Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
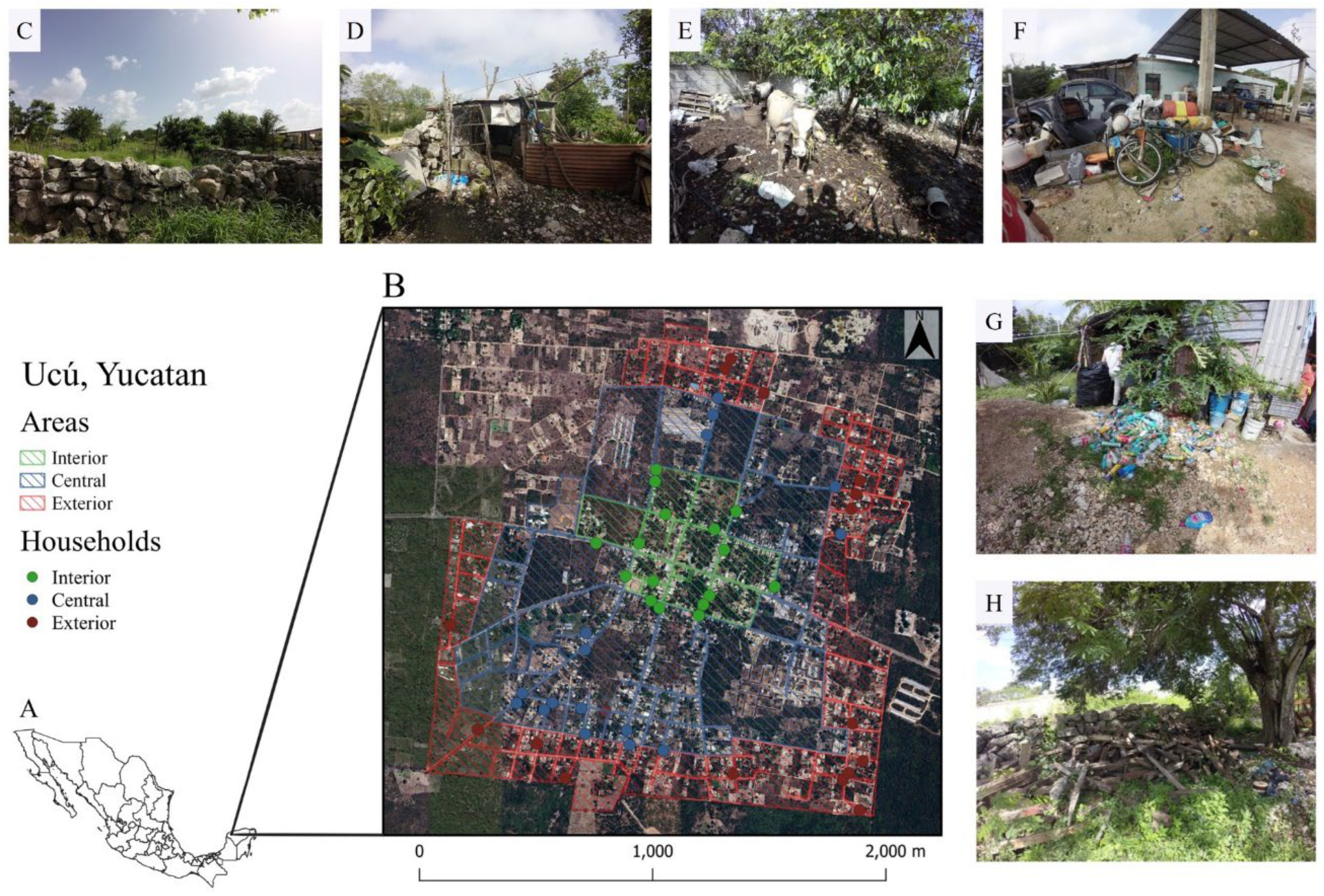
2.1. Site and Study Design
2.2. Sampling and Population Data
2.4. Microscopic Agglutination Test (MAT)
2.5. Peridomicile Data Collection
2.6. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 2009, 7, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and behavioural determinants of Leptospirosis transmission: A Systematic review. PLoS Negl Trop Dis 2015, 9, e0003843. [Google Scholar] [CrossRef] [PubMed]
- Casanovas-Massana, A.; Costa, F.; Riediger, I.N.; Cunha, M.; de Oliveira, D.; Mota, D.C.; Sousa, E.; Querino, V.A.; Nery, N. Jr; Reis, M.G.; Wunder, E.A. Jr; Diggle, P.J.; Ko, A.I. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. Water Res 2018, 130, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Casanovas-Massana, A.; Hamond, C.; Santos, L.A.; de Oliveira, D.; Hacker, K.P.; Balassiano, I.; Costa, F.; Medeiros, M.A.; Reis, M.G.; Ko, A.I.; Wunder, E.A. Leptospira yasudae sp. nov. and Leptospira stimsonii sp. nov., two new species of the pathogenic group isolated from environmental sources. Int J Syst Evol Microbiol 2020, 70, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.G.V.; Stone, N.E.; Roe, C.C.; Goris, M.G.A.; van der Linden, H.; Sahl, J.W.; Wagner, D.M.; Nally, J.E. Leptospira sanjuanensis sp. nov., a pathogenic species of the genus Leptospira isolated from soil in Puerto Rico. Int J Syst Evol Microbiol 2022, 72. [Google Scholar] [CrossRef]
- Caimi, K.; Ruybal, P. Leptospira spp., a genus in the stage of diversity and genomic data expansion. Infect Genet Evol 2020, 81, 104241. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of Leptospirosis: A systematic review. PLoS Negl Trop Dis 2015, 9, e0003898. [Google Scholar] [CrossRef]
- Browne, E.S.; Pereira, M.; Barreto, A.; Zeppelini, C.G.; de Oliveira, D.; Costa, F. Prevalence of human leptospirosis in the Americas: a systematic review and meta-analysis. Rev Panam Salud Publica 2023, 47, e126. [Google Scholar] [CrossRef]
- Yescas-Benítez, J.E.; Rivero-Perez, N.; Monntiel-Díaz, H.E.; Valladares-Carranza, B.; Peláez-Acero, A.; Morales-Ubaldo, A.L.; Zaragoza-Bastida, A. Comportamiento epidemiológico de la leptospirosis en México durante el periodo 2013-2010. Rev Salud Pública 2020, 22, 421–427. [Google Scholar] [CrossRef]
- Boletín Epidemiológico Sistema Nacional de Vigilancia Epidemiológica Sistema Único de Información 2023. Secretaría de Salud. Dirección General de Epidemiología. Available online: https://www.gob.mx/salud/documentos/boletinepidemiologico-sistema-nacional-de-vigilancia-epidemiologica-sistema-unico-de-informacion-261547 (accessed on 08 August 2024).
