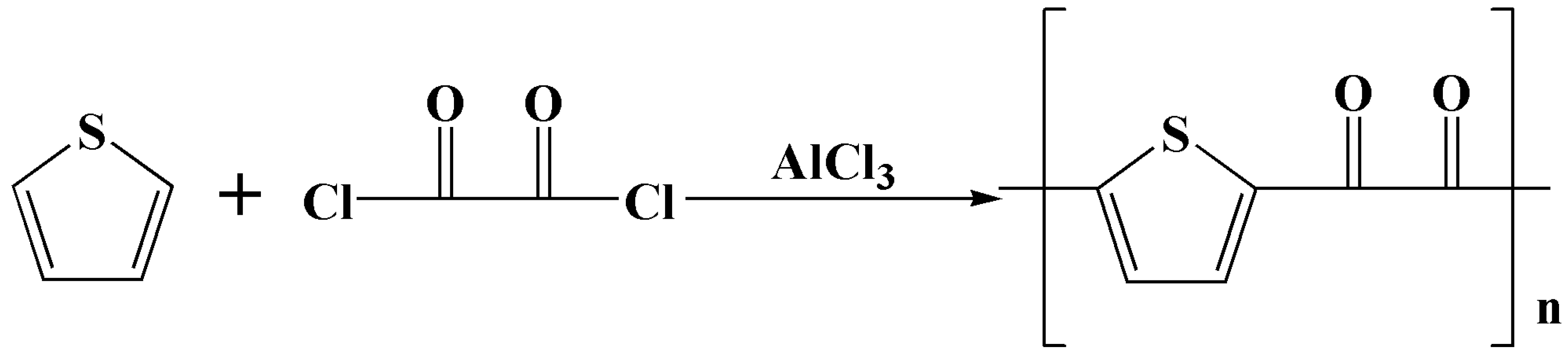1. Introduction
In recent years, with the rapid development of industry, a large amount of heavy metal wastewater containing Pb(II) and Cu(II) has been illegally discharged into rivers and other water bodies. Heavy metal ions have characteristics such as toxicity, biodegradability, and enrichment. They enter the human body through biological enrichment in drinking water or food chains, seriously endangering human health [
1,
2,
3]. At present, the main methods for treating Pb(II) and Cu(II) in water bodies include chemical precipitation, oxidation-reduction, membrane separation, adsorption, and biological methods [
4,
5,
6]. Adsorption method is an effective and excellent method for treating heavy metal wastewater. Compared with other methods, it has the characteristics of simplicity, flexibility, high efficiency, no generation of secondary pollutants, and low cost[
7,
8].
During the adsorption process, heavy metal ions in the aqueous solution combine with the active sites on the surface of the solid adsorbent and transfer to the adsorbent, thereby achieving the goal of removing heavy metal ions from wastewater. The adsorption process mainly consists of three steps: first, heavy metal ions diffuse from the solution to the surface of the adsorbent, then are adsorbed on the surface of the adsorbent, and finally diffuse within the adsorbent particles. The adsorption mechanism can be divided into physical interactions (van der Waals forces) and chemical interactions (functional group coordination, ion exchange, and redox) based on the selected adsorbent. The effect of physical adsorbents on heavy metal ions is very weak and limited, while adsorbents mainly based on chemical reactions have excellent removal performance for heavy metal ions, especially functional group coordination, which is widely used [
9,
10].
The coordination atoms of commonly used functional groups are mainly N, O, S, and P. Polymer adsorbents can be classified into four types: polymers containing N groups, O groups, S groups, and P groups [
11,
12,
13].
The commonly used functional groups for polymer adsorbents containing N functional groups include amino groups (-NH
2) and some nitrogen-containing heterocycles, such as pyridine [
14,
15]. Xu et al. [
16] functionalized poly (p-phenylenediamine) with ethylenediamine to prepare a nanoscale polymer adsorbent containing amine groups. The tiny nanostructure makes the adsorption kinetics of the adsorbent for Hg(II) very rapid, reaching adsorption equilibrium in just 15 minutes. The adsorption process conforms to the quasi second order kinetic model and Langmuir model. Liu et al. [
17] prepared two acid resistant pyridine amine based polymers, PMAA-PD and PMAD-PD, through a series of reactions. PMAA-PD adsorbent can separate Ni
2+ from acidic coexisting solutions of Co
2+ and Ni
2+ to produce cobalt with a purity of up to 99.99%. PMAD-PD adsorbent exhibits good adsorption capacity for various metal ions such as Cu(II) , Ni(II) , Co(II) , Zn(II) , Cd(II) under acidic conditions. Zou et al. [
18] successfully modified the tricyclic pyridine side group on the framework of chlorinated methylstyrene through a series of synthetic reactions. The adsorbent has good adsorption capacity for Cu(II), Ni(II), and Pb(II) in aqueous solution, with maximum adsorption capacities of 5.02, 3.38, and 1.27 mmol/g, respectively. At the same time, this adsorbent also has good reusability and can be used multiple times. The adsorbent after adsorbing Cu(II) can also serve as a catalyst for the degradation of bisphenol A.
The commonly used functional groups for organic synthetic polymer adsorbents containing O functional groups are carboxyl (-COOH) and hydroxyl (-OH) [
19,
20]. Zhang et al. [
21] prepared a polymer adsorbent rich in carboxyl and hydroxyl groups by surface initiated atom transfer radical polymerization to react chloromethylated styrene skeleton with glycidyl methacrylate, and then modified the polymer with salicylic acid. When the pH value of the solution is 4, the maximum adsorption capacities of the adsorbent for Ni(II) and Cu(II) are 138.52 mg/g and 111.21 mg/g, respectively. The adsorption process follows the quasi second order kinetic model and Langmuir model. In addition, the adsorbent also exhibits good desorption rate and reusability.
The commonly used functional groups for organic synthetic polymer adsorbents containing S functional groups are thiol (-SH) and thiophene [
22,
23]. Albakri et al. [
24] prepared a polymer adsorbent containing two sulfur-containing functional groups, thiol and thiophene, through the condensation reaction of thiol and thiophene using methanol as a crosslinking agent. This adsorbent has good thermal stability and can remain stable above 300℃. The removal ability of the adsorbent for Hg(II) in aqueous solution and methylmercury in hydrocarbon medium (decane/toluene mixture) was studied through a series of adsorption experiments. The results showed that the adsorbent had good removal ability for both Hg(II) and methylmercury. In an aqueous solution with a Hg(II) concentration of 100 mg/L, the removal rate of Hg(II) was close to 100%.
The commonly used functional groups for organic synthetic polymer adsorbents containing P functional groups are phosphonic acid groups. Al et al. [
25] used diallylaminomethylphosphonic acid and 1,1,4,4-tetraallyl piperazinium as raw materials to polymerize the two, and then treated the reacted polymer with NaOH to prepare a polymer adsorbent rich in phosphonic acid groups. This adsorbent has good adsorption effects on both Pb(II) and Cu(II) in aqueous solutions. The adsorbent has a higher adsorption capacity for Pb(II) and a faster adsorption kinetics for Cu(II). Research has shown that the adsorption process of adsorbents for two types of heavy metal ions follows both quasi second order kinetic models and Langmuir models.
