Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Microalgae and vaccines
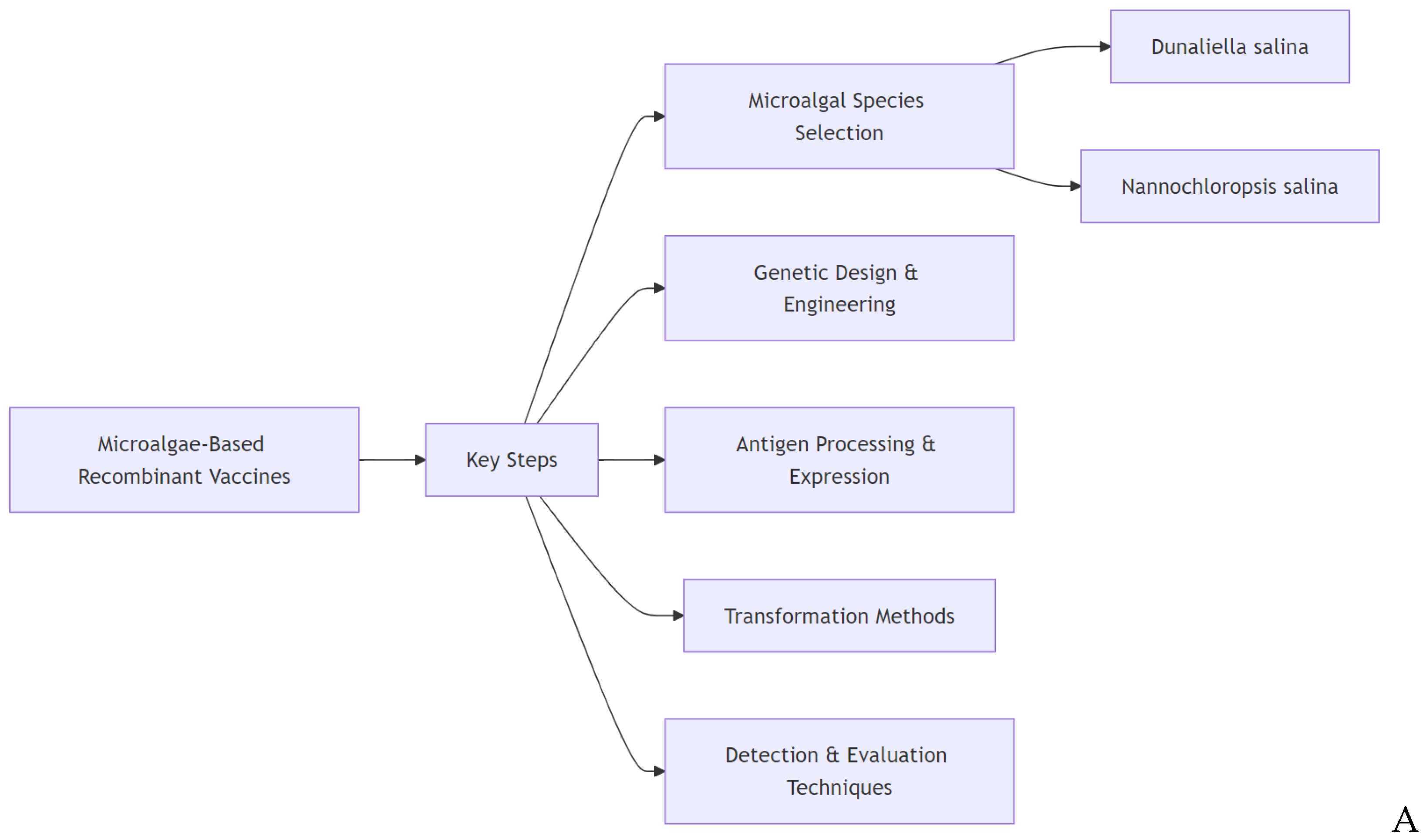
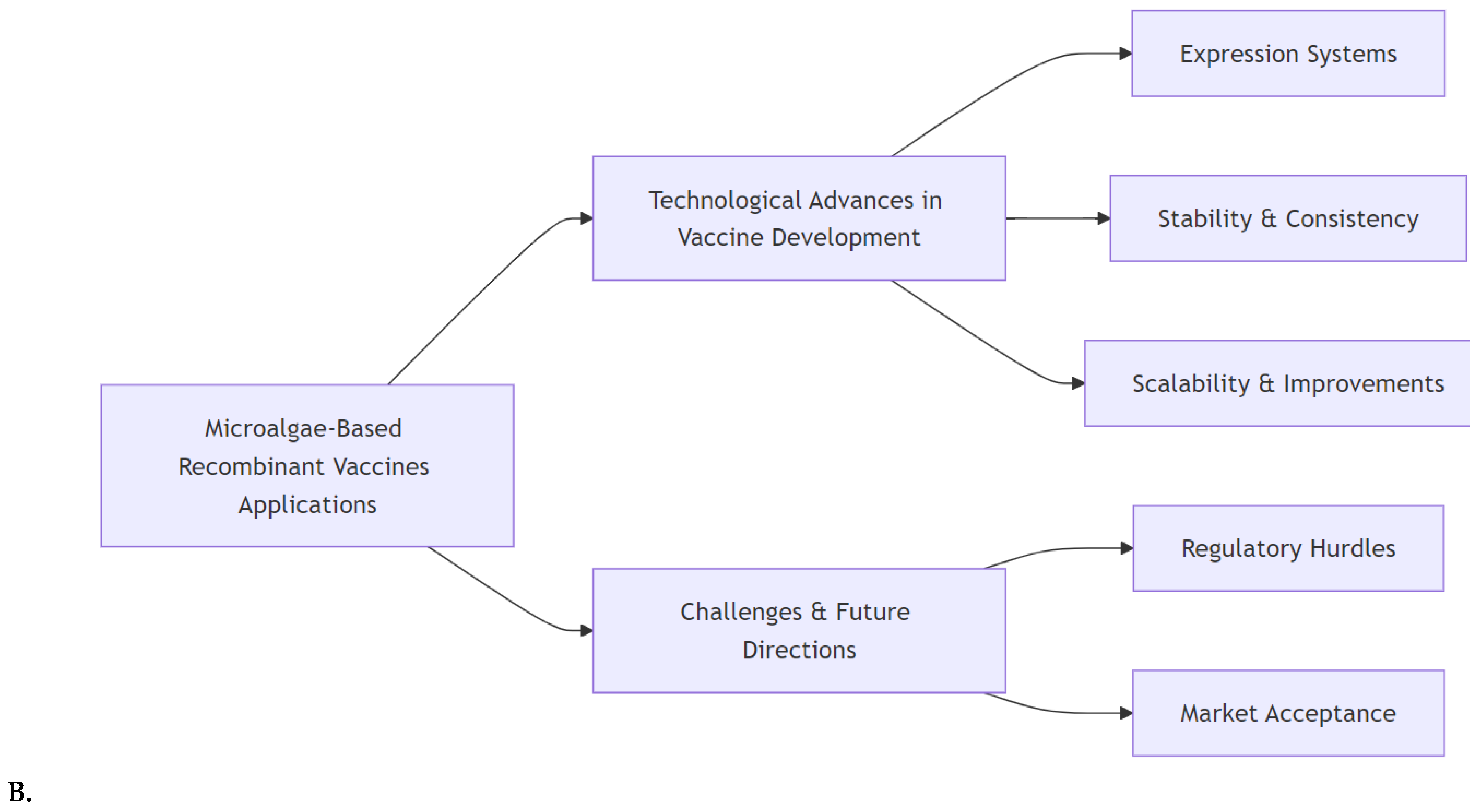
2. Microalgae-based recombinant vaccines
2.1. Development of microalgae-based recombinant vaccines
2.2. Applications of microalgae in veterinary vaccines and aquaculture
3. Marine natural products: emerging bioactive compounds and their potential as vaccine adjuvants and therapeutics
3.1. Marine-derived polysaccharides as potent vaccine adjuvants and antiviral agents
3.2. Bioactive compounds from marine sources: antiviral potential and immunomodulatory effects
3.3. Other marine-derived antimicrobials
4. Marine-derived delivery systems for vaccines

5. Marine-derived antineoplastics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anusree, M.K.; Manasa Leela, K.; Sreehari, M.; Raj, S.; Sreenikethanam, A.; Bajhaiya, A.K. Marine microalgae. In New Horizons in Natural Compound Research; 2023; pp. 251–265. [Google Scholar]
- Merlo, S.; Gabarrell Durany, X.; Pedroso Tonon, A.; Rossi, S. Marine microalgae contribution to sustainable development. Water 2021, 13, 1373. [Google Scholar] [CrossRef]
- Matsunaga, T.; Takeyama, H.; Miyashita, H.; Yokouchi, H. Marine microalgae. Marine biotechnology I 2005, 165–188. [Google Scholar]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. Journal of Functional Foods 2020, 68. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G. N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. Journal of Xenobiotics 2024, 14, 1541–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, X.; Kapoore, R. V.; Xu, C.; Vaidyanathan, S. Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Conversion and Management 2015, 106, 61–72. [Google Scholar] [CrossRef]
- Goswami, R. K.; Agrawal, K.; Verma, P. Microalgae Dunaliella as biofuel feedstock and β-carotene production: An influential step towards environmental sustainability. Energy Conversion and Management: X 2022, 13, 100154. [Google Scholar] [CrossRef]
- Liang, Z.-C.; Liang, M.-H.; Jiang, J.-G. Transgenic microalgae as bioreactors. Critical Reviews in Food Science and Nutrition 2019, 60, 3195–3213. [Google Scholar] [CrossRef]
- Ramos-Vega, A.; Angulo, C.; Bañuelos-Hernández, B.; Monreal-Escalante, E. Microalgae-made vaccines against infectious diseases. Algal Research 2021, 58. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; García-Silva, I.; González-Ortega, O.; Sandoval-Vargas, J. M.; Malla, A.; Vimolmangkang, S. The Potential of Algal Biotechnology to Produce Antiviral Compounds and Biopharmaceuticals. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Garduño-González, K. A.; Peña-Benavides, S. A.; Araújo, R. G.; Castillo-Zacarías, C.; Melchor-Martínez, E. M.; Oyervides-Muñoz, M. A.; Sosa-Hernández, J. E.; Purton, S.; Iqbal, H. M. N.; Parra-Saldívar, R. Current challenges for modern vaccines and perspectives for novel treatment alternatives. Journal of Drug Delivery Science and Technology 2022, 70. [Google Scholar] [CrossRef]
- Suraiya, S.; Ahmmed, M. K.; Haq, M. Immunity boosting roles of biofunctional compounds available in aquafoods: A review. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W. J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Khan, M. M.; Ernst, O.; Sun, J.; Fraser, I. D. C.; Ernst, R. K.; Goodlett, D. R.; Nita-Lazar, A. Mass Spectrometry-based Structural Analysis and Systems Immunoproteomics Strategies for Deciphering the Host Response to Endotoxin. Journal of Molecular Biology 2018, 430, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A. E.; Chandler, C. E.; Poli, V.; Gardner, F. M.; Tekiau, A.; Smith, R.; Bonham, K. S.; Cordes, E. E.; Shank, T. M.; Zanoni, I.; Goodlett, D. R.; Biller, S. J.; Ernst, R. K.; Rotjan, R. D.; Kagan, J. C. Deep-sea microbes as tools to refine the rules of innate immune pattern recognition. Science Immunology 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, V.; Roviello, G. N. The Potential Role of Vaccines in Preventing Antimicrobial Resistance (AMR): An Update and Future Perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M. A.; Roviello, G. N. Anti-coronavirus vaccines: Past investigations on sars-cov-1 and mers-cov, the approved vaccines from biontech/pfizer, moderna, oxford/astrazeneca and others under development against sarscov-2 infection. Current Medicinal Chemistry 2022, 29, 4–18. [Google Scholar]
- Vicidomini, C.; Borbone, N.; Roviello, V.; Roviello, G. N.; Oliviero, G. Summary of the Current Status of DNA Vaccination for Alzheimer Disease. Vaccines 2023, 11, 1706. [Google Scholar] [CrossRef]
- Gebre, M. S.; Brito, L. A.; Tostanoski, L. H.; Edwards, D. K.; Carfi, A.; Barouch, D. H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Oyston, P.; Robinson, K. The current challenges for vaccine development. Journal of medical microbiology 2012, 61, 889–894. [Google Scholar] [CrossRef]
