1. Introduction
Inflammation is the immune system’s response to harm and is an essential defense mechanism for health [
1]. Notwithstanding, uncontrollable inflammation in different parts of the body contributes to the pathogenesis of numerous chronic diseases, including diabetes, neurodegenerative diseases like Alzheimer’s, and cardiovascular diseases like atherosclerosis [
2]. Thus, some studies have concentrated on developing novel drugs to counteract inflammatory damage to cellular components, but the efficacy and adverse effects of the current medicines remain major issues [
3].
Isatin-containing heterocycles possessed favorable anti-inflammatory properties [
4,
5]. SAR analyses of
in silico studies indicated that the hydrazide moiety provides a hydrogen bonding domain that enables the structure to form a hydrogen bond essential for the interaction with amino acid residues, appending the potential of being a potent anti-inflammatory agent [
6]. Various furan-containing compounds naturally occur in plants, oils, fruits, and marine foods [
7], are reported to be biologically active, including anti-inflammatory [
8,
9], and are found in a variety of pharmaceutical medicines, such as furosemide [
10]. Here, we report the synthesis of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1), explore its in vitro anti-inflammatory potential, determine its molecular and ADMET properties, and describe its molecular behavior toward an inflammation-related receptor, namely cyclooxygenase-2 protein, using a molecular docking analysis.
2. Results and Discussion
2.1. Chemistry
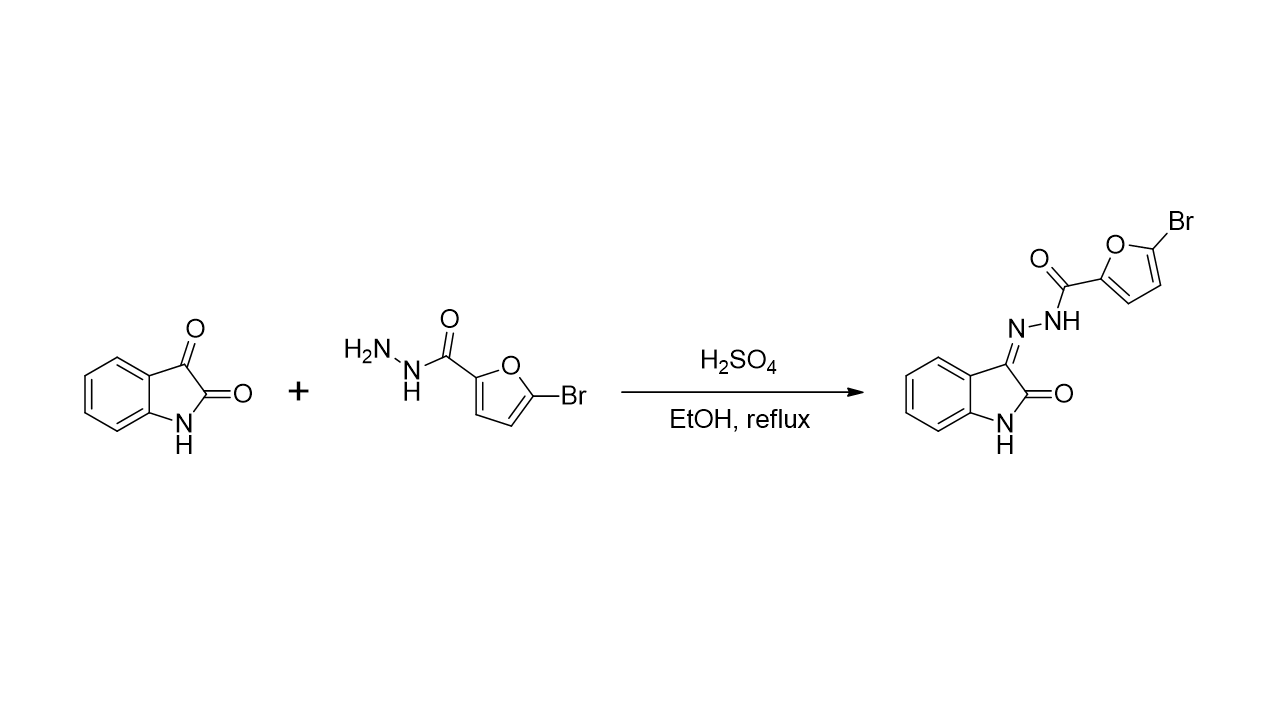
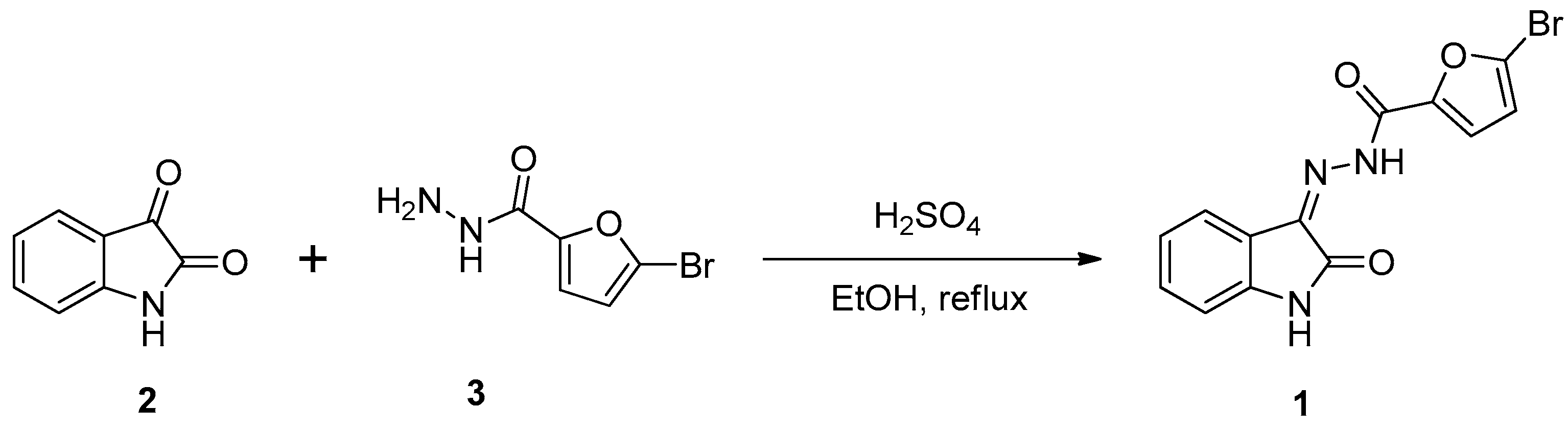
The synthesis of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) has been successfully achieved by condensation of commercially available isatin
(2) with 5-bromofuran-2-carbohydrazide
(3) under acidic conditions as shown in
Scheme 1. The reaction of isatin
(2) and 5-bromofuran-2-carbohydrazide
(3) took place for 15 minutes under reflux in ethanol as the solvent and sulfuric acid as the catalyst. The expected product was filtered and washed using dichloromethane to get compound
1 as a yellowish solid in 81% yield.
