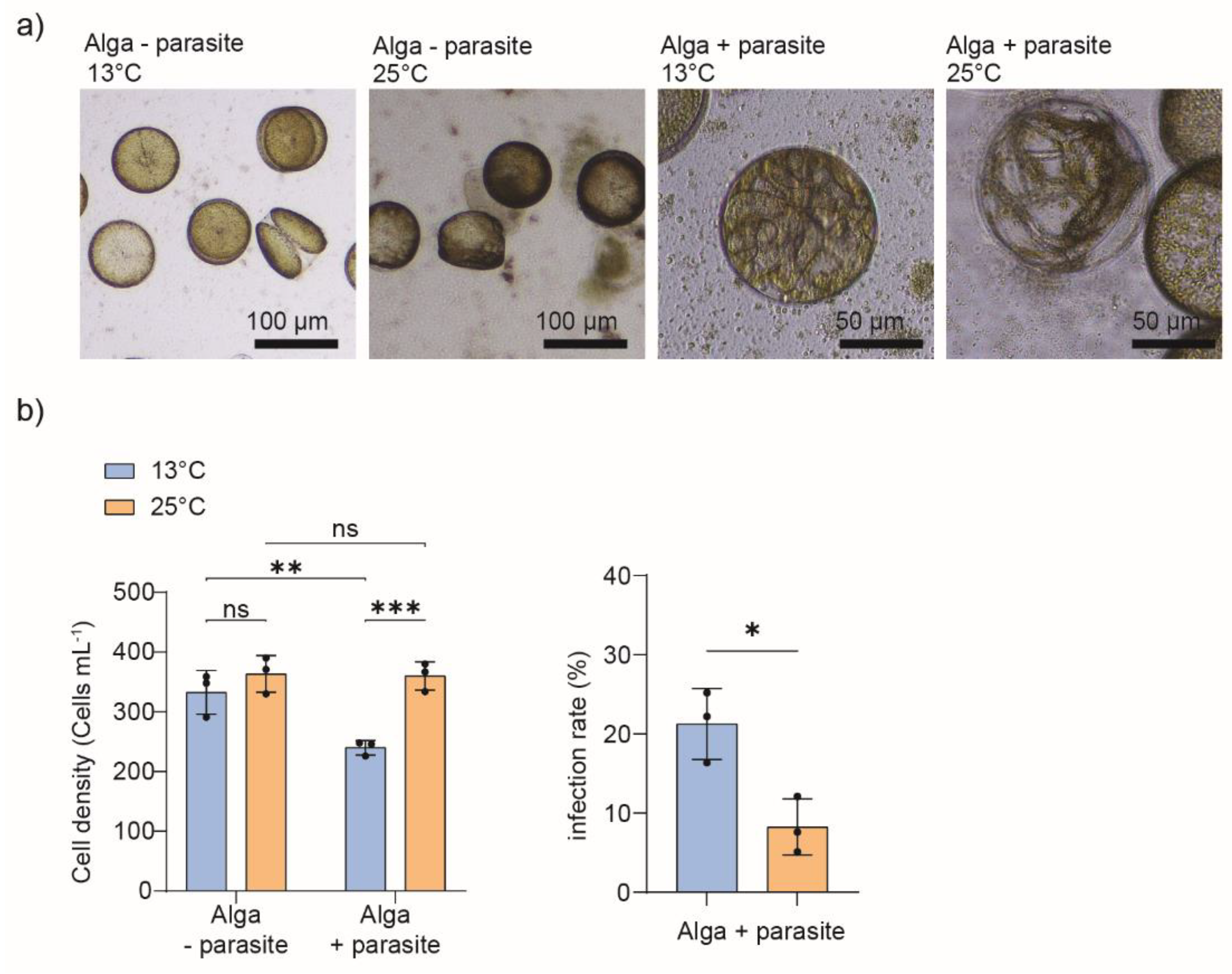
2.1. Impact of Temperature on Diatom Host Abundance and Parasite Infectivity
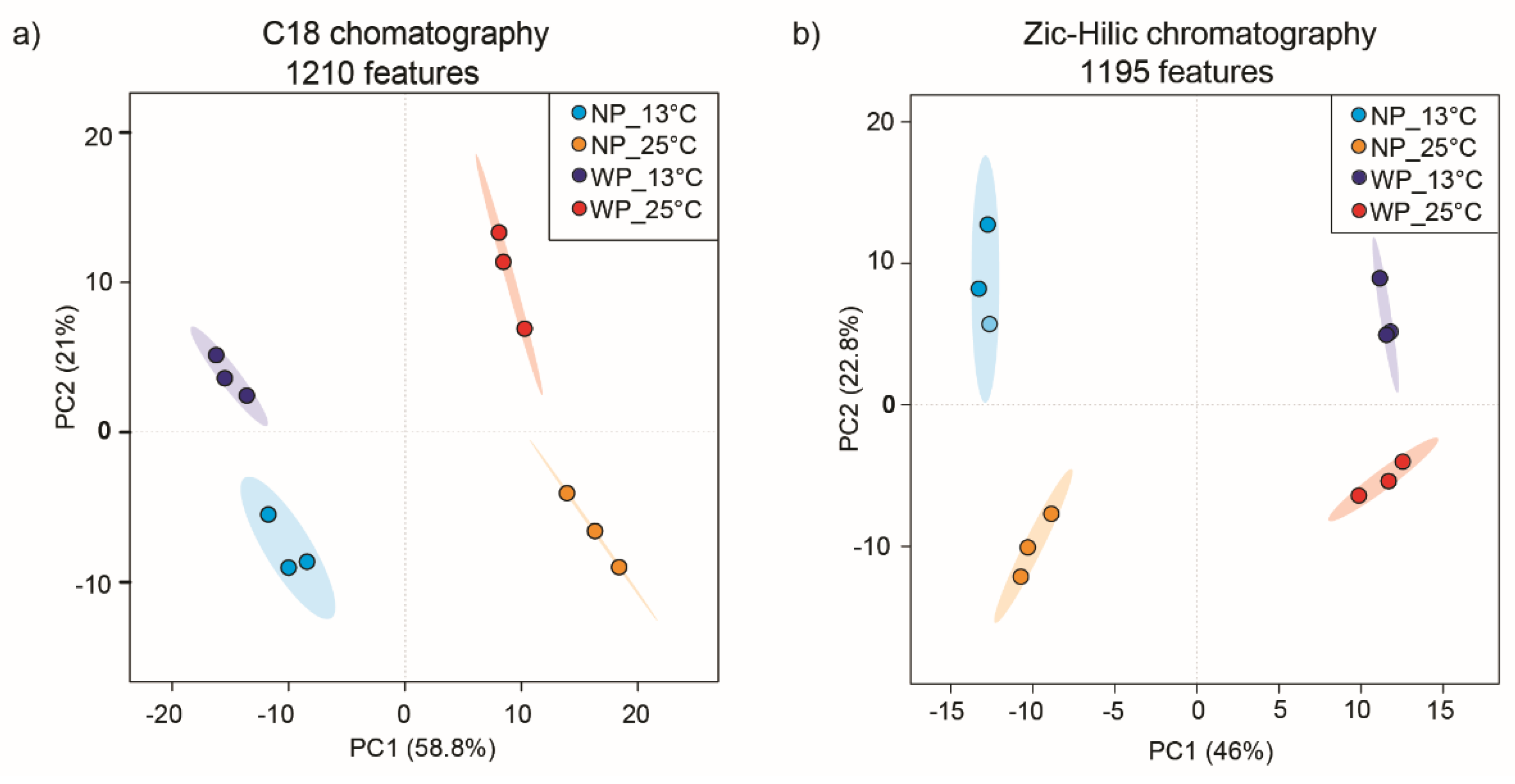
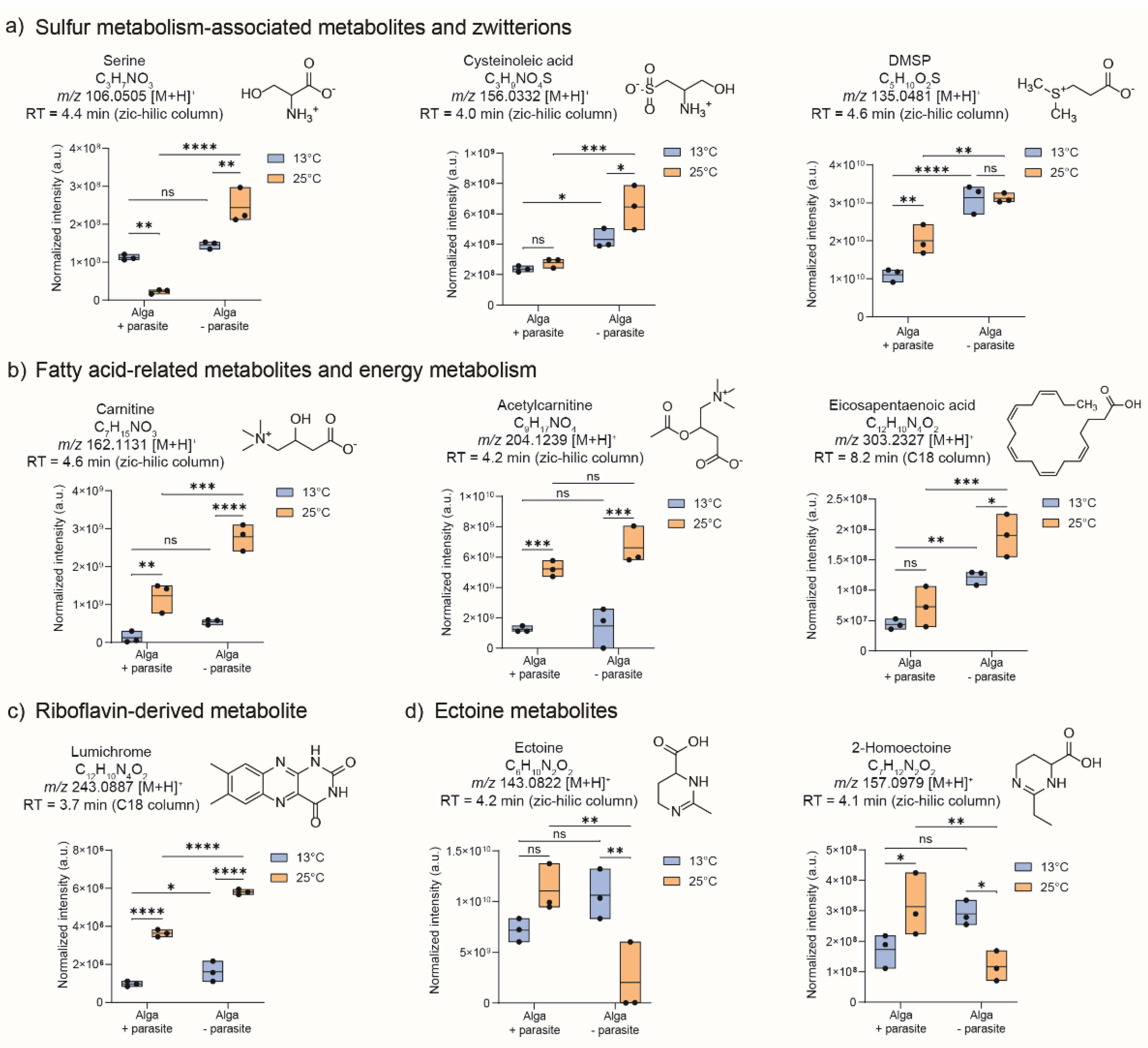
2.2. UHPLC-HRMS Analysis Reveals Altered Metabolome Patterns Associated with Different Temperatures and Parasite Infection in Diatom Cells
3. Discussion
3.1. Effect of Temperature on Cell Abundance of Bloom-Forming Diatom C. granii and Parasite Infectivity of L. coscinodisci
3.2. Effect of Temperature on Cell Metabolic Profiles of the Bloom-Forming Diatom C. granii in Interaction with the Parasitic Oomycete
4. Conclusions
5. Materials and Methods
5.1. Strains and Culture Conditions for the Biological Experiments
5.2. Culture Conditions for the Biology Experiments
5.3. Metabolic Extractions of Diatom Cultures
5.4. Chromatography
5.5. Mass Spectrometry Analysis and Data Transformation
5.6. Metabolite Identification
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Vallet, M.; Pohnert, G. Temporal and spatial signaling mediating the balance of the plankton microbiome. Annual Review of Marine Science 2022, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Cirri, E.; Pohnert, G. Algae−bacteria interactions that balance the planktonic microbiome. New Phytologist 2019, 223, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Brisson, V.; et al. Dynamic Phaeodactylum tricornutum exometabolites shape surrounding bacterial communities. New Phytologist 2023, 239, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.R.; et al. Chemical ecology of the marine plankton. Natural Product Reports 2019, 36, 1093–1116. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; et al. The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiology Reviews 2013, 37, 462–476. [Google Scholar] [CrossRef]