- Walker, B. D.; Burton, D. R. Toward an AIDS vaccine. science 2008, 320, 760–764. [Google Scholar] [CrossRef]
- Kallerup, R. S.; Foged, C. Classification of vaccines. In Subunit vaccine delivery; Springer, 2014; pp. 15–29. [Google Scholar]
- Wilde, B. B.; Park, D. J. Immunizations. Primary Care: Clinics in Office Practice 2019, 46, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Capodici, J.; Cannon, G.; Communi, D.; Boeynaems, J.-M.; Karikó, K.; Weissman, D. Extracellular mRNA Induces Dendritic Cell Activation by Stimulating Tumor Necrosis Factor-α Secretion and Signaling through a Nucleotide Receptor. Journal of Biological Chemistry 2002, 277, 12689–12696. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S. J.; Mui, B. L.; Tam, Y. K.; Karikó, K.; Barbosa, C. J.; Madden, T. D.; Hope, M. J.; Krammer, F.; Hensley, S. E.; Weissman, D. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nature Communications 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nature reviews Drug discovery 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Geng, D.; Wang, Y.; Wang, P.; Li, W.; Sun, Y. Stable expression of hepatitis B surface antigen gene inDunaliella salina(Chlorophyta). Journal of Applied Phycology 2003, 15, 451–456. [Google Scholar] [CrossRef]
- Dreesen, I. A. J.; Hamri, G. C.-E.; Fussenegger, M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. Journal of Biotechnology 2010, 145, 273–280. [Google Scholar] [CrossRef]
- Ramos-Vega, A.; Monreal-Escalante, E.; Rosales-Mendoza, S.; Bañuelos-Hernández, B.; Dumonteil, E.; Angulo, C. Trypanosoma cruzi Tc24 antigen expressed and orally delivered by Schizochytrium sp. Microalga is immunogenic in mice. Molecular Biotechnology 2024, 66, 1376–1388. [Google Scholar] [CrossRef]
- Trujillo, E.; Monreal-Escalante, E.; Angulo, C. Microalgae-made human vaccines and therapeutics: A decade of advances. Biotechnology Journal 2024, 19, 2400091. [Google Scholar] [CrossRef]
- Chen, W. N.; Demurtas, O. C.; Massa, S.; Ferrante, P.; Venuti, A.; Franconi, R.; Giuliano, G. A Chlamydomonas-Derived Human Papillomavirus 16 E7 Vaccine Induces Specific Tumor Protection. PLoS ONE 2013, 8. [Google Scholar]
- Castro-Cosio, P.; Monreal-Escalante, E.; Romero-Geraldo, R.; Angulo, C. Natural and recombinant bioactive compounds from Schizochytrium sp.: Recent advances and future prospects. Algal Research 2023, 103273. [Google Scholar] [CrossRef]
- Márquez-Escobar, V. A.; Bañuelos-Hernández, B.; Rosales-Mendoza, S. Expression of a Zika virus antigen in microalgae: Towards mucosal vaccine development. Journal of biotechnology 2018, 282, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, E.; Villegas-Zagal, R.; Ramos-Vega, A.; Bañuelos-Hernández, B.; Angulo, C.; Monreal-Escalante, E. Genetic-engineered Schizochytrium sp. expressing a multiepitopic protein based on Vibrio parahaemolyticus toxins triggers immune responses in mice. Algal Research 2024, 79, 103440. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Zhou, Y.; Xie, X.; Feng, S.; Sun, C. Anti-HBV effect of interferon-thymosin α1 recombinant proteins in transgenic Dunaliella salina in vitro and in vivo. Experimental and Therapeutic Medicine 2018, 16, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M. F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicologic Pathology 2006, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, E.; Escudero-Oñate, C. Cyanobacteria and Microalgae in the Production of Valuable Bioactive Compounds. In Microalgal Biotechnology; 2018. [Google Scholar]
- Einhaus, A.; Baier, T.; Kruse, O. Molecular design of microalgae as sustainable cell factories. Trends in Biotechnology 2024, 42, 728–738. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO 2 sequestration. International Journal of Environmental Science and Technology 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ho, S.-H. How to enhance carbon capture by evolution of microalgal photosynthesis? Separation and Purification Technology 2022, 291, 120951. [Google Scholar] [CrossRef]
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V. M.; Fernández-Siurob, I.; Garcia-Casillas, L. A.; Velázquez-Juárez, G. Dunaliella salina as a Potential Biofactory for Antigens and Vehicle for Mucosal Application. Processes 2022, 10. [Google Scholar] [CrossRef]
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V. M.; Fernández-Siurob, I.; Velázquez-Juárez, G. Immune Evaluation of Avian Influenza Virus HAr Protein Expressed in Dunaliella salina in the Mucosa of Chicken. Vaccines 2022, 10. [Google Scholar] [CrossRef]
- Chomel, B. Zoonoses. Reference Module in Biomedical Sciences 2014.