Structure identification of the synthesized compound 1 using an NMR spectrometer resulted in a 1H NMR spectrum that corresponds to the structure of 5-bromo-N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide. According to the 1H NMR spectrum, the proton of the NH group of the hydrazide moiety showed up as a broad singlet signal at δ 13.72 ppm. This NH group vibration was recorded at υ 3233 cm-1 in the FTIR spectrum. Meanwhile, the NH of the isatin ring was detected as a singlet signal at δ 11.30 ppm. In the 13C NMR spectrum, the signal of hydrazide carbonyl carbon was detected at δ 153.62 ppm. The C=O absorption reinforced this at υ 1620 cm-1 in the FTIR spectrum. Meanwhile, the signal of isatin carbonyl carbon was observed at δ 163.4 ppm. Furthermore, the absorption at υ 1680 cm-1 showed the absorption of the C=N group (imine), indicating the successful condensation of isatin (2) and 5-bromofuran-2-carbohydrazide (3). In the HRMS spectrum, since the bromine atom has two isotopes in nature, namely 79Br and 81Br, the [M+H]+ ions were recorded at m/z 333.9841 and 335.9813 which corresponds to the molecular formula for C13H879BrN3O3 and C13H881BrN3O3, respectively (calcd. 333.9822 (C13H879BrN3O3) and 335.9802 (C13H881BrN3O3)).
2.2. In vitro Anti-inflammatory Properties
The anti-inflammatory potential of 5-bromo-N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide (1) was examined through its ability to inhibit bovine serum albumin denaturation, proteinase activity, and hemolysis. The inhibiting effect of title compound 1 was investigated at several concentrations (6.25, 12.5, 25, 50, and 100 µg/mL) and was compared with diclofenac sodium, a well-known nonsteroidal anti-inflammatory drug.
2.2.1. Bovine Serum Albumin (BSA) Denaturation Effect
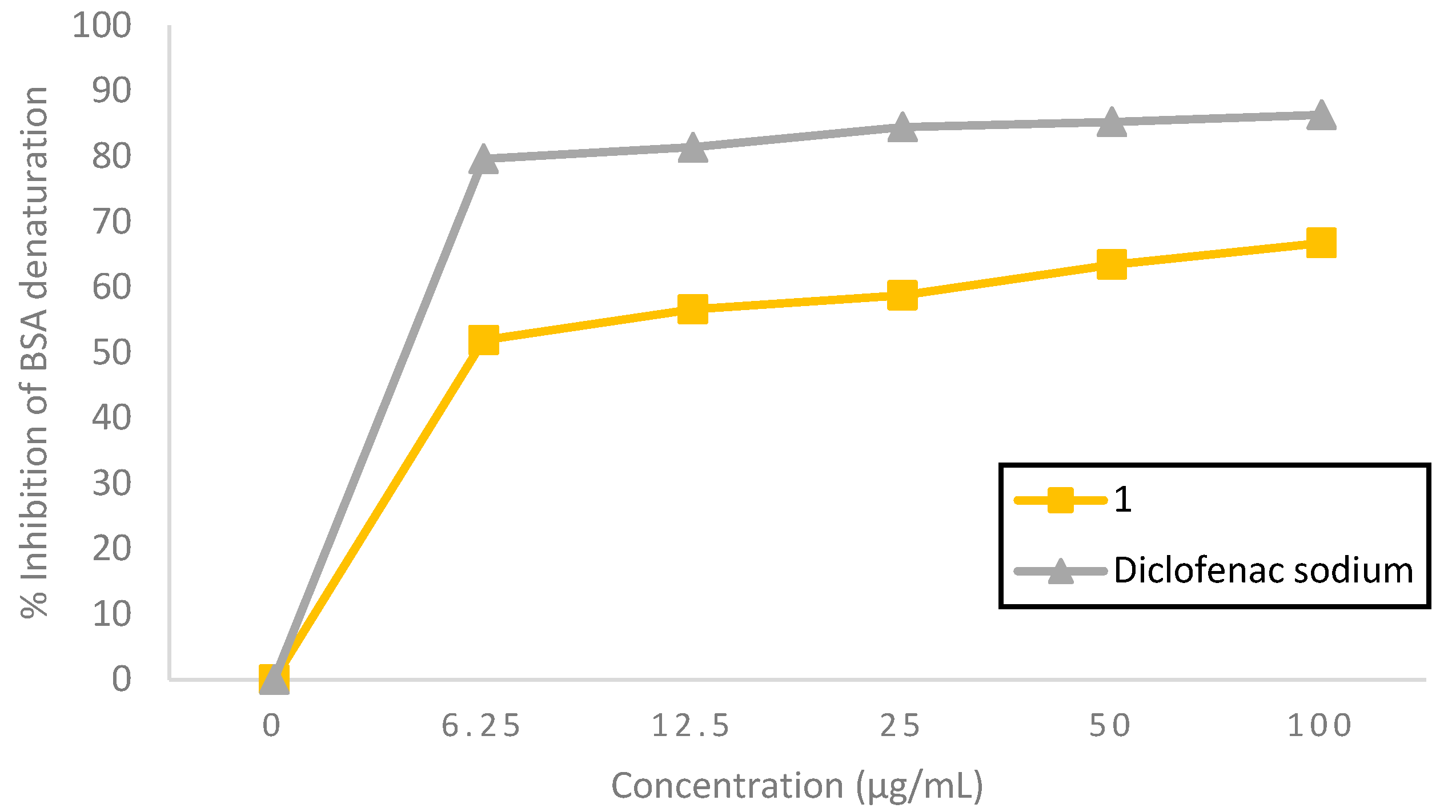
Protein denaturation, associated with proteins losing their secondary, tertiary, and quaternary structures and consequently losing their biological function, is a commonly recognized cause of inflammation; therefore, it is thought to be a marker for inflammatory disease [
11]. Hence, it was determined if the title compound
1 could inhibit heat-induced denaturation of the protein (albumin). As shown in
Figure 1, compound
1 exhibited a concentration-dependent inhibitory effect on BSA denaturation with a range of inhibition percentages from 51.89±0.001% to 66.76±0.001% at concentrations from 6.25 to 100 µg/mL, which was a lower inhibitory effect than diclofenac sodium (79.64±0.002–86.36±0.001%). As a result, compound
1 showed approximately two times lower potency than diclofenac sodium, with an IC
50 value of 3.54 and 1.99 µg/mL, respectively. However, the inhibitory effect of compound
1 on BSA denaturation could still be classified as a potent inhibitor (IC
50 < 10 µg/mL) [
20].