- Chambouvet, A.; et al. Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science 2008, 322, 1254–1257. [Google Scholar] [CrossRef]
- Ilicic, D.; Grossart, H.-P. Basal parasitic fungi in marine food webs: A mystery yet to unravel. Journal of Fungi 2022, 8, 114. [Google Scholar] [CrossRef]
- Vallet, M. Chemical ecology of plankton parasites. Botanica Marina 2024. [Google Scholar] [CrossRef]
- Ismail, S.; et al. Temperature and intraspecific variation affect host–parasite interactions. Oecologia 2023. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern ocean phytoplankton in a changing climate. Frontiers in Marine Science, 2017. Frontiers in Marine Science 2017, 4. [Google Scholar]
- Basu, S.; Mackey, K.R.M. Phytoplankton as key mediators of the biological carbon pump: their responses to a changing climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef]
- Godwin, S.C.; et al. Increasing temperatures accentuate negative fitness consequences of a marine parasite. Scientific Reports 2020, 10, 18467. [Google Scholar] [CrossRef] [PubMed]
- Hector, T.E.; et al. Symbiosis and host responses to heating. Trends in Ecology & Evolution 2022, 37, 611–624. [Google Scholar]
- Schampera, C.; et al. Parasites do not adapt to elevated temperature, as evidenced from experimental evolution of a phytoplankton–fungus system. Biology Letters 2022, 18, 20210560. [Google Scholar] [CrossRef]