- Paul-Pierre, P. Emerging diseases, zoonoses and vaccines to control them. Vaccine 2009, 27, 6435–6438. [Google Scholar] [CrossRef] [PubMed]
- Nuismer, S. L.; Bull, J. J. Self-disseminating vaccines to suppress zoonoses. Nature ecology & evolution 2020, 4, 1168–1173. [Google Scholar]
- Feng, S.; Feng, W.; Zhao, L.; Gu, H.; Li, Q.; Shi, K.; Guo, S.; Zhang, N. Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Archives of Virology 2013, 159, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Yang, J.; Karunarathna, T. K.; Qureshi, M.; Sadeyen, J.-R.; Iqbal, M. Characterization of the haemagglutinin properties of the H5N1 avian influenza virus that caused human infections in Cambodia. Emerging Microbes & Infections 2023, 12, 2244091. [Google Scholar]
- Uyeki, T. M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S. M.; Shetty, V.; Rollo, S. N.; Martinez, D. L.; Rai, S.; Gonzales, E. R. Highly pathogenic avian influenza A (H5N1) virus infection in a dairy farm worker. New England Journal of Medicine 2024. [CrossRef]
- Krammer, F.; Schultz-Cherry, S. We need to keep an eye on avian influenza. Nature Reviews Immunology 2023, 23, 267–268. [Google Scholar] [CrossRef]
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V. M.; Velázquez-Juárez, G.; Fernández-Siurob, I. Transformation of Dunaliella salina by Agrobacterium tumefaciens for the Expression of the Hemagglutinin of Avian Influenza Virus H5. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Abdelghany, M. F.; El-Sawy, H. B.; Abd El-hameed, S. A. A.; Khames, M. K.; Abdel-Latif, H. M. R.; Naiel, M. A. E. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology 2020, 107, 277–288. [Google Scholar]
- Abidin, A. A. Z.; Othman, N. A.; Yusoff, F. M.; Yusof, Z. N. B. Determination of transgene stability in Nannochloropsis sp. transformed with immunogenic peptide for oral vaccination against vibriosis. Aquaculture International 2021, 29, 477–486. [Google Scholar] [CrossRef]
- Srivastava, S.; Rahman, M. A.; Sundaram, S. Immunomodulatory Effects of Edible Microalgae. In Immune-Boosting Nutraceuticals for Better Human Health; Apple Academic Press, 2024; pp. 259–288. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S. R. Microalgae for high-value products towards human health and nutrition. Marine drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S. Microalgae and immune potential. Dietary Components and Immune Function 2010, 515–527. [Google Scholar]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Scientific reports 2017, 7, 6286. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. K.; Lee, H. H.; Seo, C. H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Marine Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A. F.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomedicine & Pharmacotherapy 2021, 134, 111091. [Google Scholar]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J. C. From sharks to yeasts: Squalene in the development of vaccine adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef]
- Lauxmann, M. A.; Santucci, N. E.; Autrán-Gómez, A. M. The SARS-CoV-2 Coronavirus and the COVID-19 Outbreak. International braz j urol 2020, 46 (suppl. 1), 6–18. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Postacute Sequelae of SARS-CoV-2 Infection in the Pre-Delta, Delta, and Omicron Eras. New England Journal of Medicine 2024, 391, 515–525. [Google Scholar] [CrossRef]
- Borbone, N.; Piccialli, I.; Falanga, A. P.; Piccialli, V.; Roviello, G. N.; Oliviero, G. Nucleic Acids as Biotools at the Interface between Chemistry and Nanomedicine in the COVID-19 Era. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M. A. R.; Roviello, G. N. Deciphering the Relationship between SARS-CoV-2 and Cancer. International Journal of Molecular Sciences 2023, 24. [Google Scholar] [CrossRef]
- Ricci, A.; Roviello, G. N. Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV. Life 2023, 13. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G. N.; Lichtfouse, E. River therapy. Environmental Chemistry Letters 2022, 20, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G. N. Evidence of Protein Binding by a Nucleopeptide Based on a Thyminedecorated L-Diaminopropanoic Acid through CD and In Silico Studies. Current Medicinal Chemistry 2021, 28, 5004–5015. [Google Scholar] [CrossRef] [PubMed]
- Autiero, I.; Roviello, G. N. Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study. International Journal of Molecular Sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Scognamiglio, P. L.; Caruso, U.; Vicidomini, C.; Roviello, G. N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. International Journal of Environmental Research and Public Health 2021, 19. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, G. N. Potential Anti-SARS-CoV-2 Molecular Strategies. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Zildzic, M.; Salihefendic, D.; Masic, I. Non-Pharmacological Measures in the Prevention and Treatment of COVID-19 Infection. Medical Archives 2021, 75. [Google Scholar] [CrossRef]
- Singh, T. U.; Parida, S.; Lingaraju, M. C.; Kesavan, M.; Kumar, D.; Singh, R. K. Drug repurposing approach to fight COVID-19. Pharmacological Reports 2020, 72, 1479–1508. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Usov, A. I. Polysaccharides of the red algae. In Advances in carbohydrate chemistry and biochemistry; Elsevier, 2011; Vol. 65, pp. 115–217. [Google Scholar]
- Duarte, M. E.; Noseda, M. D.; Cardoso, M. A.; Tulio, S.; Cerezo, A. S. The structure of a galactan sulfate from the red seaweed Bostrychia montagnei. Carbohydrate Research 2002, 337, 1137–1144. [Google Scholar] [CrossRef]
- Sánchez, R. A. R.; Saluri, K.; Tuvikene, R.; Matulewicz, M. C.; Ciancia, M. Complex sulfated galactans from hot water extracts of red seaweed Asparagopsis taxiformis comprise carrageenan and agaran structures. Carbohydrate Polymers 2023, 322, 121314. [Google Scholar] [CrossRef]
- Darko, C. N. S.; Humayun, S.; Premarathna, A. D.; Howlader, M. M.; Rjabovs, V.; Tuvikene, R. Rheology and characterization of sulfated agarans from the edible epiphytic red alga, Vertebrata lanosa (truffle seaweed). Food Hydrocolloids 2024, 151, 109770. [Google Scholar] [CrossRef]
- Pereira, J. S.; Faria, R. X. Molecular Aspects of Carrageenan in the Pharmaceutical and Food Industries. Current Nutrition & Food Science 2024, 20, 466–475. [Google Scholar]
- Gaikwad, M. Sulfated polysaccharide from marine red microalga porphyridium against SARS-CoV-2—a mini-review. Acta Scientific Microbiology 2022, 5, 128–136. [Google Scholar] [CrossRef]
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. International journal of general medicine 2018, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PloS one 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Li, M.; Miao, Z.-H.; Chen, Z.; Chen, Q.; Gui, M.; Lin, L.-P.; Sun, P.; Yi, Y.-H.; Ding, J. Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase2α by unique interference with its DNA binding and catalytic cycle. Annals of oncology 2010, 21, 597–607. [Google Scholar] [CrossRef]
- Silchenko, A. S.; Kalinovsky, A. I.; Avilov, S. A.; Andryjaschenko, P. V.; Dmitrenok, P. S.; Menchinskaya, E. S.; Aminin, D. L.; Kalinin, V. I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Natural Product Research 2013, 27, 1776–1783. [Google Scholar] [CrossRef]
- Kang, N.; Heo, S.-Y.; Kim, E.-A.; Cha, S.-H.; Ryu, B.; Heo, S.-J.; Kang, N.; Heo, S.-Y.; Kim, E.-A.; Cha, S.-H. Antiviral effect of fucoxanthin obtained from Sargassum siliquastrum (Fucales, Phaeophyceae) against severe acute respiratory syndrome coronavirus 2. Algae 2023, 38, 295–306. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.-A.; Park, A.; Heo, S.-Y.; Heo, J.-H.; Heo, S.-J. Antiviral Potential of Fucoxanthin, an Edible Carotenoid Purified from Sargassum siliquastrum, against Zika Virus. Marine Drugs 2024, 22, 247. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M. A. R.; Gilhen-Baker, M.; Roviello, G. N. The chemical basis of seawater therapies: a review. Environmental Chemistry Letters 2024, 22, 2133–2149. [Google Scholar] [CrossRef]
