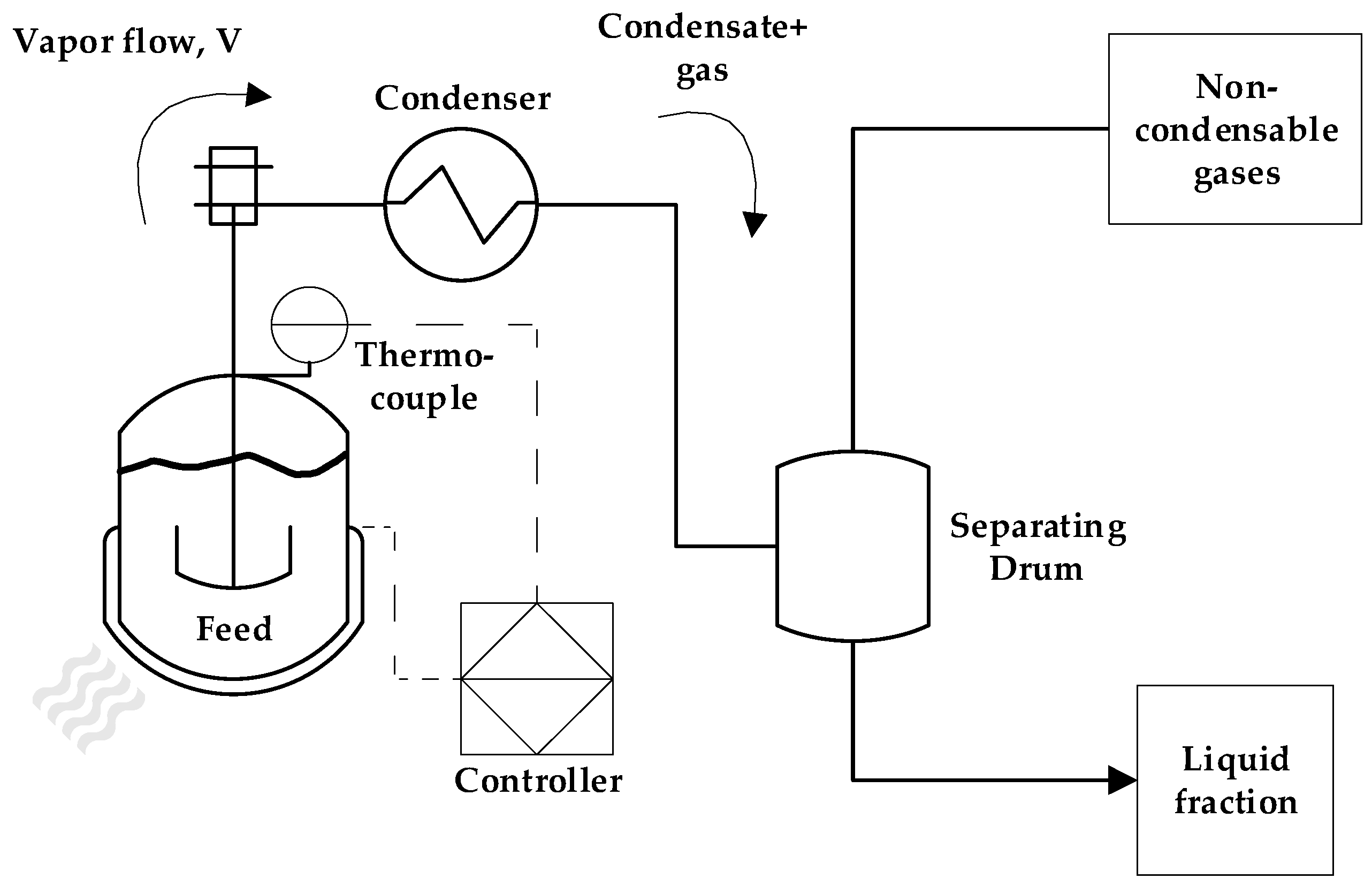
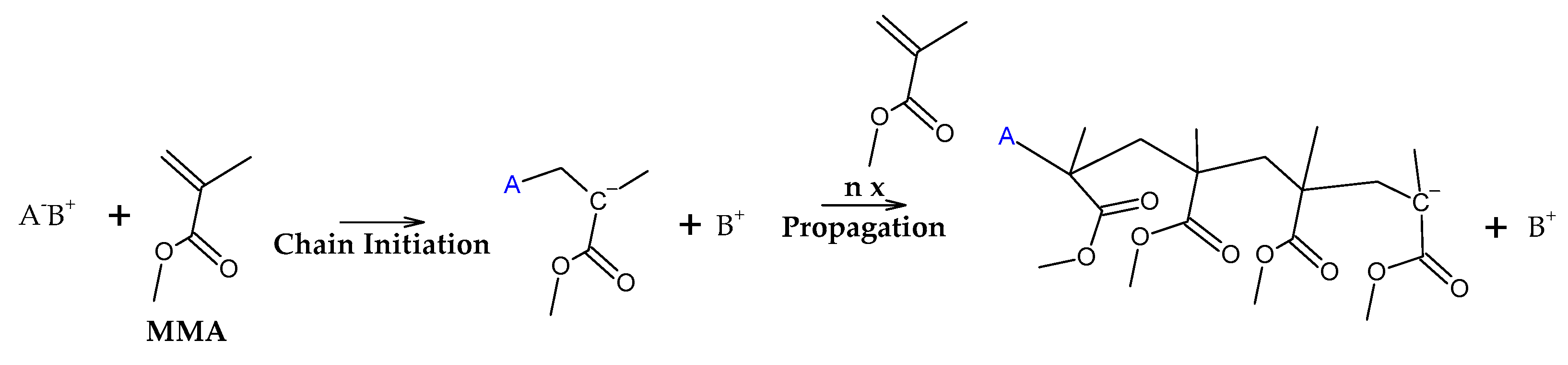
3.1. Product Yields
Table 1 presents the main findings in respect to product yields obtained by depolymerization considering different temperatures. It can be seen that liquid phase yields tend to decrease with increase in temperature, gas phase yields tend to increase and solid phase tend to decrease until a minimum is reached. Similar findings are reported in academic literature [
22,
23,
24,
26,
27]. Achilias et al. reported that increasing temperature above 450 °C increased gas yields and lowered liquid yields [
22]. Braido et. al. found only a minor increase to gas yields when polymerizing crosslinked PMMA from dental waste when increasing process temperature [
23]. The presence of crosslinking in PMMA is said to increase gas formation and unwanted carbon [
17] and Braido and collaborators reported that crosslinked PMMA did not melt during pyrolysis while the homopolymer tested did it, but they attributed the small increase in gas yields with process temperature due to the excellent properties of heat transfer of the small-scale reactor. In the larger-scale technical unit, considerable amount of char was produced (~25 wt.%) and reduced liquid yields (~65 wt.%) when compared with laboratory-scale reaction (~90 wt.% liquid yield) [
23]. Santos et al. pyrolyzed dental waste in a pilot-scale 143 L fixed-bed reactor and reported that liquid yield shows a first-order decay function with temperature and found increased gas yields (~30 wt.%) when compared to the results obtained by Braido et al. in technical scale (~6 wt.% gas yields). The char yields obtained by Santos et al. were in the range of 11-14 wt.%, lower than Braido et. al, but higher than the ones obtained in this work for a technical-scale unit, where higher temperatures were used for conversion, char yields of ~2-3 wt.% was obtained for temperatures higher than 425 °C [
24]. Braido et. al. commented that the depolymerization was not complete in technical-scale after 1 hour of reaction and a higher number of impurities was found in the reactor in technical scale when compared to lab-scale reactor, indicating that higher temperatures favors charring (polymerization to higher molecular weight hydrocarbons) of the crosslinked polymer and even though it is possible to increase liquid yield, it is not from the monomer MMA but of higher molecular weight products, such as aromatic hydrocarbons. This is not observed in lab-scale where heat transfer problems are negligible but become considerable when increasing process scale. The formed char can be further pyrolyzed by increase in process temperature, generating products in the gas phase, as it is observed in this work where higher temperatures of depolymerization (425-475 °C) was investigated when compared to Braido et al. (400 °C) [
23]. This effect is further augmented when a larger fixed bed is used as it was showed by Santos et. al, where large gas quantities and lower char yields were obtained in lower temperatures (345-420 °C) [
24]. Poudel et al. pyrolyzed artificial marble powder composed of filled PMMA and reported that increased temperature reduces the recovery of MMA from liquid-phase due to the presence of impurities, even though an increase in liquid yield occurs with temperature, pointing to the necessity of homogeneous temperature distribution in the reactor for maximization of the recovery of monomer [
26]. For fluidized bed reactors, wher
It is interesting to note that current knowledge of reaction kinetics and mechanism of PMMA depolymerization focus heavily in the type of polymerization and initiation, depropagation and termination steps of depolymerization for production of monomer, but little attention is given to why a char residue may be left in the reactor [
30]. It is known in academic literature that PMMA fabricated via a free-radical polymerization mechanism (the most common) decomposes via chain-end initiation and/or mid-chain random scission and depropagate to the monomer until a termination reaction occurs via disproportionation, recombination or chain-transfer to the solvent [
30,
31,
32,
33,
34]. The average number of depropagation events between an initiation and termination event is called the zip length and it depends on the ratio of initiation and termination to propagation reaction rates constants and can be expressed as the probability p of a depropagation event over a termination event after initiation [
30] defined by Equation (2). The zip length (1/ε) can be expressed in terms of this probability as in Equation (3).