- Wood, C.L.; et al. A reconstruction of parasite burden reveals one century of climate-associated parasite decline. Proceedings of the National Academy of Sciences 2023, 120, e2211903120. [Google Scholar] [CrossRef]
- Vallet, M.; et al. The oomycete Lagenisma coscinodisci hijacks host alkaloid synthesis during infection of a marine diatom. Nature Communications 2019, 10, 4938. [Google Scholar] [CrossRef]
- Vallet, M.; et al. Single-cell metabolome profiling for phenotyping parasitic diseases in phytoplankton. Frontiers in Analytical Science, 2023. Frontiers in Analytical Science 2023, 2. [Google Scholar]
- Kafsack, B.F.; Llinás, M. Eating at the table of another: metabolomics of host-parasite interactions. Cell Host Microbe 2010, 7, 90–99. [Google Scholar] [CrossRef]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding diatom cell wall silicification—Moving forward. Frontiers in Marine Science 2018, 5. [Google Scholar] [CrossRef]
- Kuhlisch, C.; Pohnert, G. Metabolomics in chemical ecology. Natural Product Reports 2015, 32, 937–955. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Natural Product Reports 2002, 19, 108–122. [Google Scholar] [PubMed]
- OBIS. Ocean Biodiversity Information System. 2024; Available from: www.obis.org.
- Huang, H.; et al. Genetic diversity and geographical distribution of the Red Tide species Coscinodiscus granii revealed using a high-resolution molecular marker. Microorganisms 2022, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Holfeld, H. Relative abundance, rate of increase, and fungal infections of freshwater phytoplankton. Journal of Plankton Research 2000, 22, 987–995. [Google Scholar] [CrossRef]