This article successfully synthesized a polymer adsorbent TOC containing S functional groups through thiophene and ethanedioyl chloride, with a simple method and low cost. It has a porous structure morphology, a large contact area, and the thiophene group can fully contact with heavy metal ions. It has good adsorption effect, stable physical and chemical properties, is renewable, easy to recover, and has good application prospects in the field of heavy metal ion adsorption.
2. Experimental
2.1. Synthesis of TOC
Mix 2.20g thiophene (25.4mmol) with 50mL dichloromethane and stir under ice water bath and nitrogen protection. Mix for 30 minutes. Slowly add 6.76g anhydrous AlCl3 (50.80mmol) in batches and stir for 30 minutes. Then add dropwise 3.38g of ethanedioyl chloride chloride (25.40mmol), stir for 20 minutes. Remove the ice water bath and heat to reflux for 3 hours. There is a large amount of yellow brown solid precipitates, filtered, washed with water, and dried to obtain the product.
Scheme 1.
Schematic diagrams of the preparation of TOC.
Scheme 1.
Schematic diagrams of the preparation of TOC.
3. Results and Discussion
3.1. Characterization
3.1.1. Infrared Spectral Analysis of TOC
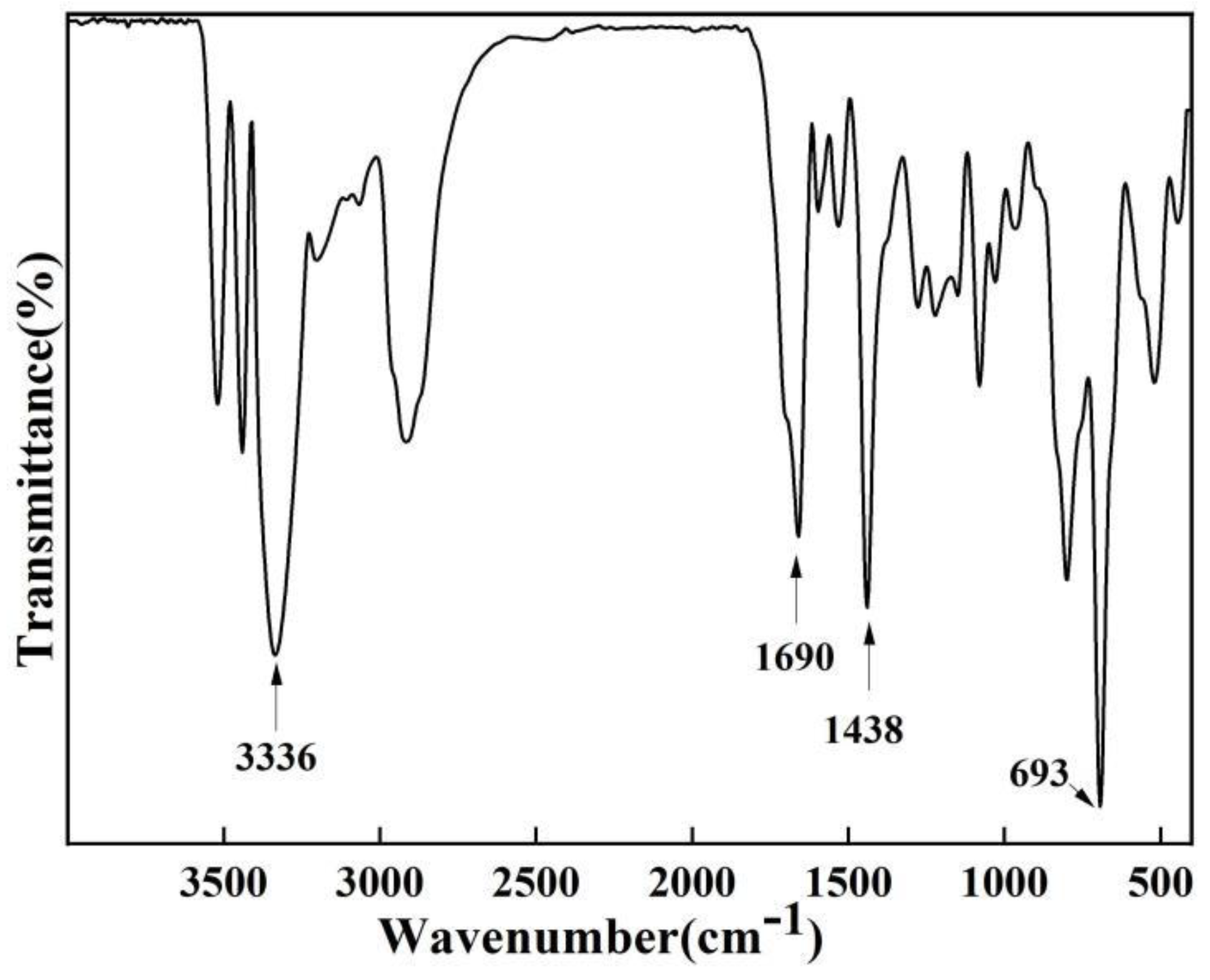
Figure 1 shows the infrared spectrum of TOC. The stretching vibration of C=O on ethanedioyl chloride at 1690cm
-1 in the figure. The absorption peaks at 693, 1438, and 3336 cm
-1 are the characteristic absorption peaks of S, C=C, and the attached H on the thiophene ring, respectively.
3.1.2. Molecular Weight Determination of TOC
Table 1 shows the molecular weight of TOC, with number-average molecular weight(Mn), weight-average molecular weight(Mw), and molecular weight distribution(PDI) of 16420, 27680, and 1.69, respectively. Mn reflects the average molecular weight of polymer molecules, while Mw focuses more on the contribution of high molecular weight components to the overall molecular weight. Through the analysis of Mn and Mw data, it can be seen that the polymer has formed, but the molecular weight is not very large. This may be due to the continuous growth of polymer carbon chains, which increases the volume of the polymer and ultimately hinders the formation of longer carbon chains. PDI=Mw/Mn=1.69 indicates that there is not much difference in the molecular weight between larger and smaller molecules in TOC, and the polymer molecular weight is relatively uniform [
26].