2.2.2. Protein Inhibition Effect
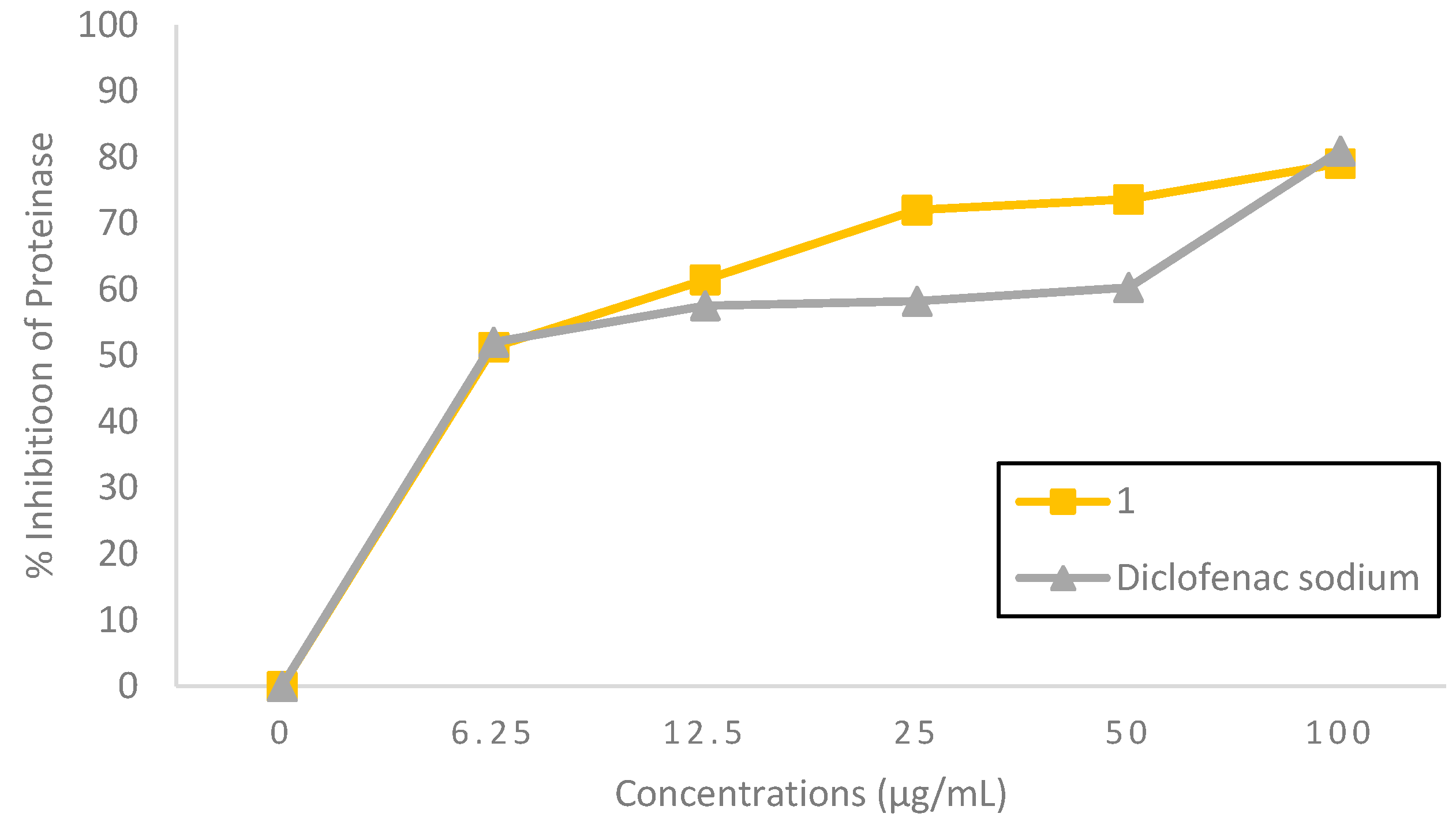
5-Bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) possessed a good proteinase inhibitory effect, as can be seen in
Figure 2. At 12.5 to 50 µg/mL concentrations, compound
1 exhibited a higher inhibitory effect on proteinase activity, with an inhibition percentage of 61.48±0.473–73.64±0.030%, compared to the standard diclofenac sodium, with an inhibition percentage of 57.57±0.002–60.31±0.001%. Meanwhile, at 6.25 and 100 µg/mL concentration, compound
1 showed proteinase inhibitory effects, with an inhibition percentage of 51.31±0.827% and 79.07± 0.001%, comparable to diclofenac sodium, with an inhibition percentage of 52.07±0.003% and 81.00±0.006%, respectively. Compound
1 was found to be 0.9 times more potent than diclofenac sodium based on their respective IC
50 value of 3.04 and 3.38 µg/mL, as shown in
Table 1, and was categorized as a potent inhibitor (IC
50 < 10 µg/mL) [
12]. It has been reported that leukocyte proteinase plays a critical role in developing tissue damage during inflammatory processes, and proteinase inhibitors give a notable degree of protection [
13]. The findings indicated that 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) might protect significantly against proteinase activity and was slightly superior to diclofenac sodium.
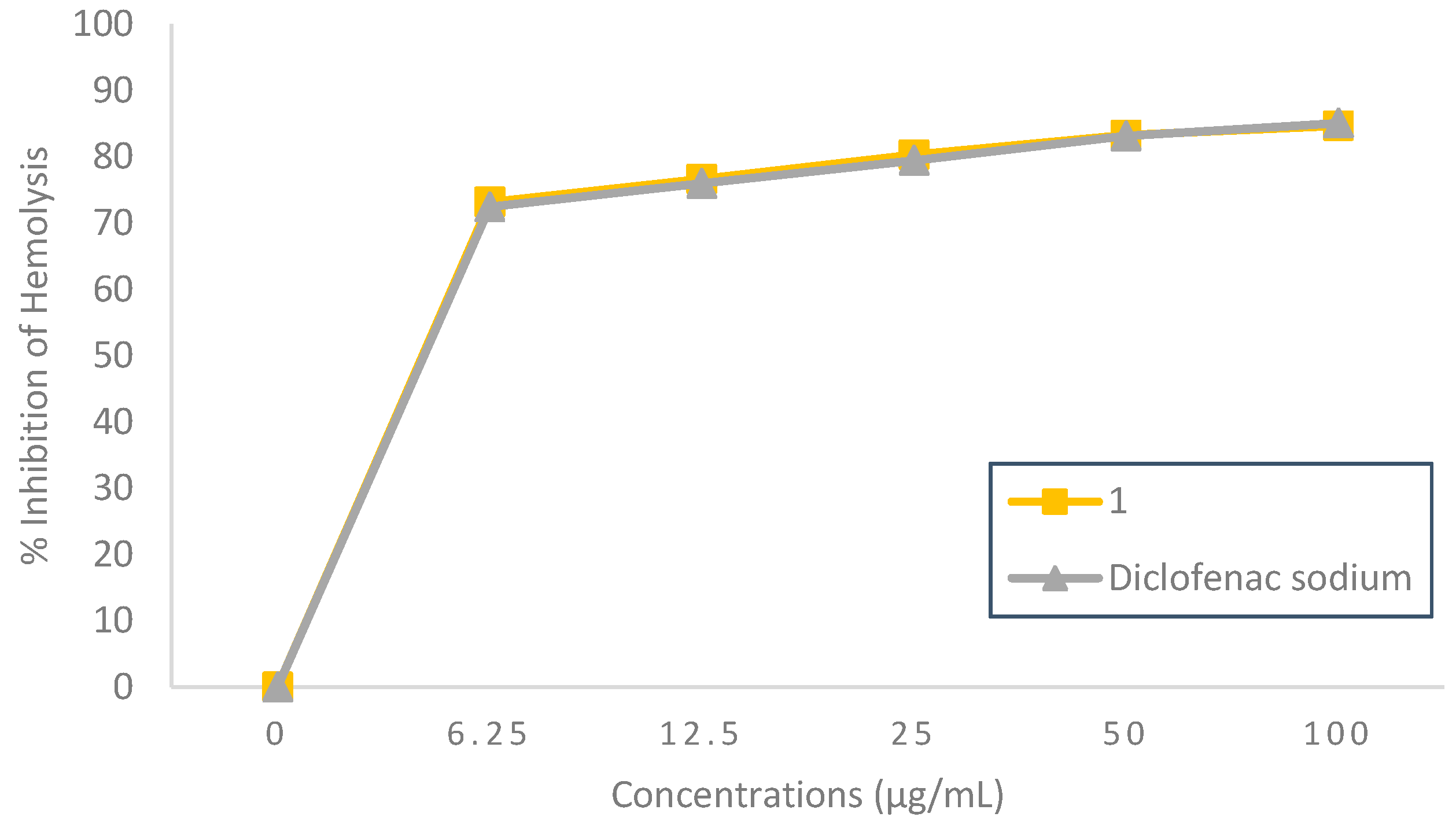
2.2.3. Membrane Stabilizing Effect
Heat-Induced Hemolysis Inhibition Effect
5-Bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) showed a noteworthy concentration-dependent anti-inflammatory activity in protecting the HRBC membrane from heat exposure, as seen in
Figure 3. At all test concentrations, the heat-induced hemolysis inhibition effect of compound
1 was similar to that of the standard diclofenac sodium, with a range of inhibition percentages 73.19±0.007–84.68±0.004% and 72.46 ±0.025–85.10±0.027%, respectively. Compound
1 concentration for inhibiting 50% of heat-induced hemolysis was found at 2.25 µg/mL, which can be considered a potent heat-induced hemolysis inhibitor (IC
50 < 10 µg/mL) [
12], while a similar inhibition effect was given by diclofenac sodium at 2.28 µg/mL. It suggested that the title compound
1 might have a significant protective effect on the RBC membrane. Since the RBC membrane is similar to the lysosomal membrane, the impact of compound
1 on RBC stabilization could be extrapolated to the lysosomal membrane stabilization, and the suppression of lysosomal material release at the site of inflammation might potentially explain the anti-inflammatory effect [
14].