- Schmitt, M.; et al. Temperature affects the biological control of dinoflagellates by the generalist parasitoid Parvilucifera rostrata. Microorganisms 2022, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; et al. Robust warming pattern of global subtropical oceans and its mechanism. Journal of Climate 2015, 28, 8574–8584. [Google Scholar] [CrossRef]
- Thomas, M.K.; et al. A global pattern of thermal adaptation in marine phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef]
- Wetsteyn, L.P.M.J.; Peperzak, L. Field observations in the Oosterschelde (The Netherlands) on Coscinodiscus concinnus and Coscinodiscus granii (Bacillariophyceae) infected by the marine fungus Lagenisma coscinodisci (Oomycetes). Hydrobiological Bulletin 1991, 25, 15–21. [Google Scholar] [CrossRef]
- Liang, Y.; et al. Molecular mechanisms of temperature acclimation and adaptation in marine diatoms. The ISME Journal 2019, 13, 2415–2425. [Google Scholar] [CrossRef]
- Case, R.J.; et al. Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environmental Microbiology 2011, 13, 529–537. [Google Scholar] [CrossRef]
- Demory, D.; et al. Temperature is a key factor in Micromonas–virus interactions. The ISME Journal 2017, 11, 601–612. [Google Scholar] [CrossRef]
- Azizah, M.; Pohnert, G. Orchestrated response of intracellular zwitterionic metabolites in stress adaptation of the halophilic heterotrophic bacterium Pelagibaca bermudensis. Mar Drugs, 2022. 20.
