Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
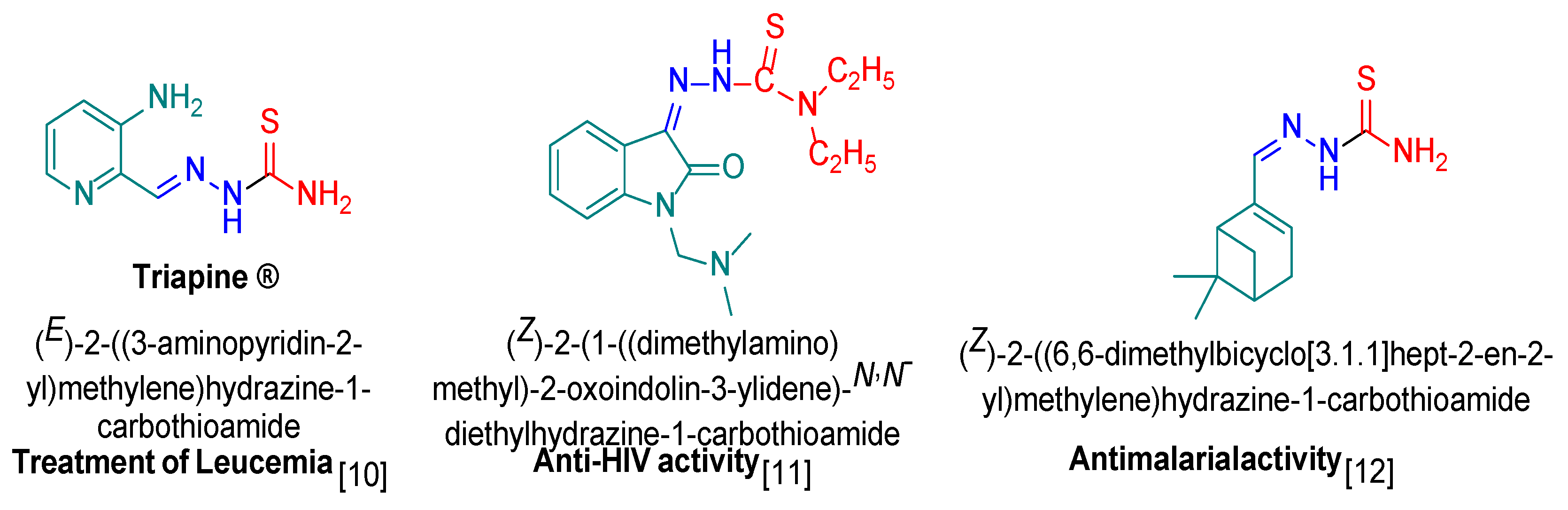
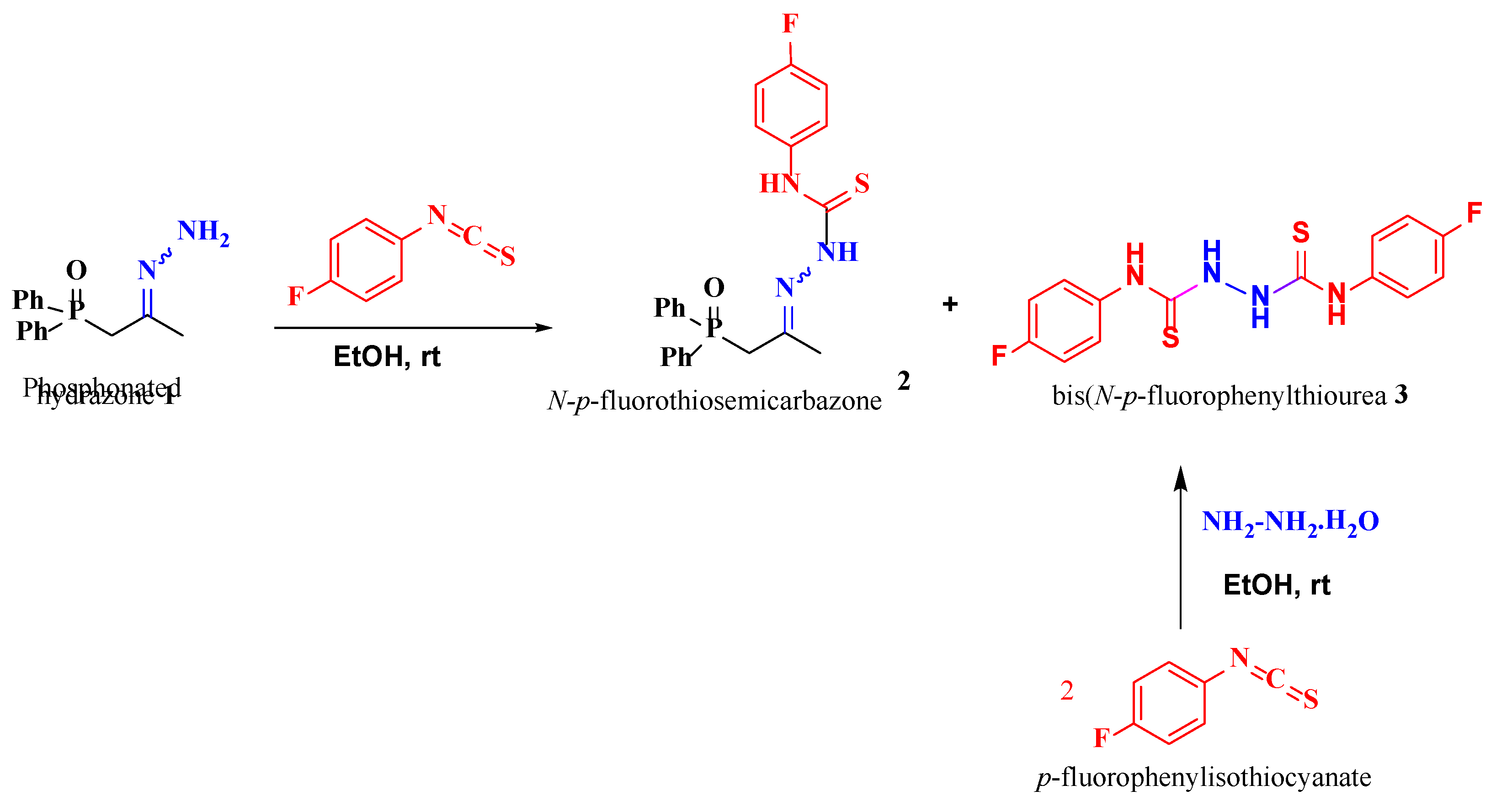
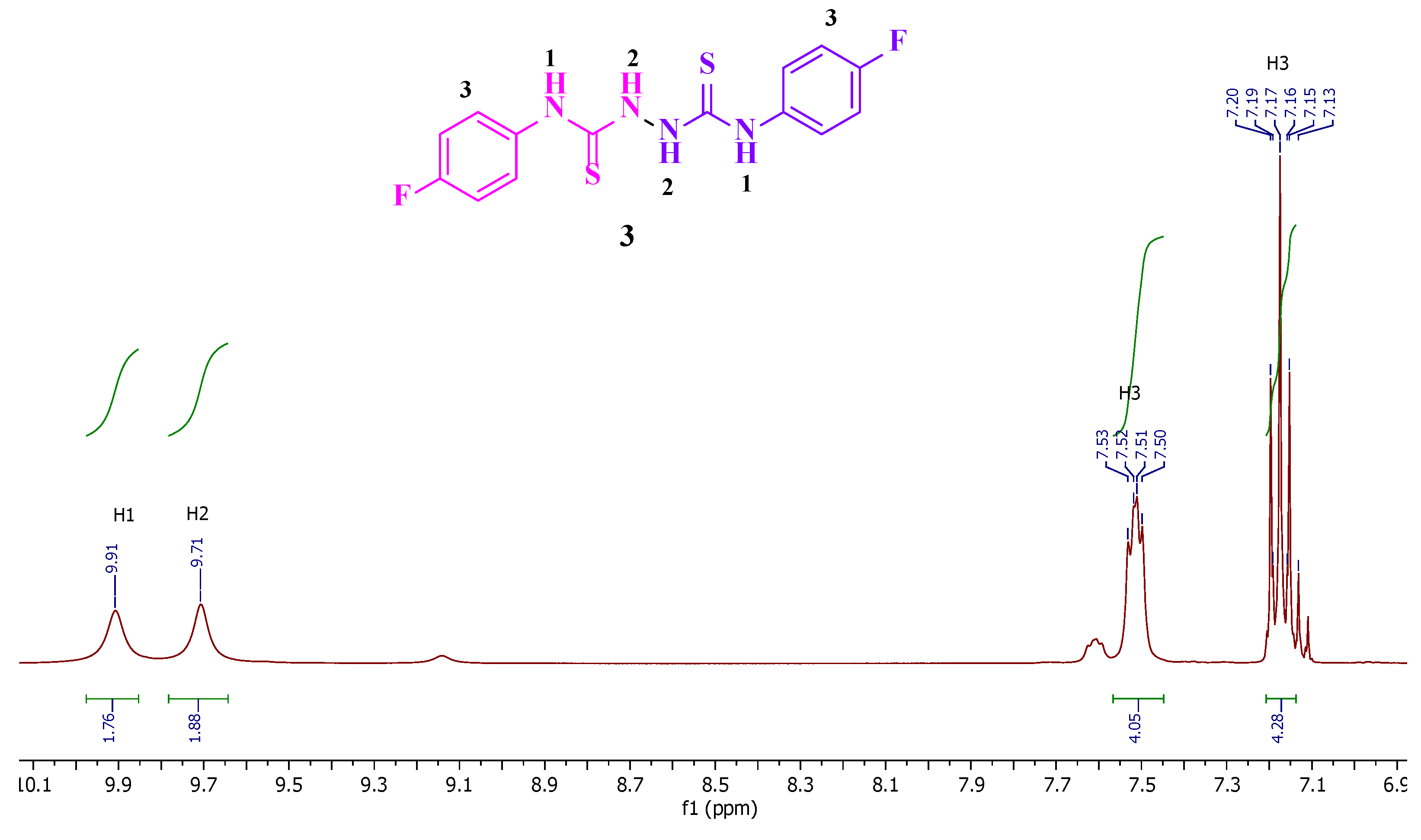
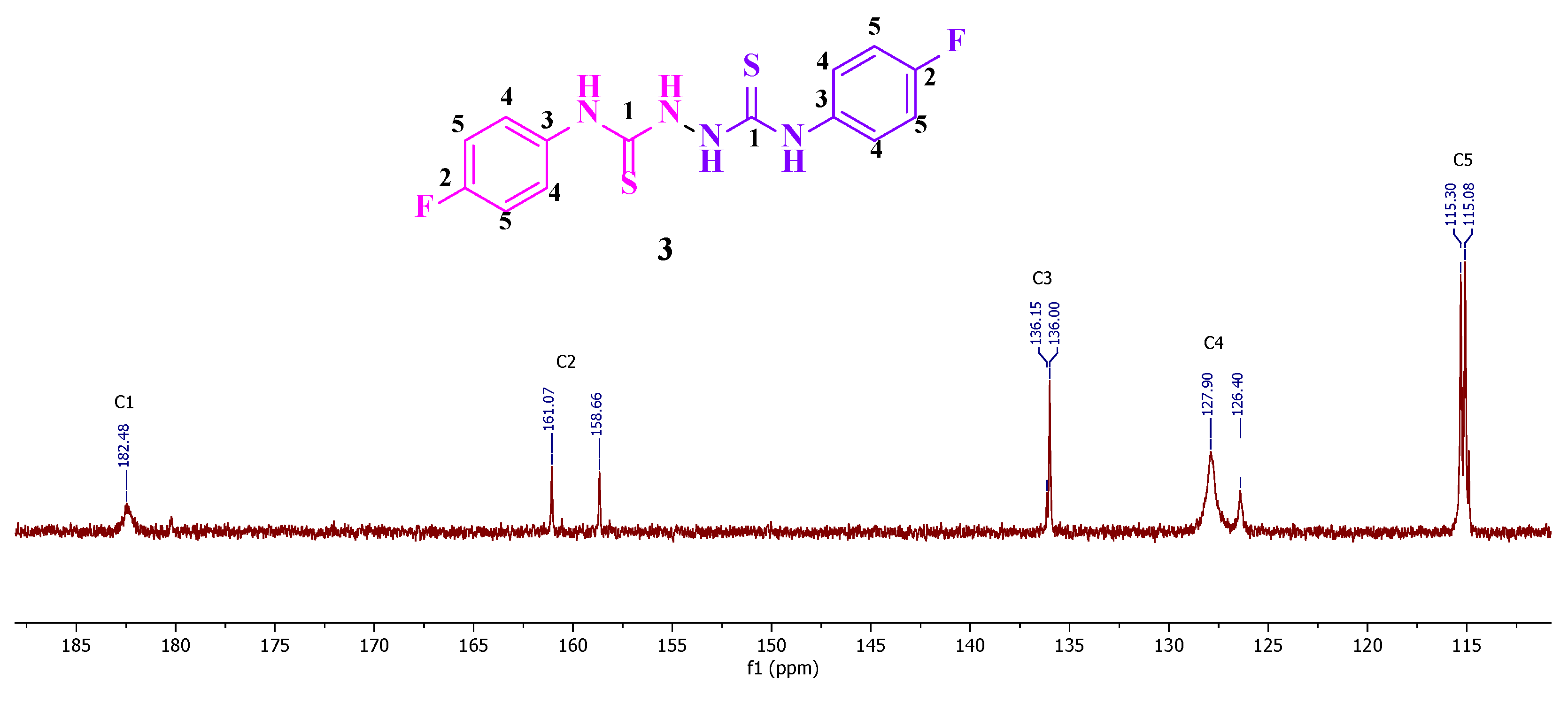
Reaction of the phosphonated hydrazone (2-hydrazineylidenepropyl) diphenylphosphine oxide 1 with p-fluorophenyl-isothiocyanate yields as major product the thiosemicarbazone Ph2P(=O)CH2{C=N-NH(C=S)-N(H)C6H4F}CH3 (2-(1-(diphenylphosphoryl)propan-2-ylidene)-N-(4-fluorophenyl)hydrazine-1-carbothioamide) 2 along with bis(N-p-fluorophenylthiourea) 3 as minor product. This latter product 3 was isolated as main product by direct treatment of p-FC6H4N=C=S with hydrazine in a 2:1 ratio. Both 2 and 3 were characterized by NMR. Furthermore, the molecular structure of 3 was elucidated by an X-ray diffraction study. A conformational DFT study, at the B3LYP/6311 G++ (d, p) level of theo-ry, confirmed a good match between the calculated structure and the experimental one.