3.1.3. EDS Analysis of TOC
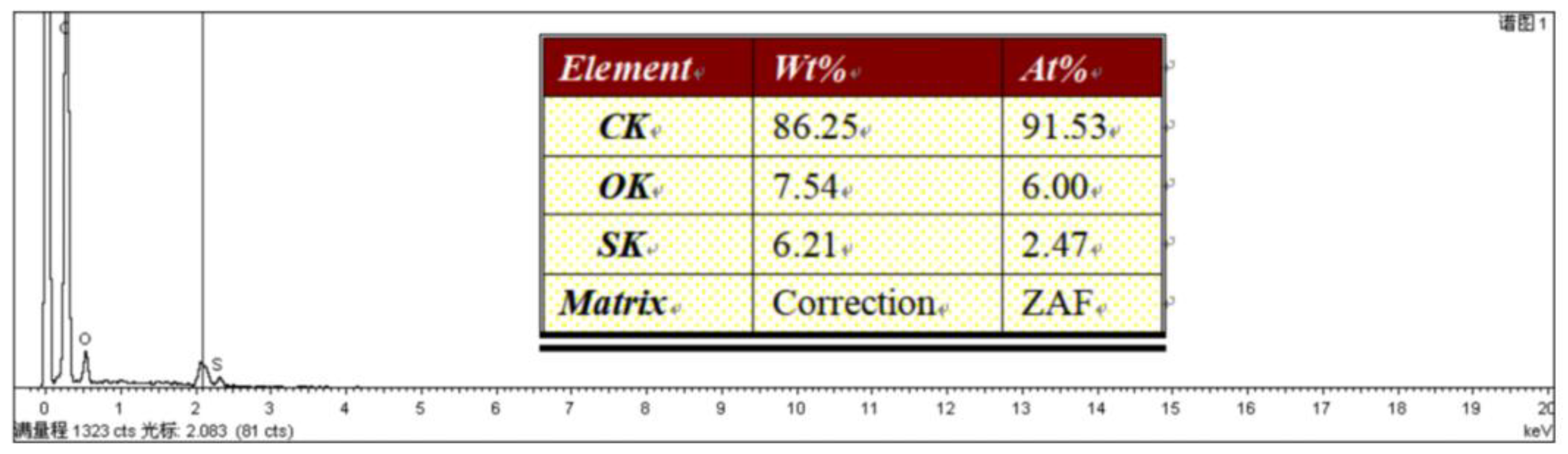
Figure 2 shows the EDS spectrum of TOC. From the figure, it can be seen that TOC only contains three elements: C, O, and S, indicating that thiophene and acetyl chloride have successfully synthesized polymer TOC. From the perspective of
Wt% and
At%, there is not much difference in the mass fractions of O and S elements, with atomic percentages close to 2:1. These data basically conform to the 2:1 ratio relationship between O and S atoms in the polymer TOC structure.
3.1.4. Scanning Electron Microscopy Analysis of TOC
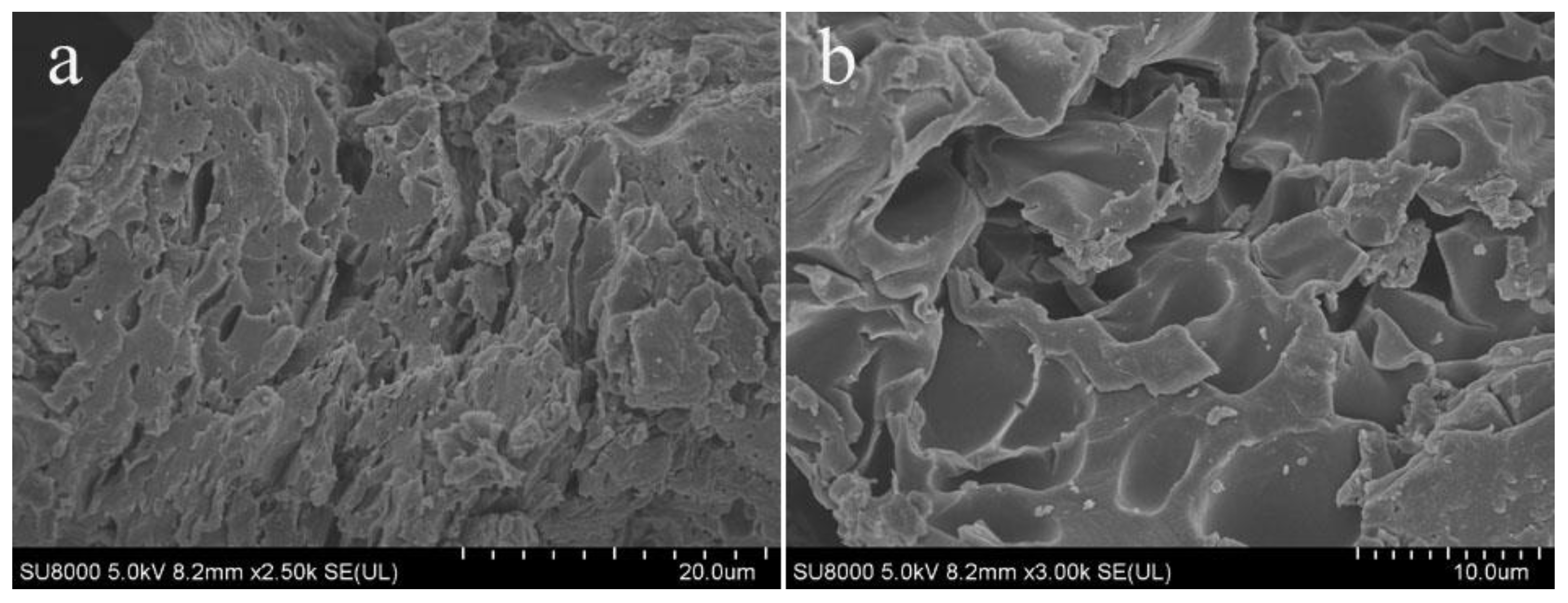
Figure 3 is a scanning electron microscope image of TOC, which shows that TOC has a porous and loose morphology. This may be due to the large volume of thiophene monomer, which may hinder the tight packing of polymer chains and form pores after the condensation reaction of thiophene and acetyl chloride. The morphology of TOC increases the contact area with heavy metal ions in aqueous solution, which can effectively enhance the adsorption of heavy metal ions.