- Vado-Solis, I.; Cárdenas-Marrufo, M.F.; Jiménez-Delgadillo, B.; Alzina-López, A.; Laviada-Molina, H.; Suarez-Solís, V.; Zavala-Velázquez, J.E. Clinical-epidemiological study of leptospirosis in humans and reservoirs in Yucatán, México. Rev Inst Med Trop Sao Paulo 2002, 44, 335–340. [Google Scholar] [CrossRef]
- Torres-Castro, M.A.; Gutiérrez-Ruíz, E.; Hernández-Betancourt, S.; Peláez-Sánchez, R.; Agudelo-Flórez, P.; Guillermo-Cordero, L.; Puerto, F.I. First molecular evidence of Leptospira spp. in synanthropic rodents captured in Yucatan, Mexico. Revue Méd Vét 2014, 165, 213–218. [Google Scholar]
- Torres-Castro, M. .; Cruz-Camargo, B.; Medina-Pinto, R.; Reyes-Hernández, B.; Moguel-Lehmer, C.; Medina, R.; Ortiz-Esquivel, J.; Arcila-Fuentes, W.; López-Ávila, A.; Noh-Pech, H.; Panti-May, A.; Rodríguez-Vivas, I.; Puerto, F.I. Detección molecular de leptospiras patógenas en roedores sinantrópicos y silvestres capturados en Yucatán, México. Biomedica, 2018, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Panti-May, J.A.; DE Andrade, R.R.C.; Gurubel-González, Y.; Palomo-Arjona, E.; Sodá-Tamayo, L.; Meza-Sulú, J.; Ramírez-Sierra, M.; Dumonteil, E.; Vidal-Martínez, V.M.; Machaín-Williams, C.; DE Oliveira, D.; Reis, M.G.; Torres-Castro, M.A.; Robles, M.R.; Hernández-Betancourt, S.F.; Costa, F. A survey of zoonotic pathogens carried by house mouse and black rat populations in Yucatan, Mexico. Epidemiol Infect 2017, 145, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Galaz, A.; Reyes-Novelo, E.; Hernández-Betancourt, S.; Panti-May, A.; Estrella, E.; Sánchez-Montes, S.; Noh-Pech, H.; Lugo-Caballero, C.; Colunga-Salas, P.; Peláez-Sánchez, R.; Sosa-Escalante, J.; Herrera-Flores, B.G.; Rodríguez-Vivas, R.I.; Torres-Castro, M. Study on the relation of the characteristics of the capture sites with the Leptospira spp. occurrence in bats and rodents from Yucatan, Mexico. Acta Trop 2024, 249, 107072. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, M.; Febles-Solís, V.; Hernández-Betancourt, S.; Noh-Pech, H.; Estrella, E.; Peláez-Sánchez, R.; Panti-May, A.; Herrera-Flores, B.; Reyes-Hernández, B.; Sosa-Escalante, J. Leptospira patógenas en murciélagos de Campeche y Yucatán, México. Rev MVZ Córdoba 2020, 25, e1815. [Google Scholar] [CrossRef]
- Torres–Castro, M.; Panti–May, J.A.; MacSwiney-González, M.C.; Lugo–Caballero, C.; Suárez–Galaz, A.; Suárez–Galaz, M.; Yeh–Gorocica, A.; Cruz–Camargo, B. Detección de Leptospira spp. en murciélagos de la península de Yucatán, México. Rev Cient-Fac Cien V 2023, 33, 6. [Google Scholar] [CrossRef]
- Ruiz-Piña, H.A.; Puc-Franco, M.A.; Flores-Abuxapqui, J.; Vado-Solis, I.; Cardenas-Marrufo, M.F. Isolation of Salmonella enterica and serologic reactivity to Leptospira interrogans in opossums (Didelphis virginiana) from Yucatán, México. Rev Inst Med Trop Sao Paulo, 2002, 44, 235–237. [Google Scholar] [CrossRef]
- Suárez-Galaz, A.R.; Hernández-Betancourt, S.; Panti-May, J.A.; Manrique-Saide, P.; Torres-Castro, M. Evidencia de Leptospira spp. en musarañas Cryptotis mayensis. Nuevo hospedero en Yucatán, México. Rev Biomedica 2021, 32, 161–165. [Google Scholar] [CrossRef]
- Jimenez-Coello, M.; Vado-Solis, I.; Cárdenas-Marrufo, M.F.; Rodríguez-Buenfil, J.C.; Ortega-Pacheco, A. Serological survey of canine leptospirosis in the tropics of Yucatan Mexico using two different tests. Acta Trop 2008, 106, 22–26. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Vado-Solís, I.; Jiménez-Delgadillo, B.; Cárdenas-Marrufo, M.; Pérez-Osorio, C.; Puerto-Solís, M.; Jiménez-Coello, M. Serological survey of Leptospira interrogans, Toxoplasma gondii and Trypanosoma cruzi in free roaming domestic dogs and cats from a marginated rural area of Yucatan Mexico. Vet Med Sci 2017, 3, 40–47. [Google Scholar] [CrossRef]
- Torres-Castro, M.; Díaz-Aceves, D.; Suárez-Galaz, A.; Reyes-Novelo, E.; Rodríguez-Vivas, R.I. Evidencia de Leptospira spp. en sangre de perros de una comunidad rural de Yucatán, México. Rev MVZ Córdoba 2021, 26, e2098. [Google Scholar] [CrossRef]