- Hu, C. Marine natural products and human immunity: novel biomedical resources for anti-infection of SARS-CoV-2 and related cardiovascular disease. Natural Products and Bioprospecting 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M. J.; Di Pilato, M.; Garris, C.; Mempel, T. R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; Ferrera, F.; Bernardi, C.; Parodi, A.; Pasquale, G.; Leonardi, A.; Filaci, G.; De Palma, R.; Fontana, A. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Scientific Reports 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Saleem, M.; Yaseen, H. S.; Yehya, A. H. S.; Saadullah, M.; Zubair, H. M.; Oon, C. E.; Khaniabadi, P. M.; Khalid, S. H.; Khan, I. U. ; Mahrukh, Potential Role of Marine Species-Derived Bioactive Agents in the Management of SARS-CoV-2 Infection. Future Microbiology 2021, 16, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Fontanella, F.; D’Alessandro, T.; Roviello, G. N. A Survey on Computational Methods in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2024, 14. [Google Scholar] [CrossRef]
- Fabre, J.-F.; Niangoran, N.; Gaignard, C.; Buso, D.; Mouloungui, Z.; Valentin, R. Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. European Food Research and Technology 2022, 248, 1583–1599. [Google Scholar] [CrossRef]
- Nelson, K. M.; Dahlin, J. L.; Bisson, J.; Graham, J.; Pauli, G. F.; Walters, M. A. The essential medicinal chemistry of curcumin: miniperspective. Journal of medicinal chemistry 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Glaser, J.; Holzgrabe, U. Focus on PAINS: false friends in the quest for selective anti-protozoal lead structures from Nature? MedChemComm 2016, 7, 214–223. [Google Scholar] [CrossRef]
- Baell, J. B. Feeling nature’s PAINS: natural products, natural product drugs, and pan assay interference compounds (PAINS). Journal of natural products 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food & Function 2020, 11, 7415–7420. [Google Scholar]
- Aghasadeghi, M. R.; Zaheri Birgani, M. A.; Jamalimoghadamsiyahkali, S.; Hosamirudsari, H.; Moradi, A.; Jafari-Sabet, M.; Sadigh, N.; Rahimi, P.; Tavakoli, R.; Hamidi-Fard, M. Effect of high-dose Spirulina supplementation on hospitalized adults with COVID-19: a randomized controlled trial. Frontiers in Immunology 2024, 15, 1332425. [Google Scholar] [CrossRef] [PubMed]
- Quimque, M. T. J.; Notarte, K. I. R.; Fernandez, R. A. T.; Mendoza, M. A. O.; Liman, R. A. D.; Lim, J. A. K.; Pilapil, L. A. E.; Ong, J. K. H.; Pastrana, A. M.; Khan, A. Virtual screening-driven drug discovery of SARS-CoV2 enzyme inhibitors targeting viral attachment, replication, post-translational modification and host immunity evasion infection mechanisms. Journal of Biomolecular Structure and Dynamics 2021, 39, 4316–4333. [Google Scholar] [CrossRef] [PubMed]
- Khan, M. T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M. T.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2—a molecular dynamic study. Journal of Biomolecular Structure and Dynamics 2021, 39, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P. C.; Richards, D.; Tanner, H. L.; Feldmann, M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. The Lancet Rheumatology 2020, 2, e653–e655. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X 2020, 2, 100029. [Google Scholar] [CrossRef]
- Kim, S.-K. Handbook of marine macroalgae: biotechnology and applied phycology; John Wiley & Sons, 2011. [Google Scholar]
- Abdelrheem, D. A.; Ahmed, S. A.; Abd El-Mageed, H.; Mohamed, H. S.; Rahman, A. A.; Elsayed, K. N.; Ahmed, S. A. The inhibitory effect of some natural bioactive compounds against SARS-CoV-2 main protease: insights from molecular docking analysis and molecular dynamic simulation. Journal of Environmental Science and Health, Part A 2020, 55, 1373–1386. [Google Scholar] [CrossRef]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef]
- Surti, M.; Patel, M.; Adnan, M.; Moin, A.; Ashraf, S. A.; Siddiqui, A. J.; Snoussi, M.; Deshpande, S.; Reddy, M. N. Ilimaquinone (marine sponge metabolite) as a novel inhibitor of SARS-CoV-2 key target proteins in comparison with suggested COVID-19 drugs: designing, docking and molecular dynamics simulation study. RSC advances 2020, 10, 37707–37720. [Google Scholar] [CrossRef]
- Vijayaraj, R.; Altaff, K.; Rosita, A. S.; Ramadevi, S.; Revathy, J. Bioactive compounds from marine resources against novel corona virus (2019-nCoV): in silico study for corona viral drug. Natural Product Research 2021, 35, 5525–5529. [Google Scholar] [CrossRef]
- O'Keefe, B. R.; Giomarelli, B.; Barnard, D. L.; Shenoy, S. R.; Chan, P. K.; McMahon, J. B.; Palmer, K. E.; Barnett, B. W.; Meyerholz, D. K.; Wohlford-Lenane, C. L. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. Journal of virology 2010, 84, 2511–2521. [Google Scholar] [CrossRef]
- Okechukwu, Q. N.; Adepoju, F. O.; Kanwugu, O. N.; Adadi, P.; Serrano-Aroca, Á.; Uversky, V. N.; Okpala, C. O. R. Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies. Pharmaceuticals 2024, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Banday, A. H.; ul Azha, N.; Farooq, R.; Sheikh, S. A.; Ganie, M. A.; Parray, M. N.; Mushtaq, H.; Hameed, I.; Lone, M. A. Exploring the potential of marine natural products in drug development: A comprehensive review. Phytochemistry Letters 2024, 59, 124–135. [Google Scholar] [CrossRef]
- Carroll, A. R.; Copp, B. R.; Grkovic, T.; Keyzers, R. A.; Prinsep, M. R. Marine natural products. Natural Product Reports 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Arnáiz, V.; Rufo, D.; Arroyo, Y. Current Status of Indole-Derived Marine Natural Products: Synthetic Approaches and Therapeutic Applications. Marine Drugs 2024, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Hu, C. Marine natural products and human immunity: novel biomedical resources for anti-infection of SARS-CoV-2 and related cardiovascular disease. Natural Products and Bioprospecting 2024, 14, 12. [Google Scholar] [CrossRef]
- Lu, X.; Yuan, F.; Qiao, L.; Liu, Y.; Gu, Q.; Qi, X.; Li, J.; Li, D.; Liu, M. AS1041, a novel derivative of marine natural compound Aspergiolide A, induces senescence of leukemia cells via oxidative stress-induced DNA damage and BCR-ABL degradation. Biomedicine & Pharmacotherapy 2024, 171, 116099. [Google Scholar]
- Hovhannisyan, A. M.; Tovmasyan, A. S.; Mkrtchyan, A. F.; Ghazaryan, K. R.; Minasyan, E. V.; Dallakyan, O. L.; Chobanyan, M. S.; Zakaryan, H.; Roviello, G. N.; Saghyan, A. S. Synthesis and evaluation of new mono- and binuclear salen complexes for the Cα-alkylation reaction of amino acid substrates as chiral phase transfer catalysts. Molecular Catalysis 2024, 569. [Google Scholar] [CrossRef]
- Odeleye, T.; White, W. L.; Lu, J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: a review. Food & function 2019, 10, 2278–2289. [Google Scholar]
- Yakoot, M.; Salem, A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterology 2012, 12. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J. i.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Science & Nutrition 2020, 8, 5195–5205. [Google Scholar]
- Sansone, C.; Brunet, C.; Noonan, D. M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A. L.; Lee, N.; Kim, S.; Kim, Y.-E.; Christabel, C.; Yu, H.; Kim, E.-J.; Oh, Y.-K. Enhanced astaxanthin production in Haematococcus lacustris by electrochemical stimulation of cyst germination. Bioresource Technology 2024, 411, 131301. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S., Microalgae and Immune Potential. In Dietary Components and Immune Function; 2010; pp. 515–527.