3.1.5. Thermogravimetric (TG) Analysis
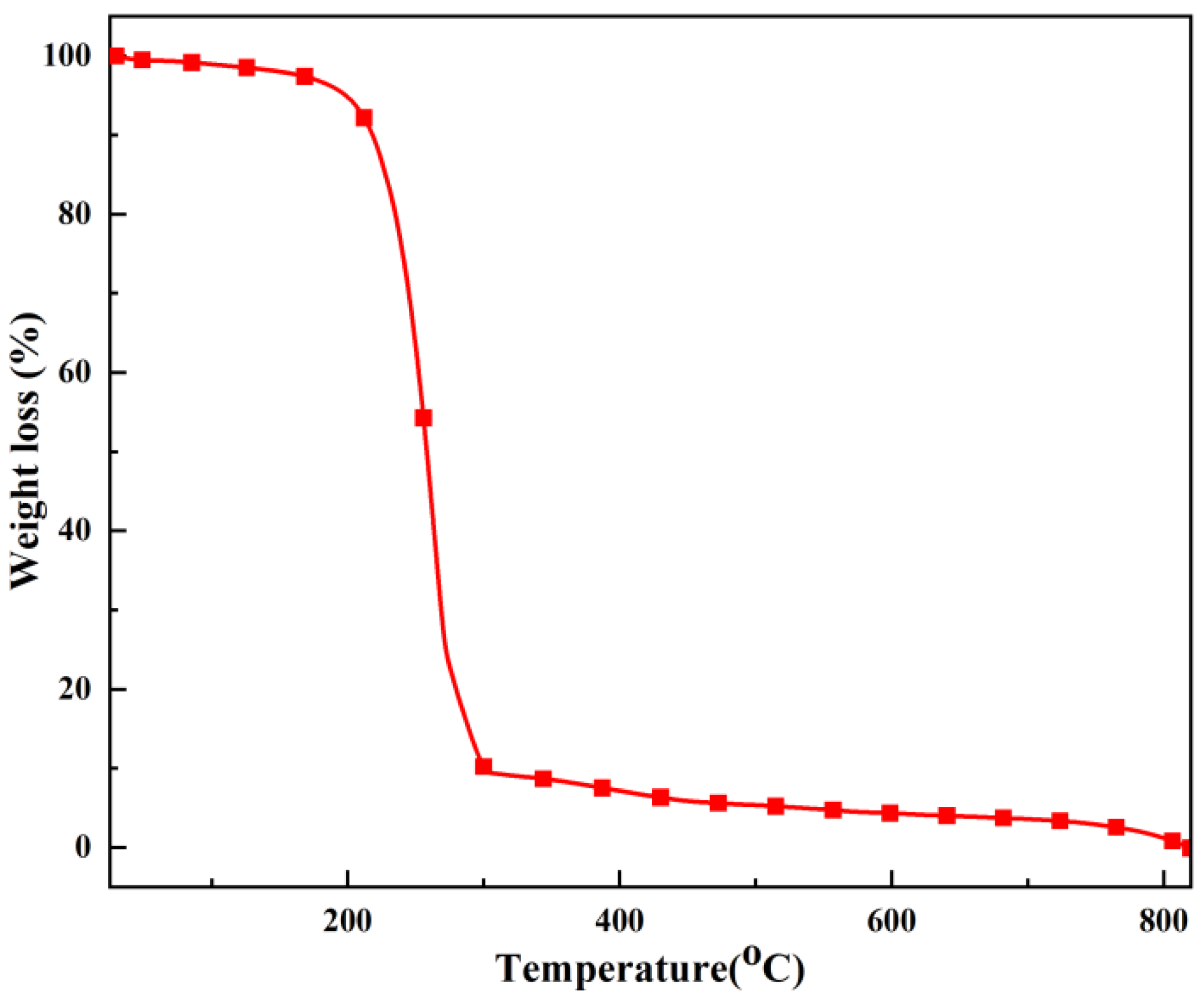
Figure 4 is a thermogravimetric diagram of TOC. From the figure, it can be seen that the thermogravimetric curve of TOC reaches a basic constant weight state after undergoing a significant weight loss process. Between 0-180℃, there is approximately 2% weight loss, mainly caused by the evaporation of adsorbed water in the sample. Between 180-450℃, the weight loss of the sample is about 95%, and the reason for this weight loss is related to the thermal decomposition and oxidation of the polymer. After 450℃, there is basically only a small amount of residue left in TOC, and the weight change is minimal and tends to stabilize.
The weight of the TOC adsorbent sample did not show significant changes before 180 ℃, indicating that the TOC adsorbent has good thermal stability.
Figure 4.
TG curve of TOC.
Figure 4.
TG curve of TOC.
3.2. Study on Adsorption Performance
3.2.1. Saturated Adsorption Capacity
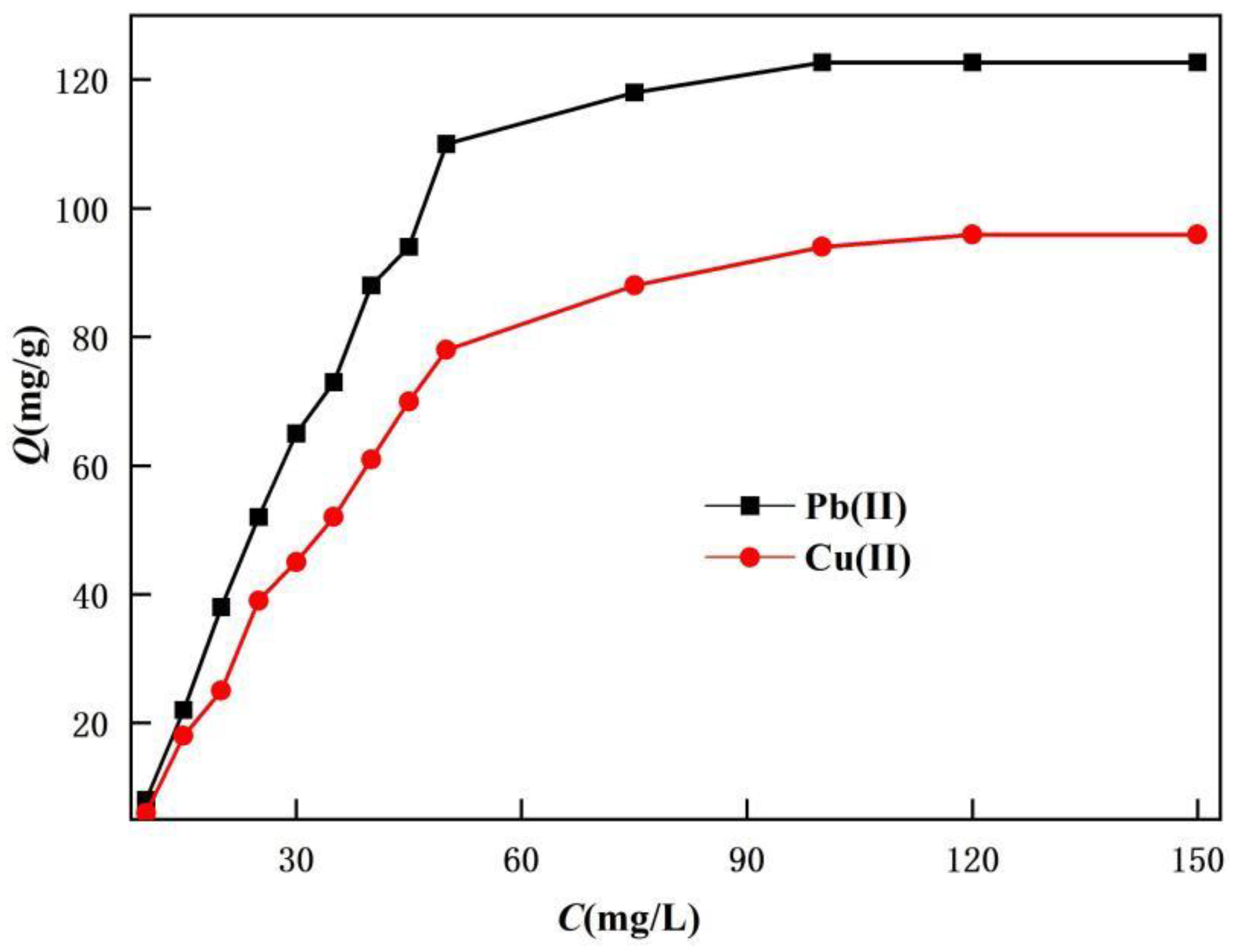
Take 0.02g TOC and add it to 50 mL of solutions with different concentrations of heavy metal ions. Stir at room temperature for 5 hours and test the concentration of heavy metal ions in the filtrate after adsorption. Under optimized conditions, the adsorption capacity of the adsorbent was examined.
It can be clearly seen from Figure 4 that the adsorption capacity of TOC increases with the initial concentration of the solution, and tends to saturate at high concentrations. The maximum adsorption capacities of TOC for Pb(II) and Cu(II) were calculated to be 122.7mg/g (0.593mmol/g) and 95.9mg/g (1.498mmol/g), respectively. TOC has a large adsorption capacity, which may be related to the porous surface of TOC, fully exposed coordination sites, and the ability to maximize contact with adsorbed ions for the most effective adsorption of these ions. The maximum number of ions adsorbed by TOC of the same mass for Cu(II) is greater than that for Pb(II), which may be related to the small particle radius of Cu(II) resulting in a larger number of particles accommodated in the same space.