Keywords:
1. Introduction
2. Results and Discussion
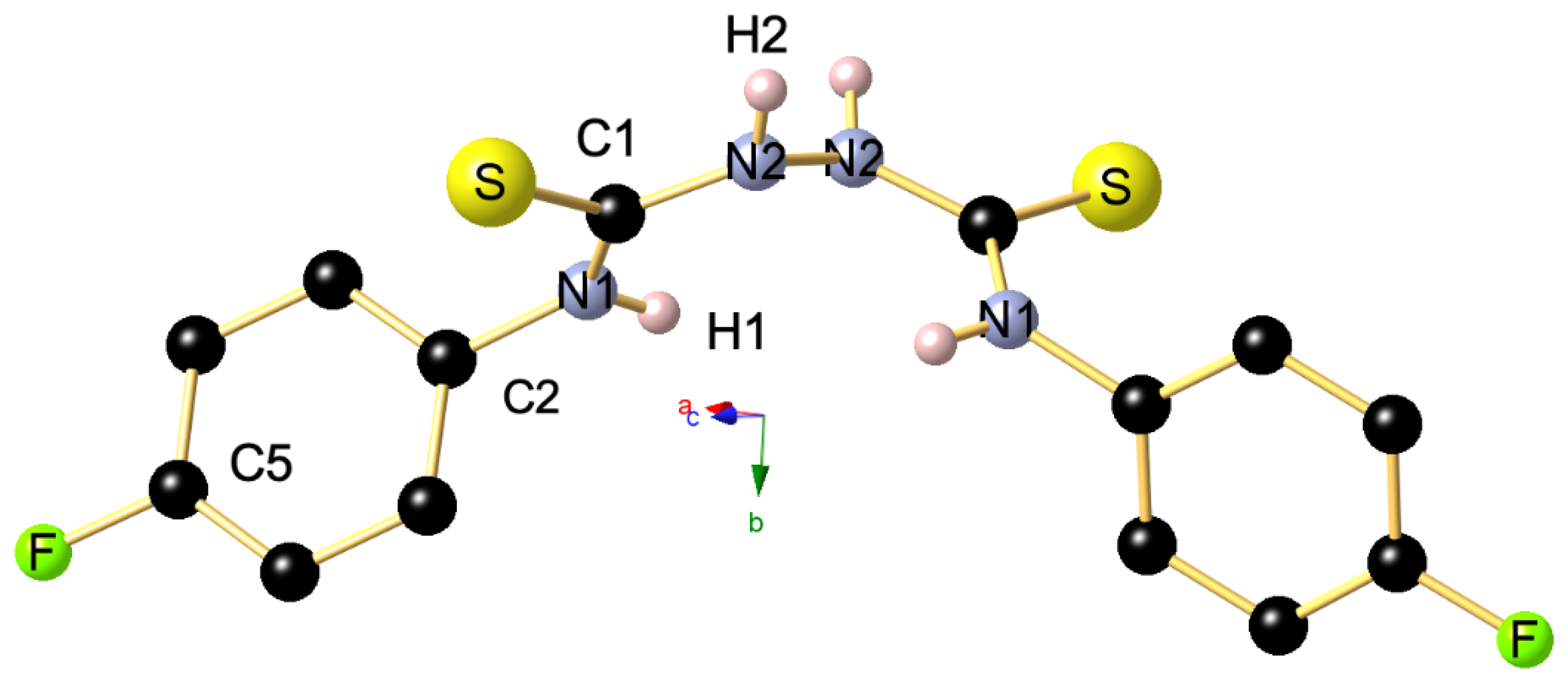
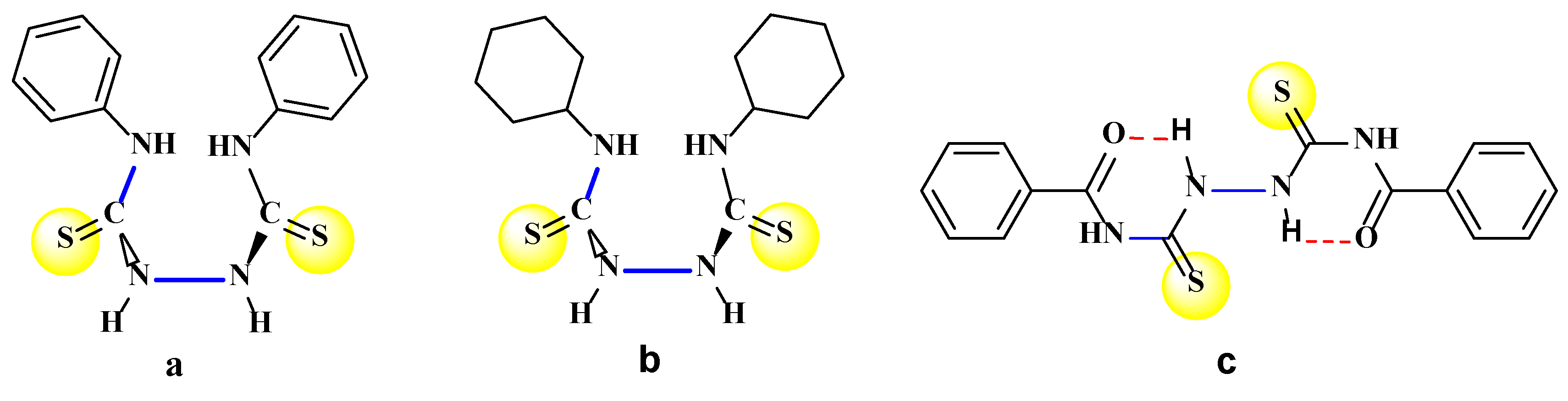
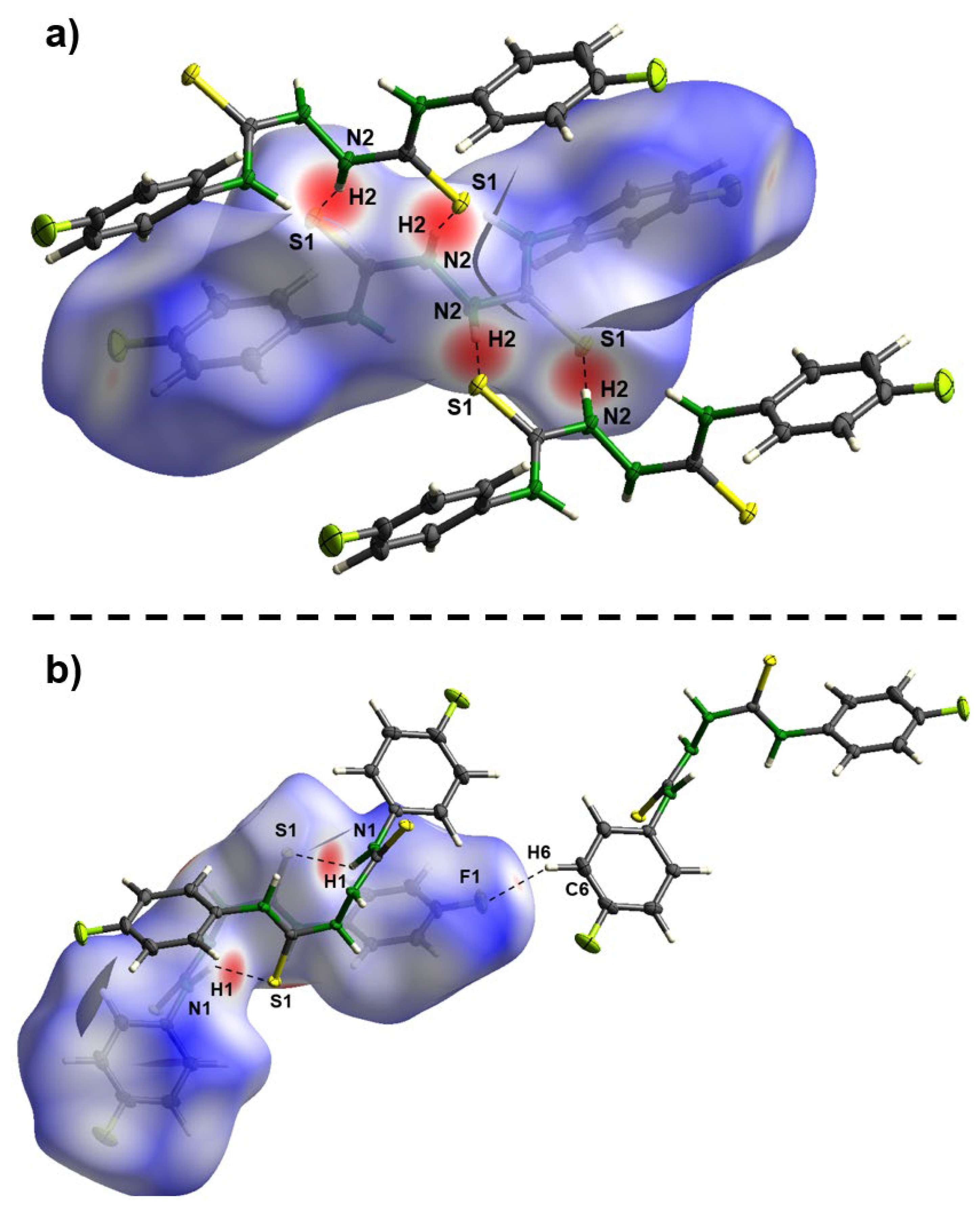
3. Materials and Methods
Theoretical calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanzari-Mnallah, D.; Salhi, S.; Knorr, M.; Kirchhoff, J. L.; Strohmann, C.; Efrit, M. L.; Akacha, A. B. Synthesis of Isomeric β-Cycloalkoxyphosphonated Hydrazones Containing a Dioxaphosphorinane Ring: Conformational and Conformational Investigation and Molecular Docking Analysis. J. Mol. Struct. 2024, 1317, 139035. DOI: 10.1016/j.molstruc.2024.139035. [CrossRef]
- Ben Akacha, A.; Barkallah, S.; Zantour, H. ¹³C NMR and ³¹P NMR Spectral Assignment of New β-Phosphonylated Hydrazones. Magn. Reson. Chem. 1999, 37, 916–920. DOI: 10.1002/(SICI)1097-458X(199912)37:12<916::AID-MRC547> 3.0.CO;2-W. [CrossRef]