- Garcés, E.; et al. Host-released dimethylsulphide activates the dinoflagellate parasitoid Parvilucifera sinerae. The ISME Journal 2013, 7, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Barak-Gavish, N.; et al. Bacterial virulence against an oceanic bloom-forming phytoplankter is mediated by algal DMSP. Science Advances 2018, 4, eaau5716. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; et al. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science 2010, 329, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Garren, M.; et al. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. The ISME Journal 2013, 8, 999–1007. [Google Scholar] [CrossRef]
- Fenizia, S.; Weissflog, J.; Pohnert, G. Cysteinolic acid is a widely distributed compatible solute of marine microalgae. Marine Drugs 2021, 19, 683. [Google Scholar] [CrossRef]
- Azizah, M.; Pohnert, G. 2-Homoectoine: An additional member of the ectoine family from phyto- and bacterioplankton involved in osmoadaptation. Journal of Natural Products 2024, 87, 50–57. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I. , Lipid metabolism in microalgae, in The Physiology of Microalgae, M.A. Borowitzka, J. Beardall, and J.A. Raven, Editors. 2016, Springer International Publishing: Cham. p. 413-484.
- Nikitashina, V.; Stettin, D.; Pohnert, G. Metabolic adaptation of diatoms to hypersalinity. Phytochemistry 2022, 201, 113267. [Google Scholar] [CrossRef]
- Imazaki, A.; et al. Contribution of peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata. Eukaryotic Cell 2010, 9, 682–694. [Google Scholar] [CrossRef]
- Blasio, M.; Balzano, S. Fatty acids derivatives from eukaryotic microalgae, pathways and potential applications. Front Microbiol 2021, 12, 718933. [Google Scholar] [CrossRef]
- Wen, Z.-Y.; Chen, F. Application of statistically-based experimental designs for the optimization of eicosapentaenoic acid production by the diatom Nitzschia laevis. Biotechnology and Bioengineering 2001, 75, 159–169. [Google Scholar] [CrossRef]
- Hu, H.; Gao, K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnology Letters 2006, 28, 987–992. [Google Scholar] [PubMed]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agriculture and Natural Resources 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Jeyakumar, B.; Asha, D.; Varalakshmi, P.; Kathiresan, S. Nitrogen repletion favors cellular metabolism and improves eicosapentaenoic acid production in the marine microalga Isochrysis sp. CASA CC 101. Algal Research 2020, 47, 101877. [Google Scholar]
- Decelle, J.; et al. Intracellular development and impact of a marine eukaryotic parasite on its zombified microalgal host. The ISME Journal 2022, 16, 2348–2359. [Google Scholar] [CrossRef]
- Dakora, F.D.; Matiru, V.; Kanu, A.S. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants. Frontiers in Plant Science, 2015. 6.
- Rajamani, S.; et al. The vitamin riboflavin and its derivative lumichrome activate the LasR bacterial quorum-sensing receptor. Molecular Plant-Microbe Interactions® 2008, 21, 1184–1192. [Google Scholar] [CrossRef]
- Tsukamoto, S.; et al. Lumichrome. European Journal of Biochemistry 1999, 264, 785–789. [Google Scholar] [CrossRef]
- Ahmad, I.; et al. Photolysis of riboflavin in aqueous solution: a kinetic study. International Journal of Pharmaceutics 2004, 280, 199–208. [Google Scholar] [CrossRef]
- Brisson, V.; et al. Identification of effector metabolites using exometabolite profiling of diverse microalgae. mSystems 2021, 6, e00835–e21. [Google Scholar] [CrossRef]
- Lopez, B.R.; et al. Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal Research 2019, 44, 101696. [Google Scholar] [CrossRef]
- Peng, H.; Bashan, L.E.D.; Higgins, B.T. Comparison of algae growth and symbiotic mechanisms in the presence of plant growth promoting bacteria and non-plant growth promoting bacteria. Algal Research 2021, 53, 102156. [Google Scholar] [CrossRef]
- Kanu, S.; Dakora, F.D. Thin-layer chromatographic analysis of lumichrome, riboflavin and indole acetic acid in cell-free culture filtrate of Psoralea nodule bacteria grown at different pH, salinity and temperature regimes. Symbiosis 2009, 48, 173–181. [Google Scholar] [CrossRef]
- Maier, I.; Calenberg, M. Effect of extracellular Ca2+ and Ca2+-antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (Phaeophyceae). Botanica Acta 1994, 107, 451–460. [Google Scholar] [CrossRef]
- Wang, M.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature Biotechnology 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