- Zavala-Velázquez, J.; Pinzón-Cantarell, J.; Flores-Castillo, M. La leptospirosis en Yucatán. Estudio serológico en humanos y animales. Salud Publica Mex 1984, 26, 254–259. [Google Scholar] [PubMed]
- Agudelo-Flórez, P.; Restrepo-Jaramillo, B.N.; Arboleda-Naranjo, M. Situación de la leptospirosis en el Urabá antioqueño colombiano: estudio seroepidemiológico y factores de riesgo en población general urbana. Cad Saude Publica 2007, 23, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.B.; Ribeiro, G.S.; Felzemburgh, R.D.; Santana, F.S.; Mohr, S.; Melendez, A.X.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; Carvalho, M.S.; Reis, M.G.; Ko, A.I. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis 2008, 2, e228. [Google Scholar] [CrossRef]
- Espinoza-Gómez, F.; Maldonado-Rodríguez, A.; Coll-Cardenas, R.; Hernandez-Suárez, C.M.; Fernández-Salas, I. Presence of triatominae (Hemiptera, Reduviidae) and risk of transmission of Chagas disease in Colima, Mexico. Mem Inst Oswaldo Cruz 2002, 97, 25–30. [Google Scholar] [CrossRef]
- Reyes-Novelo, E.; Ruiz-Piña, H.; Canché-Pool, E.B.; Panti-May, J.A.; Escobedo-Ortegón, F.J. El peridomicilio y las zoonosis en Yucatán. Hacia la búsqueda de Una Salud. Trop Subtrop Agroecosystems 2022, 25, 011. [Google Scholar] [CrossRef]
- Dzib-Paredes, G.; Rodríguez-Vivas, R.I.; Panti-May, A.; Noh-Pech, H.; Rosado-Aguilar, J.A.; Torres-Castro, M. Frecuencia de Borrelia burgdorferi sensu lato y Leptospira spp. en pequeños roedores de Yucatán, México. Rev Cient-Fac Cien V 2022, 32, 1–8. [Google Scholar] [CrossRef]
- Ruiz-Piña, H.A.; Reyes-Novelo, E.A. El huerto familiar yucateco y las zoonosis. In Huertos Familiares de la Península de Yucatán,1rst ed.; Salvador-Flores, J., Ed.; Universidad Autónoma de Yucatán: Mérida, México, 2012; Volume 1, pp. 359–374. [Google Scholar]
- Instituto Nacional de Estadística y Geografía. Compendio de Información Geográfica Municipal 2010. Ucú. Yucatán. Available online: https://www.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/31/31100.pdf#page=2.09 (accessed on 08 August 2024).
- Instituto Nacional de Estadística y Geografía. Espacio y Datos de México. Available online: https://www.inegi.org.mx/app/mapa/espacioydatos/default.aspx?ag=311000001 (accessed on 08 August 2024).
- Panti-May, J.A.; Hernández-Betancourt, S.; Ruíz-Piña, H.; Medina-Peralta, S. Abundance and population parameters of commensal rodents present in rural households in Yucatan, Mexico. Int Biodeterior Biodegradation 2012, 66, 77–81. [Google Scholar] [CrossRef]
- Panti-May, J.A.; Hernández-Betancourt, S.F.; Torres-Castro, M.A.; Parada-López, J.; López-Manzanero, S.; Herrera-Meza, M. A population study of the house mouse, Mus musculus (Rodentia: Muridae), in a rural community of Mérida, México. Caribb Nat 2018, 46, 1–13. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology. Presenting numerical data, 4th ed.; Blackwell Science: Oxford, United Kingdom, 2018; pp. 251–269. [Google Scholar]
- Ponce-Saavedra, J.; Quijano-Ravell, A.F.; Valdez-Mondragón, A. Técnicas para la recolección de arañas y otros arácnidos en ambientes antrópicos. In Manual de Técnicas para el Estudio de Fauna Nativa en Ambientes Urbanos, 1st ed.; Zuria, I., Olvera-Ramírez, A.M., Ramírez-Bastida, P., Eds.; Universidad Autónoma de Querétaro: Querétaro, México, 2019; Volume 1, pp. 59–76. [Google Scholar]
- Hernández-Camacho, N.; Muñoz-García, C.I.; Ruiz-Piña, H.A.; Reyes-Novelo, E.A.; Olvera-Ramírez, A.M. Técnicas de manejo de hospederos y colecta de parásitos de vertebrados urbanos. In Manual de Técnicas para el Estudio de Fauna Nativa en Ambientes Urbanos, 1rst ed.; Zuria, I., Olvera-Ramírez, A.M., Ramírez-Bastida, P., Eds.; Universidad Autónoma de Querétaro: Querétaro, México, 2019; Volume 1, pp. 173–186. [Google Scholar]
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 09 August 2024).