- Kanzari-Mnallah, D.; Efrit, M. L.; Ben Akacha, A. Synthèse de 1,3,2-Dioxaphosphorinanes Diastéréoisomères: Influence de la Conformation des 1,3-Diols de Départ sur Leurs Structures et Conformations. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 665–673. DOI: 10.1080/10426507.2017.1314156. [CrossRef]
- Salah, N.; Arfaoui, Y.; Bahri, M.; Efrit, M. L.; Ben Akacha, A. Synthèse et Étude Conformationnelle par RMN (¹H, ¹³C, ³¹P) et DFT des Cycloalcoxyphosphinallenes et des Hydrazones β-Cycloalcoxyphosphonatées. Phosphorus, Sulfur Silicon Relat. Elem. 2013, 188, 609–622. DOI: 10.1080/10426507.2012.737117. [CrossRef]
- Ben Akacha, A.; Ayed, N.; Baccar, B.; Charrier, C. Phospho-4-Pyrazoles. Synthesis and Proton, Phosphorus-31, and Carbon-13 NMR. Phosphorus Sulfur Relat. Elem. 1988, 40, 63–68. DOI: 10.1080/03086648808072894. [CrossRef]
- Nurkenov, O. A.; Ibrayev, M. K.; Satpaeva, Zh. B.; Dauletzhanova, Zh. T.; Seilkhanov, T. M. Synthesis and Study of Antioxidant Activity of Hydrazone and Thiosemicarbazide Based on N-Morpholinoacetic Acid Hydrazide. Vestnik Karaganda Univ. Ser. Chem. 2018, 1, 22–26.
