1. Introduction
The human gastrointestinal tract consists of distinct regions, including the stomach, small intestine and colon, each comprising a unique anatomy, physiology, and microenvironment [
1,
2]. This intricate gut ecosystem hosts trillions of microbes collectively known as the gut microbiota, with bacteria being the most abundant and prominent species [
3,
4]. The gut microbiota significantly impacts human health by metabolizing undigested nutrients, producing beneficial molecules, preventing pathogen colonization, and training the immune system [
5,
6,
7,
8]. While the composition of gut microbiota remains generally stable, its dramatic change in response to unhealthy factors may lead to various mental and physical diseases [
9,
10,
11]. To better understand how the varying gastrointestinal environment contribute to health outcomes,
in vivo studies are crucial. However, such studies often pose challenges due to ethical concerns, limited accessibility to intestinal samples, and high costs [
12,
13]. Moreover, the inherent complexity and variability among individuals often result in non-uniform data [
14]. Hence, the development of robust
in vitro models of the ileal microbiota has become vital.
In vitro fermentation models designed to simulate various sections of the human gastrointestinal tract enable the investigation of microbial communities with programmable control over physiological parameters, such as nutrient availability and pH levels [
15,
16]. A series of systems with varying complexity have been developed and implemented in the field of gut microbiome research [
17,
18,
19]. These simulated models can be classified as dynamic and static, with the dynamic models more closely reflecting the inherent condition of human gut environment [
16,
20,
21]. Among the dynamic simulators, the Simulator of Human Intestinal Microbial Ecosystem (SHIME
®) is the most extensively used and validated
in vitro dynamic model by featuring a continuous and real-time monitoring setup and entire gastrointestinal tract integration (
Figure 1) [
22,
23]. This review will outline the recent key developments of SHIME
®, emphasizing its ability to accurately mimic the human gastrointestinal environment and its diverse applications in food and nutritional science, drug development, and gut health research. We will begin by providing a general overview of the SHIME
® technology, including its history, working principles, and versional developments. Next, we will discuss the current status and practical applications of SHIME
® in various research contexts. Finally, we will explore future trends and challenges facing the SHIME
® platform, and further consider how ongoing advancements may enhance its effectiveness and accuracy in appreciating gut microbiota interactions and the implication for human health.
2. Overview of SHIME®
SHIME, an acronym for the Simulator of the Human Intestinal Microbial Ecosystem, has been a widely used tool for studying gut microbiota dynamics [
22]. In 2010, the name SHIME
® was jointly registered by ProDigest and Ghent University, making a formal collaboration to further advance this technology. Such a simulator allows for the cultivation of a complex intestinal microbial ecosystem over an extended period while maintaining representative conditions of different intestinal regions, to closely mimic
in vivo gut conditions.
24
2.1. History of SHIME®
The Simulator of the Human Intestinal Microbial Ecosystem (SHIME
®) is a multicompartment dynamic simulator of the human gut, originally developed in 1993 by Verstraete and co-workers from Ghent University [
22]. The creation of the model was driven by the realization that the fecal microbiota, often used as a proxy for gut microbiota research, significantly differs from the
in vivo colon microbiota in terms of community composition and metabolic activity. Initial efforts to mimic the colon environment, such as inoculation of fecal microbiota into a single-stage chemostats, were only effective for shorter periods [
24,
25]. This limitation arose because key environmental parameters such as pH, redox potential, available nutrients and microbial population dynamics change constantly within the reactors. In 1981, Miller and Wolin collectively addressed some of these challenges by developing a semi-continuous fermenter, which could simulate the intermittent replenishment of nutrient media, allowing for a more sustained microbial environment [
26]. However, the complexity of colon, together with its varying regions and unique conditions, makes it challenging to simulate a representative culture of colon microbiota in a single compartment. To overcome the limitations of single-compartment models, Macfarlane et al. introduced a multi-compartment reactor system that could simulate the distinct conditions of different regions of colon [
27]. Upon these advancements, SHIME
® was developed as one of the most representative gut simulators of this generation [
17,
28]. Technically, the SHIME
® is an evolution of the gut simulator introduced by Macfarlane from University of Reading. While the original Reading model provided valuable insights into colonic microbiota, the SHIME differentiates it by incorporating the conditions of the upper digestive tract (stomach, small intestine), thereby offering a more comprehensive simulation of the entire gastrointestinal system [
22,
24].