For depolymerization reactions where the zip length is larger than the degree of polymerization, the polymer chain reacts fully, leaving no smaller species behind and consequently no char. If the zip length is lower, then a longer polymer residue is left behind. Depending on the dispersity of molecular mass of the reacting polymer and type of termination (disproportionation or recombination), this residue may be collected in solid, liquid or gas phases [
30,
32]. But this mechanism does not take into account competitive side reactions, such as polymer charring in the case of thermal decomposition of PMMA. The overall reaction represents a complex sequence of reactions occurring during high-temperature pyrolysis of PMMA, generating solid carbon and gases such as aromatic hydrocarbons, CO, CO
2 and H
2. These reactions occur between pyrolysis products, mainly through aromatization and condensation reactions but also gasification and reduction reactions in high-temperature zones. To some extent, may occur with any carbonaceous feedstock in the reactor [
35,
36,
37,
38]. This phenomenon changes the composition of the polymeric chain during semi-batch depolymerization and virtually stops the unzipping and depropagation to the monomer when a certain temperature is reached. This could explain why some polymers tend to stop the depolymerization reaction before even the monomer equilibrium concentration, i.e., the point where propagation and depropagation reactions reach the same reaction rate [
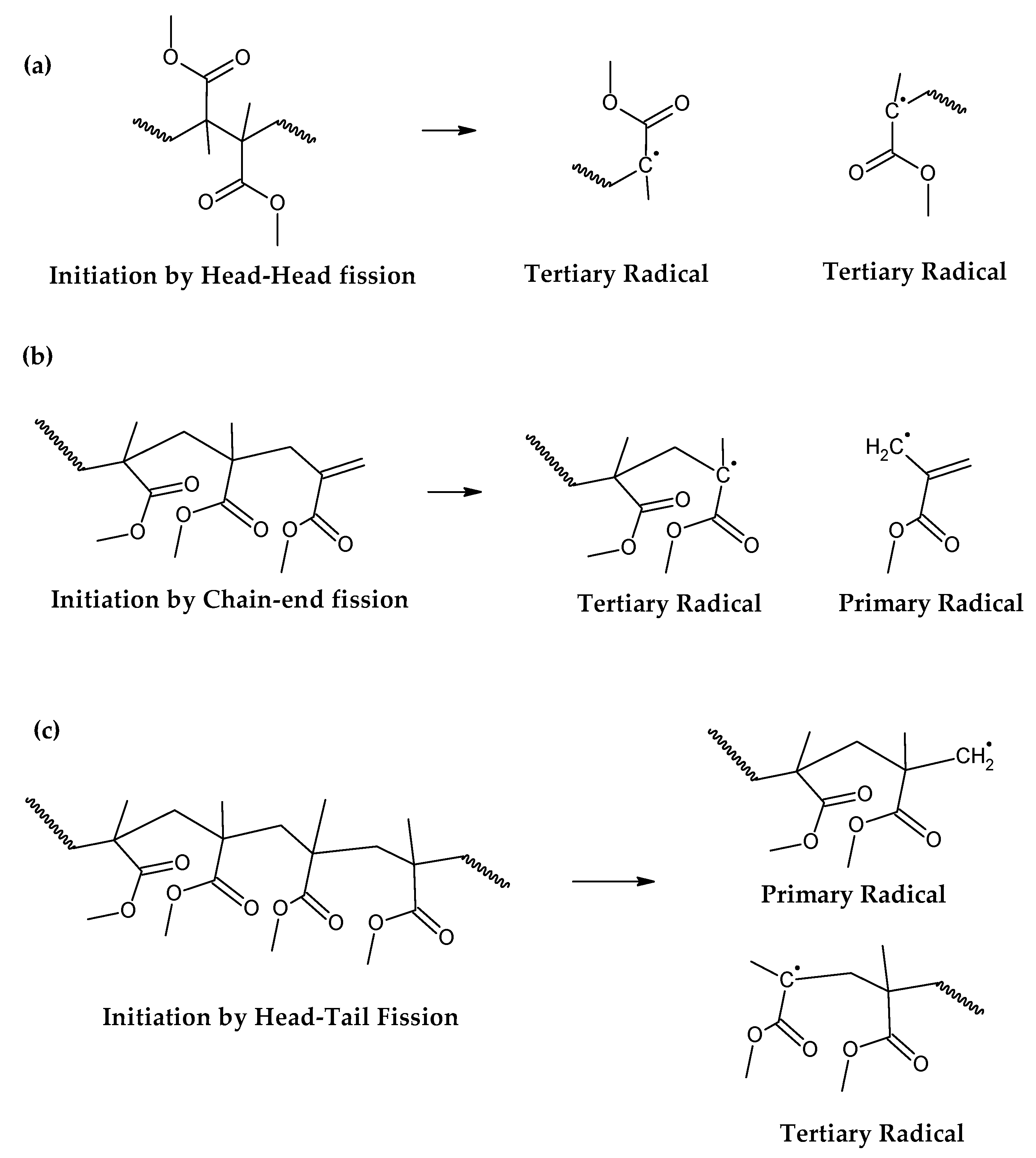
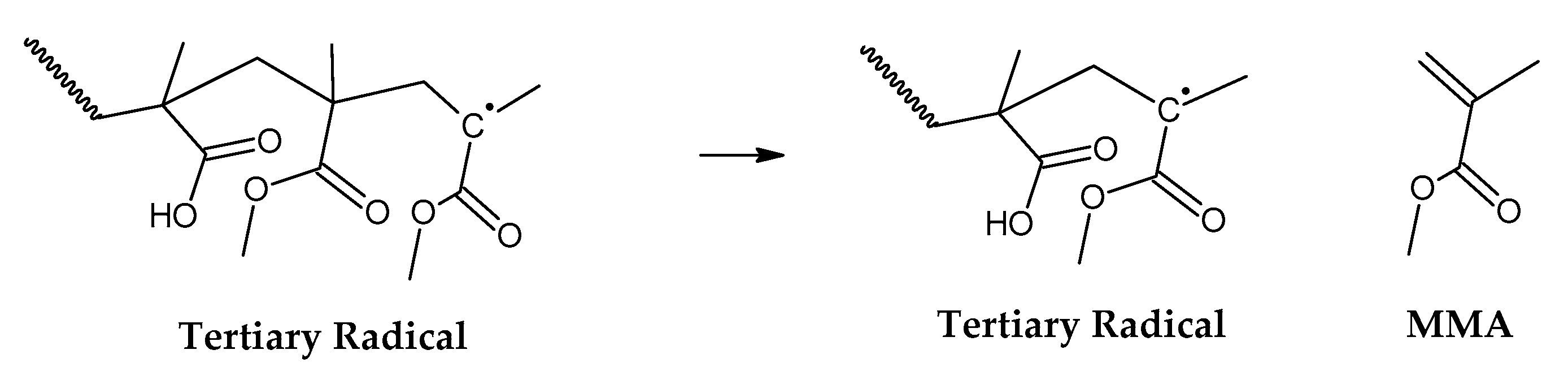
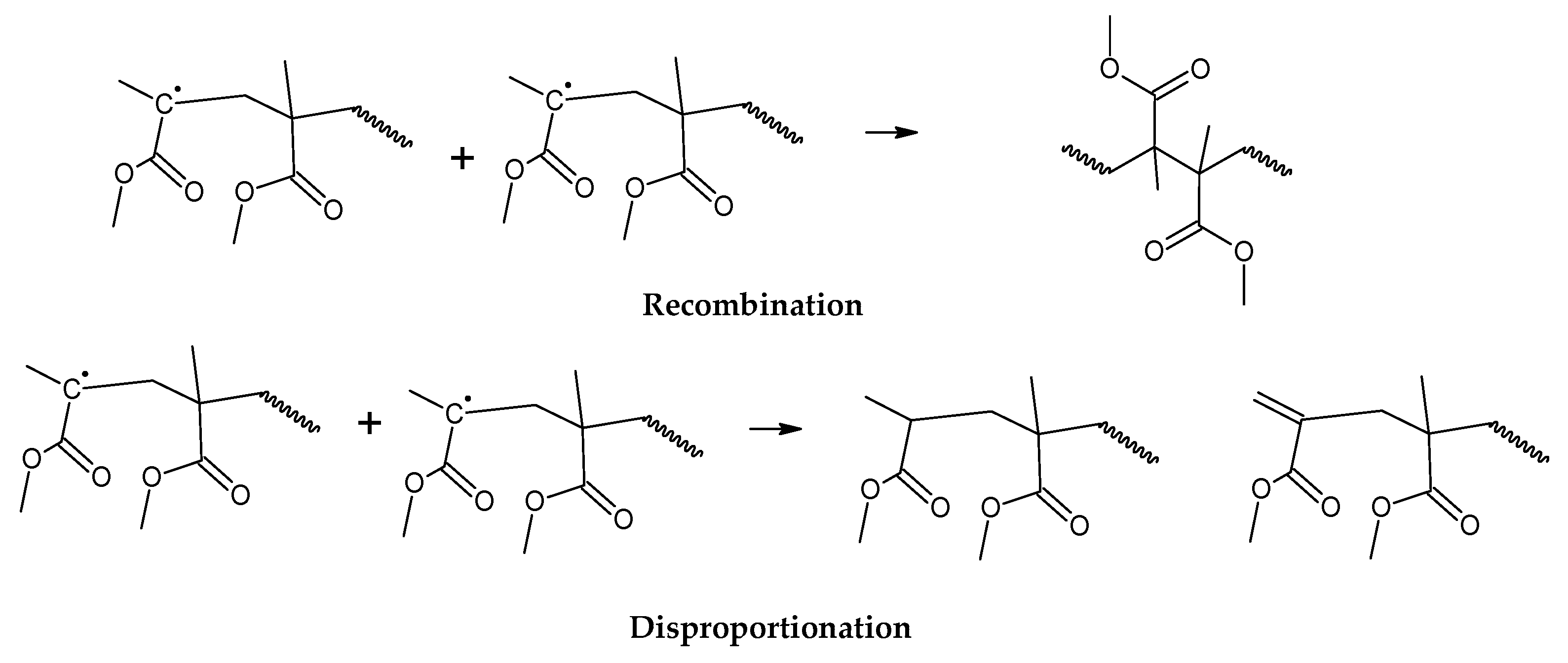
30]. The mechanism of thermal decomposition of PMMA, composed of initiation, depropagation and termination reactions is shown in
Figure 2,
Figure 3 and
Figure 4, respectively. The most popular charring reactions are shown in
Figure 5. It is important to note that termination of reaction via chain-transfer to the solvent is not possible during thermal depolymerization of PMMA in solid phase and it is not shown in mechanism of
Figure 4.
The initiation reactions are shown in increasing bond energy. A head-to-head linkage shown as (a) in
Figure 2, is the lower in bond energy and it is the most likely to break during thermal decomposition. It is formed by termination by recombination of the polymer chain and it happens 20% of the time during FRP polymerization [