- Barrera-Tolosa, M.S. Elaboración de un Manual de Rehabilitación para la Especie Didelphis marsupialis Alojados en el CAV-CEARFS de la CDMB. Bachelor Thesis, Universidad Cooperativa de Colombia, Facultad de Ciencias de la Salud, Medicina Veterinaria y Zootecnia, Bucaramanga, Colombia, 18 May 2020. [Google Scholar]
- World Organisation for Animal Health. Manual of Diagnosis Tests and Vaccines for Terrestrial Animals, thirteenth edition 2024. Chapter 3.1.12. Leptospirosis. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.12_LEPTO.pdf (accessed on 09 August 2024).
- Donald, D.M. Manual de Métodos para el Diagnóstico de Laboratorio de la Leptospirosis, 1rst ed.; Organización Panamericana de la Salud, Centro Panamericano de Zoonosis 1983.
- Carrillo-Peraza, J.R.; Manrique-Saide, P.; Rodríguez-Buenfil, J.C.; Escobedo-Ortegón, J.F.; Rodríguez-Vivas, R.I.; Bolio-González, M.E.; Barrera-Pérez, M.; Reyes-Novelo, E.; Sauri-Arceo, C.H. Estudio serológico de la tripanosomiasis americana y factores asociados en perros de una comunidad rural de Yucatán, México. Arch Med Vet 2014, 46, 75–81. [Google Scholar] [CrossRef]
- Koyoc-Cardeña, E.; Medina-Barreiro, A.; Escobedo-Ortegón, F.J.; Rodríguez-Buenfil, J.C.; Barrera-Pérez, M.; Reyes-Novelo, E.; Chablé-Santos, J.; Selem-Salas, C.; Vazquez-Prokopec, G.; Manrique-Saide, P. Chicken coops, Triatoma dimidiata infestation and its infection with Trypanosoma cruzi in a rural village of Yucatán, Mexico. Rev Inst Med Trop Sao Paulo 2015, 57, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, M.; Reyes-Novelo, E.; Noh-Pech, H.; Tello-Martín, R.; Lugo-Caballero, C.; Dzul-Rosado, K.; Puerto-Manzano, F.; Rodríguez-Vivas, RI. Personal and household factors involved in recent Rickettsia exposure in a rural population from Yucatán, Mexico. Zoonoses Public Health 2020, 67, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Rosado, K.R.; Reyes-Novelo, E.; Lugo-Caballero, C.; Cuxim-Koyoc, A.D.; Collí-Padrón, F.; Tello-Martín, R.; López-Ávila, K.B.; Palma-Chan, A.; Peniche-Lara, G.; Ruiz-Piña, H.A. Urban ecology of hosts and vectors of Rickettsia in a rickettsiosis-endemic city of the Yucatan peninsula, Mexico. Acta Trop 2021, 216, 105832. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists - A primer to Quantitative Parasitology. Trends Parasitol 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA. Available online: https://posit.co/ (accessed on 09 August 2024).
- R Core Team. _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. Available on: https://www.r-project.org/ (accessed on 09 August 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Tousand Oaks CA, USA, 2019; 608p. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A.; ExpDes: Pacote Experimental Designs. Version 1.2.2. Available online: https://cran.r-project.org/web/packages/ExpDes/ExpDes.pdf (accessed on 09 August 2024).
- Gross, J.; Ligges, U.; Tests for Normality. Package ‘nortest’. Version 1.0-4. Available online: https://cran.r-project.org/web/packages/nortest/nortest.pdf (accessed on 09 August 2024).
- Russell, L.; Least-Squares Means. Package ‘lsmeans’. Version 2.30-0. Available online: https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf (accessed on 09 August 2024).
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; Riebl, H.; Singmann, H.; Estimated Marginal Means, aka Least-Squares Means. Package ‘emmeans’. Version 1.10.4. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 09 August 2024).
- De Rosario-Martínez, H.; Fox, J.; R Core Team; Phil, C. Post-Hoc Interaction Analysis. Package ‘phia’. Version 0.3-1. Available online: https://cran.r-project.org/web/packages/phia/phia.pdf (accessed on 10 August 2024).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T.; PBC, Posit. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Version 3.5.1. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 10 August 2024).
- Ripley, B.; Venables, W.; Feed-Forward Neural Networks and Multinomial Log-Linear Models. Package ‘nnet’. Version 7.3-19. Available online: https://cran.r-project.org/web/packages/nnet/nnet.pdf (accessed on 10 August 2024).
- Wickham, H.; reshape2: Flexibly Reshape Data: A Reboot of the Reshape Package. Package ‘reshape2’. Version 1.4.4. Available online: https://cran.r-project.org/web/packages/reshape2/reshape2.pdf (accessed on 10 August 2024).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; EISPACK authors; Heisterkamp, S.; Van Willigen, B.; Ranke, J.; R Core Team. Linear and Nonlinear Mixed Effects Models. Package ‘nlme’. Version 3.1-166. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 10 August 2024).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Support Functions and Datasets for Venables and Ripley’s MASS. Version 7.3-61. Available online: https://cran.r-project.org/web/packages/MASS/MASS.pdf (accessed on 10 August 2024).