Figure 4.
The effect of initial concentration on the adsorption quantity.
Figure 4.
The effect of initial concentration on the adsorption quantity.
3.2.2. Influence of pH Value
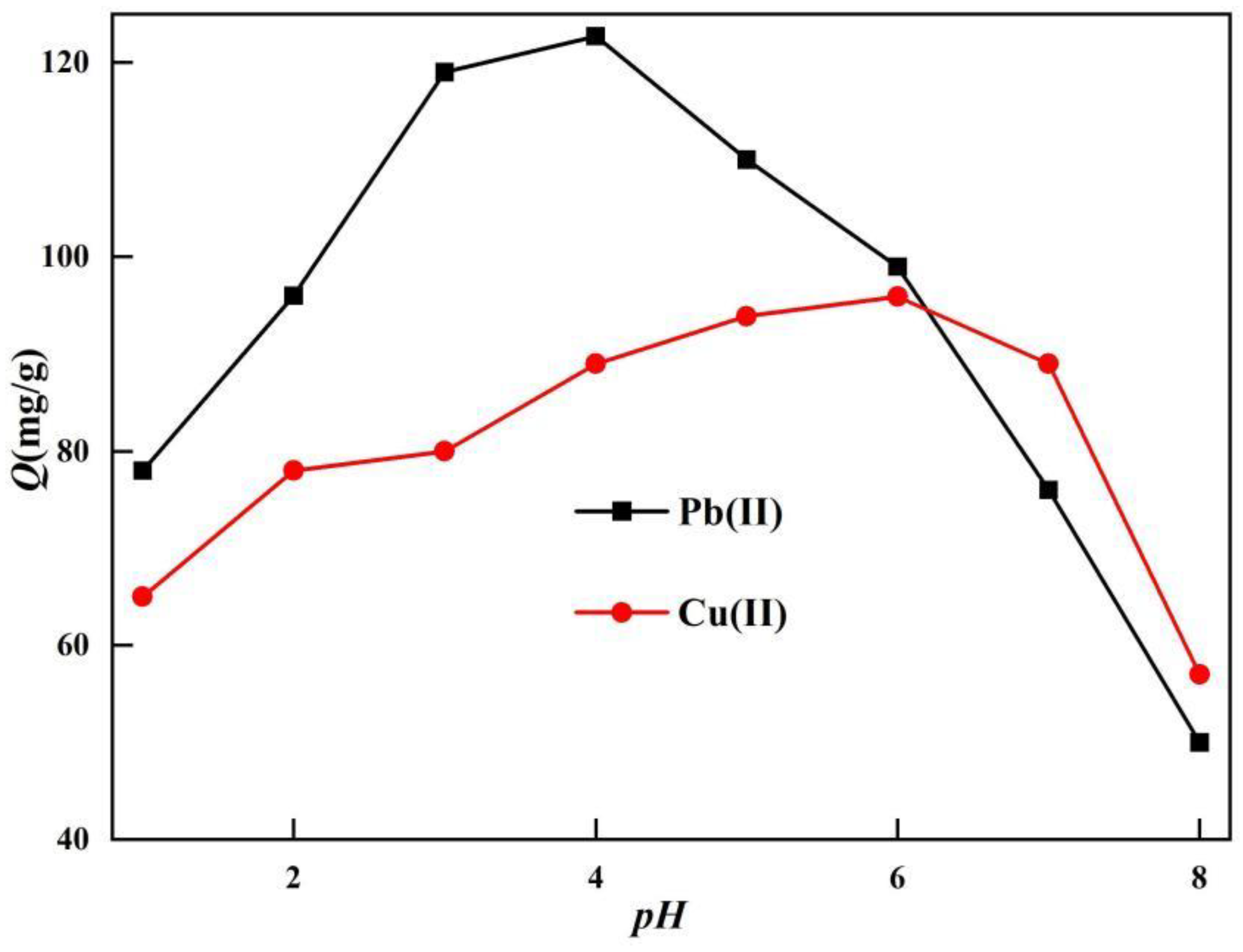
Add 0.02g TOC to solutions with different concentrations of heavy metal ions at different pH values, stir at room temperature for 5 hours, test the concentration of heavy metal ions in the filtrate after adsorption, and investigate the effect of adsorbents on adsorption capacity at different pH values.
The pH value of the solution has a significant impact on the performance of adsorbents in adsorbing metal ions, as it can affect the protonation degree of polar functional groups on the surface of the adsorbent, thereby affecting the surface charge of the adsorbent and the types of heavy metal ions. Appropriate solution pH can not only reduce the interference of environmental factors, but also increase the adsorption capacity of adsorbents [
27].
The ionic form of lead ions varies at different pH values. When the pH is low, lead ions are the main form of Pb(II), When 2<pH<6, Pb(II) and Pb(OH)
+ are the main forms of existence. When pH>6, Pb(II) hydrolyzes to form Pb(OH)
2 precipitate. Similarly, copper ions also exist in different ionic forms at different pH. When the pH is low, copper ions are the main form of Cu(II), and When pH>6, Cu(OH)
2 precipitation will occur in the solution. After repeated experimental testing, it was determined that TOC had the best adsorption effect on lead ions and copper ions when the pH was 4 and 6, respectively [
25].
Figure 5.
Effect of pH on adsorption.
Figure 5.
Effect of pH on adsorption.
3.2.3. Effect of Contacting Time on the Adsorption Capacity
Take 100 mL of Pb(II) and Cu(II) water samples at 50 mg/L each, add 0.02g of TOC to each, stir, and take samples at different times to determine the concentration of heavy metal ions. Calculate the adsorption capacity Q at the corresponding time.