2.2. The Modules and Working Principles of SHIME®
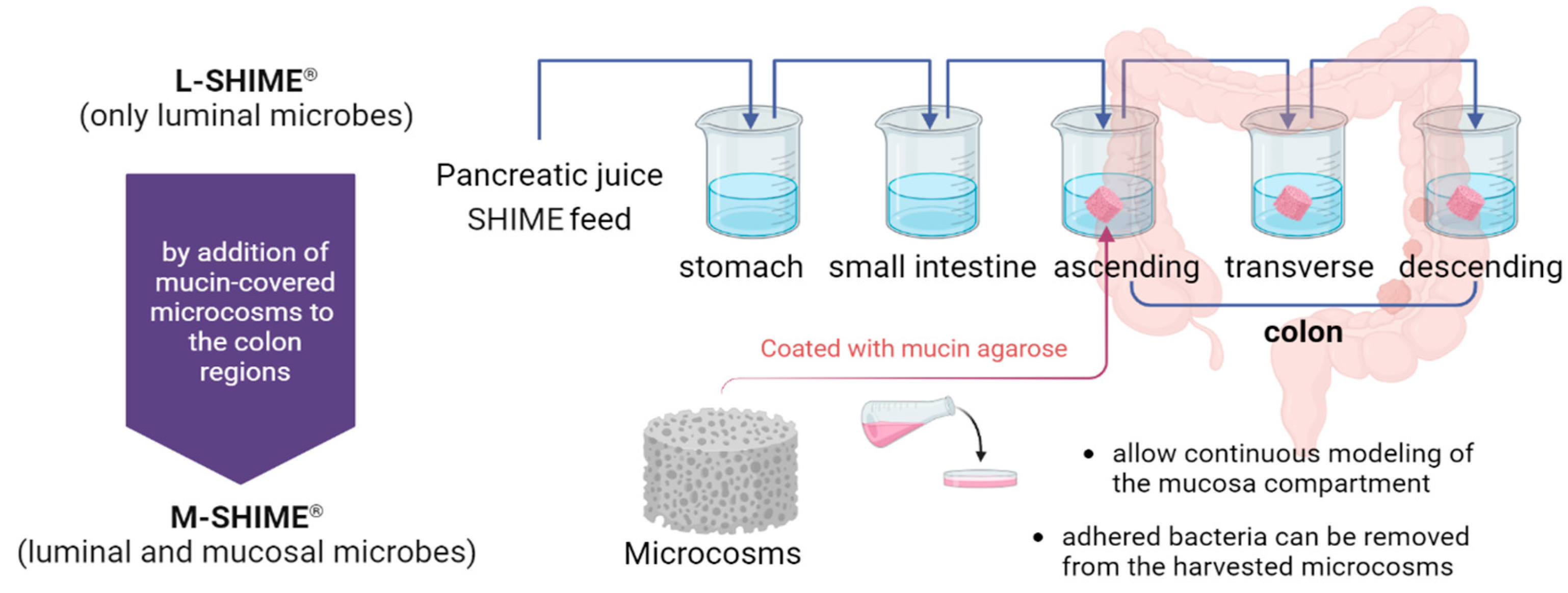
SHIME
® consists of a succession of five compartments that replicate different sections of the digestive tract (
Figure 1). It contains double-jacketed glass vessels that are connected through peristaltic pumps. The first two compartments simulate the upper digestive tract (stomach and small intestine), where factors like acidity, digestive enzymes, and nutrient absorption play significant roles. These compartments are crucial for creating the appropriate conditions for downstream microbial colonization and fermentation in the colon. The next three compartments simulate the lower digestive tract (the ascending, transverse, and descending colon), where the gut microbiota is most active. In these compartments, SHIME accurately mimics the pH gradients, nutrient availability, and anaerobic conditions in the real human colon, which allows for the investigation of region-specific microbial dynamics, including fermentation processes and the production of metabolites like short-chain fatty acids [
22,
24].
A typical SHIME experiment consists of four stages, each meticulously designed to evaluate the dynamics of the gastrointestinal microbial community under controlled condition [
24]. The initial stabilization period (normally 2 weeks), allows the microbial community to adapt to the specific environmental conditions established in the respective colon regions. This adaptation is crucial, as it allows for the stabilization of microbial populations to provide a balanced ecosystem for the subsequent measurements. For the second basal period (~ 2 weeks), the reactor operates under nominal conditions and baseline parameters such as microbial composition, pH, and metabolic activity, are measured. This data serves as a vital reference point for evaluating the effects of any interventions introduced later. In the third stage (around 2–4 weeks), a specific treatment (e.g., probiotic, prebiotic, or bioactive molecules) is introduced to assess the impact on the gastrointestinal microbial community. The final stage of washout (2 weeks) aims to assess how long the effects induced by the tested substance persist in the absence. This washout period is essential for understanding the stability and longevity of any changes observed in the microbial community following the treatment.
24
2.3. Parameters Setup of SHIME®
The SHIME’s stomach and small intestine modules follow a fill-and-draw principle, wherein a defined nutritional medium is added to the gastric compartment three times a day, along with pancreatic and bile liquid to the small intestine compartment. Following the digestion process in the gastric and intestine compartments, the resulting slurry is pumped into the ascending colon vessel, initiating colon digestion. The three colon compartments are continuously stirred to ensure thorough mixing with constant volume and pH control to simulate natural digestive conditions. The system’s retention times, which represent the time it takes for medium to move through the modules, can be adjusted by altering the flow rates from the gastric and small intestine, or by changing the volumes of the colon. The retention times can vary from 24 h to 72 h, depending on the human target group being simulated (e.g., individuals with different health conditions). To promote the growth of diverse microbial community, a specifically optimized gut microbiota medium is often required as no such a universal medium can fit to culture all the gut microorganisms. The composition of a culture medium for microbiological studies typically composes of water, carbon source, nitrogen source, and some mineral salts [
29]. Temperature is typically set to physiological levels (about 37°C) to simulate human body temperature. Nutrient availability is controlled through the addition of specific growth media tailored for each stage of digestion, introduced at defined intervals [
22].