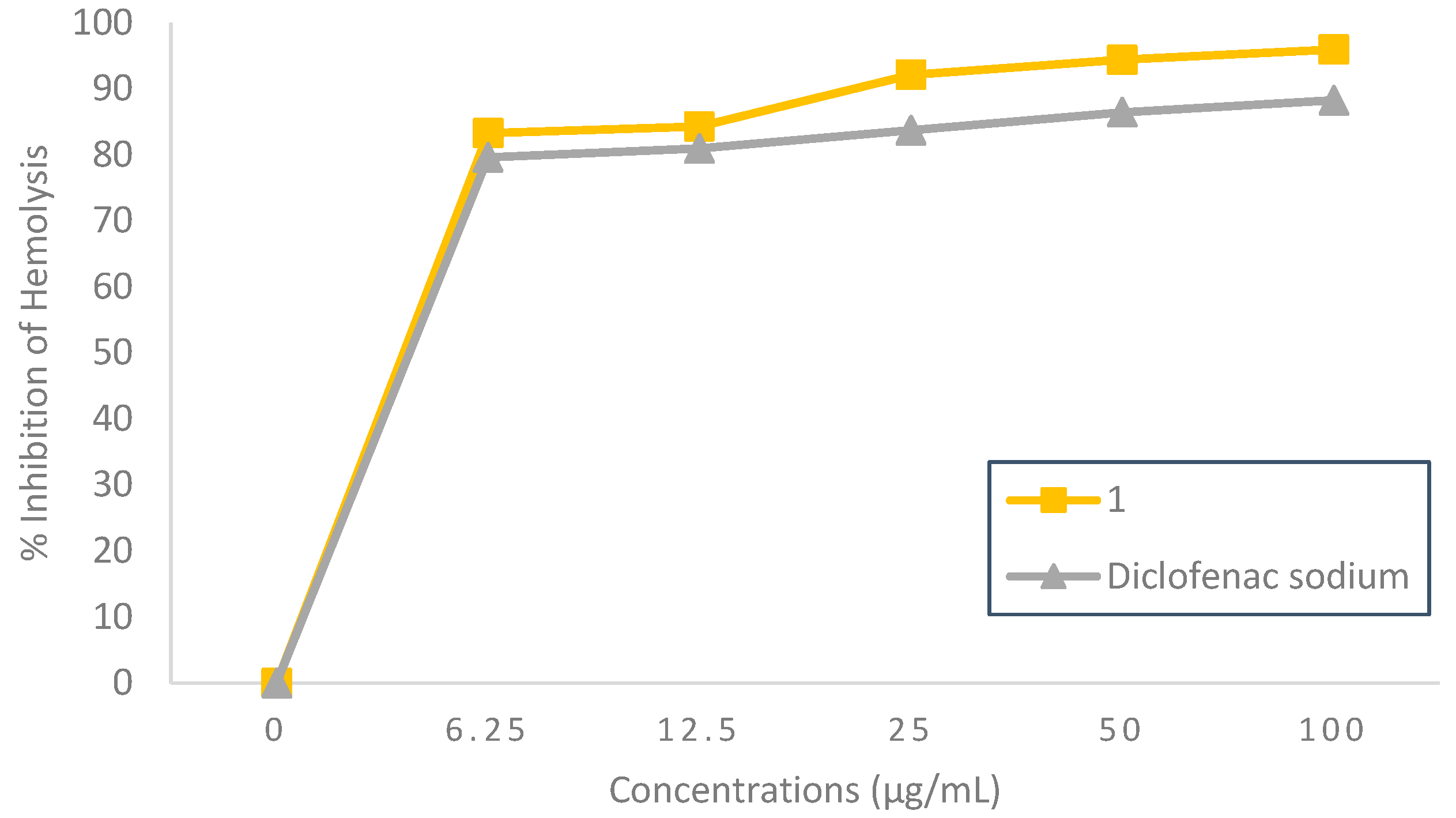
Hypotonicity-Induced Hemolysis Inhibition Effect
The outstanding anti-inflammatory activity of 5-bromo-
N’-(2-oxoindolin-3-ylidene)-furan-2-carbohydrazide
(1) was demonstrated through the high percentage of hypotonicity-induced hemolysis inhibition, as can be seen in
Figure 4. It was observed that compound
1 could inhibit hemolysis in a concentration-dependent manner with an inhibition range of 83.30±0.002% to 95.95±0.001% at concentrations of 6.25 to 100 µg/mL. Meanwhile, the standard diclofenac sodium at the same concentration showed a hemolysis inhibition range of 79.63±0.001% to 88.27±0.001%. Compound
1 concentration inhibited 50% of hypotonicity-induced hemolysis at 1.82 µg/mL, as shown in
Table 1, which increased the potency by 0.9-fold compared to diclofenac sodium (IC
50 = 2.00 µg/mL). This showed that 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) could be considered a potent hemolysis inhibitor (IC
50 < 10 µg/mL) [
12]. Hemolysis can occur due to excessive fluid accumulation in RBCs, resulting in the rupture of the RBC membrane, leaving it vulnerable to secondary damage from lipid peroxidation induced by free radicals [
15]. Based on the results, 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) might be very effective in maintaining the stability of RBC membrane from hemolysis due to hypotonicity and was better than diclofenac sodium.