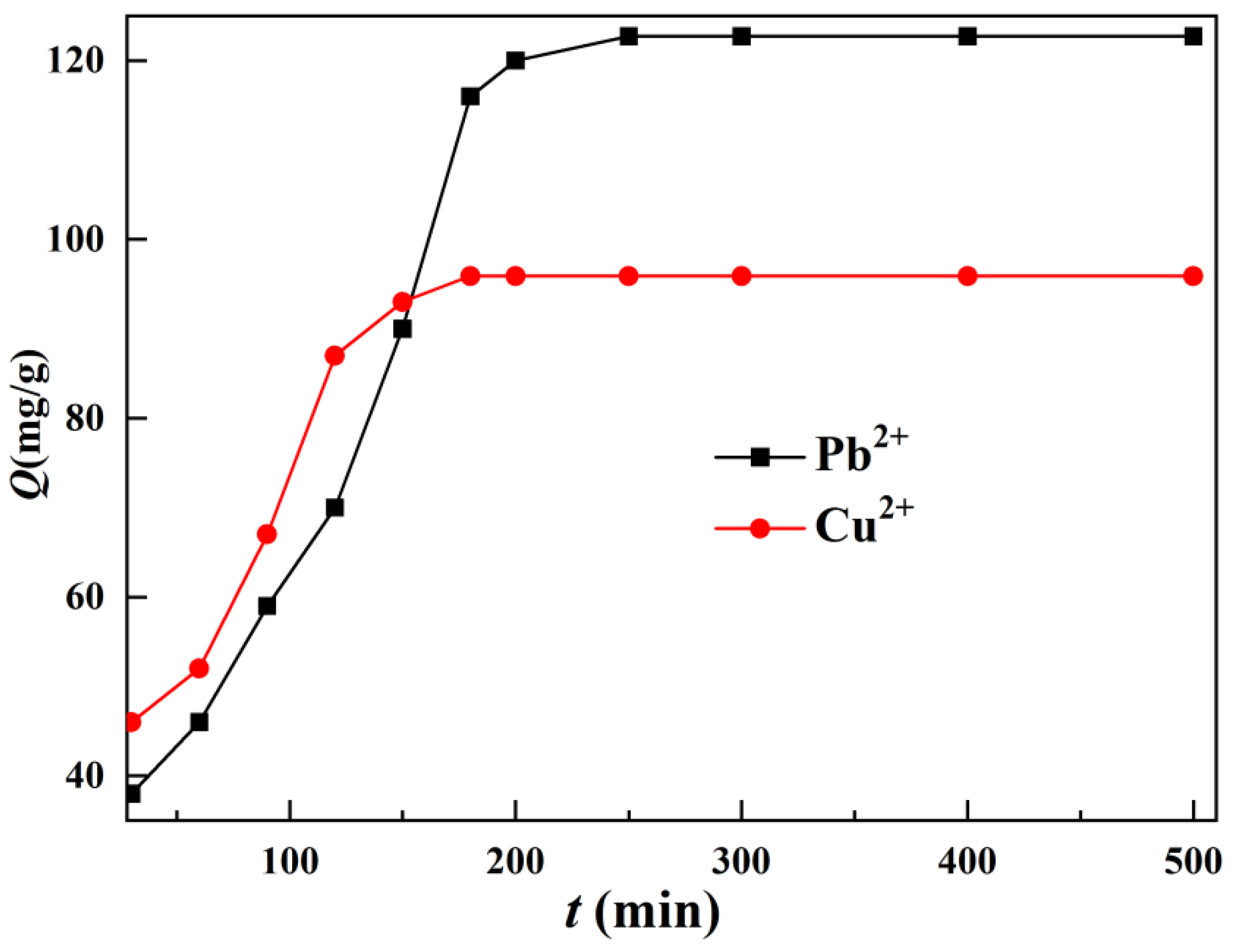
From
Figure 6, it can be seen that the adsorption capacity of TOC increases with the increase of adsorption time, and its adsorption capacity continues to rise. Adsorption equilibrium can be reached within 250 minutes, with high adsorption efficiency. This may be related to the porous surface morphology of TOC, which increases the surface area of TOC, exposes adsorption sites more fully, and accelerates the adsorption of heavy metal ions. The adsorption time of Cu(II) by TOC is faster than that of Pb(II), which may be related to the smaller particle radius of Cu(II) and its faster movement speed in porous polymers. In order to fully react the adsorbent with heavy metal ions, the time for TOC adsorption of Pb(II) is set to 250 minutes, and the time for adsorption of Cu(II) is set to 180 minutes.
3.2.4. Selection of Eluents
Desorption of Pb(II) or Cu(II) adsorbents using acid solutions of different concentrations. The results are shown in
Table 2. At room temperature, 1.00 mol/L HCl and 0.50 mol/L HNO
3 have the best desorption effect on Pb(II) and Cu(II). Therefore, this experiment chose 1.00 mol/L HCl to desorb Pb(II) and 0.50 mol/L HNO
3 to desorb Cu(II).
3.2.5. Reusability of Adsorbents
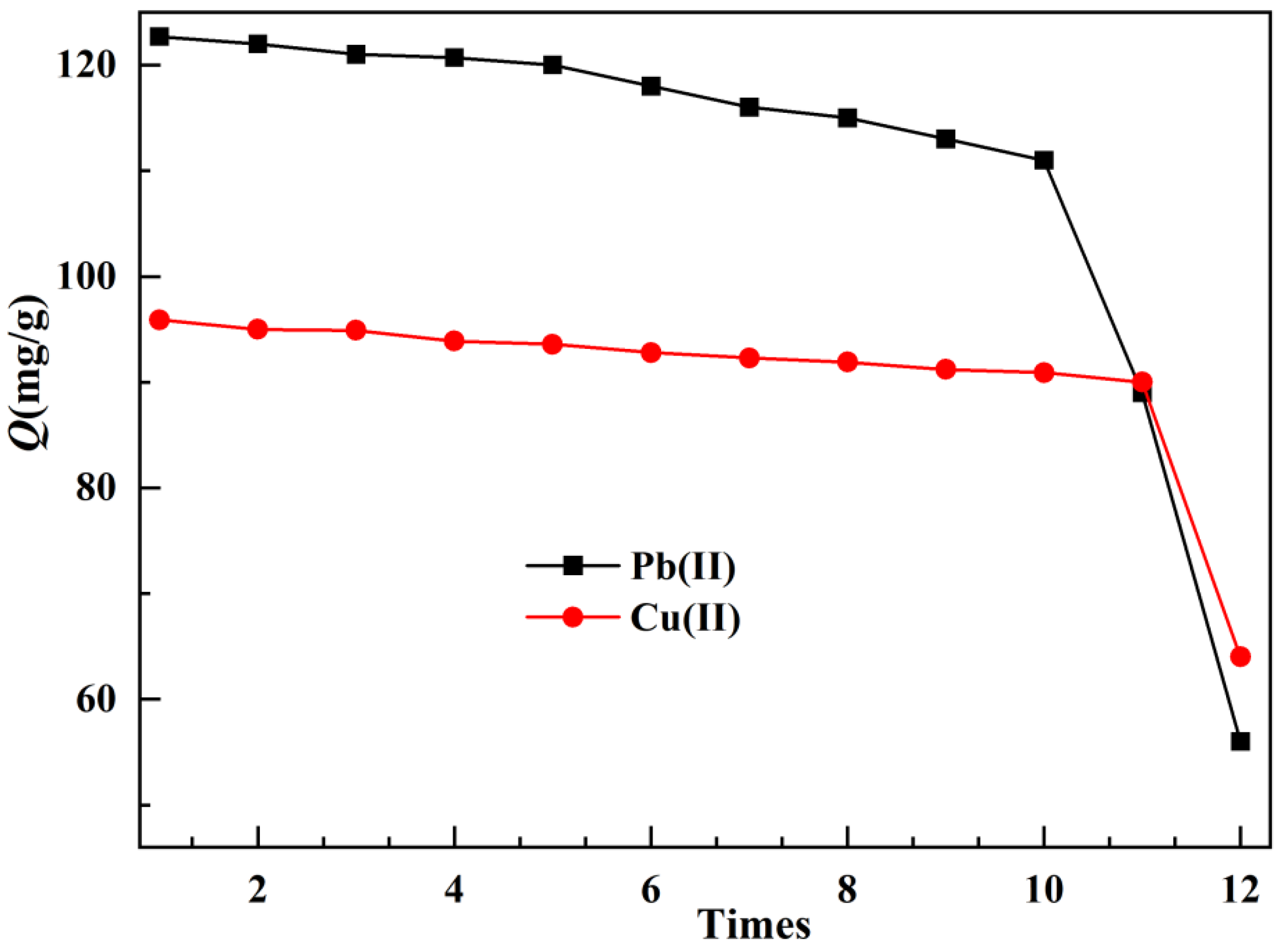
The effect of repeated use of the adsorbent on the adsorption capacity was tested, and the results are shown in
Figure 7. During the 12 cycles of adsorption and elution, the maximum adsorption capacity decreased with increasing usage, which may be due to a small amount of residual heavy metal ions occupying the adsorption sites, hindering the results of readsorption. From the results in the figure, it can be seen that for Pb(II), after 10 repeated uses, the adsorption capacity of TOC is 111mg/g; For Cu(II), after 11 repeated uses, the adsorption capacity of TOC was 90mg/g, and the reuse efficiencies were 90.46% and 93.85%, respectively. This indicates that TOC has excellent reusability, which may be related to its good structural stability. The number of repeated uses of Pb(II) by TOC is slightly smaller than that of Cu(II), which may be related to the larger particle radius of Pb(II), which makes it difficult to elute, and the higher density of lead ions, which damages the adsorbent to a greater extent during elution.