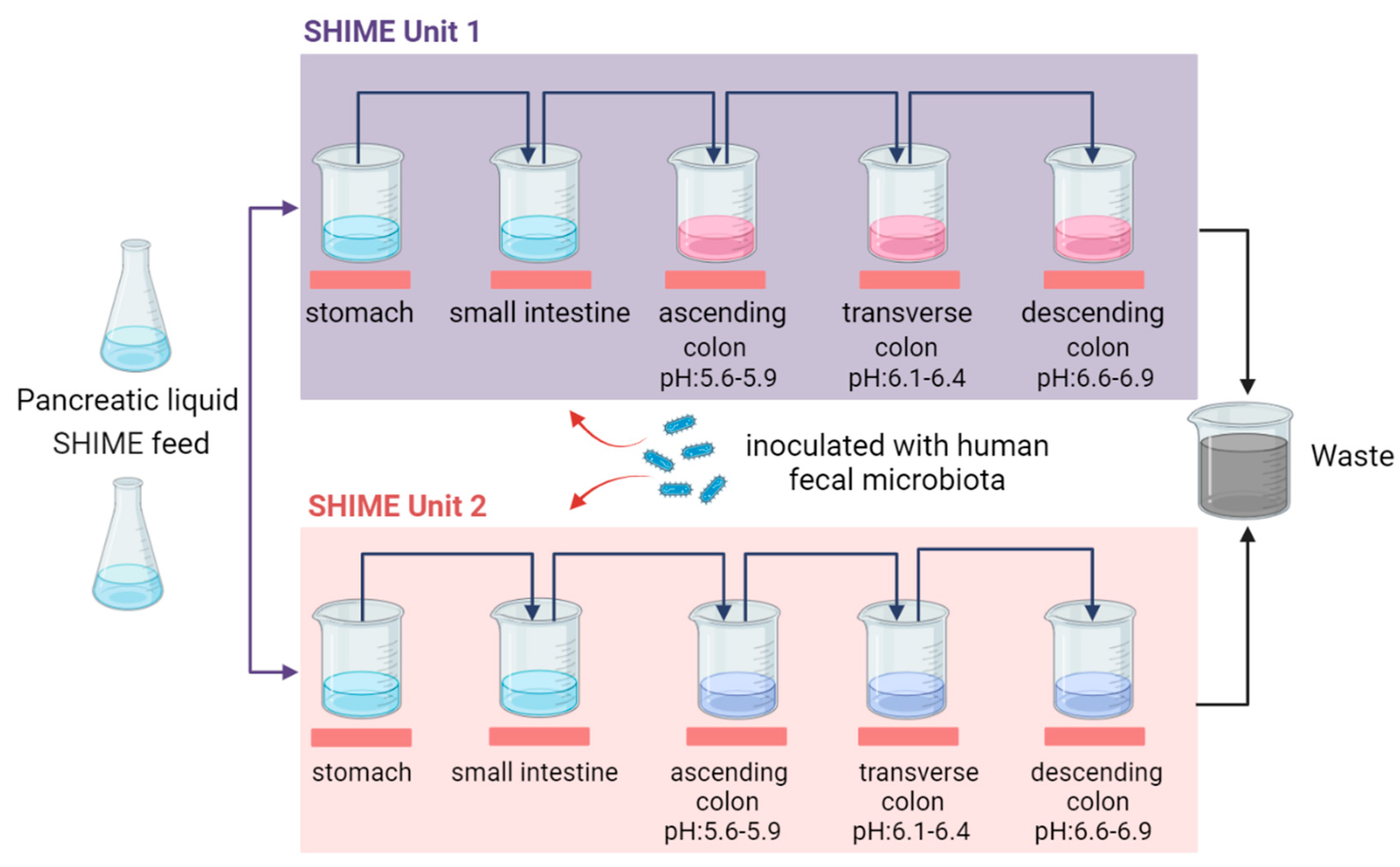
Additionally, the gastric compartment was maintained at a fixed pH of 2.0, but with the introduction of a fully computer-controlled SHIME system, specific pH profiles can now be established to more accurately replicate the dynamic changes in pH during digestion in both gastric and intestine modules. While the small intestine typically operates at slightly acidic to neutral pH conditions, the pH in the colon compartments is maintained with specific ranges, that is between 5.6 and 5.9 in the ascending colon, 6.1–6.4 in the transverse colon, and 6.6–6.9 in the descending colon (
Figure 2) [
30]. The pH gradient closely simulates the conditions found in the real human colon and is critical for understanding the impact of microbial activity in different gut regions. Mixing of the digestive slurry in each compartment is achieved using magnetic stir bars to ensure homogeneous conditions throughout the compartments. The entire SHIME system is kept in anaerobic conditions by flushing the headspace of each compartment daily with N
2 gas or a N
2/CO
2 (9:1) gas mixture, mimicking the low-oxygen environment of the gastrointestinal tract [
31]. By carefully setting these parameters, SHIME
® provides a robust platform for studying the complex interactions within the gut microbiome and their implications for human health and disease.
2.4. Development of SHIME®
Currently, the SHIME
® model is employed to investigate the physical, chemical, enzymatic, and microbiological parameters of the human gut under controlled conditions. Researchers have optimized and adapted the SHIME
® model for various experimental purposes, resulting in specialized versions such as M-SHIME
®, [
32] Twin-SHIME
®, [
33] and Triple-SHIME
® (
Table 1) [
34]. The conventional SHIME
® focuses on observing luminal microorganisms, hence also referred to as L-SHIME
® [
22]. Yet, the human gut harbours a vast and intricate microbial community that plays a crucial role in maintaining health by preventing pathogen colonization and producing essential nutrients, and these microorganisms are not randomly distributed in the intestinal tract. Those adhering to the mucosa of the intestinal wall are particularly significant, as they act as a "barrier" to influence the mucosal immune response and occupy specific ecological niches [
35].