2.3. Drug-likeness, Molecular and ADMET Properties
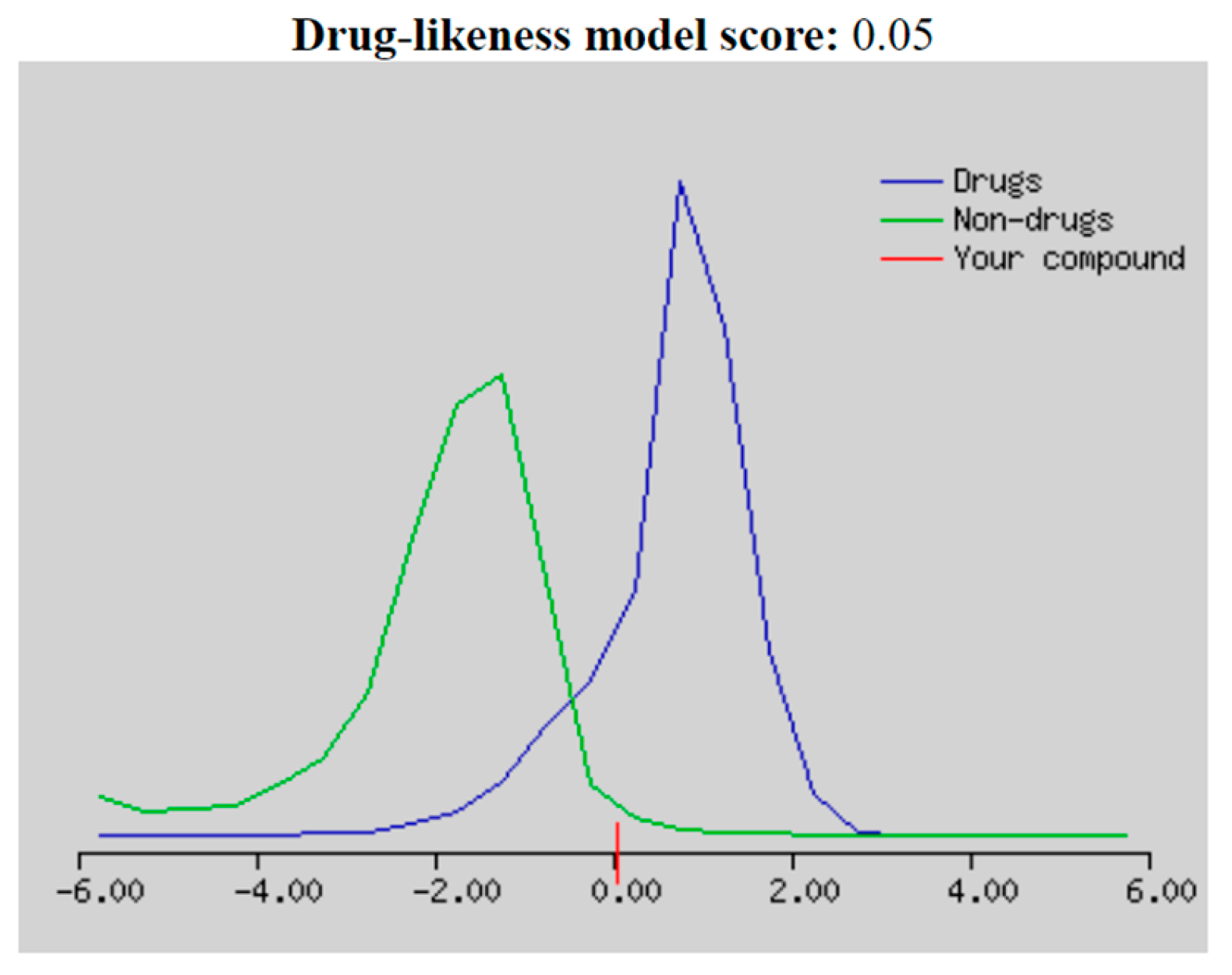
"Drug-likeness" is a balance of various structural and molecular characteristics determining if a given molecule is similar to known drugs. According to the Molsoft drug-likeness score distribution, compounds that scored between -2.0 and 2.0 were regarded as drug-like candidates [
16]. Looking at the drug-likeness model score plotting of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) in
Figure 5, compound
1 fell below the blue curve (drug molecules) with a score of 0.05, indicating it could be considered as a drug-like compound. Regarding the model score, the molecular properties of compound
1 did not violate Lipinski’s Rule of Five (Ro5), as seen in
Table 2, which obeyed the following parameters: molecular weight ≤ 500, log P ≤ 5, hydrogen bond donors (HBD) ≤ 5, and hydrogen bond acceptors (HBA) ≤ 10 [
16]. Compound
1 had a molecular weight of 332.97 g/mol, a molLogP value of 2.44, and the number of HBD and HBA was 2 and 4, respectively. Hence, it could be considered as an orally active candidate. Compound
1 had good solubility reflected by a logS value of no more than 6 [
17]. In addition, compound
1 also showed good absorption through the gut based on its molPSA (molecular polar surface area) value of 68.29 A
2, which was within the optimal polarity range of 20-130 A
2 [
16]. It was strengthened with a human intestinal absorption percentage of 91.993%, considered highly absorbed, as shown in
Table 3. However, compound
1 might not be feasible in the stomach’s acidic environment due to its poor solubility at gastric pH, as indicated by the lower pKa value (pKa < 5.0), which might affect its absorption properties. Nevertheless, it could be fully absorbed in the small and large intestines due to its high solubility at intestinal pH [
18].
Compound
1 was found to be a non-substrate for P-glycoprotein, the most essential member among ABC-transporters. Cytochromes P450 (CYP) were isoenzymes consisting of five primary isoforms, namely CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4, which played an essential role in the metabolic biotransformation process leading to drug elimination. Inhibition of these isoenzymes was a significant factor in pharmacokinetics-related drug-drug interactions that resulted in toxic or other undesirable side effects due to metabolite accumulation or lower drug clearance [
17]. As shown in
Table 3, compound
1 did not inhibit these isoforms. Organic Cation Transporter 2 (OCT2) had a crucial role in the renal clearance of xenobiotics and endogenous compounds, raising the possibility of adverse interactions between OCT2 substrates and coadministered OCT2 inhibitors. Examining whether a compound was a potential OCT2 substrate gave helpful information on its clearance and potential contradictions [
19]. The result showed that compound
1 was not a renal OCT2 substrate. Furthermore, the excretion value of compound
1 was estimated using the total clearance (CLtot) model with a log mL/min/kg value of -0.286, which indicated a combination of renal and hepatic clearance and was essential for determining dose level to achieve steady-state volume [
20]. The steady-state volume of distribution (VDss) of compound
1 was found to be at a log L/kg value of -0.375, equivalent to 0.42 L/kg, and considered low (log VDss < -0.15) [
21].
According to the toxicity prediction results, compound
1 was shown to be non-mutagenic (Ames-negative), not an inhibitor of hERG I/II, and non-toxic to the liver. Compound
1 was found to have a low maximum recommended tolerated dose (MRTD, log(mg/kg/day) ≤ 0.477 [
22]) with a log(mg/kg/day) value of -0.2. Acute oral toxicity in rats of compound
1 was estimated at a potential dose to kill 50% of the rat population (LD
50), which obtained an LD
50 value of 2.197 mol/kg. Moreover, compound
1 was found to be not toxic to Flathead Minnows (log(LC
50) < 0.3), with a log(LC
50) value of 1.463, indicating that this compound was not hazardous to the underwater environment [
21].
2.4. Molecular Docking Study
Cyclooxygenase (COX) is an essential enzyme for converting arachidonic acid into prostaglandins [33]. Its inducible isoform, called cyclooxygenase-2 (COX-2), is responsible for the elevated production of prostaglandins during inflammation [
23]. COX-2 complexed with SC-558 (PDB ID: 6COX) was chosen for investigating the possible molecular behavior of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) at the COX-2 active site. SC-558 was redocked at the COX-2 active site before the title compound
1 docking simulation to validate the docking protocol. The results showed that the docking protocol produced a root mean square deviation (RMSD) value of 0.89 Å. This RMSD value lower than 2 Å indicated that the binding pose of the redocked SC-558 is close to the native binding pose of SC-558 [
21]. The binding interactions of the reduced SC-558 are shown in
Table 4.