- Gihsoyl, A.; Terzioglu, N.; Bik, G. Synthesis of Some New Hydrazide-Hydrazones, Thiosemicarbazides, and Thiazolidinones as Possible Antimicrobials. Eur. J. Med. Chem. 1997, 32, 753–757. DOI: 10.1016/S0223-5234(97)88918-0. [CrossRef]
- Salah, N.; Zribi, S.; Efrit, M. L.; Ben Akacha, A. Hydrazones β-Phosphonates: Nouvelles Voies d’Accès aux Thiosemicarbazones, 4-Phosphopyrazoles et Indoles 2-Phosphonates. J. Soc. Chim. Tunisie 2013, 15, 133–141.
- Ragab, A.; Ammar, Y. A.; Mahmoud, M.; Mohamed, B. I.; El-Tabl, S. Abdou; Farag, S. S. Synthesis, Characterization, Thermal Properties, Antimicrobial Evaluation, ADMET Study, and Molecular Docking Simulation of New Mono Cu(II) and Zn(II) Complexes with 2-Oxoindole Derivatives. Comput. Biol. Med. 2022, 145, 105473. DOI: 10.1016/j.compbiomed.2022.105473. [CrossRef]
- Dharmasivam, M.; Kaya, B.; Wijesinghe, T.; Azad, M. G.; Gonzálvez, M. A.; Hussaini, M.; Chekmarev, J.; Bernhardt, P. V.; Richardson, D. R. Designing Tailored Thiosemicarbazones with Bespoke Properties: The Styrene Moiety Imparts Potent Activity, Inhibits Heme Center Oxidation, and Results in a Novel “Stealth Zinc(II) Complex.” J. Med. Chem. 2023, 66, 1426–1453. DOI: 10.1021/acs.jmedchem.2c01600. [CrossRef]
- Bal, T. R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and Evaluation of Anti-HIV Activity of Isatin β-Thiosemicarbazone Derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455. DOI: 10.1016/j.bmcl.2005.07.046. [CrossRef]
- Pelosi, G. Thiosemicarbazone Metal Complexes: From Structure to Activity. Open Crystallogr. J. 2010, 3, 16–28. DOI: .2174/1874846501003010016. [CrossRef]
- Syadza, F.; Hasbullah, S. A.; Yamin, B. M. Synthesis, Structural Elucidation, and Antioxidant Study of Ortho-Substituted N,N′-bis(benzamidothiocarbonyl)hydrazine Derivatives. IOP Conf. Ser.: J. Phys.: Conf. Ser. 2018, 979, 012010. DOI: 10.1088/1742-6596/979/1/012010. [CrossRef]
- Mészáros Szécsényi, K.; Leovac, V. M.; Radosavljević Evans, I. Synthesis and Characterisation of a Novel Polymeric Cd Complex, Catena-(μ-Thio)[bis(N-Phenylthiourea)]bis(dimethylsulfoxide)dichlorocadmium(II). J. Coord. Chem. 2006, 59, 171–180. DOI: 10.1080/00958970500171240. [CrossRef]
- Akinchan, N.T.; Drożdżewski, P.M.; Battaglia, L.P. Crystal structure and spectroscopic characterization of bis(N-phenylthiourea). J. Chem. Crystallogr. 2002, 32, 91–97. DOI: 10.1023/A:1015616911355. [CrossRef]
- Jaiswal, S.; Gond, M.K.; Bharty, M.K.; Maiti, B.; Krishnamoorthi, S.; Butcher, R.J. Manganese(II) catalyzed synthesis of bis (N-cyclohexylthiourea) derived from thiosemicarbazide: Structural characterization, fluorescence, cyclic voltammetry, Hirshfeld surface analysis and DFT calculation. J. Mol. Struct. 2021, 1246, 131060-131070. DOI: 10.1016/j.molstruc.2021.131060. [CrossRef]
- Yamin, B. M. Y.; Yusof, M. S. M. N,N'-Bis(benzamidothiocarbonyl)hydrazine Dimethyl Sulfoxide Disolvate. Acta Cryst. 2003, E59, o358-o359. DOI: 10.1107/S1600536803003635. [CrossRef]
- Firdausiah, S.; Hasbullah, S.A.; Yamin, B. M.; Synthesis, structurale elucidation and antioxidant study of Ortho-substituted N,N’-bis(benzamidothiocarbonyl)hydrazine derivatives. J. Phys.: Conf. Ser. 2018, 979, 012010.