- Fox, J.; Marquez, M.; Muenchen, R.; Putler, D.; R Commander Miscellaneous Functions. Package ‘RcmdrMisc’. Version 2.9-1. Available online: https://cran.r-project.org/web/packages/RcmdrMisc/RcmdrMisc.pdf (accessed on 10 August 2024).
- Garcia-Lopez, M.; Lurier, T.; Bouilloud, M.; Pradel, J.; Tatard, C.; Sepulveda, D.; Anfray, G.; Dussert, J.; Bourhy, P.; Charbonnel, N.; Djelouadji, Z. Prevalence, genetic diversity and eco-epidemiology of pathogenic Leptospira species in small mammal communities in urban parks Lyon city, France. PLoS One 2024, 19, e0300523. [Google Scholar] [CrossRef]
- Adler, B.; de la Peña-Moctezuma, A. Leptospira and leptospirosis. Vet Microbiol 2010, 140, 287–96. [Google Scholar] [CrossRef]
- Méndez, C.; Benavides, L.; Esquivel, A.; Aldama, A.; Torres, J.; Gavaldón, D.; Meléndez, P.; Moles, L. Pesquisa serológica de Leptospira en roedores silvestres, bovinos, equinos y caninos en el noreste de México. Rev Salud Animal 2013, 35, 25–32. [Google Scholar]
- Levett, PN. Leptospirosis. Clin Microbiol Rev 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Fornazari, F.; Langoni, H.; Marson, P.M.; Nóbrega, D.B.; Teixeira, CR. Leptospira reservoirs among wildlife in Brazil: Beyond rodents. Acta Trop 2018, 178, 205–212. [Google Scholar] [CrossRef]
- Horta, M.C.; Ragozo, A.M.A.; Casagrande, R.A.; Reiko, E.; Gennari, S.M. Occurrence of anti-Toxoplasma gondii, Neospora caninum and Leptospira spp. antibodies in opossums (Didelphis spp.) in São. Braz J Vet Res Anim Sci 2016, 53, 1–9. [Google Scholar] [CrossRef]
- Jorge, S.; Hartleben, C.P.; Seixas, F.K.; Coimbra, M.A.; Stark, C.B.; Larrondo, A.G.; Amaral, M.G.; Albano, A.P.; Minello, L.F.; Dellagostin, O.A.; Brod, C.S. Leptospira borgpetersenii from free-living white-eared opossum (Didelphis albiventris): first isolation in Brazil. Acta Trop 2012, 124, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.D.S.; Braga-Domingos, S.C.; Azevedo, M.I.N.D.; Peruquetti, R.C.; de Albuquerque, N.F.; D’Andrea, P.S.; Botelho, A.L.M.; Crisóstomo, C.F.; Vieira, A.S.; Martins, G.; Teixeira, B.R.; Carvalho-Costa, F.A.; Lilenbaum, W. Small mammals as carriers/hosts of Leptospira spp. in the western Amazon forest. Front Vet Sci 2020, 7, 569004. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.J.; de Lima-Peixoto, A.; de Farias, A.S.S.; Junior-Pinheiro, T.; da Costa, D.F.; Silva, M.L.C.R.; Júnior, J.P.A.; Malossi, C.D.; Ullmann, L.S.; de Azevedo, S.S.; Alves, C.J.; Dos Santos-Higino, S.S. Didelphis albiventris as a carrier of Leptospira sp. in the central nervous tissue in the semiarid region of Northeast, Brazil. Comp Immunol Microbiol Infect Dis 2020, 73, 101560. [Google Scholar] [CrossRef]
- Vieira, A.S.; D’Andrea, P.S.; Vilela, R.D.V.; Loretto, D.; Jaeger, L.H.; Carvalho-Costa, F.A.; Lilenbaum, W. Pathogenic Leptospira species are widely disseminated among small mammals in Atlantic Forest biome. Transbound Emerg Dis 2019, 66, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Piña, H.; Pacheco-Castro, J.; Lugo-Pérez, J.A. El “zorro” de Yucatán y su relación con la población humana. In Estudios multidisciplinarios de las enfermedades zoonóticas y ETVs en Yucatán, 1rst ed.; Pacheco-Castro, J., Lugo-Pérez, J.A., Tzuc-Canché, L., Ruíz-Piña, H.A., Eds.; Universidad Autónoma de Yucatán: Mérida, México, 2013; Volume 1, pp. 215–232. [Google Scholar]
- Ortega-Pacheco, A.; Colin-Flores, R.F.; Gutiérrez-Blanco, E.; Jiménez-Coello, M. Frequency and type of renal lesions in dogs naturally infected with Leptospira species. Ann N Y Acad Sci 2008, 1149, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Marrufo, M.F.; Vado-Solis, I.; Perez-Osorio, C.E.; Correa, J.S. Seropositivity to leptospirosis in domestic reservoirs and detection of Leptospira spp. from water sources, in farms of Yucatan, Mexico. Trop Subtrop Agroecosystems 2011, 14, 185–189. [Google Scholar]