38]. FRP-made polymers degrade in lower temperatures (140-200 °C) but this type of termination is not present in anionic polymerized PMMA, where propagation occurs almost exclusively by H-T propagation and termination only occurs deliberately and no H-H linkages are formed [
38]. The mechanism of anionic polymerization is shown in
Figure 5. The second most likely bond cleavage is at vinyl chain-ends, formed during termination by disproportionation, also not present in anionic polymerization. This type of initiation (
Figure 2b) usually occurs around 300-323 °C, lower than for random H-T fission (327-402 °C) (
Figure 2c), where usually most of the decomposition is observed in mass loss of thermogravimetry experiment.
Independently of the type of polymerization used to fabricate the polymer, due to the different energies present in different linkages, it is possible to obtain an approximate proportion of linkage types in determined polymer by thermogravimetry experiments, where a controlled mass of polymer is degraded in different temperatures at an inert atmosphere and it is observed the mass loss (or its derivative) at each respective temperature of decomposition (TGA/DTG). Due to the small mass used (~5 mg), heat transfer and diffusion effects are negligible in thermal decomposition and chemical effects are maximized, i.e., the parameters observed are largely due to linkages and substances present in the sample [
39].
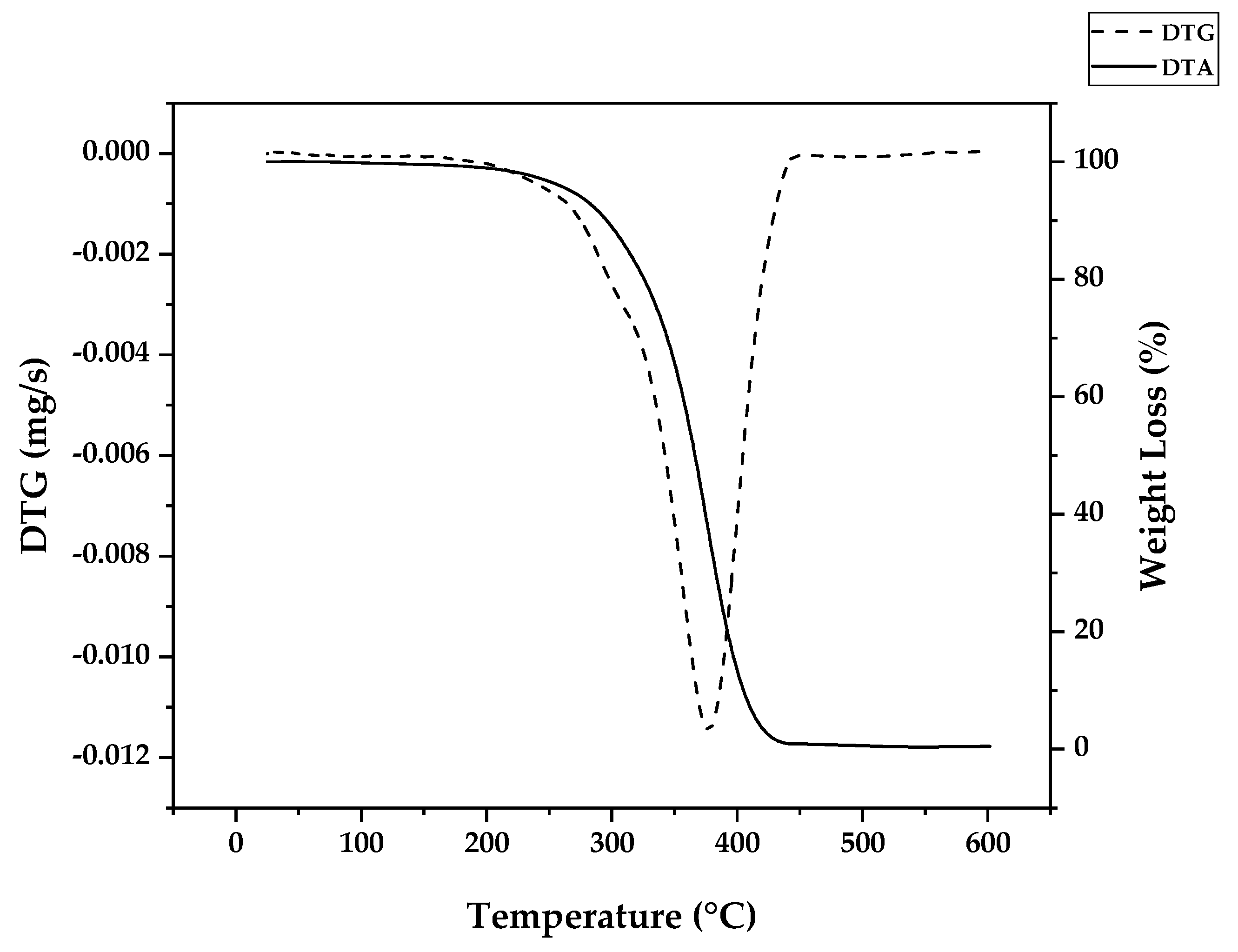
Figure 6 shows the weight loss of PMMA dental waste obtained by conducting a TGA/DTG experiment using 10 °C/min of heating rate, nitrogen atmosphere and maximum temperature of 600 °C. The DTG curve shows a peak of maximum decomposition at temperature of 376 °C indicating that most of the thermal degradation happens because of random H-T fission. Indeed, for FRP or anionic polymerization, most of the bonds in high molecular weights polymer chains are H-T linkages. But, from the weight loss curve, one can observe that thermal degradation started as early as 300 °C, pointing to the presence of vinyl chain-ends formed due to disproportionation in FRP, supposedly not present for anionic polymerization. Nevertheless, no decomposition was observed before this temperature, indicating that no H-H linkages were present due to termination by recombination, supposedly happening around 20% of the time with FRP. This effect could be suppressed by the presence of crosslinking agent EGDMA, giving thermal stability to the linear chain of polymer [
31].