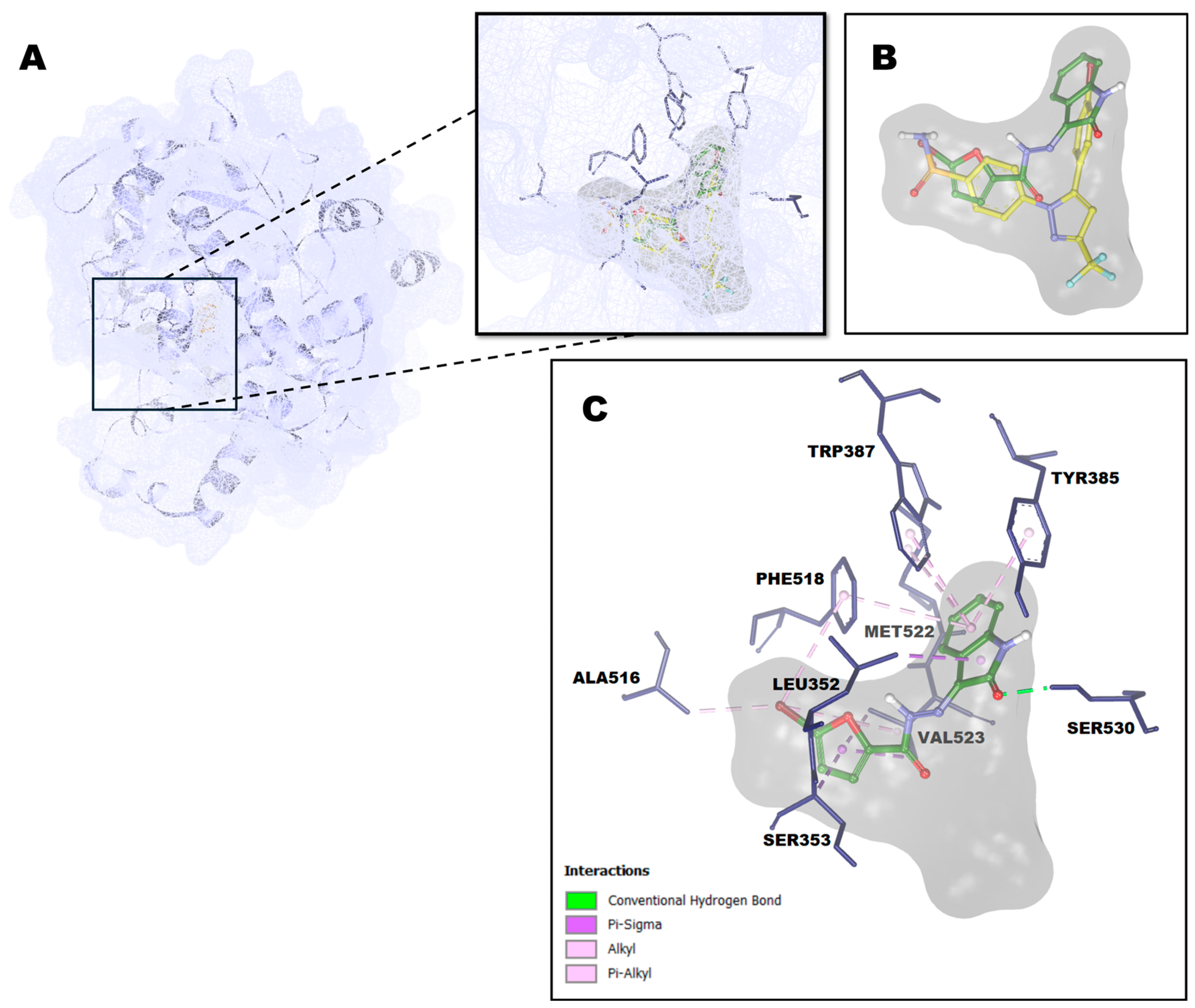
The molecular docking results disclosed that compound
1 has a good affinity with a binding energy value of -9.63 kcal/mol, as seen in
Table 4. The features of compound
1 have some equivalents in the binding region of SC-558 to the cyclooxygenase active site of COX-2 protein. SC-558 has occupied three areas of the cyclooxygenase active site of the COX-2 protein, while compound
1 only occupied two regions: the isatin skeleton of compound
1 bound in the same cavity as the bromophenyl ring of SC-558 and the bromofuranyl ring of compound
1 overlapped with the phenylsulphonamide moiety of SC-558 in the channel leading from the cyclooxygenase cite branch at the SC-558 binding site to the surface of COX-2 protein, as shown in
Figure 6B. This unique channel contributed significantly to selectivity inhibitor binding in the COX-2 protein. In contrast, it did not appear in the COX-1 due to the difference in the size of the side chain at 434, thus precluding the possibility of a molecular gate to a new hydrophilic pocket [
23]. The isatin skeleton was bound with some hydrophobic residues formed by alkyl hydrophobic interaction with Met552, π-σ hydrophobic interaction with Leu352, and π-alkyl hydrophobic interactions with Tyr385, Trp387, and Phe518. Besides, the C=O group of the isatin skeleton formed a hydrogen bond with Ser530 in the branch area of the cyclooxygenase active site. The furan ring of compound
1 formed two hydrophobic interactions: π-σ interaction withVal523 and the backbone of Ser353. Meanwhile, the Bromo group embedded in the furan ring formed three hydrophobic interactions: one π-alkyl interaction with Phe518 and two alkyl interactions with Val523 and Ala516, a residue that contributed to the specificity of COX-2 protein [34]. In addition, compound
1 also formed van der Waals interactions with Ala527, Val349, Tyr348, Phe381, Gly526, Leu384, Ile517, Gln192, Arg513, and His90.
3. Materials and Methods
3.1. Materials
Materials utilized in this study were purchased from Tokyo Chemical Industry and Sigma-Aldrich and were not purified before use. Thin-layer chromatography (TLC) was used to monitor the reaction, which was seen under UV at 254 nm. Melting poin was determined on Fisher-John melting point apparatus and has not been corrected. The 1H and 13C NMR spectra were taken at 400 and 100 MHz on a Jeol JNM-ECS400 spectrometer in DMSO-d6, with tetramethylsilane (TMS) serving as an internal standard. Reports are given in parts per million (ppm) for the chemical shifts (δ) and in Hertz for the coupling constants (J). FTIR spectrum was captured using a Shimadzu 8400S FTIR spectrometer. Mass spectra were recorded in a Xevo G2-S Qtof Mass Spectrometer with an ESI ionization in positive mode. The absorbance of the sample was measured using a Thermo Scientific Genesys 10S UV-VIS spectrophotometer.