- Blum-Domínguez, S del C. ; Chi-Dzib, M.Y.; Maldonado-Velázquez, M.G.; Nuñez-Oreza, L.A.; Gómez-Solano, M.I.; Caballero-Poot, R.I.; Tamay-Segovia P. Detection of reactive canines to Leptospira in Campeche City, Mexico. Rev Argent Microbiol 2013, 45, 34–38. [Google Scholar]
- Cruz-Romero, A.; Gil-Alarcón, G.; Ochoa-Valencia, J.L.; Ramos-Vásquez, J.R.; Romero-Salas, D.; Becker, I.; Sánchez-Montes, S.; Arenas, P. Seroprevalencia de Leptospira en perros ferales de la Reserva Ecológica del Pedregal de San Ángel, México. Rev Cient-Fac Cien V 2024, 34, 6. [Google Scholar] [CrossRef]
- Jimenez-Coello, M.; Ortega-Pacheco, A.; Guzman-Marin, E.; Guiris-Andrade, D.M.; Martinez-Figueroa, L.; Acosta-Viana, K.Y. Stray dogs as reservoirs of the zoonotic agents Leptospira interrogans, Trypanosoma cruzi, and Aspergillus spp. in an urban area of Chiapas in southern Mexico. Vector Borne Zoonotic Dis 2010, 10, 135–141. [Google Scholar] [CrossRef]
- Goldstein, RE. Canine leptospirosis. Vet Clin North Am Small Anim Pract 2010, 40, 1091–101. [Google Scholar] [CrossRef]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract 2015, 56, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Mazzotta, E.; Boniotti, M.B.; Bertasio, C.; Bellinati, L.; Lucchese, L.; Battilani, M.; Ceglie, L.; Marchione, S.; Esposito, G.; Natale, A. Outbreak of Leptospira borgpetersenii serogroup Sejroe infection in kennel: The role of dogs as sentinel in specific environments. Int J Environ Res Public Health 2022, 19, 3906. [Google Scholar] [CrossRef] [PubMed]
- Ellis, WA. Animal leptospirosis. Curr Top Microbiol Immunol 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Francey, T.; Schuller, S.; Stoddard, R.A.; Cowgill, L.D.; Moore, G.E. Updated ACVIM consensus statement on leptospirosis in dogs. J Vet Intern Med 2023, 37, 1966–1982. [Google Scholar] [CrossRef]
- Zamri, M.I.M.; Shafie, N.J.; Ali, M.R.M.; Awoniyi, A.M.; Argibay, H.D.; Costa, F. Socio-environmental factors associated with small mammal assemblage and Leptospira prevalence in Suburban Areas of Terengganu, Malaysia. Asian Pac J Trop Med 2024, 17, 400–407. [Google Scholar] [CrossRef]
- Salazar-Barrientos, L de L.; Magaña-Magaña, M.A.; Latourneirie-Moreno, L. Importancia económica y social de la agrodiversidad del traspatio en una comunidad rural de Yucatán, México. Agric Soc Desarro 2015, 12, 1-14.
- Ruiz-Piña, H.A.; Reyes-Novelo, E.; Escobedo-Ortegón, F.J.; Barrera-Pérez, M.A. Mamíferos sinantrópicos y la transmisión de enfermedades zoonóticas en el área rural de Yucatán. In Huertos Familiares de la Península de Yucatán,1rst ed.; Salvador-Flores, J., Ed.; Universidad Autónoma de Yucatán: Mérida, México, 2012; Volume 1, pp. 183–194. [Google Scholar]
- Herrera-Flores, B.G.; Santos-Fita, D.; Naranjo, E.J.; Hernández-Betancourt, S. Importancia cultural de la fauna silvestre en comunidades rurales del norte de Yucatán, México. Peninsula 2019, 14, 27–55. [Google Scholar] [CrossRef]
- Nahuat-Cervera, P.E.; Estrada-Riaño, I.A.; Peraza-Romero, F.; Uitzil-Collí, M.O.; Basora-Dorantes, R.A. ; Buenfil-Morales, S de los Á. Conocimiento y aprovechamiento tradicional de vertebrados silvestres en la comunidad maya de Zavala, municipio de Sotuta, Yucatán, México. Estudios de Cultura Maya 2021, LVII, 275–304. [Google Scholar]
- Richardson, D.J.; Gauthier, J.L. A serosurvey of leptospirosis in Connecticut peridomestic wildlife. Vector Borne Zoonotic Dis 2003, 3, 187–193. [Google Scholar] [CrossRef]
- Helman, S.K.; Tokuyama, A.F.N.; Mummah, R.O.; Stone, N.E.; Gamble, M.W.; Snedden, C.E.; Borremans, B.; Gomez, A.C.R.; Cox, C.; Nussbaum, J.; Tweedt, I.; Haake, D.A.; Galloway, R.L.; Monzón, J.; Riley, S.P.D.; Sikich, J.A.; Brown, J.; Friscia, A.; Sahl, J.W.; Wagner, D.M.; Lynch, J.W.; Prager, K.C.; Lloyd-Smith, J.O. Pathogenic Leptospira are widespread in the urban wildlife of southern California. Sci Rep 2023, 13, 14368. [Google Scholar] [CrossRef]