Da Ros et al. modelled the kinetics of thermal depolymerization of PMMA dental waste containing 5 wt.% EGDMA as crosslinking agent. They compared thermograms with pure PMMA and concluded that EGDMA-crosslinked PMMA degrades mainly through random H-T fission, suppressing early degradation by H-H fission. While pure PMMA showed DTG curves with 3 clearly distinct peaks, for EGDMA-PMMA only two peaks of degradation were observed, a small peak between 250-300 °C (vinyl chain-end fission) and a much higher peak at 376 °C (random H-T fission) and the kinetics of depolymerization could be modelled using a consecutive 2-step reaction, dominated by the random H-T fission mechanism [
31]. This correlates well with the necessity of high degree of polymerization, i.e., a high molecular weight polymer, in dental cement to obtain a polymer with desired mechanical properties [
40,
41,
42]. In such a polymer, most of the bonds in the branched polymer chain are H-T bonds formed during monomer propagation. In fact, Da Ros et al. could model the depolymerization kinetics even when considering a one-step reaction due to dominance of random scission in the mechanism [
31]. When observing the thermogram from
Figure 6 (this work), one can observe that for this dental waste cement, only a clear distinct peak was observed but it was observed thermal degradation of 22 wt.% before a temperature of 330 °C was reached (temperature of vinyl chain-ends fission), indicating that most termination reactions in the FRP process of crosslinked PMMA was due to disproportionation. It seems that the branching of the polymer chain minimizes the formation of H-H linkages formation due to recombination reactions (or at least suppress its early breaking in thermal decomposition) and allows that only 22 % of the polymerization reactions are terminated and 78 % occurs until all polymers is consumed in the polymerization approximating the process of depolymerization of FRP PMMA to anionic polymerized PMMA and conferring thermal stability to the polymer. This makes it possible that the branched chain of PMMA decomposes through the depropagation mechanism (unzipping of the polymer chain) until all the polymer is converted in monomer. Since most of the depolymerization occurs due to random fission, it is speculated that the FRP allied to crosslinking minimizes the formation of chain-end groups.
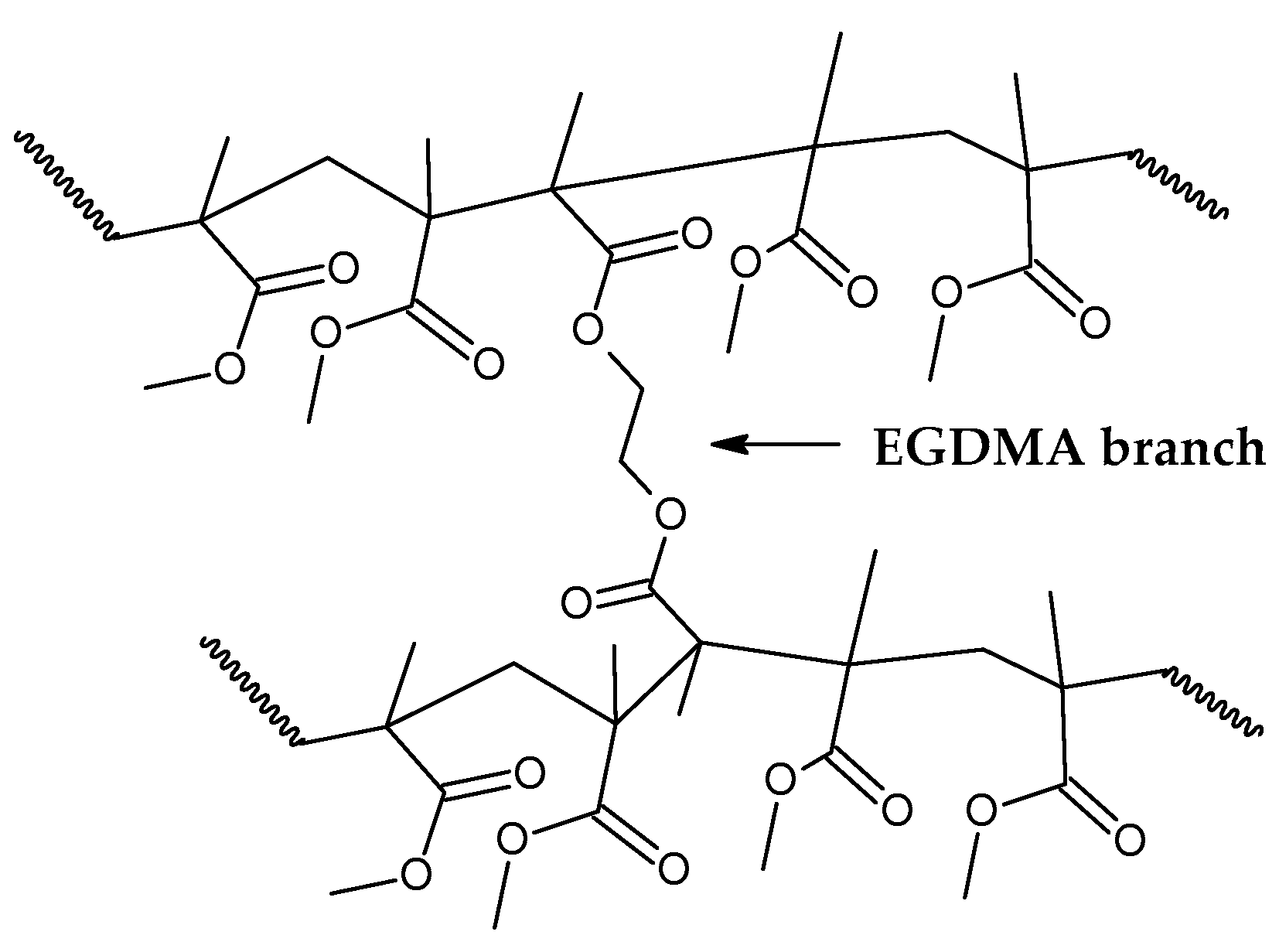
Figure 7 shows how the EGDMA inserts itself and create the increased elasticity and strength PMMA polymer [
40,
41,
42].
Indeed, branching of the polymer chain increases its molecular weight and improve properties such as low solubility in organic solvents and improved strength and elasticity [
41]. As it was suggested by thermal analysis and application of PMMA as dental cement, a high degree of polymerization is expected for this polymer, since most of the decomposition is happens due to H-T fission of the polymeric chain, indicating a high polymerization degree. This parameter (equation 4) is the number of monomer units in a polymer or oligomer molecule and it can be represented by the ratio of the number-average molecular weight of the polymer (
) to the molecular weight of the monomer unit (M
0). Dental resins are usually prepared by FRP of prepolymerized PMMA beads with benzoyl peroxide as initiator and liquid MMA as monomer. The prepolymerized beads usually presents high molecular weight (120000-996000) that is further increased after repolymerization [
41]. Since molecular weight of MMA is 100.177 g/mol, dental resins usually present degrees of polymerization higher than 120. For saturated chains of PMMA polymer, degradation rate (mass loss) by random scission is described by equations (5) and (6) for zip lengths higher and lower than the degree of polymerization, respectively. K
s is the rate constant for random chain scission (H-T fission) and γ is the reciprocal of the average zip length between initiation and termination reactions [
43].
If the zip length is lower than the degree of polymerization, it means that depropagation of polymer into monomer does not happen to completion, i.e., it terminates forming intermediary products of depolymerization. Observation of the thermogram from
Figure 6 reveals that virtually all PMMA dental waste is converted to gases (99.5% of weight loss) suggesting that, even if zip length of this depolymerization process is lower than DP, all terminated products are converted to gases before a temperature of 450 °C. If the zip length is lower than DP, the vapors composition should change from an enriched-MMA vapor to the pyrolysis products of the terminated products of the depolymerization reaction at later stages (higher temperatures). Beyond that, there is also the possibility of side-reactions between depolymerization products [
33] and charring reactions [
35,
36,
37,
38].