3.2.6. Adsorption Isotherm Model
The adsorption behavior of TOC is described using Langmuir and Freundlich adsorption isotherm equations, and the adsorption processes of Hg(II) and Pb(II) by TOC are fitted using Langmuir and Freundlich adsorption isotherm models, respectively. The adsorption isotherm equation is as follows [
28].
In the formula, Qe is the equilibrium adsorption capacity (mg/g), Ce is the concentration of metal ions after adsorption reaches equilibrium (mg/L), Qmax is the saturation adsorption capacity (mg/g), and b, kF, and n are constants.
The fitting results are shown in
Table 3. The adsorption equilibrium of Pb(II) and Cu(II) ions by TOC can be well described by the Freundlich model, because Freundlich (
R2=0.9912, 0.9851) can better fit the experimental data than Langmuir (
R2=0.6796, 0.8217). At the same time, it was found that the theoretical values of saturated adsorption capacity calculated according to the Langmuir model were 89.68 and 71.52 mg/g, which were significantly different from the experimental values of 122.7 and 95.9 mg/g. This also indicates that the Langmuir model cannot be used to fit the experimental data.
According to the Freundlich model data in
Table 3, the
n values for the adsorption of Pb(II) and Cu(II) by TOC are 5.32 and 3.75, respectively. Therefore, 1/n is between 0 and 1, indicating that adsorption is easy to occur, which is consistent with the faster adsorption rate of TOC. The fitting results are related to the structure of TOC. Due to the stable, porous, and layered structure of TOC, heavy metal ions will dissociate in aqueous solution and fully contact with the adsorption sites hidden deep inside the block solid to form complexes. Therefore, the process of TOC adsorbing heavy metal ions is a multi-layer adsorption process.
3.2.7. Adsorption Kinetics Model
For the adsorption behavior of TOC, pseudo-first-order kinetic models and pseudo-second-order kinetic models are used for fitting. The pseudo-first-order kinetic model describes [
29]:
In the equation, Qt is the adsorption capacity at time t (mg/g), Qeq is the equilibrium adsorption capacity (mg/g), and k1 (min-1) is the pseudo-first-order rate constant.
The pseudo-second-order kinetic model is as follows:
In the equation, Qt is the adsorption capacity at time t (mg/g), Qeq is the equilibrium adsorption capacity (mg/g), and k2 is the pseudo-second-order rate constant g/(mg·min).
As shown in
Table 4, the pseudo-second-order kinetic models (
R2=0.9879, 0.9827) of TOC for Pb(II) and Cu(II) ions can better fit the adsorption kinetics data than pseudo-first-order kinetic models (
R2=0.8924, 0.6952), and the predicted equilibrium adsorption amounts of 125.27, 98.75mg/g and experimental values of 122.7, 95.9mg/g using the pseudo-second-order kinetic models are also relatively close. This indicates that the adsorption process of Pb(II) and Cu(II) ions by TOC follows a chemical adsorption process controlled by pseudo-second-order kinetic reactions. The fitting results indicate that the adsorption process of Pb(II) and Cu(II) ions by TOC is achieved through the formation of coordination bonds between the functional groups of TOC and heavy metal ions, which is consistent with experimental analysis and results.
4. Conclusion
(1) In this article, the polymer adsorbent TOC containing S functional group was successfully synthesized by thiophene and ethanedioyl chloride. The successful synthesis of TOC was confirmed by IR, molecular weight determination, EDS, SEM, and TG .
(2) For Pb(II), the maximum adsorption capacity of TOC can reach 122.7 mg/g (0.593 mmol/g) at pH=4, 250 minutes. After adsorption, it can be desorbed with 1.00 mol/L HCl, and the adsorbent can be reused 10 times; For Cu(II), the maximum adsorption capacity of TOC can reach 95.9mg/g (1.498mmol/g) at pH=6, 180min. After adsorption, it can be desorbed with 0.50 mol/L HNO3, and the adsorbent can be reused 11 times. The adsorption process of TOC on Pb(II) and Cu(II) conforms to the Freundlich model of multi-layer adsorption and the chemical adsorption process controlled by pseudo-second-order kinetic reactions.
(3) Adsorbents of the same quality adsorb more substances of Cu(II) than Pb(II), with shorter time and more repeated use. The reason may be that Cu(II) has a smaller radius, can accommodate more in a limited space, moves faster, and is easier to desorb.