To address this concern, the M-SHIME
® (i.e., Mucosal-SHIME
®) was developed by augmenting the L-SHIME
® with microstructures that mimic the mucin cap across the three colonic regions (
Figure 1) [
32]. The innovative setup allows bacteria adhering to the mucosal layer to colonize these structures, forming a mucosal microcosm within the reactor. By replacing half of this mucosal microecology every around three days, researchers can effectively simulate the natural renewal process of the intestinal mucosal layer, enabling continuous modelling of mucosal dynamics overtime. Furthermore, the model permits the harvesting of adherent bacteria from the replaced mucosal microcosm, facilitating the detailed characterization of the mucosal community. This capability is crucial for investigating the specific interactions between mucosal-localized microbiota and host epithelial cells, which are essential for maintaining gut health and immune function [
36].
The Twin-SHIME
® system (
Figure 2), developed by Van den Abbeele and co-workers, innovatively connects two SHIME
® systems in parallel, facilitating liquid transfer between reactors while maintaining identical pH and temperature conditions through a double-ended pump [
33]. This configuration is particularly advantageous for simultaneous studies of different interventions on gut microbiome, ensuring consistent environmental conditions across both systems and enhancing the reliability of experimental outcomes. When focusing on a single colonic region, it is feasible to set up several parallel SHIME
® units, allowing for a comprehensive analysis of microbial dynamics. However, when the area of interest spans three colonic regions, the Twin-SHIME
® limits the capacity for handling repetitions, making the Triple-SHIME
® a more suitable solution. Unlike the Twin-SHIME
®, which often permits the simulation the stomach and small intestine within a single compartment, the Triple-SHIME
® necessitates separate compartments for the gastrointestinal tract [
34,
37]. This separation enhances the simulation’s fidelity, accommodating the unique physiological characteristics of each digestive segment.
Furthermore, Bondue et al. adapted the SHIME
® model to study microbiota in young children, replicating their unique digestive processes and colonic environments [
38]. This modified model (i.e., toddle SHIME
®) is particularly effective for assessing the effects of prebiotics and probiotics on young children’s microbiota, as well as for evaluating the metabolism of drugs and endocrine disruptors that may affect their health. The Mini-Colon Model (MiCoMo), a benchtop multi-bioreactor system that consists of triplicate bioreactors working independently, has gained popularity in laboratory settings due to its high throughput and multiplexing capabilities, providing cost-effective and superior simulation outcomes for gut microbiota research [
39]. To further enhance the SHIME
® model, Vasquez et al. integrated a chemical gas sensor utilizing carbon nanotubes for continuous monitoring of gaseous biomarkers within the simulator [
40]. This advancement establishes a comprehensive sensor platform that facilitates real-time monitoring of gaseous biomarkers in a cost-effective manner, improving the model’s utility for studying gut microbiota and their metabolic activities. Through these innovations and adaptations, the SHIME
® model is continuing to be a vital tool in understanding gut microbiota interactions and their implications for human health.
2.5. Advantages and Limitations of SHIME®
The Simulator of the Human Intestinal Microbial Ecosystem offers unique advantages, making it a valuable tool in gut microbiota research.
Table 2 provides a brief summary of the advantages and limitations of the SHIME
® technology [
22,
24]. One of the primary strengths is its multicompartment design, which simulates and integrates the entire gastrointestinal tract, allowing for more accurate representation of microbial dynamic across the stomach, small intestine, and the colons. Real-time monitoring setup enables the continuous measurement of various parameters, such as pH, temperature, and gas production, providing detailed insights into microbial activity. Additionally, SHIME’s long-term stability and controlled condition facilitate extended experimental periods, allowing researchers to manipulate defined environmental factors like nutrient availability and fermentation conditions. The model can also be modified for specific research needs, with adaptation like M-SHIME
® for studying mucosal interactions and Twin- or Triple-SHIME for parallelly conducting simultaneous studies across multiple compartments. This flexibility enhances its applicability in various research contexts.
As for the limitations of the SHIME® system, one significant drawback is the absorption unit employs mainly semi-permeable membrane, which limits its ability to accurately simulate active absorption processes. Besides, the entire gastrointestinal simulation system lacks a bionic peristatic mechanism, resulting in a less realistic representation of gut motility and its effects on microbial dynamics and nutrient transport. Meanwhile, the absence of host cells within the system makes it challenging to replicate crucial interactions such as those between microbiota and epithelial cells. Consequently, while SHIME® provides valuable insights into gut microbiota research, these limitations highlight the need for complementary methods to better understand the multifaceted hallmarks of human gut ecosystem.
3. Applications of SHIME®
At present, SHIME
® has become a significant technological platform for investigating the effect of food or drugs on intestinal microecology. Researchers have leveraged this technology to study the metabolism of food bioactive substances, the dynamics of drug release, and the function and diversity of bacteria within the gut ecosystem. Its sophisticated design allows for real-time monitoring and flexible manipulation of the gastrointestinal conditions, making it an indispensable tool in the fields of food safety and nutrition science, pharmacology, and microbial ecology [
41,
42,
43]. Below we will present some key examples of SHIME
® technology applied in these fields.