When one compares the results obtained in thermal analysis with the yields of products of depolymerization of PMMA dental waste in the technical unit of IME, some char is formed in the larger-scale unit (~2 wt.%), indicating that the overall depolymerization mechanism includes more reactions than what it is initially supposed for PMMA thermal degradation. Indeed, mechanisms of depolymerization are usually kinetically controlled and depends upon heat transfer and diffusion coefficients, being influenced by sample thickness [
33]. This means that other reactions could be involved and be determinant to final product distribution and composition. Experimentation with pyrolysis of carbonaceous materials usually point to higher char yields when using low temperature and heating rates [
35,
36,
37,
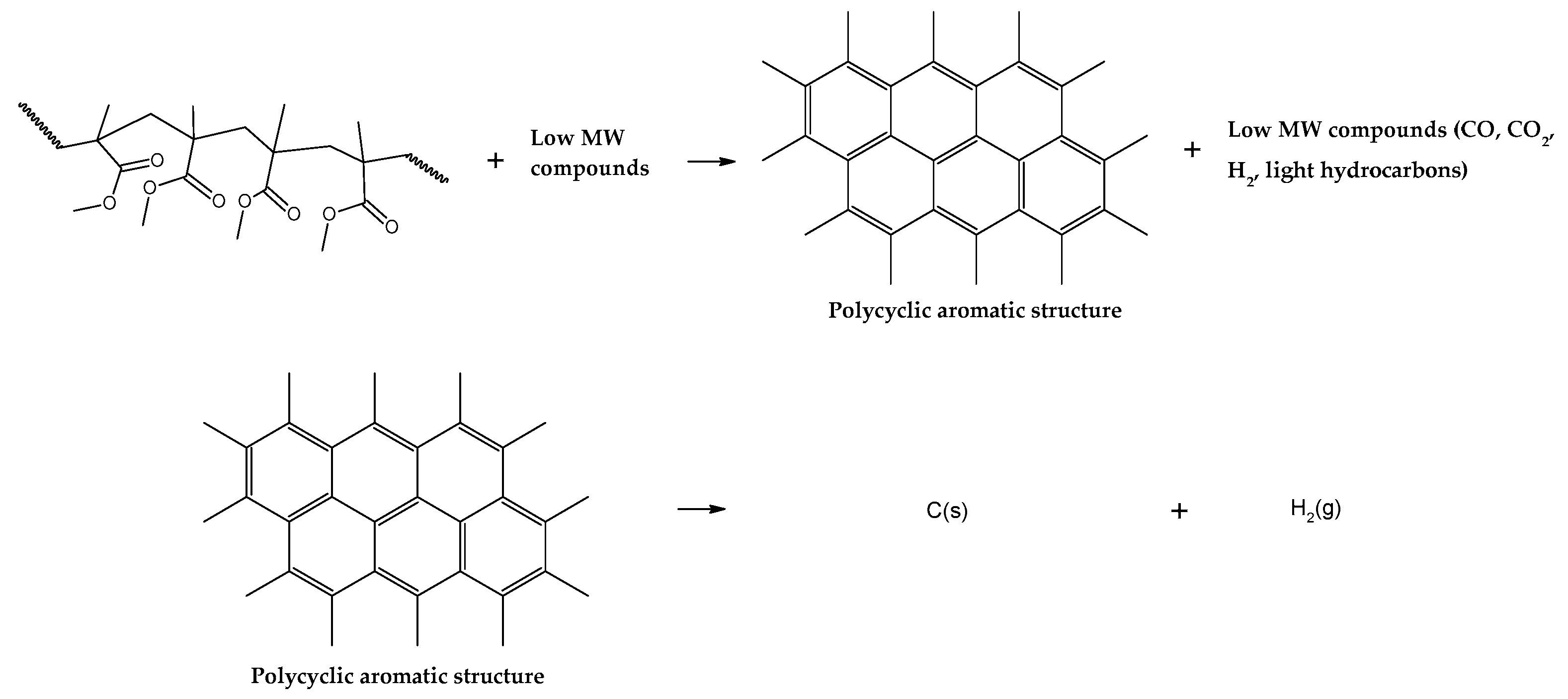
38]. Although not fully known, it is estimated that char is formed due to inter- and intramolecular rearrangements reactions, forming a polycyclic aromatic structure as in
Figure 8. Further reactions include hydrogen abstraction events, forming hydrogen gas and graphitic carbon. Due to the lower actual heating rate through the polymeric bed in the technical scale reactor, more char is formed than in the small-scale thermal reactor of TGA analysis.
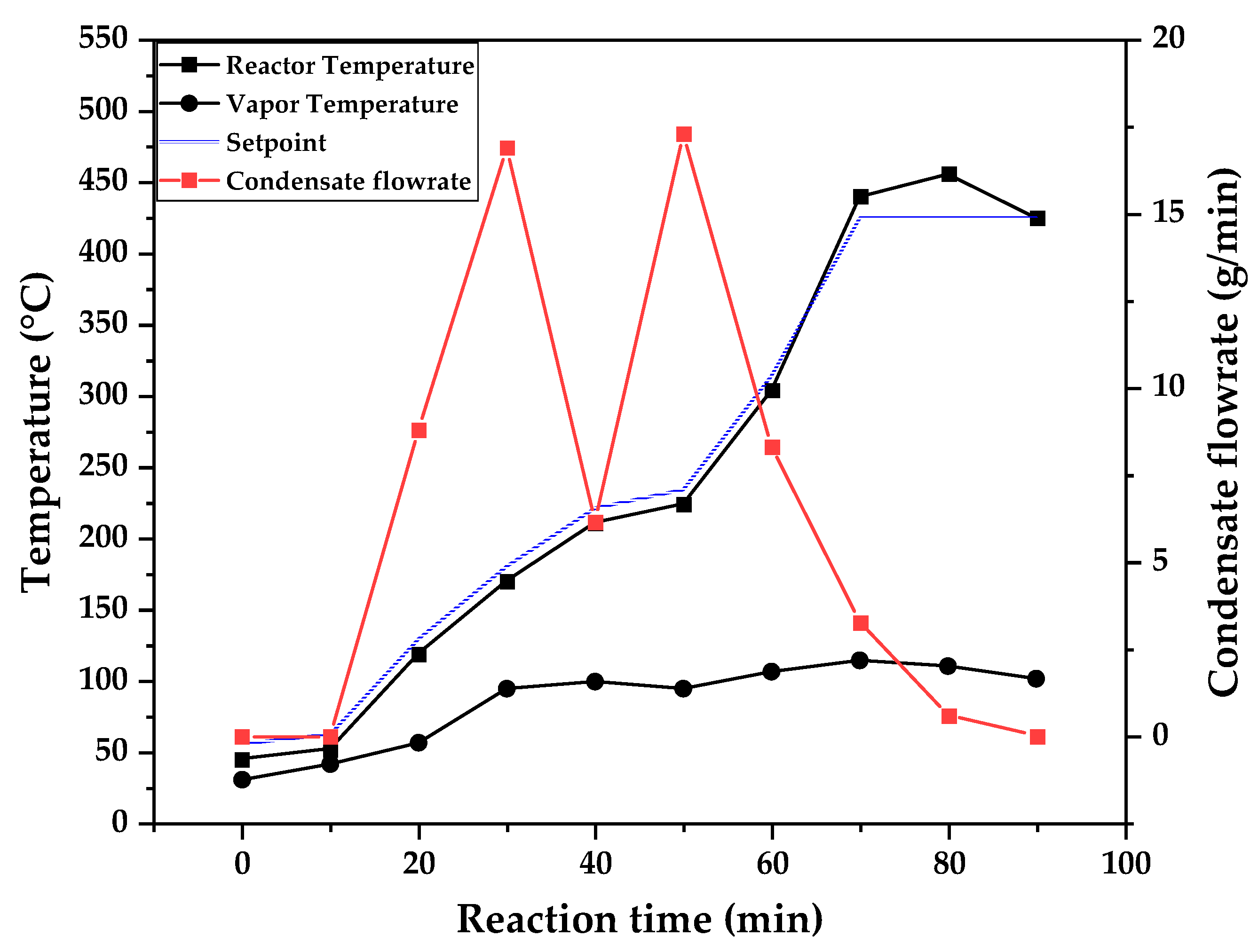
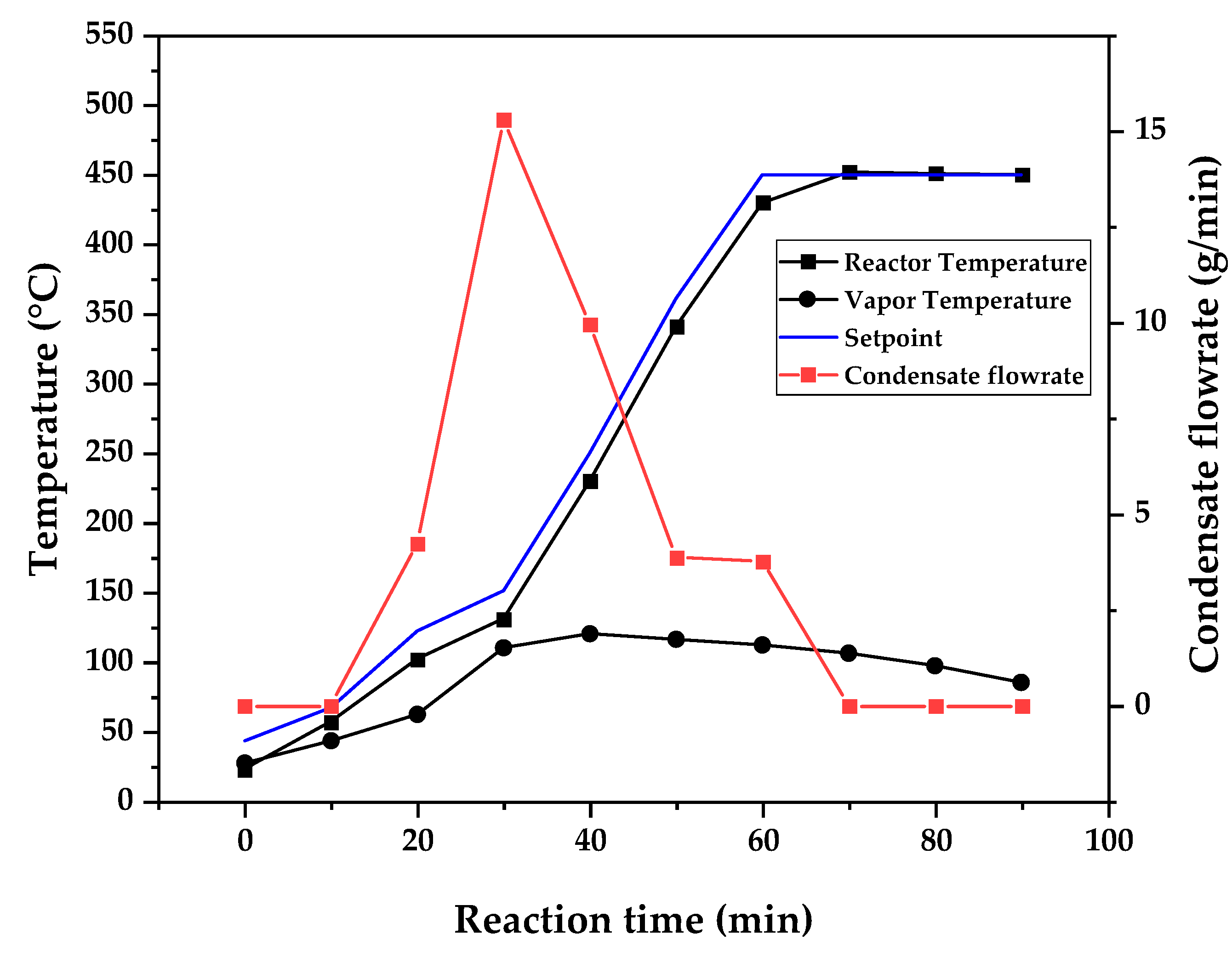
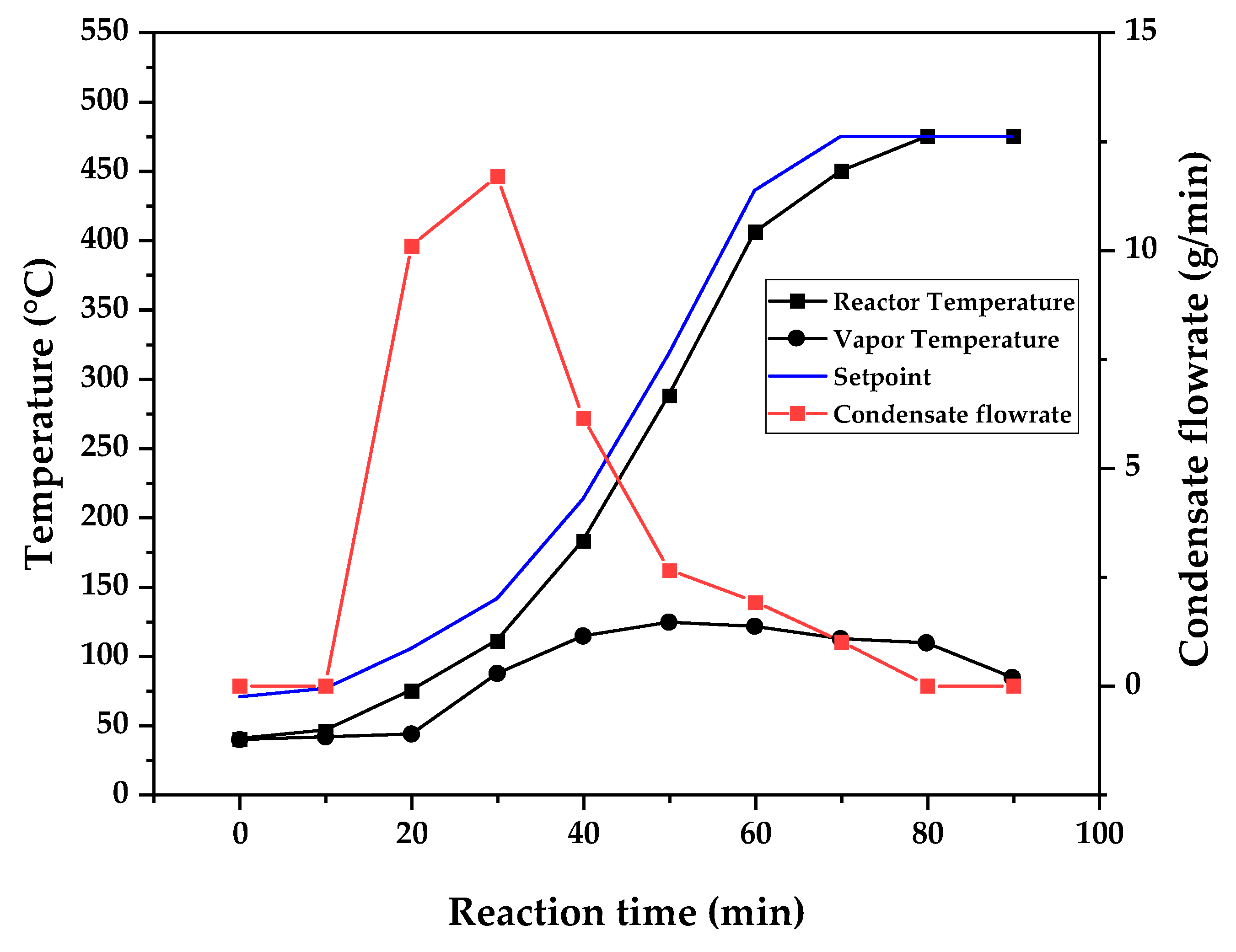
Figure 9,
Figure 10 and
Figure 11 shows the temperature profile of reactor and vapor temperature according to reaction time for PMMA dental waste depolymerization at 425, 450 and 475 °C, respectively. In order to effectively analyze the semi-batch depolymerization process, it was also included in the graphs of