3.2. Synthesis of 5-bromo-N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide (1)
A solution of isatin
(2) (0,074 g; 0,50 mmol), 5-bromofuran-2-carbohydrazide
(3) (0,10 g; 0,49 mmol), and a catalytic amount of sulphuric acid in ethanol (10 mL) was refluxed for 15 minutes (reaction was monitored by TLC using ethyl asetate as eluent). After the reaction was complete, the mixture was cooled to room temperature. The precipitate was filtered off, washed with dichloromethane, and dried to yield the title compound as a yellowish solid (0.13 g, 81%); mp: 212-213
oC; FTIR (KBr) υ (cm
-1) 3233 (N-H), 1680 (C=N), 1620 (C=O amide);
1H NMR (400 MHz, DMSO-
d6) δ 6.91-6.96 (m, 2H, ArH); 7.06-7,11 (m, 1H, ArH), 7.35-7.44 (m, 2H, ArH), 7.59 (t,
J = 7,6 Hz, 1H, ArH), 11.31 (1H, s, NH), 13.72 (1H, bs NH);
13C NMR (100 MHz, DMSO-
d6) δ 111.7, 115.7, 119.9, 120.2, 121.6, 123.2, 127.3, 132.4, 138.9, 143.1, 148.1, 153.6, 163.4. HRESIMS
m/z (pos): 333.9841 (C
13H
879BrN
3O
3) and 335.9813 (C
13H
881BrN
3O
3) (calcd. 333.9827 (C
13H
879BrN
3O
3) and 335.9807 (C
13H
881BrN
3O
3)) (
Supplementary Materials).
3.3. In vitro Anti-inflammatory Activity Assay
The in vitro anti-inflammatory activities of 5-bromo-N’-(2-oxoindolin-3-ylidene)-furan-2-carbohydrazide (1) were evaluated using bovine serum albumin (BSA) denaturation assay, proteinase activity inhibition assay, and hemolysis inhibition assay.
3.3.1. Bovine Serum Albumin Denaturation Assay
The inhibition of BSA denaturation was examined by preparing a 5 mL reaction mixture consisting of 0.2 mL of 1% bovine albumin, 4.78 mL of phosphate buffer saline (pH 6.4), and 0.02 mL of compound
1 at various concentrations (6.25, 12.5, 25, 50, 100 ppm). The mixtures were incubated at 37℃ for 15 minutes, followed by heating at 70℃ for 5 minutes. After cooling the mixtures, the absorbance was measured at 660 nm using a UV-VIS spectrophotometer. A phosphate buffer solution was used as a control. Diclofenac sodium was used as a reference standard drug at the same concentration as the test sample. The percent inhibition of BSA denaturation was calculated using the following equation [
15]:
where
= the absorbance of the control and
= the absorbance of the test sample. The IC
50 value was calculated using linear regression by plotting compound
1 concentration as the x-axis and the denaturation inhibitory percent as the y-axis [
24].
3.3.2. Proteinase Activity Inhibition Assay
Anti-proteinase activity was carried out by preparing a 2 mL reaction solution, which consisted of 0.06 mg trypsin, 1 mL of 20 mM buffer Tris-HCl (pH 7.4), and 1 mL of compound
1 at different concentrations (6.25, 12.5, 25, 50, 100 µg/mL). The solutions were incubated at 37℃ for 5 minutes and added 1 mL of 0.8% casein (w/v). The reaction mixtures were then incubated for a further 20 minutes at 37℃. At the end of the incubation, the mixtures were given 2 mL of 70% perchloric acid to stop the reaction and centrifuged. The absorbance of the supernatant was measured at 210 nm using a UV-VIS spectrophotometer against the buffer serving as the blank [
25]. A phosphate buffer solution was used as a control. Diclofenac sodium was used as a reference standard drug at the same concentration as compound
1. The inhibition percentage of proteinase activity was calculated using Equation 1. The IC
50 value was calculated using linear regression by plotting compound
1 concentration as the x-axis and the proteinase inhibitory percent as the y-axis [
24].
3.3.3. Hemolysis Inhibition Assay
Preparation of Human Red Blood Cells (HRBCs) Suspension
Blood samples were taken from a healthy human volunteer who had abstained from NSAID use for 14 days prior to the experiment, centrifuged at 3000 rpm for 5 minutes in a heparinized centrifuge tube, and the supernatant was removed. The obtained precipitate was washed three times with the same volume as normal saline (0.9% NaCl). Then, the resulting HRBCs were diluted using a 10 mM isotonic buffer solution consisting of 0.2 g/L NaH
2PO
4, 1.15 g/L Na
2HPO
4, and 9.0 g/L NaCl to produce a 10% suspension (v/v) [
25].
Heat-Induced Hemolysis Inhibition
A mixture of 0.05 mL of HRBCs suspension, 2.95 mL of phosphate buffer solution (pH 7.4), and 0.05 mL of test compound at different concentrations (6.25, 12.5, 25, 50, 100 µg/mL) was prepared. The mixture was incubated at 54℃ for 20 minutes in a shaken water bath. The resulting mixture was centrifuged for 5 minutes at 2500 rpm, and the absorbance of the supernatant was measured at 540 nm using a UV-VIS spectrophotometer [
25]. Diclofenac sodium was used as a reference standard drug at the same concentration compound
1. The percent inhibition of hemolysis was calculated using Equation 1. The IC
50 value was calculated using linear regression by plotting compound
1 concentration as the x-axis and the hemolysis inhibitory percent as the y-axis [
24].
Hypotonicity-Induced Hemolysis Inhibition
The hypotonicity-induced hemolysis inhibition test is based on a study described by Aidoo et al. [
15] with some modifications. A mixture of 0.5 mL of HRBCs suspension, 2 mL of hyposaline solution, 1 mL of phosphate buffer, and compound
1 at different concentrations (6.25, 12.5, 25, 50, 100 µg/mL) was prepared. The mixture was then incubated at 37℃ for 30 minutes and was centrifuged for 5 minutes at 3000 rpm. The resulting supernatant was measured at 560 nm using a UV-VIS spectrophotometer. Diclofenac sodium was used as a reference standard drug at the same concentration as compound
1. The percent inhibition of hemolysis was calculated using Equation 1. The IC
50 value was calculated using linear regression by plotting compound
1 concentration as the x-axis and the hemolysis inhibitory percent as the y-axis [
24].
3.4. Drug-Likeness, Molecular and ADMET Prediction
The two-dimensional structure of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) was converted to its chemical identifier code in the form of a Simplified Molecular-Input Line-Entry System (SMILES) using MarvinSketch 20.18 [40]. The SMILES was then used to predict drug-likeness, molecular, and ADMET properties. Drug-likeness and molecular properties, and ADMET prediction was performed using web servers, namely Molsoft [41] and pKCSM [
21], respectively.
3.5. Molecular Docking Study
A molecular docking study of 5-bromo-
N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide
(1) was performed using the procedure conducted by Santoso et al. [
21]. The three-dimensional structure of cyclooxygenase-2 (COX-2) complexed with a selective inhibitor called SC-558 was downloaded from the protein data bank (PDB ID: 3W37) and produced using MGLTools 1.5.6. The three-dimensional structure of compound
1 was created with the MarvinSketch 20.18. Docking simulations were carried out using the Autodock4.2 software package. The docking pocket was determined using a grid box with XYZ dimensions of 40x40x40 centered at x: 23.049, y:23.526, and z:46.984. The docking result was analyzed using BIOVIA Discovery Studio.
4. Conclusions
In this study, 5-bromo-N’-(2-oxoindolin-3-ylidene)furan-2-carbohydrazide (1) was synthesized and evaluated for its in vitro anti-inflammatory properties, and determined its in silico molecular and ADMET properties, as well as performing its molecular docking study. Compound 1 showed concentration-dependent inhibition with a more than 50% inhibition percentage at all test concentrations (6.25 to 100 µg/mL) in inhibiting BSA denaturation, proteinase activity, and hemolysis. According to the results, compound 1 was a potent anti-inflammatory agent with a range of IC50 value 1.82–3.54 µg/mL, comparable to the standard diclofenac sodium with a range of IC50 value 1.99–3.38 µg/mL. Compound 1 was regarded as a drug-like candidate without violation of Lipinski’s rule of five and had good absorption, distribution, metabolism, and elimination properties. In addition, it was considered safe due to its non-toxic properties in the human body and underwater environment. A molecular docking study showed compound 1 bound in a specific channel found only on the cyclooxygenase active site of the COX-2 protein (PDB ID: 6COX), indicating compound 1 might be a selective COX-2 inhibitor. This data suggested that compound 1 might be developed into an anti-inflammatory agent.