3.1. SHIME® Application in Food and NUTRITION Science
The SHIME
® technology is an extensively utilized in the field of food safety and nutrition research. Its principal applications include the simulation of food digestion and absorption processes, investigation of nutrient metabolic pathways, and the assessment of their impact on intestinal microbiota. The model allows for the examination of the production and role of metabolites like short-chain fatty acids (SCFAs), primarily butyrate, acetate, and propionate, which are crucial metabolites produced by the gut microbiota and play various important roles in the gut health [
44]. Amont these, butyrate is especially important for maintaining the integrity of the gut barrier as an energy source for intestinal epithelial cells. Several studies have utilized the SHIME
® model to investigate the effects of different food additives, fibers, and probiotics on gut microbiota and their metabolic outcomes [
45,
46]. For instance, Gonza et al. employed the SHIME
® model to investigate the effects of several food additives, such as polysorbate 80, sucralose, maltodextrin, and sodium nitrite, on the gut microbiota and metabolic activity of individuals under health and unhealth conditions. They found that polysorbate 80 and sucralose reduced butyrate–producing bacteria (e.g.,
Roseburia,
Faecalibacterium prausnitzii) while increasing bacterial species such as
Enterococcus and
Veillonella, which are positively correlated with intestinal inflammation and fibrosis [
47].
Van den Abbeele et al. demonstrated that the intake of long-chain arabinoxylan and inulin significantly increased the production of propionate and butyrate in the gut. The study further showed
Bifidobacterium longum was stimulated by long-chain arabinoxylan, while
Bifidobacterium adolescentis responded to inulin intake [
48]. Similarly, Tails Fernanda et al explored the effect of fermented milk with fruit pulp on the
in vitro intestinal microbiota using the SHIME
® model. They reported that this probiotic product increased the production of beneficial SCFAs while reducing harmful ammonium ions, indicating its potential to promote gut health [
49].
The SHIME
® technology is also valuable for assessing the digestion and metabolism of nutrients in the gastrointestinal system, particularly in the presence of intestinal microbiota. Liu and co-workers employed SHIME
® to investigate the digestion and bioavailability of soybean components, polysaccharides and isoflavones [
50,
51]. Their findings revealed that both polysaccharides and isoflavones were primarily broken down and utilized by the gut microbiota in the colon. Moreover, soybean polysaccharides can promote the growth of beneficial probiotics and enhance the ability to inhibit pathogenic bacteria, underscoring its potential role in being functional food to improve gut microbiota.
3.2. SHIME® Application in DRUG Development
Given the functional characteristics, the SHIME
® technology is also adept at simulating the release, absorption, and metabolism of drugs in the gut, as well as investigating the interactions between gut microorganisms and pharmaceutical compounds [
43,
52] This capacity makes SHIME
® an important platform for drug development. Tabare et al. utilized SHIME
® technology to evaluate the release profile of Eudragit
® FS microparticles that contain bacteriophage LUZ19, aiming to develop phage oral dosage forms for targeting bacteria in the colon [
53]. Similarly, Martínez-López et al. assessed the degradation of insulin microcapsules prepared by enzymatic gelation of arabinoxylan using SHIME
®, and found that arabinoxylans microcapsules can inhibit the simulated conditions of the upper gut system and act as a barrier for insulin delivery to the colon [
54].
In another study, Derave and co-workers employed SHIME
® to evaluate the magnesium bioavailability of 15 commercial magnesium supplement formulations through dissolution assays. The findings demonstrated a wide variation in absorption and dissolution among the magnesium products, and provide a valid
in vitro SHIME-based methodology for predicting
in vivo bioavailability and effectiveness of micronutrients [
55]. Grootaert et al. explored the prebiotic effects of aglycone-based xyloglucan oligosaccharides and inulin using Twin-SHIME
®, revealing distinct degradation patterns occurred in the different regions of colon and demonstrating the potential of this differentiation to deeply understand specific drug metabolism and reaction conditions [
56].
Additionally, the integration of SHIME
® technology also provides extensive data that inform the study of drug combination and delivery strategies. Li et al. employed the SHIME
® model to examine the
in vitro effects of a combination of colistin and amoxicillin on intestinal flora and its antibiotic resistance, as well as to evaluate the recovery of gut microbiota upon fecal microbiota transplantation (FMT) treatment [
57]. The study highlights a potential administration of antibiotics and application of FMT in the clinic settings. Besides, Mccoubrey et al. leveraged SHIME
® to compare the efficacy of two colonic delivery strategies for ulcerative colitis (UC) by measuring the release of mesalazine from both prodrug-mediated and formulation-mediated approaches. The findings underscored the approach selected for colonic drug delivery could significantly impact the effectiveness of UC treatment [
58]. Another relevant example is that, Cesar et al. investigated the effects of a citrus flavonoid mixture combined with metformin for treating pre-diabetes, and the results demonstrated the potential of combination administration to lower blood glucose levels and enhance glucagon-like peptide-1 (GLP-1) in pre-diabetic patients [
59]. Upon these applications, the SHIME
® technology has provided critical insights into drug metabolism, efficacy, and the complex interactions between drugs and gut microbiota.