Figure 9,
Figure 10 and
Figure 11, the vapor flow formed during the heated depolymerization reaction for each temperature. It can be observed that vapors started to form and condense in considerably lower temperature than what was observed in thermal analysis of
Figure 6, where thermal degradation (weight loss) started above 200 °C and for the technical scale unit, vapors started to form and condense in liquid state at 75 °C. We believe it to be an effect of the greater dimensions of the particles bed of polymer inside the technical reactor when compared to the thermobalance of the TGA analysis. Since the PMMA dental waste present poor heat transfer properties, a larger fixed bed of polymer creates a temperature gradient between the rim and center of the reactor, i.e., when the center presents a certain temperature, it is expected that the rims of the fixed bed are at higher temperatures. Since the reactor thermocouple is located at the center of the reactor, it probably showed lower temperatures at reaction beginning, stabilizing at a correct value in higher reaction times. Ribeiro et al. conducted PMMA depolymerization at different production scales and concluded that this effect is augmented for larger semi-batch reactors [
27]. By observing
Table 1, one can perceive that it also affects the products distribution in different phases. Even though it is possible to obtain high liquid phase yields in lab-scale (30g) PMMA dental waste depolymerization [
22,
23], only 81 wt.% liquid phase yield could be obtained for temperature of 425 °C in the technical scale unit and for higher temperatures (450 and 475 °C), this yield was much lower, around 62 wt.%.