Author Contributions
Conceptualization, D.C.; writing—original draft preparation, D.C.; investigation, D.C.; resources, D.C.; validation, W.Z.; writing—review and editing, W.Z.; supervision, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (grant number, 2024D01A96).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoda, G. , Mohsen, T. Efficient adsorption of lead and copper from water by modification of sand filter with a green plant-based adsorbent: Adsorption kinetics and regeneration. Environmental Research 2024, 259, 119529. [CrossRef]
- Jin, C. , Hua, Z. , Asfandyar, S. , Shehnaz, A. F. A. , Shengbo, G. , Christian, S. , Zhen, Mo. , Chao, H. Efficient removal of heavy metals using 1,3,5-benzenetricarboxylic acid-modified zirconium-based organic frameworks. Environmental Technology & Innovation 2024, 33, 103516. [CrossRef]
- Tan, S. , Zhang, T. , Cheng, C. , Wang, Z. , Li, H. , Zhao, Y. Efficient removal and stepwise recovery of various heavy metals from water by using calcium carbonate with different activity. Separation and Purification Technology 2025, 354, 129142. [CrossRef]
- Sonali, R. D. , Satyajit, M. D. , Ajinkya, K. , Amaya, S. A review outlook on methods for removal of heavy metal ions from wastewater. Separation and Purification Technology 2024, 350, 127868. [CrossRef]
- Luca, B. , Emanuela, S. , Federica, B. , Francesco, G. Metal nanostructures in polymeric matrices for optical detection and removal of heavy metal ions, pesticides and dyes from water. Chemosphere 2024, 362, 142636. [CrossRef]
- Motomu, S. , Eri, N. , Masahiko, M. Rejection of heavy metal ions in water by zeolite forward osmosis membrane. Separation and Purification Technology 2024, 357, 130163. [CrossRef]
- Wang, B. , Wang, J. , Mao, R. , Li, Z. , Cheng, Y. , Niu, Y. , Chen, H. Synthesis of bifunctional silica aerogels for robust and simultaneous removal of Hg(II) and malachite green: Performance and mechanism. Separation and Purification Technology 2025, 355, 129773. [CrossRef]
- Chen, Z. , Su, X. , Li, K. , Niu, S. , Shen, Z. , Li, X. , Chen, S. , Wu, W. A thiolated TiO2-based degradable superhydrophobic wood for oil–water separation and heavy metal treatment. Separation and Purification Technology 2024, 354, 128949. [CrossRef]
- Young, G. K. Hybrid method integrating adsorption and chemical precipitation of heavy metal ions on polymeric fiber surfaces for highly efficient water purification. Chemosphere 2024, 363, 142909. [CrossRef]
- Wang, Y. , Tamaki, N. , Chen, X. , Xu, Y. L. , He, Y. , Wu, Y. X. , Zhang, J. Q. , Tian, W. , Zhou, M. H. , Wang, S. X. Studies on adsorption properties of magnetic composite prepared by one-pot method for Cd(II), Pb(II), Hg(II), and As(III): Mechanism and practical application in food. Journal of Hazardous Materials 2024, 466, 133437. [CrossRef]
- Fu, R. , Jiao, R. , Cao, X. , Zhang, H. , Chen, Y. , Sun, H. , Zhu, Z. , Li, J. , Li, An. Capture of volatile iodine by novel conjugated microporous polymers hollow spheres containing N, S electro-rich groups. Surfaces and Interfaces 2024, 46, 104025. [CrossRef]
- Wang, L. , Liu, L. , Chen, R. , Jiao, Y. , Zhao, K. , Liu, Y. , Zhu, G. Carbonized polymer dots-based molecular imprinting: An adsorbent with enhanced selectivity for highly efficient recognition and removal of ceftiofur sodium from complex samples. Journal of Hazardous Materials 2024, 473, 134637. [CrossRef]
- Luo, G. , Jiang, J. , Wei, S. , Huang, C. , Chen, D. , Zhu, B. , Zhang, S. Introducing sulfonic acid polymers into MOF nanochannels for ultra-high Ba2+ adsorption capacity and proton conductivity. Separation and Purification Technology 2024, 343, 127133. [CrossRef]
- Yuan, L. , Guo, H. , Li, Q. , Zhang, H. , Xu, M. , Zhang, W. , Zhang, Y. , Hua, M. , Lv, L. , Pan, B. Machine-Learning-Assisted Material Discovery of Pyridine-Based Polymers for Efficient Removal of ReO4–. Environmental Science & Technology 2024, 58, 15298. [CrossRef]
- Chen, S. , Zhao, L. , Li, X. , Chen, Z. , Hu, X. , Zi, F. Constructing a cationic pyridine for the highly selective and efficient recovery of gold from waste printed circuit boards. Chemical Engineering Journal 2024, 483, 149325. [CrossRef]
- Xu, C. , Qu, R. , Li, S. , Sun, C. , Zhang, Y. , Gao, J. , Niu , Y. , Ma, Q. , Song, X. , Wang, S. , Li, C. Preparation, Characterization, and Rapid Adsorption of Hg2+ on Nanoscale Aramid-based Adsorbent. Journal of Polymers and the Environment 2016, 24, 206. [CrossRef]
- Liu, Z. , Wang, L. , Lv, Y. , Xu, X. , Zhu, C. , Liu, F. , Li, A. Impactful modulation of micro-structures of acid-resistant picolylamine-based chelate resins for efficient separation of heavy metal cations from strongly acidic media. Chemical Engineering Journal 2021, 420, 129684. [CrossRef]
- Zou, B. , Zhang, S. , Sun, P. , Zhao, Q. , Zhang, W. , Zhang, X. , Ran, L. , Zhou, L. , Ye, Z. Synthesis of a novel Poly-chloromethyl styrene chelating resin containing Tri-pyridine aniline groups and its efficient adsorption of heavy metal ions and catalytic degradation of bisphenol A. Separation and Purification Technology 2021, 275, 119234. [CrossRef]
- Sebastian, B. , Pepijn, S. , Kim R. , Saumey, J. , Renee, K. , Christian, M. , Mahiar, M. , Hamedi, E. Z. , Anna H. In Situ Functionalization of Polar Polythiophene-Based Organic Electrochemical Transistor to Interface In Vitro Models. ACS Applied Materials & Interfaces 2024, 27, 54292. [CrossRef]
- Fan, S. , Liu, Yao. , Zhang, Z. , Huang, M. , Wang, Y. , Gao, J. , Xiong, Y. A green synthesis method of a mussel-inspired polyphenol-functionalized silica-based material and its highly efficient adsorption of gallium. Separation and Purification Technology 2024, 349, 127670. [CrossRef]
- Zhang, J., Chen, Y. Uptake of Fe(iii), Ag(i), Ni(ii) and Cu(ii) by salicylic acid-type chelating resin prepared via surface-initiated atom transfer radical polymerization. RSC Adv. 2016, 6, 69370. [CrossRef]
- Li, X. , Jia, Z. , Tan, H. W. , Yang, Y. , Hou, L. A. Enhanced simultaneous adsorption and detection of mercury (II) using functionalized metal − organic framework with defect structures. Separation and Purification Technology 2024, 354, 129110. [CrossRef]
- Wang, L. , Liu, J. , Wang, J. , Zhang, D. , Huang, J. Thiophene-based porphyrin polymers for Mercury (II) efficient removal in aqueous solution. Journal of Colloid and Interface Science 2023, 653, 405. [CrossRef]
- Muhammad, A. A. , Tawfik A. S. , Youcef, M. , Thomas, F. G. , Othman, C. S. A. H. Synthesis of a new thiophenol-thiophene polymer for the removal of mercury from wastewater and liquid hydrocarbons. Journal of Colloid and Interface Science 2021, 582, 428–438. [CrossRef]
- Othman, C. S. A. H. , Shaikh, A. A. Removal of heavy metal ions using a novel cross-linked polyzwitterionic phosphonate. Separation and Purification Technology 2012, 98, 94–101. [CrossRef]
- Chen, J.; Seko, N. Cleavage of the Graft Bonds in PVDF–g–St Films by Boiling Xylene Extraction and the Determination of the Molecular Weight of the Graft Chains. Polymers 2019, 11, 1098. [CrossRef]
- Tang, J. , Chen, Y. , Zhao, M. , Wang, S. , Zhang, L. Phenylthiosemicarbazide-functionalized UiO-66-NH2 as highly efficient adsorbent for the selective removal of lead from aqueous solutions. Journal of Hazardous Materials 2021, 413, 125278. [CrossRef]
- Abdullah, N. , Mohd, F. A. , Jun, H. S. Recovery of waste cooking palm oil as a crosslinker for inverse vulcanized adsorbent to remove iron (Fe3+) ions. Journal of Environmental Chemical Engineering 2024, 12, 111853. [CrossRef]
- Akar, T. , Alim, S. , Akar, S. S. T. A novel sustainable and eco-friendly biosourced hybrid sorbent for toxic Pb2+ decontamination: Nano metal oxide functionalized salt-tolerant plant biomass. Journal of Cleaner Production 2024, 439, 140838. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).