- Devkar, H. U.; Thakur, N. L.; Kaur, P. Marine-derived antimicrobial molecules from the sponges and their associated bacteria. Canadian Journal of Microbiology 2023, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E.; Cheng, Y.-C. Antiviral agents. Encyclopedia of Microbiology 2019, 176. [Google Scholar]
- Mayer, A. M.; Glaser, K. B.; Cuevas, C.; Jacobs, R. S.; Kem, W.; Little, R. D.; McIntosh, J. M.; Newman, D. J.; Potts, B. C.; Shuster, D. E. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends in pharmacological sciences 2010, 31, 255–265. [Google Scholar] [CrossRef]
- El-Hawary, S. S.; Sayed, A. M.; Mohammed, R.; Hassan, H. M.; Rateb, M. E.; Amin, E.; Mohammed, T. A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A. Bioactive brominated oxindole alkaloids from the Red Sea sponge Callyspongia siphonella. Marine drugs 2019, 17, 465. [Google Scholar] [CrossRef]
- Wietz, M.; Mansson, M.; Gotfredsen, C. H.; Larsen, T. O.; Gram, L. Antibacterial compounds from marine Vibrionaceae isolated on a global expedition. Marine drugs 2010, 8, 2946–2960. [Google Scholar] [CrossRef]
- Fischbach, M. A.; Walsh, C. T.; Clardy, J. The evolution of gene collectives: How natural selection drives chemical innovation. Proceedings of the National Academy of Sciences 2008, 105, 4601–4608. [Google Scholar] [CrossRef]
- Mahajan, G.; Thomas, B.; Parab, R.; Patel, Z. E.; Kuldharan, S.; Yemparala, V.; Mishra, P. D.; Ranadive, P.; D'Souza, L.; Pari, K. In vitro and in vivo activities of antibiotic PM181104. Antimicrobial agents and chemotherapy 2013, 57, 5315–5319. [Google Scholar] [CrossRef]
- Kumar, R.; Subramani, R.; Feussner, K.-D.; Aalbersberg, W. Aurantoside K, a new antifungal tetramic acid glycoside from a Fijian marine sponge of the genus Melophlus. Marine Drugs 2012, 10, 200–208. [Google Scholar] [CrossRef]
- Martínez-Poveda, B.; Quesada, A. R.; Medina, M. Á. Pleiotropic role of puupehenones in biomedical research. Marine Drugs 2017, 15, 325. [Google Scholar] [CrossRef]
- Deutsch, C.; Penn, J. L.; Lucey, N. Climate, oxygen, and the future of marine biodiversity. Annual Review of Marine Science 2024, 16, 217–245. [Google Scholar] [CrossRef]
- Dube, K. A comprehensive review of climatic threats and adaptation of marine biodiversity. Journal of Marine Science and Engineering 2024, 12, 344. [Google Scholar] [CrossRef]
- Rocha, R.; Azevedo, F.; Oliveira, U.; Cardoso, M.; Clerier, P.; Fortes, R.; Lopes-Filho, E.; Lorini, M.; Miranda, L.; Moura, R. West Atlantic coastal marine biodiversity: the contribution of the platform iNaturalist. Aquatic Ecology 2024, 58, 57–71. [Google Scholar] [CrossRef]
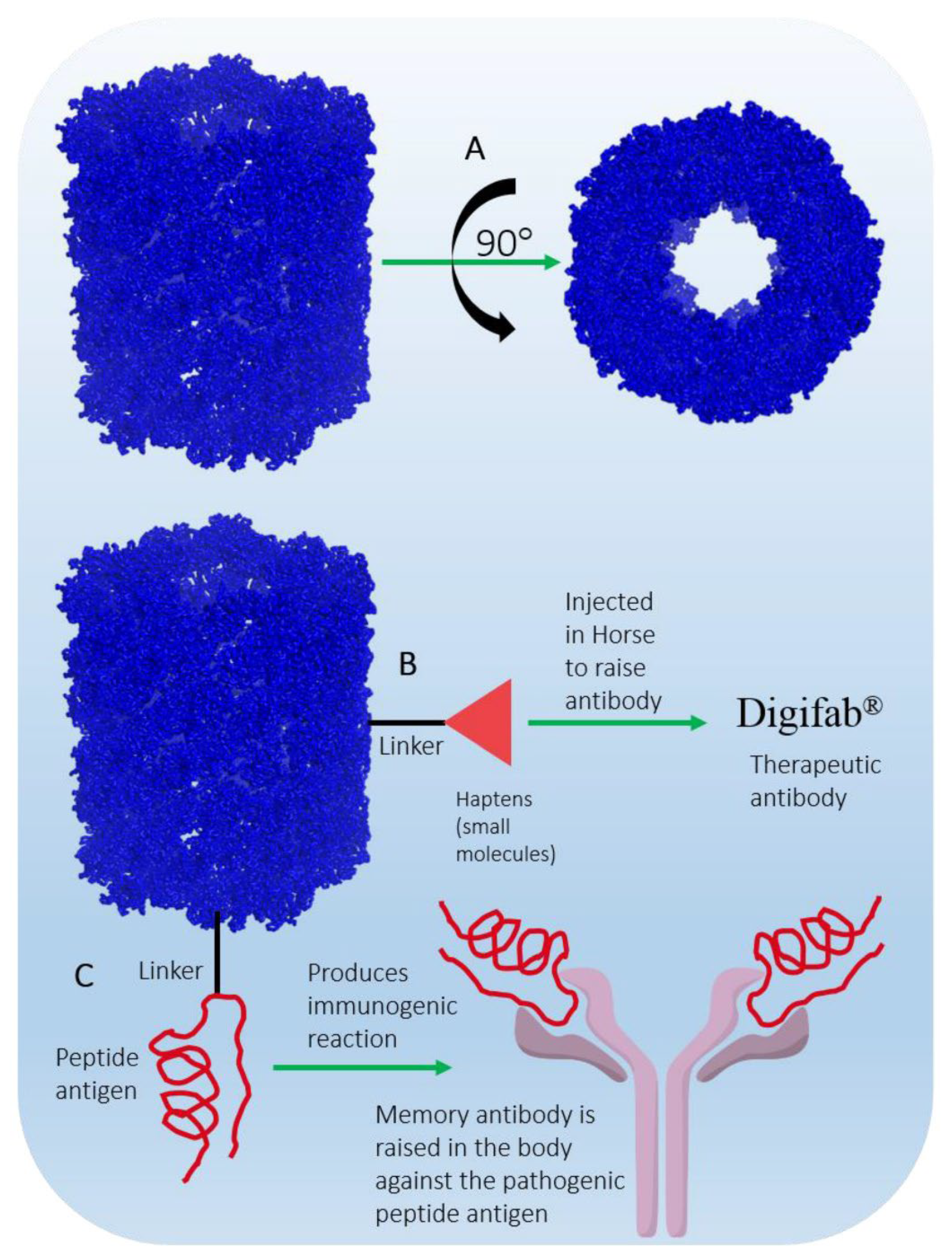
- Sanina, N. Vaccine adjuvants derived from marine organisms. Biomolecules 2019, 9, 340. [Google Scholar] [CrossRef]
- Jo, S.-H.; Kim, C.; Park, S.-H. Novel marine organism-derived extracellular vesicles for control of anti-inflammation. Tissue Engineering and Regenerative Medicine 2021, 18, 71–79. [Google Scholar] [CrossRef]
- Sheikhhossein, H. H.; Iommelli, F.; Di Pietro, N.; Curia, M. C.; Piattelli, A.; Palumbo, R.; Roviello, G. N.; De Rosa, V. Exosome-like Systems: From Therapies to Vaccination for Cancer Treatment and Prevention—Exploring the State of the Art. Vaccines 2024, 12, 519. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: functional biopolymers from marine crustaceans. Marine biotechnology 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Marzano, M.; Borbone, N.; Amato, F.; Oliviero, G.; Fucile, P.; Russo, T.; Sannino, F. 3D Chitosan-Gallic Acid Complexes: Assessment of the Chemical and Biological Properties. Gels 2022, 8. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Tsai, M.-H.; Yen, H.-j.; Shyu, H.-F.; Cheng, K.-m.; Chen, X.-a.; Chen, C.-c.; Young, J.-j.; Kau, J.-H. A fucoidan-quaternary chitosan nanoparticle adjuvant for anthrax vaccine as an alternative to CpG oligodeoxynucleotides. Carbohydrate polymers 2020, 229, 115403. [Google Scholar] [CrossRef]
- Tsai, M.-h.; Chuang, C.-c.; Chen, C.-c.; Yen, H.-j.; Cheng, K.-m.; Chen, X.-a.; Shyu, H.-f.; Lee, C.-y.; Young, J.-j.; Kau, J.-h. Nanoparticles assembled from fucoidan and trimethylchitosan as anthrax vaccine adjuvant: In vitro and in vivo efficacy in comparison to CpG. Carbohydrate polymers 2020, 236, 116041. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environmental chemistry letters 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Ege, H.; Ege, Z. R.; Gunduz, O., Marine-Derived Materials for the Development of Advanced Drug Delivery Systems. In Handbook of the Extracellular Matrix; 2024; pp. 1–15.