3.3. SHIME® Application in Gut Health Research
Gut microbiota significantly impacts human health and physiology, playing a vital role in establishing the mucosal barrier and maintenance of gut homeostasis. These gut microbes influence the intestinal barrier by regulating expression and distribution of tight junction proteins, as well as modulating mucus secretion and mucin glycosylation [
60]. The study from Piche et al indicated that an impaired gut barrier was a critical factor in the predisposition to intestinal diseases, such as irritable bowel syndrome (IBS), and the results demonstrated that the mucosal soluble mediators can reproduce functional permeability changes and molecular alterations, including ZO-1 mRNA expression, as observed in patients with IBS [
61]. Suligoj and co-workers utilized the SHIME model, along with the Caco2 cell line and gut organisms, to investigate adult gut microbiota and gut barrier function. They demonstrated that the human milk oligosaccharides (HMOs) are able to shape the infant gut microbiota by selectively stimulating the growth of bifidobacteria and also have the ability to modulate immune function and enhance the gut barrier integrity, indicating that HMOs may confer health benefits even in adults [
62].
Importantly, investigating gut microbiota could serve as an alternative strategy for prevention clinical disease. Salgaco et al. examined the effect of infant cereals containing
Bifidobacterium animalis ssp.
lactis BB-12
® on the gut microbiota of infants using SHIME
®. After inoculating the fecal microbiota of three young children, those consuming BB-12
® cereals experienced beneficial growth
of Lactobacillaceae gasseri, linked to a reduced risk of allergic rhinitis in children, and
L. kefiri, associated with the obesity prevention [
63]. In another study, Marzorati et al. employed SHIME
® for the first time to assess the effect of the spore-based oral probiotic MegaSporeBiotic™ on gut microbial activity and community composition, highlighting the potential of probiotic treatment to alter gut metabolism and increase bacterial diversity [
64].
Additionally, existing studies have employed the M-SHIME
® model to investigate the inhibition of invasive
Escherichia coli in mucus by specific probiotics and prebiotics, such as
Lactobacillus reuteri 1063 and arabinoxylans. The interventions help limit the adverse effects of pathogens on the host by reducing their proximity to epithelial cells, which in turn minimizes the potential for infection and inflammation.
48 Lambrecht et al. used the M-SHIME
® model to evaluate the transfer of a resistance plasmid containing multiple antibiotic resistance genes from commensal
Escherichia coli to the human gut microbiota. This study highlighted the risks of horizontal gene transfer, which can promote the spread of antibiotic resistance within the gut, posing a significant challenge for treatment options in humans [
65].
3.4. SHIME® Application in Traditional Chinese Medicine
The study of the metabolic process of Chinese herbs in the digestive tract is crucial to reveal the medicinal substances and their intrinsic therapeutic mechanisms. Currently, research on the efficacy of traditional Chinese medicine largely relies on animal experiments, but the results cannot be directly applied to humans due to species-specific differences. Additionally, the complexity of traditional Chinese medicines compositions makes it challenging to uncover the exact metabolic process in vivo. Therefore, constructing an in vitro bionic digestive system for the traditional Chinese medicine can provide a more effective approach to discovering their mechanism of action.
Currently, SHIME
® technology is still in the exploratory stage within the realm of Chinese medicine research. Wu and co-workers combined the SHIME
® technology with co-culture techniques involving intestinal flora and endothelial cell to investigate the anti-inflammatory effects of black heather (
Aronia melanocarpa) polyphenols and their modulation of the intestinal microbiota, and they found that the aronia polyphenols modulated the gut microbial composition though inducing beneficial SCFAs production and preventing inflammatory stress of endothelial cells [
66]. Similarly, Liu et al. utilized the SHIME
® model to reveal how orange-peel soup modulates intestinal flora and short-chain fatty acid metabolites, offering insights into its potential clinical application for treating vomiting and eructation [
67]. Yao and co-workers also made a significant discovery using SHIME
®, proving that the classical Chinese medicine preparation (Chaihu Shugan San) can significantly improve the intestinal mucosal barrier function of individuals with liver qi stagnation syndrome, along with potent immunomodulatory effect on intestinal tumors [
68]. This provides new perspectives for exploring the mechanism of traditional Chinese medicine on gut microbial ecosystems, particularly for some chronic diseases (e.g., obesity, diabetes and cardiovascular). Another specific example is Chenpi, a well-known Chinese medicine that has been clinically used for cholesterol reduction and various gastrointestinal symptoms. Maria Falduto et al. employed the SHIME
® model to examine how the microbial community changes during the treatment of two Chenpi extracts, thereby revealing the anti-obesity effects of Chenpi extracts rich in polymethoxyflavonoids and laying the groundwork for future identification of obesity-related biomarkers within the gut microbiota [
69].
4. Future Trends and Challenges of SHIME®
Generally, the SHIME
® systems lack feedback mechanism from the host, making the development of sophisticated host-microbe essential, thus an intriguing tendency for SHIME
® is the integration of continuous dynamic models with additional host cells to enhance its relevant nature to the real gut environment. In fact, studies have conducted to explore the effect of samples from
in vitro model on Caco2 cells and immune cells THP-1 and U937, to study adherence, cytokine production, or gene expression [
70,
71]. In the future, expanding the host cell types (e.g., enterocytes, goblet, and paneth cells) within the SHIME
® system, along with incorporating innovative technologies such as omics (e.g., genomics, metabolomics), will provide deeper insights into gut microbe-host interactions and related metabolic pathways.