- Della Rossa, P.; Tantrakarnapa, K.; Sutdan, D.; Kasetsinsombat, K.; Cosson, J.F.; Supputamongkol, Y.; Chaisiri, K.; Tran, A.; Supputamongkol, S.; Binot, A.; Lajaunie, C.; Morand, S. Environmental factors and public health policy associated with human and rodent infection by leptospirosis: a land cover-based study in Nan province, Thailand. Epidemiol Infect 2016, 144, 1550–1562. [Google Scholar] [CrossRef]
- McMahon, B.J.; Morand, S.; Gray, J.S. Ecosystem change and zoonoses in the Anthropocene. Zoonoses Public Health 2018, 65, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Bourhy, P.; Picardeau, M.; Moulin, L.; Wurtzer, S. Effect of disinfection agents and quantification of potentially viable Leptospira in freshwater samples using a highly sensitive integrity-qPCR assay. PLoS One 2021, 16, e0251901. [Google Scholar] [CrossRef] [PubMed]
- Canul-Bacab, F.; May-Hoil, P.E. El problema de la basura en el interior del estado de Yucatán. Reaxion 2016, 3, 0–0. [Google Scholar]
- Pacheco-Castro, J.; Lugo-Pérez, J.A.; Tzuc-Canché, L.M. Relación de variables socioeconómicas y culturales con la prevalencia de enfermedades zoonóticas y ETVs en Molas. In Huertos Familiares de la Península de Yucatán,1rst ed.; Salvador-Flores, J., Ed.; Universidad Autónoma de Yucatán: Mérida, México, 2012; Volume 1, pp. 97–122. [Google Scholar]

| Species | n | Sex | Age (%) | MAT | Seroprevalence (IC 95 %) |
|
|---|---|---|---|---|---|---|
| ♂ (%) | ♀ (%) |
|||||
| Mus musculus (synanthropic) | 32 | 22 (68.8) | 10 (31.2) | J: 5 (15.6) A: 27 (84.4) |
23/29 | 81.8% (64.5–93)* |
| Peromyscus yucatanicus (wild) | 3 | 2 (66.7) | 1 (33.3) | A: 3 (100) |
3/3 | |
| Ototylomys phyllotis (synanthropic) | 1 | -- | 1 (100) | A: 1 (100) |
1/1 | |
| Didelphis virginiana (synanthropic) | 24 | 11 (45.8) | 13 (54.2) | J: 8 (33.4) A: 16 (66.6) |
5/16 | 31.2% (11–58.7) |
| Canis lupus familiaris | 66 | 39 (59.1) | 27 (40.9) | C: 12 (18.2) A: 47 (71.2) G: 7 (10.6) |
35/62 | 56.5% (43.3–69) |
| Total | 118 | - | - | - | 67/111 | 60.4% (50.6–69.5) |
| Species | Individuals/Analyzed sera | |||
|---|---|---|---|---|
| Interior | Central | Exterior | Total | |
| Mus musculus (synanthropic) | 10/10 | 5/3 | 17/16 | 32/29 |
| Peromyscus yucatanicus (wild) | -- | 1/1 | 2/2 | 3/3 |
| Ototylomys phyllotis (synanthropic) | -- | -- | 1/1 | 1/1 |
| Rodent total | 10/10 | 6/4 | 20/19 | 36/33 |
| Didelphis virginiana (synanthropic) | 11/7 | 8/5 | 5/4 | 24/16 |
| Canis lupus familiaris | 19/18 | 21/20 | 26/24 | 66/62 |
| Total | 40/35 | 35/29 | 51/47 | 126/111 |
|
Species |
Serogroup |
Hosts |
Total n (%) |
||
|---|---|---|---|---|---|
| Rodents n (%) ≥ 1:100 |
Opossums n (%) ≥ 1:200 |
Dogs n (%) ≥ 1:400 |
|||
| L. noguchii | Panama | 2 (7.4) | -- | -- | 2 (3) |
| L. borgpetersenii | Tarassovi | -- | 1 (20) | 3 (8.6) | 4 (6) |
| Ballum | -- | 1 (20) | -- | 1 (1.5) | |
| Sejroe | 6 (22.2) | 1 (20) | 8 (23) | 15 (22.4) |
|
| L. interrogans | Pyrogenes | -- | -- | 5 (14.2) | 5 (7.5) |
| Bataviae | 3 (11.1) | -- | 1 (2.8) | 4 (6) | |
| Canicola | 7 (26) | -- | 12 (34.2) | 19 (28.3) | |
| Australis | -- | -- | -- | -- | |
| Icterohaemorrhagiae | -- | -- | 2 (5.8) | 2 (3) | |
| Hardjo | 1 (3.7) | -- | -- | 1 (1.5) | |
| Pomona | 4 (14.8) | -- | 1 (2.8) | 5 (7.4) |
|
| L. kirschneri | Grippotyphosa | 3 (11.1) | -- | 2 (5.8) | 5 (7.4) |
| Cynopteri | -- | 2 (40) | -- | 2 (3) |
|
| More than one serogroup | 1 (3.7) | -- | 1 (2.8) | 2 (3) |
|
| Total | 27 | 5 |
35 | 67 | |
| Peridomicile Characteristics | Number of peridomiciles (%) | Bivariate analysis (P ≤ 0.3) |
Multivariate analysis (P ≤ 0.05) |
RR (CI 95%) |
|---|---|---|---|---|
|
Presence of seropositive animal |
38 (79.2) | |||
|
Locality’s area Interior Central Exterior |
16 (33.3) 16 (33.3) 16 (33.3) |
1 |
|
|
|
Geographic polygon Northeast Northwest Southeast Southwest |
10 (20.8) 9 (18.8) 12 (25) 17 (35.4) |
0.24* |