- Barbosa, A. I.; Coutinho, A. J.; Costa Lima, S. A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Marine Drugs 2019, 17. [Google Scholar] [CrossRef]
- Carroll, E. C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H. B.; Mansouri, S.; McEntee, C. P.; Lambe, E.; Agger, E. M. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Riteau, N.; Sher, A. Chitosan: an adjuvant with an unanticipated STING. Immunity 2016, 44, 522–524. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Marine Drugs 2022, 20. [Google Scholar] [CrossRef]
- Suleria, H.; Osborne, S.; Masci, P.; Gobe, G. Marine-Based Nutraceuticals: An Innovative Trend in the Food and Supplement Industries. Marine Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef]
- Odeleye, T.; Zeng, Z.; White, W. L.; Wang, K. S.; Li, H.; Xu, X.; Xu, H.; Li, J.; Ying, T.; Zhang, B.; Feng, T.; Lu, J. Effects of preparation method on the biochemical characterization and cytotoxic activity of New Zealand surf clam extracts. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Guo, K.; Su, L.; Wang, Y.; Liu, H.; Lin, J.; Cheng, P.; Yin, X.; Liang, M.; Wang, Q.; Huang, Z. Antioxidant and anti-aging effects of a sea cucumber protein hydrolyzate and bioinformatic characterization of its composing peptides. Food & Function 2020, 11, 5004–5016. [Google Scholar]
- Dalisay, D. S.; Tenebro, C. P.; Sabido, E. M.; Suarez, A. F. L.; Paderog, M. J. V.; Reyes-Salarda, R.; Saludes, J. P. Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies. Marine Drugs 2024, 22, 114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Sun, S. Prospects of marine-derived compounds as potential therapeutic agents for glioma. Pharmaceutical Biology 2024, 62, 513–526. [Google Scholar] [CrossRef]
- Chien, S.; Reiter, L. T.; Bier, E.; Gribskov, M. Homophila: human disease gene cognates in Drosophila. Nucleic acids research 2002, 30, 149–151. [Google Scholar] [CrossRef]
- Pandey, U. B.; Nichols, C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological reviews 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Rand, M. D.; Tennessen, J. M.; Mackay, T. F.; Anholt, R. R. Perspectives on the Drosophila melanogaster model for advances in toxicological science. Current Protocols 2023, 3, e870. [Google Scholar] [CrossRef]
- Cagan, R., Drug screening using model systems: some basics. The Company of Biologists Ltd: 2016; Vol. 9, pp 1241-1244.