This behavior seems correlated to the different regimes of heating used for each experiment. Even though temperature profiles of the three experiments were alike, including reaction time, condensate flowrates reduced with temperature increase due to formation of gaseous non-condensable substances, reducing liquid phase yield and increasing gaseous yields, as in
Table 1. It can be observed that for endothermic reactions being conducted in semi-batch reactors, almost no temperature increase will occur when an endothermic process is in place, in fact, added energy will be used to increase the rate of the endothermic processes, such as the depolymerization reaction and vaporization of formed products [
28,
29]. Only after those processes occur to completion, temperature starts to rise again to desired setpoint. In fact, the different temperatures used in the experiments actually represent different heating rates and final temperatures of reaction. In
Figure 9,
Figure 10 and
Figure 11, the setpoint curve represents the temperature chosen by the operator to indirectly set the heating rate used. This is done by choosing setpoints with different temperature errors from actual reactor temperature, of 10, 20 and 30 °C for the experiments of 425, 450 and 475 °C, respectively. This was done in order to achieve different profiles of heating from the heating element providing the driving force of the chemical reaction happening. From the relay indication of the control panel, it is possible to visualize if power is supplied to the heating element. The amount of power of the heating element is controlled by the amount of time it receives electrical current and due to the configuration of the controller, is set to operate at different levels depending the temperature error difference (T
e) between setpoint and actual reactor temperature. For the technical scale reactor, this error difference was set to 20 °C, causing it to operate under three different modes:
1 – Te < 10 °C → Heating element provides power to increase slowly the temperature and maintain it once reaction starts;
2 – 10 °C < Te < 20 °C → Heating element provides power more frequently, increasing reaction rate;
3 – Te > 20 °C → Heating element is always on;
In
Figure 9,
Figure 10 and
Figure 11, liquid condensate flowrate reduced with increased processing temperature but the actual flowrate of vapors probably increased due to formation of more non-condensable gases. Increased heating rate was able to further react the MMA formed during PMMA dental waste depropagation into different reaction products, mostly in the gas phase, probably in the form of carbon dioxide and light hydrocarbons. Further testing of the composition of the gas phase is needed in order to obtain detailed characteristics of these secondary reactions.
3.2. Chemical Composition Analysis
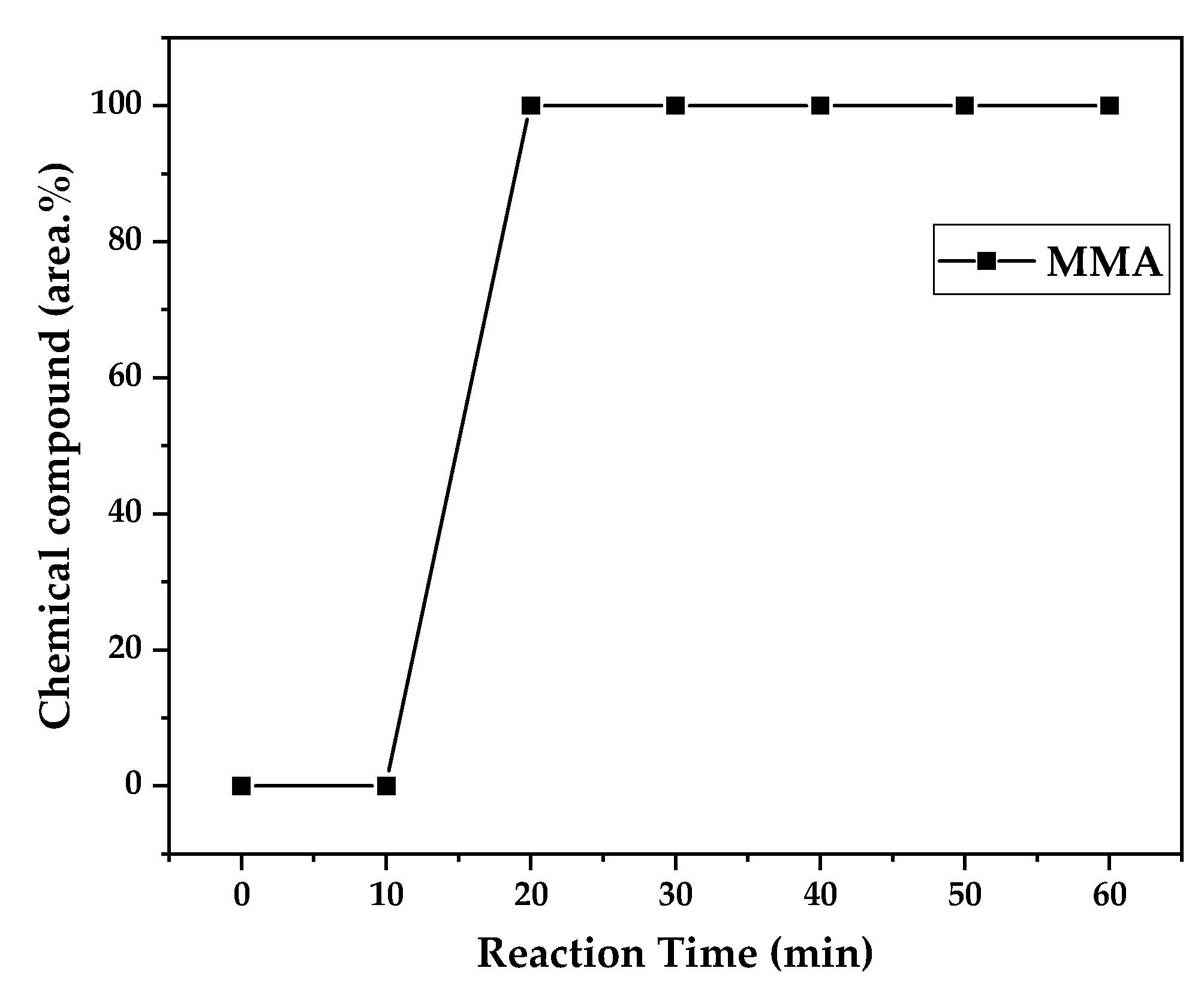
Figure 12,
Figure 13 and
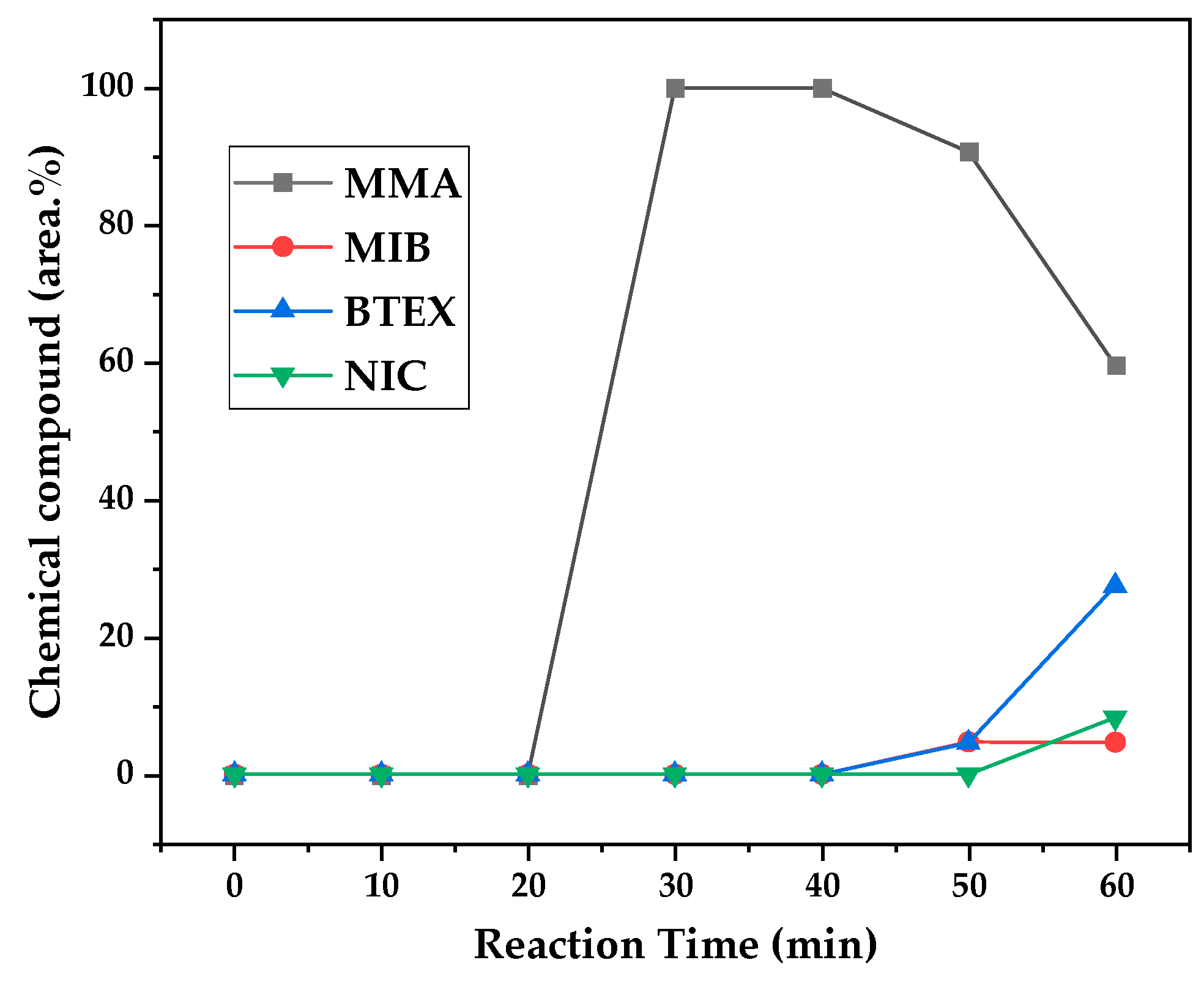
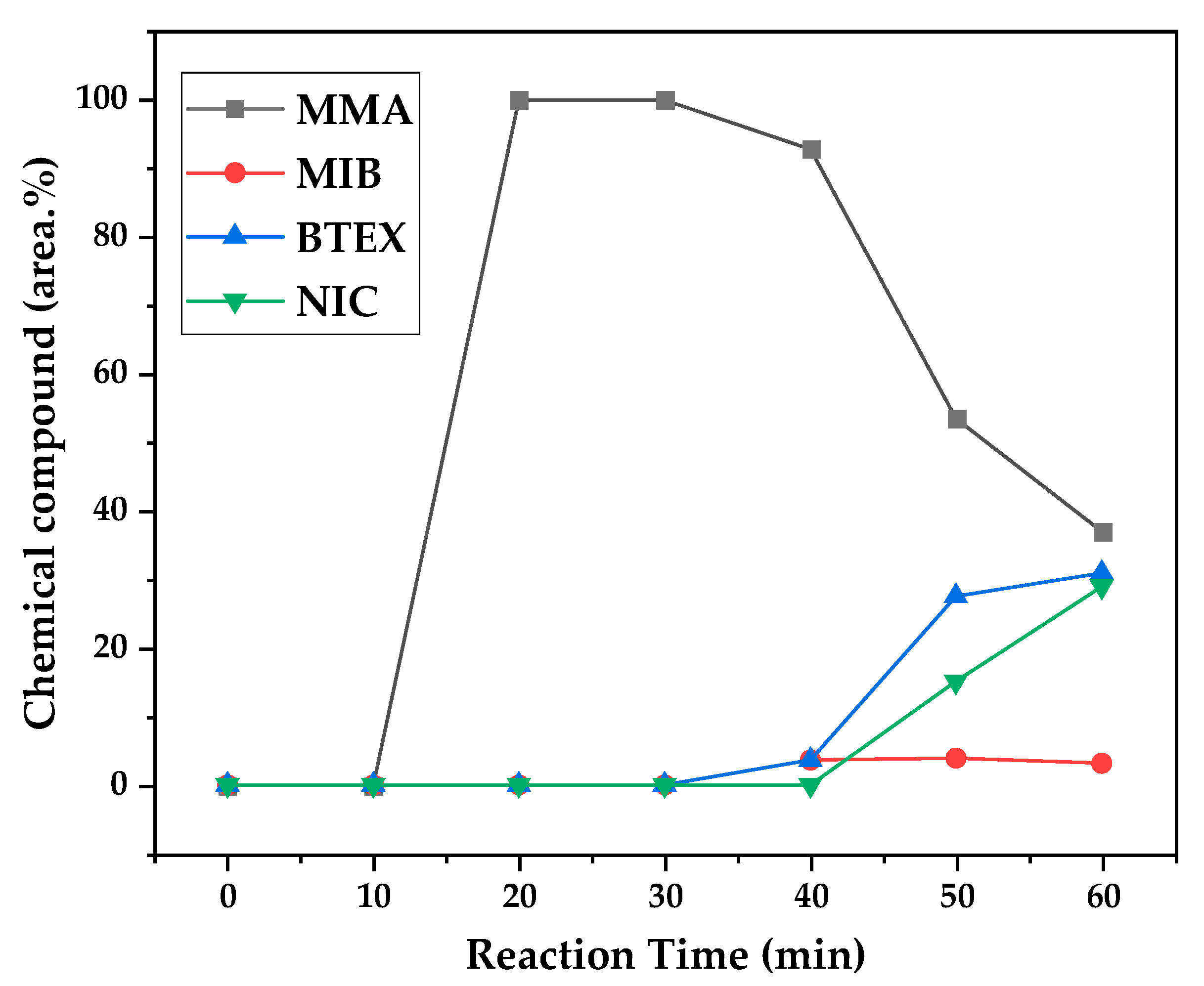
Figure 14 presents graphs of how chemical composition of the liquid product formed during PMMA dental waste depolymerization changed over reaction time in the three temperatures of depolymerization. Initially, liquid product is composed by the monomer MMA, being the only product of depolymerization in low temperature and reaction times, corroborating the established depolymerization mechanisms of PMMA, where most of the vapor product formed is due to the depropagation reaction, producing monomer MMA. As the reaction proceeds, since it is a semi-batch reactor, the degree of polymerization of the remaining feed in the reactor reduces over time but also the zip length due to the diminishing length of the polymer chain. Besides, the polymer chains are also transformed into other products due to condensation and aromatization reactions, turning into char and other cracking products of the char pyrolysis. Higher heating rates also can increase or accelerate some side-reactions, increasing product complexity and distribution. These reactions compete with the depropagation of the polymer chain into monomer and eventually other products are formed in the reactor and vaporize, condensing into the liquid product of depolymerization. This can be seen in