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: IR spectrum of compound 1; Figure S2: 1H NMR spectrum of compound 1; Figure S3: 13C NMR spectrum of 1; Figure S4: High Resolution Mass spectrum of compound 1.
Author Contributions
Conceptualization, methodology, resoures, funding acquisition, M.S. and S.N.; investigation, software, formal analysis, visualization, and writing—original draft preparation, N.P.A.; validation, data curation, writing—review and editing, supervision, M.S. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Riset dan Inovasi untuk Indonesia Maju (RIIM) Batch 4 of National Research and Innovation Agency (BRIN) and Indonesia Endowment Fund for Education Agency (LPDP), grant number 161/IV/KS/11/2023 and 3183/PKS/ITS/2023.
Data Availability Statement
All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors acknowledge Ministry of Finance and Ministry of Education and Culture, Research, and Technology of Indonesia Republic for supporting fund.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Md.M.; Islam, Md.E.; Hossain, Md.S.; Akter, M.; Rahman, Md.A.A.; Kazi, M.; Khan, S.; Parvin, M.S. Unveiling the Therapeutic Potential: Evaluation of Anti-Inflammatory and Antineoplastic Activity of Magnolia champaca Linn’s Stem Bark Isolate through Molecular Docking Insights. Heliyon 2024, 10, e22972. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.A.; Foster, J.G.; Wood, S.K.; Hebert, P.R.; Hennekens, C.H. Benefits and Risks of Nonsteroidal Anti-Inflammatory Drugs: Methodologic Limitations Lead to Clinical Uncertainties. Drug. Inf. J. 2019, 53, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Jarapula, R.; Gangarapu, K.; Manda, S.; Rekulapally, S. Synthesis, In Vivo Anti-Inflammatory Activity, and Molecular Docking Studies of New Isatin Derivatives. Int. J. Med. Chem. 2016, 2016, 2181027. [Google Scholar] [CrossRef]
- Hassanzadeh, F.; Jafari, E.; Khayambashi, N.; Hajhashemi, V. Synthesis and Anti-Inflammatory Effects Evaluation of 1,3 Substituted Isatin Derivatives:(TJPS-2020-0290.R1). TJPS 2021, 45, 248–252. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals (Basel) 2022, 15, 272. [Google Scholar] [CrossRef]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Pourteymour Fard Tabrizi, F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef]
- Manolov, S.; Ivanov, I.; Bojilov, D.; Nedialkov, P. Synthesis, In Silico, and In Vitro Biological Evaluation of New Furan Hybrid Molecules. Processes 2022, 10, 1997. [Google Scholar] [CrossRef]
- Yang, L.; He, J. Traditional Uses, Phytochemistry, Pharmacology and Toxicological Aspects of the Genus Hosta (Liliaceae): A Comprehensive Review. J. Ethnopharmacol. 2021, 265, 113323. [Google Scholar] [CrossRef]
- Banerjee, R.; Kumar, H.K.S.; Banerjee, M. Medicinal Significance of Furan Derivatives: A Review. Int. J. Rev. Life. Sci. 2012, 2, 7–16. [Google Scholar]
- Derbel, H.; Elleuch, J.; Mahfoudh, W.; Michaud, P.; Fendri, I.; Abdelkafi, S. In Vitro Antioxidant and Anti-Inflammatory Activities of Bioactive Proteins and Peptides from Rhodomonas sp. Appl. Sci. 2023, 13, 3202. [Google Scholar] [CrossRef]
- Karjalainen, M.J.; Neuvonen, P.J.; Backman, J.T. In Vitro Inhibition of CYP1A2 by Model Inhibitors, Anti-Inflammatory Analgesics, and Female Sex Steroids: Predictability of In Vivo Interactions. BCPT 2008, 103, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, J.C.; Matus-Basto, A.J.; Acereto-Escoffié, P.; Segura-Campos, M.R. Antioxidant and Anti-Inflammatory Activities of Phenolic Compounds Isolated from Melipona beecheii Honey. Food Agr. Immunol. 2017, 28, 1424–1437. [Google Scholar] [CrossRef]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and Anti-Inflammatory Activities of Arbutus unedo Aqueous Extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Aidoo, D.B.; Konja, D.; Henneh, I.T.; Ekor, M. Protective Effect of Bergapten against Human Erythrocyte Hemolysis and Protein Denaturation In Vitro. Int. J. Inflam. 2021, 2021, 1279359. [Google Scholar] [CrossRef]
- Anwar, M.M.; Shalaby, M.; Embaby, A.M.; Saeed, H.; Agwa, M.M.; Hussein, A. Prodigiosin/PU-H71 as A Novel Potential Combined Therapy for Triple Negative Breast Cancer (TNBC): Preclinical Insights. Sci. Rep. 2020, 10, 14706. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Tsume, Y.; Langguth, P.; Garcia-Arieta, A.; Amidon, G.L. In Silico Prediction of Drug Dissolution and Absorption with Variation in Intestinal pH for BCS Class II Weak Acid Drugs: Ibuprofen and Ketoprofen. Biopharm. Drug Disp. 2012, 33, 366–377. [Google Scholar] [CrossRef]
- Hacker, K.; Maas, R.; Kornhuber, J.; Fromm, M.; Zolk, O. Substrate-Dependent Inhibition of the Human Organic Cation Transporter OCT2: A Comparison of Metformin with Experimental Substrates. PloS One 2015, 10, e0136451. [Google Scholar] [CrossRef]
- Domínguez-Villa, F.X.; Durán-Iturbide, N.A.; Ávila-Zárraga, J.G. Synthesis, Molecular Docking, and In Silico ADME/Tox Profiling Studies of New 1-aryl-5-(3-azidopropyl)indol-4-ones: Potential Inhibitors of SARS CoV-2 Main Protease. Bioorg. Chem. 2021, 106, 104497. [Google Scholar] [CrossRef]
- Aijijiyah, N.P.; Wati, F.A.; Rahayu, R.; Srilistiani, A.; Mahzumi, F.; Aulia, T.; Santoso, L.; Pamela, E.; Ramadhani, E.Y.; Ilfahmi, Y.A.; et al. Synthesis, α-Glucosidase Inhibitory Activity, and Molecular Docking of Cinnamamides. Med. Chem. Res. 2023, 32, 723–735. [Google Scholar] [CrossRef]
- Ejeh, S.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E. Computational Insight to Design New Potential Hepatitis C Virus NS5B Polymerase Inhibitors with Drug-Likeness and Pharmacokinetic ADMET Parameters Predictions. Futur. J. Pharm. Sci. 2021, 7, 219. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Elisha, I.L.; Dzoyem, J.-P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The Anti-Arthritic, Anti-Inflammatory, Antioxidant Activity and Relationships with Total Phenolics and Total Flavonoids of Nine South African Plants Used Traditionally to Treat Arthritis. BMC Complement. Altern. Med. 2016, 16, 307. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).