- Millet-Boureima, C.; Selber-Hnatiw, S.; Gamberi, C. Drug discovery and chemical probing in Drosophila. Genome 2021, 64, 147–159. [Google Scholar] [CrossRef]
- DeLoriea, J.; Millet-Boureima, C.; Gamberi, C. Protocol to build a drug-testing pipeline using large populations of Drosophila melanogaster. STAR protocols 2023, 4, 102747. [Google Scholar] [CrossRef]


| Marine-derived compound (type) | Application/Effect |
|---|---|
| Fucoidan (adjuvant from brown seaweed) | Stimulates both humoral and cellular immune responses, enhancing vaccine efficacy. |
| Saponins (adjuvant from sea cucumbers) | Enhances antigen presentation, promoting a robust immune response in vaccines. |
| Microalgae-derived lipids (adjuvant) | Creates stable emulsions, improving bioavailability and offering immunomodulatory properties. |
| Carrageenan (antiviral polysaccharide) | Exhibits antiviral activity, especially against SARS-CoV-2, used in nasal sprays and hygiene products. |
| Iota-carrageenan (antiviral polysaccharide) | Reduces symptoms of common cold and inactivates viral glycoproteins. |
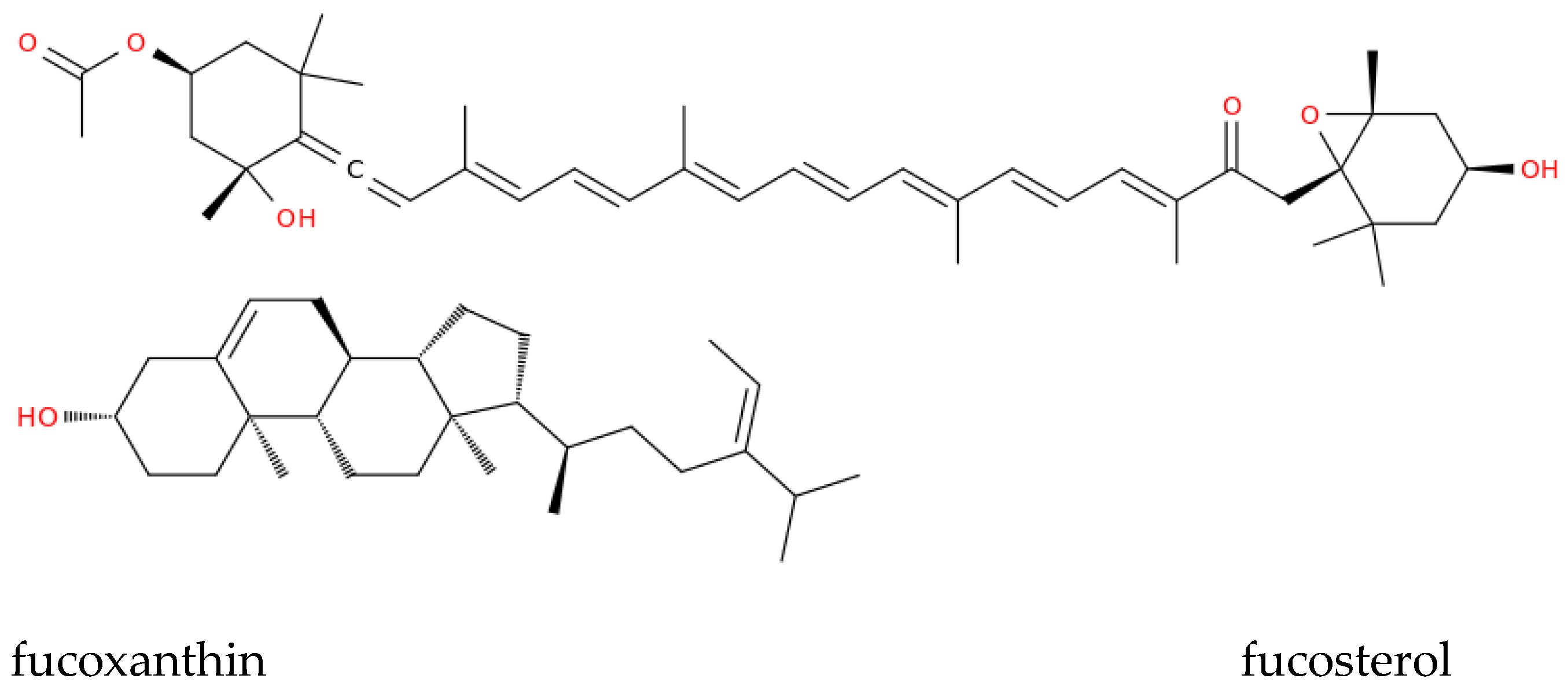
| Fucoxanthin (antioxidant from Sargassum siliquastrum) | Mitigates DNA damage, enhances antioxidant enzyme levels, and protects cells from oxidative stress. |
| Fucosterol (antioxidant from marine sources) | Boosts cellular antioxidant defenses and protects human hepatic cells from oxidative damage. |
| Compound | Marine source | SARS-CoV-2 protein target | Potential activity | Ref. |
|---|---|---|---|---|
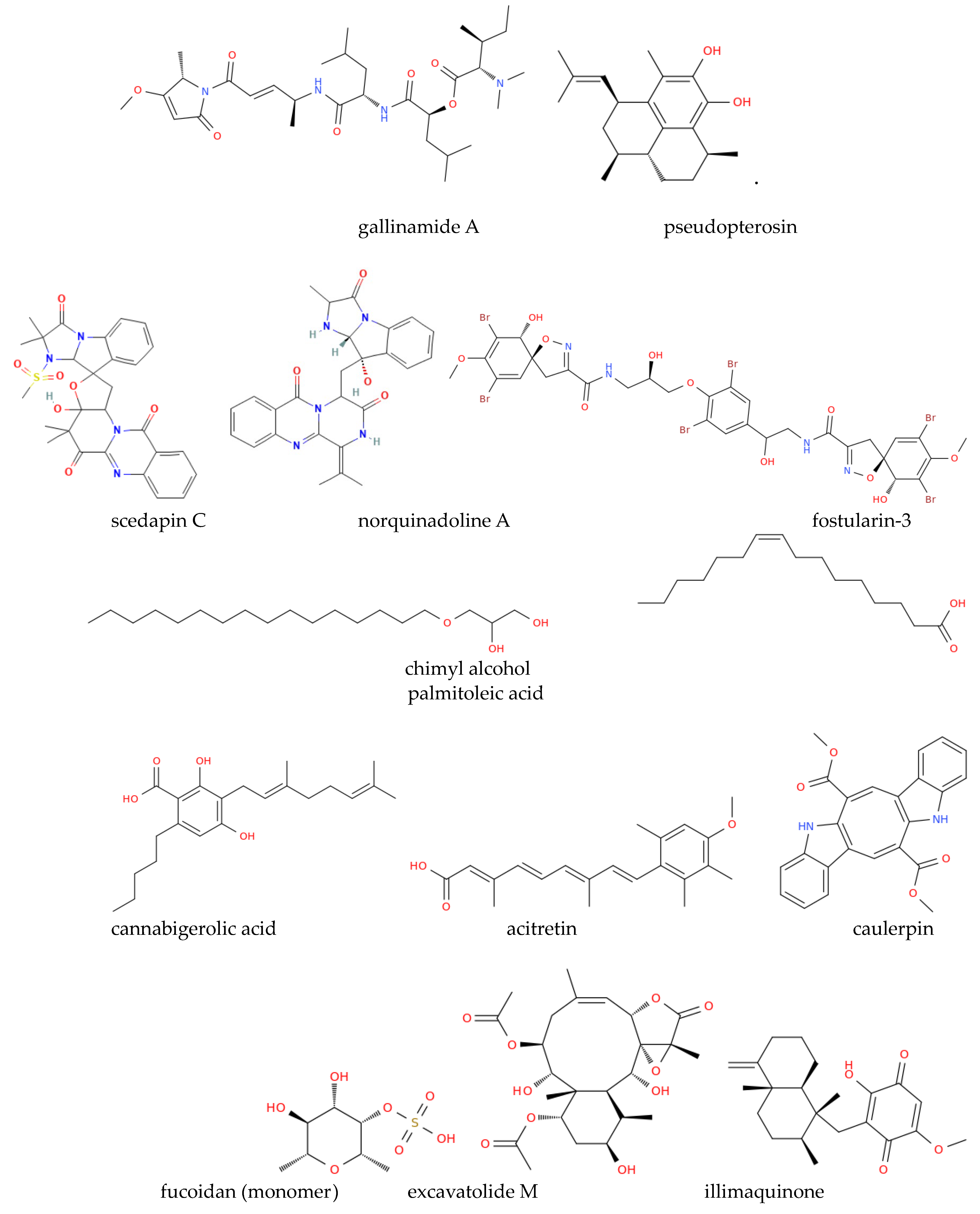
| Scedapin C | Scedosporium apiospermum | PLpro | Inhibits viral replication and activates immune responses by blocking PLpro activity | [97] |
| Norquinadoline A | Scedosporium apiospermum | PLpro | Inhibits PLpro, potentially blocking viral replication and boosting immune response | [97] |
| Fostularin-3 | Alpysinidae | Mpro | Forms hydrogen bonds and hydrophobic interactions with Mpro, potentially inhibiting virus | [98] |
| Caulerpin | Caulerpa racemosa | Mpro | Inhibits virus life cycle, anti-inflammatory properties by down-regulating cytokines | [99,100] |
| Quercetin | Brown algae (Sargassum genus) | ACE2 receptor | Disrupts ACE2 receptor interactions, reduces respiratory symptoms and inflammation | [101] |