Recently, organoids have emerged as a promising 3D culture system, bridging the gap between 2D and in vivo models. These multicellular structures can recapitulate the complex architecture and cellular interactions found in real tissues, overcoming the limitation of reductionist
in vitro models [
72]. Intestinal organoids hence offer a valuable alternative for modelling the gastrointestinal tract and to explore the interactions between the gut microbiota and human epithelium. Organoids are frequently derived from adult stem cells isolated from the small intestine or the colonic epithelium, giving rise to sophisticated constructs known as enteroids or colonoids [
73]. However, challenges remain in effectively combining the organoids with a microbiota, as trapping microbes within organoids has proven difficult. Another related advancement is the organ-on-chip model of the colon, which represented a breakthrough in studying human colonic mucosa. This model can produce a thick mucous layer with a bilayer structure that closely mirrors
in vivo observations, enabling a more precise studies of microbial interaction and real-time responses within the SHIME
® system [
74]. The development of computational ‘‘in silico” models that integrate artificial intelligence and machine learning techniques also represents a future direction for SHIME
®. These technologies, along with digital twin systems, will improve the design and optimization of
in vitro system and provide a smarter and faster evaluation of the SHIME
® models. Moreover, the growing interest in personalized medicine can leverage SHIME
® technology to model individual variations, paving the way for tailored treatments based on unique microbial profiles [
75].
While the SHIME® system has gained dramatic insights into the human gut microbiota, it is crucial to acknowledge the inherent limitation of in vitro simulator. The complexity of the human gut environment is challenging to fully replicate, making it essential to continually refine SHIME® models to better reflect actual gut conditions. Additionally, standardizing methodologies across different studies is also necessary to facilitate comparison and reproducibility, ensuring that SHIME® continues to be a valuable tool in microbiota research.
5. Conclusions
Overall, the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) is an exceptional tool for researchers seeking to delve into the intricacies of gut microbiota and their interference on human health. Its ability to replicate the human gut environment with high accuracy makes it invaluable for studying the intestinal microbial dynamics across the fields of food and nutritional science, pharmacology, and gut ecosystem. Despite the inherent limitations and technical challenges associated within in vitro models, the insight gained from SHIME® into gastrointestinal processes are significant and impactful. As research advances, the integration of SHIME® with advanced technologies, such as omics, organoid and organ-on-chip systems, or potential personalized medicines, promises to enhance its relevance in microbiota research and clinical applications.
Author Contributions
Conceptualization and methodology, W.Z., D.W. and X.F.; manuscript writing, W.Z., D.W. and X.F.; review and editing, X.Z., Q.Y., G.L.M. and X.F.; supervision, X.F. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. U23A20513, X.F.), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (No. 2024C03106, X.F.), Ningbo Top Medical and Health Research Program (No. 2022030309, X.F.), and the Fundamental Research Funds for the Central Universities (226-2024-00001, X.F.; 226-2024-00149, G.-L.M.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474(11), 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998, 42(1), 2–7. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: a proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 2016, 14(8), e1002533. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307(5717), 1915–1920. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54(9), 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535(7610), 85–93. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352(6285), 539–544. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136(1), 65–80. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8(1), 51. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; Chen, Z.S. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7(1), 135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, H.; Lan, C.; Ren, J. Help, hope and hype: ethical considerations of human microbiome research and applications. Protein Cell 2018, 9(5), 404–415. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current sampling methods for gut microbiota: A call for more precise devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489(7415), 220–230. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; Mackie, A.R.; Marzorati, M.; Menard, O.; Minekus, M.; Miralles, B.; Recio, I.; Van den Abbeele, P. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59(10), 1546–1562. [Google Scholar] [CrossRef]
- Biagini, F.; Daddi, C.; Calvigioni, M.; De Maria, C.; Zhang, Y.S.; Ghelardi, E.; Vozzi, G. Designs and methodologies to recreate in vitro human gut microbiota models. Bio-Des. Manuf. 2022, 6(3), 298–318. [Google Scholar] [CrossRef]
- O’Farrell, C.; Stamatopoulos, K.; Simmons, M.; Batchelor, H. In vitro models to evaluate ingestible devices: Present status and current trends. Adv. Drug Deliv. Rev. 2021, 178, 113924. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation in vitro models to study food-induced gut microbiota shift: an updated review. FEMS Microbiol. Lett. 2020, 367(12), fnaa097. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Gonza, I.; Bondue, P.; Douny, C.; Taminiau, B.; Daube, G.; Scippo, M.L.; Delcenserie, V. Human Adult Microbiota in a Static Colon Model: AhR Transcriptional Activity at the Crossroads of Host-Microbe Interaction. Foods 2022, 11(13), 1946. [Google Scholar] [CrossRef]