- Both preparation of bis(N-p-chlorophenylthiourea and bis(N-p-methoxyphenylthiourea have been reported, but these derivatives are not crystallographically characterized: Herrero, J. M.; Fabra, D.; Matesanz, A. I.; Hernández, C.; Sánchez-Pérez, I.; Quiroga, A. G. Dithiobiureas Palladium(II) Complexes’ Studies: From Their Synthesis to Their Biological Action. J. Inorg. Biochem. 2023, 246, 112261. DOI: 10.1016/j.jinorgbio.2023.112261. [CrossRef]
- Spackman, P. R.; Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Jayatilaka, D.; Spackman, M. A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization, and Quantitative Analysis of Molecular Crystals. J. Appl. Cryst. 2021, 54, 1006–1011. DOI: 10.1107/S1600576721003292. [CrossRef]
- Bruker. Apex 4; Bruker AXS Inc.: Madison, WI, USA, 2021.
- Sheldrick, G. M. A Short History of SHELX. Acta Cryst. A 2008, 64, 112–122. DOI: 10.1107/S0108767307043930. [CrossRef]
- Sheldrick, G. M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. DOI: 10.1107/S2053273314026370. [CrossRef]
- Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. DOI: 10.1107/S2053229614024218. [CrossRef]
- Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. DOI: 10.1107/S0021889808042726. [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. et al., Gaussian 09. 2009, Revision A.1, Gaussian Inc, Wallingford, CT.
- Dennington, R. D.; Keith, T.A.; Millam, J.M. GaussView 5.0.8. 2008. Gaussian Inc.
- Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 1372-1377. DOI: 10.1063/1.464913. [CrossRef]
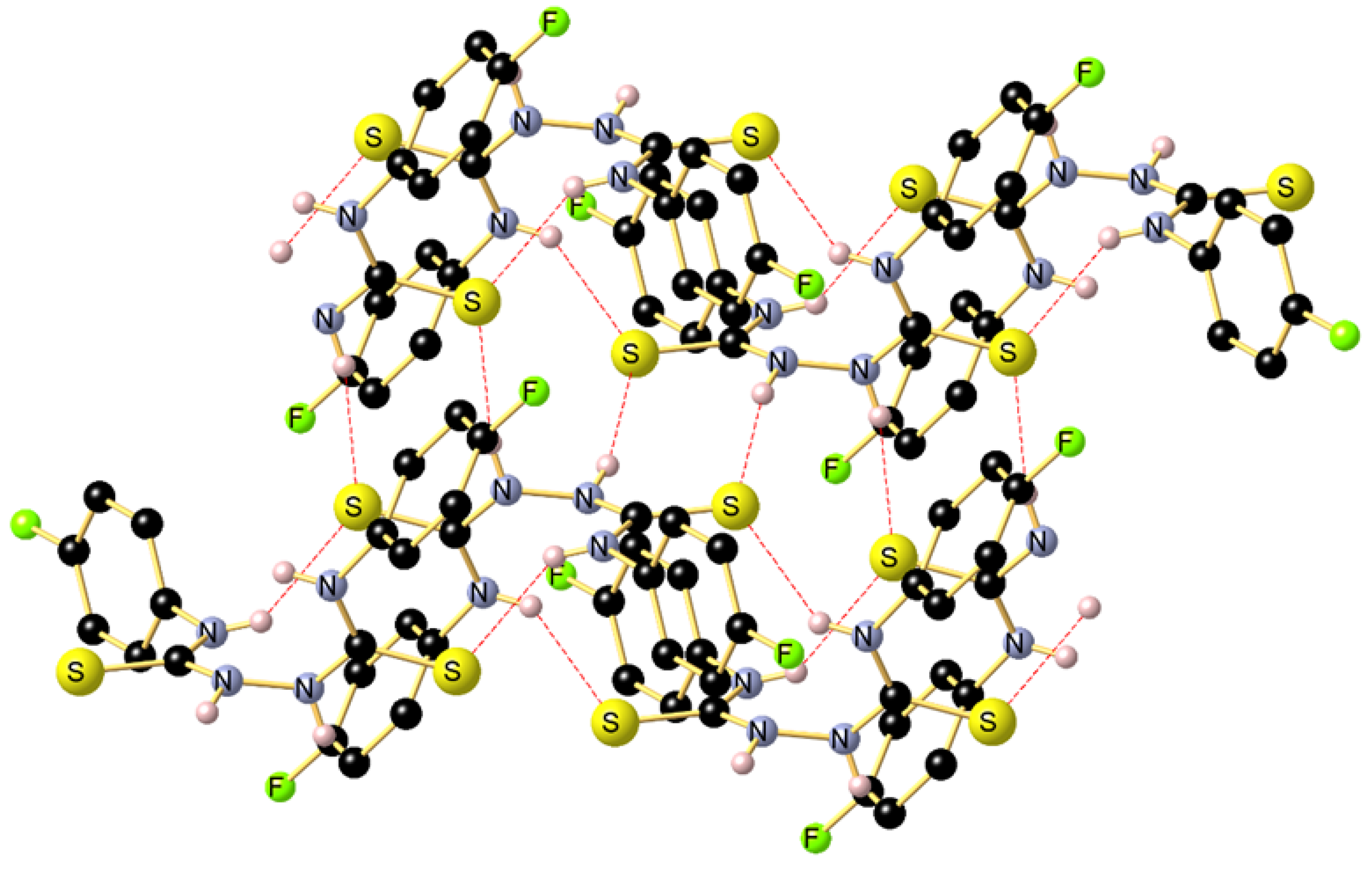
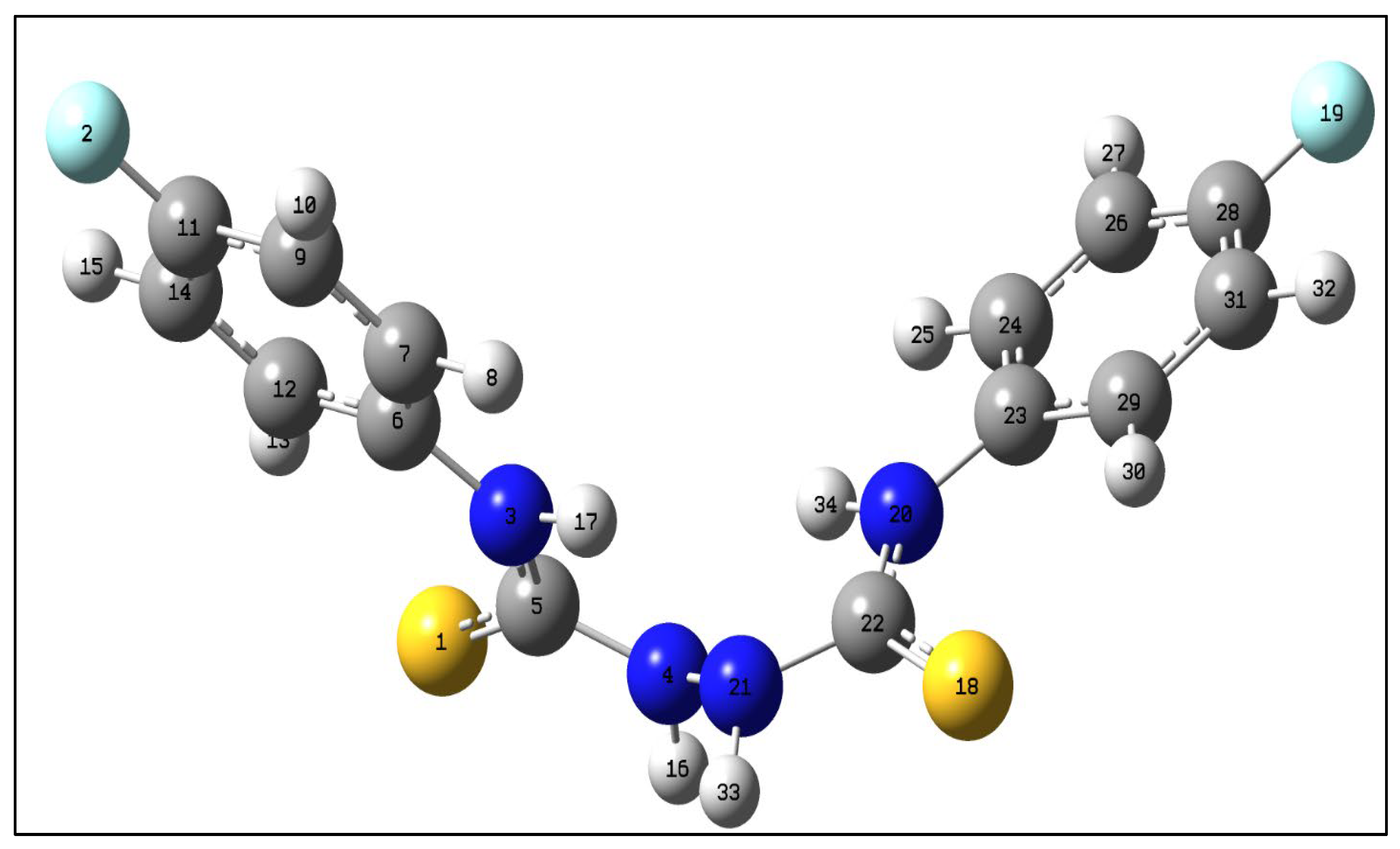
| Bond lengths (Å) | ExpSCXRD | Calc.in gas phase | Calc.in EtOH | Angles (°) | Exp.SCXRD | Calc.in gas phase | Calc.in EtOH |
|---|---|---|---|---|---|---|---|
| S1-C5 | 1.697 (17) | 1.666 | 1.684 | C5-N4-N21 | 119.70 (16) | 120.35 | 120.84 |
| F2-C11 | 1.366 (2) | 1.354 | 1.361 | C5-N3-C6 | 122.29 (14) | 132.86 | 127.48 |
| N4-N21 | 1.404 (3) | 1.396 | 1.392 | N4-C5-S1 | 118.08 (12) | 117.25 | 118.46 |
| N4-C5 | 1.367 (2) | 1.415 | 1.388 | N3-C5-S1 | 123.81 (13) | 130.02 | 126.85 |
| N3-C5 | 1.332 (2) | 1.349 | 1.343 | C7-C6-N3 | 119.35 (16) | 115.79 | 118.59 |
| N3-C6 | 1.440 (2) | 1.416 | 1.426 | N3-C5-N4 | 118.08 (15) | 112.69 | 114.68 |
| C6-C7 | 1.394 (2) | 1.402 | 1.395 | F2-C11-C9 | 118.36 (19) | 118.94 | 118.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