| Fucoidan | Brown algae | Viral entry proteins | Inhibits the S-glycoprotein of SARS-CoV-2 and disrupts the ERK signaling pathway. Anti-inflammatory and enhances vaccine response | [95] |
| Iota-carrageenan | Red algae | Viral entry proteins | Potential to block viral entry | [102] |
| Chondroitin sulfate C | Sharks | Viral entry proteins | Potential to block viral entry | [95] |
| Excavatolide M | Gorgonian (Briareum excavatum) | TMPRSS2 | Shows potential to inhibit TMPRSS2 | [103] |
| Illimaquinone | Marine sponge | PLpro | Inhibits papain-like protease, antiviral potential | [104] |
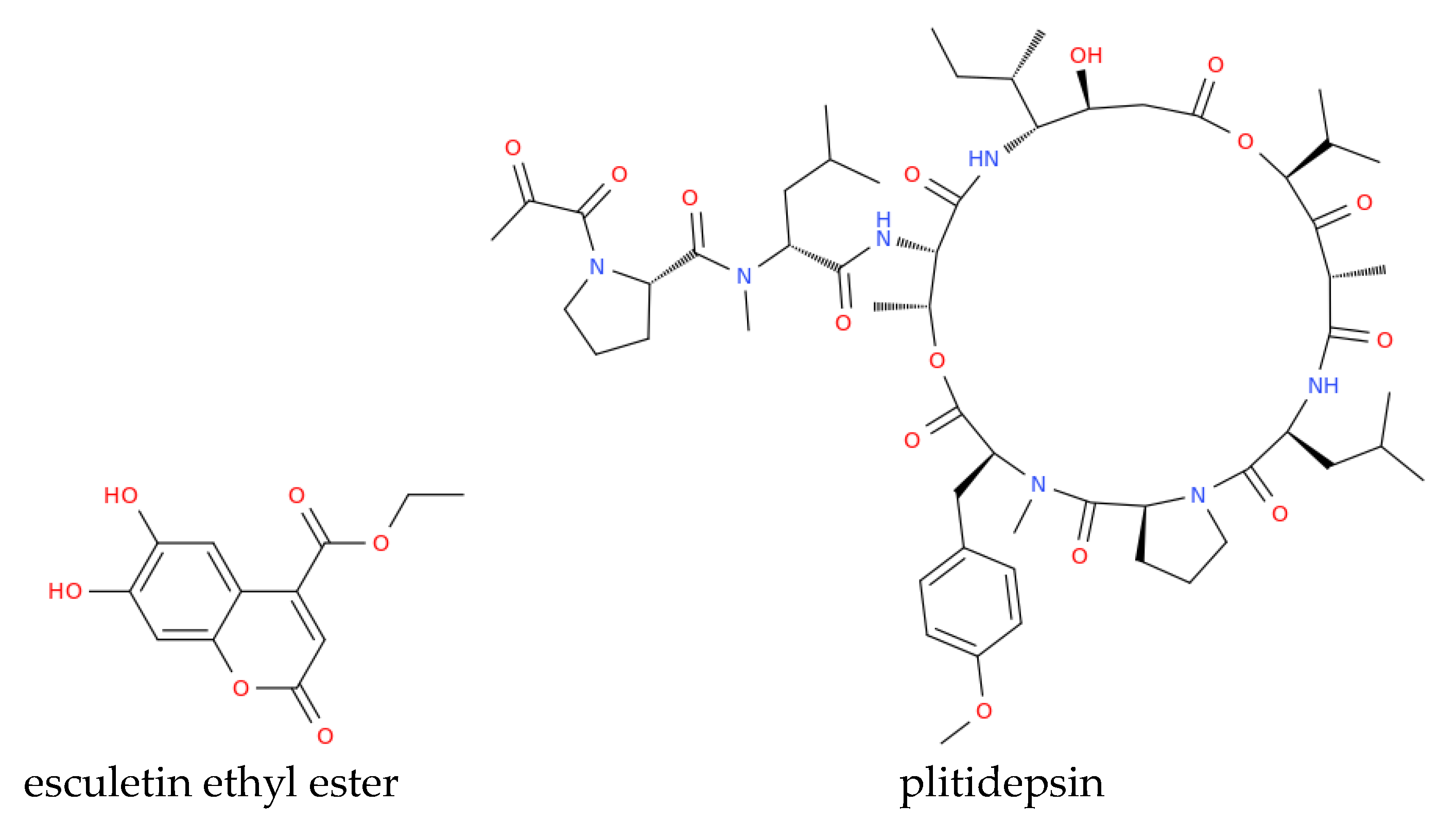
| Esculetin ethyl ester | Marine sponge (Axinella cf. corrugata) | N3 protease | Strong binding affinity to N3 protease | [105] |
| Griffithsin | Griffithsia sp. (seaweed) | Spike glycoprotein | Blocks spike glycoprotein, preventing viral entry into host cells | [105,106] |
| Marine-Derived Antimicrobial | Marine Source | Activity | Organism(s) Active Against | References | |
|---|---|---|---|---|---|
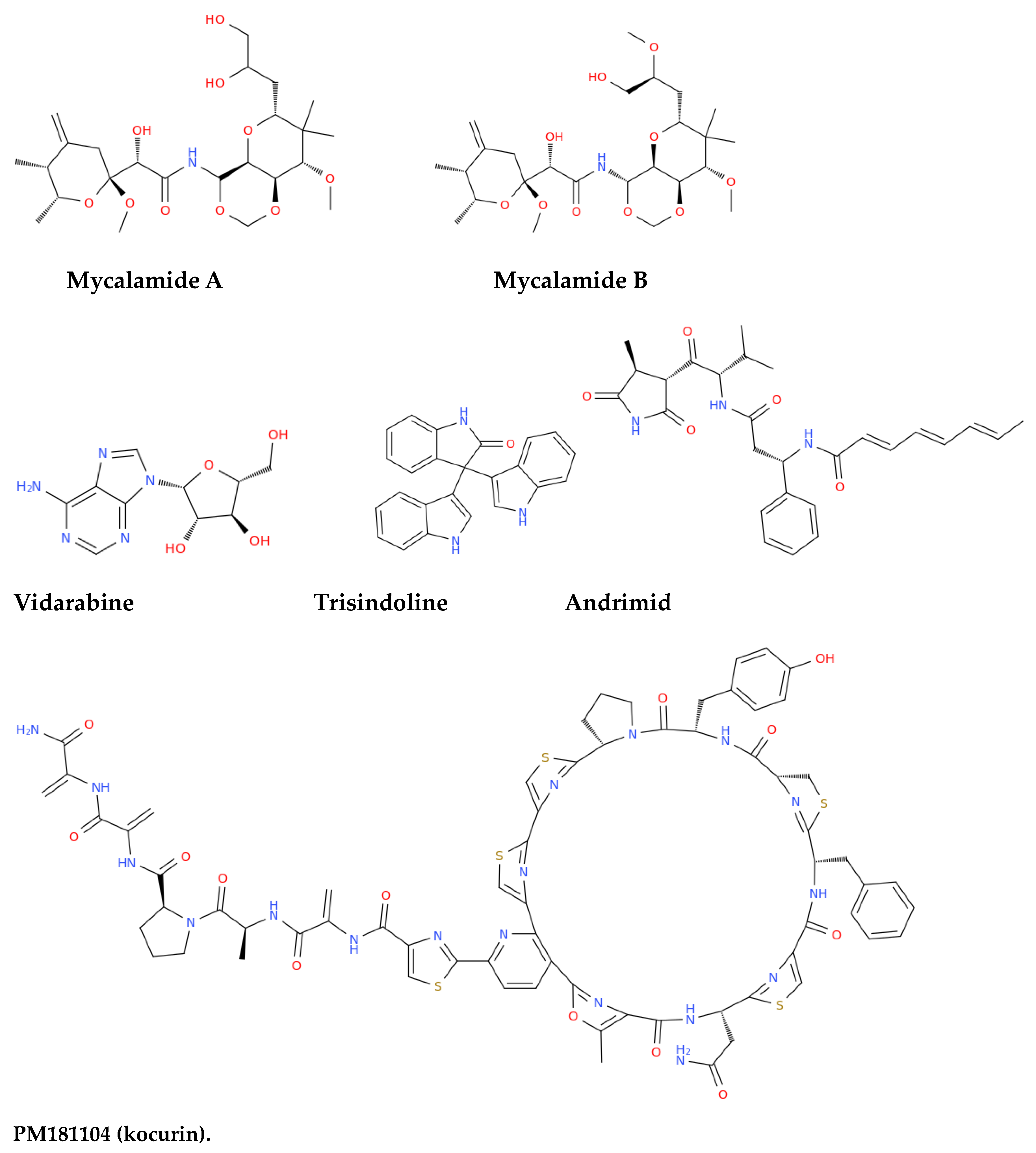
| Mycalamide A & B | New Zealand sponge (Mycale sp.) | Antiviral, antitumor, protein synthesis inhibition | Murine coronavirus A59, HSV, Polio, Influenza virus | [120] | |
| Vidarabine | Sponge (Cryptotethya crypta) | Antiviral | Herpes simplex virus (HSV), Cytomegalovirus, Varicella zoster virus (VZV) | [121] [122] | |
| Trisindoline | Sponge (Callyspongia siphonella) | Antibacterial, cytotoxic | S. aureus, Bacillus subtilis | [123] | |
| Andrimid | Sponge (Hyatella sp.) and bacteria (Pseudomonas fluorescens) | Broad-spectrum antibacterial | Methicillin-resistant Staphylococcus aureus (MRSA), Salmonella enteritidis, Vibrio harveyi, Yersinia ruckeri | [124] [125] | |
| PM181104 | Marine sponge (Spirastrella inconstans var. digitata) | Antibacterial, protein synthesis inhibition | MRSA, Enterococci, S. aureus (resistant and sensitive strains) | [126] | |
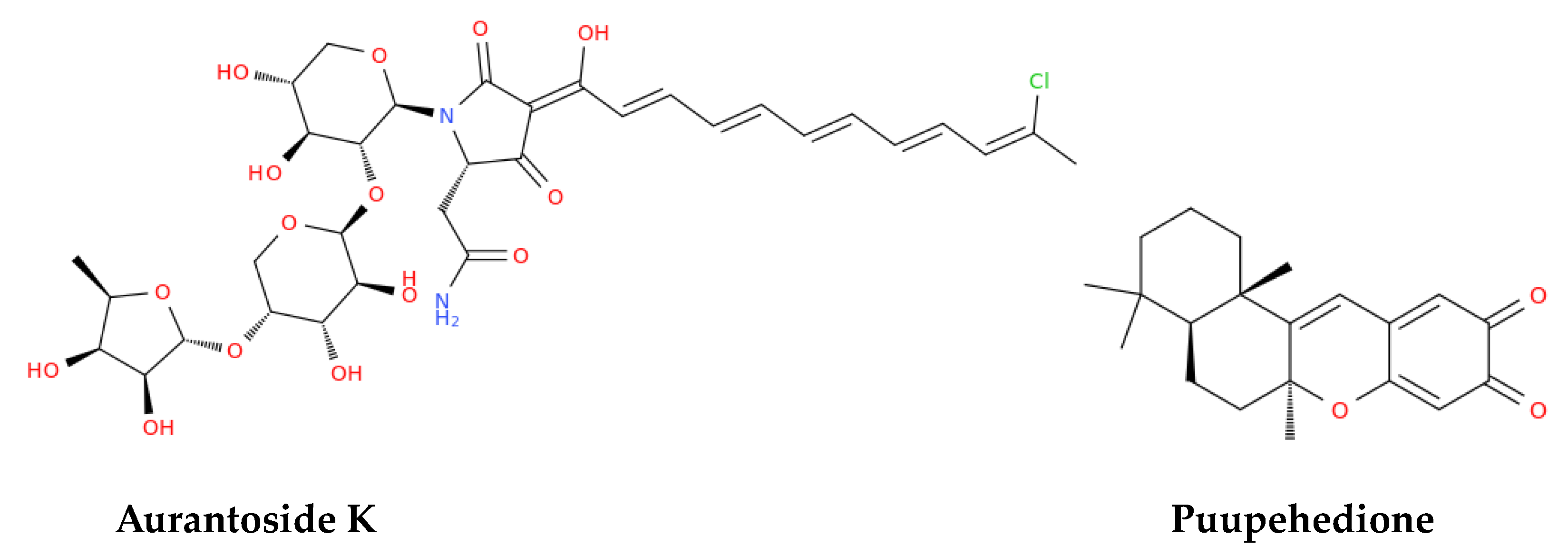
| Aurantoside K | Sponge (Melophlus sp.) | Antifungal | Amphotericin B-resistant C. albicans, Cryptococcus neoformans, A. niger, Penicillium sp., others | [127] | |
| Puupehedione | Verongid sponge | Antitumor, anti-angiogenic, antimicrobial, immunomodulatory | Various microbial pathogens | [120,128] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