Reference 0.24 0.06 0.74 |
0.16 (0.005 - 2.94) 0.07 (0.002 - 0.80) 1.59 (0.06 – 25.98) |
|
Type of neighboring Houses Inhabited properties Public areas |
11 (22.9) 23 (48) 14 (29.1) |
1 |
||
|
Peridomicile delimiter Block wall Stone wall Wall built with diverse materials Without delimiter |
4 (8.4) 15 (31.2) 15 (31.2) 14 (29.2) |
0.12* |
||
|
Peridomicile area (m2) |
1129.02 ± 741.48 | 0.03* | 0.01 | 1.004 (1.001 - 1.008) |
|
Type of floor Dirt Dirt and concrete |
26 (54.2) 22 (45.8) |
1 |
||
|
Main vegetation cover Trees Schrubs Herbaceus |
21 (43.7) 6 (12.5) 6 (12.5) |
0.48 1 1 |
|
|
|
Dominant vegetation Trees Herbaceous |
28 (58.3) 20 (41.7) |
1 |
||
| Number of trees | 23.31 ± 14.87 |
0.48 | ||
|
Diversity of trees |
8.10 ± 3.74 |
0.08 | ||
|
Buildings in the peridomicile Warehouse Barn Buildings for animal husbandry |
21 (43.7) 10 (20.8) 23 (47.9) |
0.48 1 0.29* |
||
|
Production animals Absence One species More than one species |
24 (50) 13 (27.1) 11 (22.9) |
0.46 |
||
|
Captive wildlife Absence One species More than one species |
26 (54.2) 18 (37.5) 4 (8.4) |
0.3* |
|
|
|
Accumulation of miscellaneous items PET Cans Cardboard Pots Firewood Stones Construction materials Cement blocks Wood Construction clay Construction rubbish Gravel |
35 (72.9) 34 (70.8) 9 (18.8) 43 (89.5) 39 (81.2) 34 (70.8) 32 (66.7) 24 (50) 4 (8.3) 10 (20.8) 6 (12.5) 4 (8.3) |
0.1* 1 1 1 0.37 0.45 1 0.72 1 0.66 1 0.56 |
|
|
| Water containers | 45 (93.7) | 0.51 |
| Models | AIC | Residual deviance |
df | Deviance |
P |
|---|---|---|---|---|---|
| Model 1. Null model | 44.47 | 49.13 |
|||
| Model 2. Peridomicile area | 52.61 | 40.47 | 1 | 8.65 | 0.003 |
| Model 3. Number of trees | 49.34 | 48.61 | 0 | -8.14 |
|
| Model 4. Tree diversity | 46.17 | 45.34 | 0 | 3.27 |
|
| Model 5. Area + tree diversity | 51.13 | 40.2 | 1 | 5.17 | 0.02 |
| Model 6. Area + geographic polygon | 41.62 | 31.62 | 2 | 8.55 | 0.01 |
| Peridomicile Characteristics | Number of peridomiciles (%) | Bivariate Analysis (P ≤ 0.3) |
Multinomial analysis (P < 0.05) |
RR (95%CI) |
|---|---|---|---|---|
|
Number of seropositive species per peridomicile Zero One Two |
10 (20.8) 25 (52) 13 (27) |
|||
|
Locality’s area Interior Central Exterior |
16 (33.3) 16 (33.3) 16 (33.3) |
0.007* | Reference One species: 0.96 Two species: 0.41 One species: 0.10 Two species: 0.14 |
1.05 (0.14 – 7.55) 3.16 (0.19 – 51.41) 0.17 (0.02 – 1.41) 6.62 (0.51 – 85.44) |
|
Geographic polygon Northwest Northeast Southest Southwest |
9 (18.8) 10 (20.8) 12 (25) 17 (35.4) |
0.61 | ||
|
Type of neighboring Houses Inhabited properties Public areas |
11 (22.9) 23 (48) 14 (29.1) |
0.59 | ||
|
Peridomicile delimiter Block wall Stone wall Wall builded with diverse materials Without delimiter |
4 (8.4) 15 (31.2) 15 (31.2) 14 (29.2) |
0.30* | ||
|
Type of floor Dirt Dirt and concrete |
26 (54.2) 22 (45.8) |
0.28* | ||
|
Main vegetation cover Trees Schrubs Herbaceus |
21 (43.7) 6 (12.5) 6 (12.5) |
0.24* 0.88 0.05* |
|
|
|
Dominant vegetation Trees Herbaceous |
28 (58.3) 20 (41.7) |
0.20* | ||
|
Buildings in the peridomicile Warehouse Barn Buildings for animal husbandry |
21 (43.7) 10 (20.8) 23 (47.9) |
0.24* 0.58 0.40 |
||
|
Production animals Absence One species More than one species |
24 (50) 13 (27.1) 11 (22.9) |
0.71 | ||
|
Captive wildlife Absence One species More than one species |
26 (54.2) 18 (37.5) 4 (8.4) |
0.47 |
|
|
|
Accumulation of miscellaneous items PET Cans Cardboard Pots Firewood Stones Construction materials Cement blocks Wood Construction clay Construction rubbish Gravel Water containers |
35 (72.9) 34 (70.8) 9 (18.8) 43 (89.5) 39 (81.2) 34 (70.8) 32 (66.7) 24 (50) 4 (8.3) 10 (20.8) 6 (12.5) 4 (8.3) 45 (93.7) |
0.03* 0.005* 0.54 0.15* 0.62 0.76 0.92 0.86 0.41 0.46 1 0.80 0.77 |
One species: 0.02* Two species: 0.67 |
8.52 (1.38 – 52.56) 1.46 (0.25 – 8.52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