Figure 13 and
Figure 14 for the reactions conducted at 450 and 475 °C, respectively. After 20 minutes of liquid condensation, the composition starts to change from 100 area.% of MMA to side products as Methyl isobutyrate (MIB), aromatic hydrocarbons as benzene, toluene, xylene (BTEX) and other complex compounds that could not be accurately identified, supposedly hydrocarbons and oxygenated compounds with long chains of carbons (char and tar precursors) [
35].
As it was seen in the previous section, the mechanism of depolymerization is affected by the heating rate used and it is related to the final temperature of reaction in the technical scale unit. By observing the temperature profiles of
Figure 10 and
Figure 11, higher heating rates produced lower liquid condensate yields but the chemical composition of the obtained liquid fraction shows that for most of the time, it was majorly composed of MMA, indicating that, initially, most of the secondary products formed were gases, being in the non-condensable gases phase and were formed due to secondary cracking reactions of the diffusing MMA through the heated polymeric bed. For the experiment conducted at 425 °C, liquid product was composed of 100 area.% of MMA and no side products were formed in liquid phase with a char yield of 9.44 wt.%. For the experiments using higher heating rate and final temperature of
Figure 13 and
Figure 14 (450 and 475 °C), lower char yields were obtained (~2.5 wt.%) but increased presence of side-products, showing that both the final temperature and heating rate influences the formation of char and side-products.
The presence of these side-products in the vapor composition of PMMA dental waste depolymerization can also be visualized by the vapor temperature showed in
Figure 9,
Figure 10 and
Figure 11. It is possible to observe that vapor temperature tends to maintain its value near the boiling point of the vaporized mixture of substances coming out of the heated reactor. Since most of the liquid condensate is MMA, values around 101 °C should be observed (boiling point of MMA). For the experiment of
Figure 9 (425 °C), maximum vapor temperature was 114 °C (70 minutes of reaction), but most of the liquid was formed under 100 °C. For
Figure 10 (450 °C) and
Figure 11 (475 °C), vapor temperature increased to over 110 °C at 30 and 40 minutes of reaction, respectively and maximum temperatures were 120 and 124 °C. Chemical composition of
Figure 13 and
Figure 14 reveal that vapor temperature increased due to the presence of side-products in liquid condensate, formed after 30 minutes of reaction.
The mechanism of depolymerization is also affected by the existing kinetics in the reactor, i.e., the heat transfer and diffusion of formed products through the polymer particle’s bed. In small-scale and miniaturized reactors (such as the thermobalance of TGA/DTG analysis), the geometry of the particle’s bed is small compared to the heat transfer and diffusion needed. As the size of the reactor increases, the effect of heat transfer and diffusion also increases, changing reaction rates and product composition [
27]. Since the temperature controller thermocouple is located in a thermal well near the center of the particle bed, there is probably a temperature gradient from the rim to the center of the cylindrical reactor, meaning that the actual temperature at the rims should be higher than the setpoint. This can explain why semi-batch fixed bed reactors produce more gas and less liquid phase yields when compared to small-scale semi-batch reactors. The higher temperature at the rim can further crack the products of depolymerization, forming non-condensable gases such as CO, CO
2, H
2 and light hydrocarbons (CH
4, C
2H
2 and others), increasing gas yields and reducing the actual recovery of MMA. Since formed products are part of the gas phase, there is no change of chemical composition of the liquid phase until most of the feed converts into char and the char pyrolysis dominates the products distribution and composition.