- Singh, V.; Son, H.; Lee, G.; Lee, S.; Unno, T.; Shin, J.H. Role, relevance, and possibilities of in vitro fermentation models in human dietary, and gut-microbial studies. Biotechnol. Bioeng. 2022, 119(11), 3044–3061. [Google Scholar] [CrossRef] [PubMed]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39(2), 254–258. [Google Scholar] [CrossRef] [PubMed]
- Venema, K.; van den Abbeele, P. Experimental models of the gut microbiome. Best. Pract. Res. Clin. Gastroenterol. 2013, 27(1), 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wiele, T.V. d.; Abbeele, P.V. d.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In The Impact of Food Bioactives on Health: In vitro and ex vivo models; SpringerLink: New York, NY, USA, 2015; pp. 305–317. [Google Scholar]
- McDonald, J.A.; Schroeter, K.; Fuentes, S.; Heikamp-Dejong, I.; Khursigara, C.M.; de Vos, W.M.; Allen-Vercoe, E. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J. Microbiol. Methods 2013, 95(2), 167–174. [Google Scholar] [CrossRef] [PubMed]
- MILLER, T.L.; WOLIN, M.J. Fermentation by the human large intestine microbial community in an in vitro semicontinuous culture system. Appl. Environ. Microbiol. 1981, 400–407. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H.; Macfarlane, S.; Gibson, G.R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989, 67, 521–527. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Smet, I.D.; Verstraete, W. Validation of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) reactor Using microorganism-associated activities. Microb. Ecol. Health Dis. 2009, 7(4), 191–200. [Google Scholar]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: the evolution of culture media in clinical microbiology. New Microbes New Infect. 2020, 34, 100622. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11(8), 2112–2122. [Google Scholar] [CrossRef]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; Van de Wiele, T. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb. Biotechnol. 2011, 5(1), 106–115. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; Zoetendal, E.; Kleerebezem, M.; Smidt, H.; Van de Wiele, T. Microbial community development in a dynamic gut model Is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium Cluster IX. Appl. Environ. Microbiol. 2010, 76(15), 5237–5246. [Google Scholar] [CrossRef] [PubMed]
- Duysburgh, C.; Verstrepen, L.; Broeck, M.V. d.; Righetto, Z.; Perez, M. Investigation of enterogermina’s protective and restorative mechanisms on the gut microbiota with PPI, Using SHIME technology. Nutrients 2023, 15(3), 653. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017, 4(1), 33–46. [Google Scholar] [CrossRef]
- Kaur, H.; Ali, S.A.; Yan, F. Interactions between the gut microbiota-derived functional factors and intestinal epithelial cells - implication in the microbiota-host mutualism. Front. Immunol. 2022, 13, 1006081. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Gonza, I.; Bondue, P.; Druart, G.; Al-Chihab, M.; Boutaleb, S.; Douny, C.; Taminiau, B.; Daube, G.; Scippo, M.-L.; Thonart, P.; Delcenserie, V. Unveiling the influence of a probiotic combination of Heyndrickxia coagulans and Lacticaseibacillus casei on healthy human gut microbiota using the TripleSHIME® system. Microbiol. Res. 2024, 285, 127778. [Google Scholar] [CrossRef]
- Bondue, P.; Lebrun, S.; Taminiau, B.; Everaert, N.; LaPointe, G.; Crevecoeur, S.; Daube, G.; Delcenserie, V. A toddler SHIME® model to study microbiota of young children. FEMS Microbiol. Lett. 2020, 367(16), fnaa135. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ng, A.; Maurice, C.F.; Juncker, D. The Mini Colon Model: a benchtop multi-bioreactor system to investigate the gut microbiome. Gut Microbes 2022, 14(1). [Google Scholar] [CrossRef]
- Vasquez, S.; Angeli, M.A.C.; Polo, A.; Costantini, A.; Petrelli, M.; Avancini, E.; Di Cagno, R.; Gobbetti, M.; Gaiardo, A.; Valt, M.; Lugli, P.; Petti, L. In vitro gastrointestinal gas monitoring with carbon nanotube sensors. Sci. Rep. 2024, 14(1), 825. [Google Scholar] [CrossRef]
- Moon, J.S.; Li, L.; Bang, J.; Han, N.S. Application of in vitro gut fermentation models to food components: A review. Food Sci. Biotechnol. 2016, 25(S1), 1–7. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. In vitro models to study human gut-microbiota interactions: Applications, advances, and limitations. Microbiol. Res. 2023, 270, 127336. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Bhat, S.; Lee, J.C. Potential implications of gut microbiota in drug pharmacokinetics and bioavailability. Pharmacotherapy 2020, 40(7), 704–712. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; Cammarota, G.; Ianiro, G. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15(9), 2211. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.C.; Laurie, I.; Rotsaert, C.; Marzorati, M.; Risso, D.; Karnik, K. Age-Dependent Prebiotic Effects of Soluble Corn Fiber in M-SHIME® Gut Microbial Ecosystems. Plant Foods Human. Nutr. 2023, 78(1), 213–220. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Gonza, I.; Goya-Jorge, E.; Douny, C.; Boutaleb, S.; Taminiau, B.; Daube, G.; Scippo, M.L.; Louis, E.; Delcenserie, V. Food additives impair gut microbiota from healthy individuals and IBD patients in a colonic in vitro fermentation model. Food Res. Int. 2024, 182. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Marzorati, M.; Derde, M.; De Weirdt, R.; Joan, V.; Possemiers, S.; Van de Wiele, T. Arabinoxylans, inulin and Lactobacillus reuteri 1063 repress the adherent-invasive Escherichia coli from mucus in a mucosa-comprising gut model. npj Biofilms Microbiomes 2016, 2(1), 16016. [Google Scholar] [CrossRef]
- Borgonovi, T.F.; Salgaco, M.K.; Oliveira, G.L.V.; Carvalho, L.A.L.; Pinheiro, D.G.; Todorov, S.D.; Sivieri, K.; Casarotti, S.N.; Penna, A.L.B. Functional fermented milk with fruit pulp modulates the In Vitro intestinal microbiota. Foods 2022, 11(24), 4113. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Liang, Z.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res. Int. 2022, 152. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Hao, L.; Du, P.; Li, C.; Han, H.; Xu, H.; Liu, L. The bioavailability of soybean polysaccharides and their metabolites on gut microbiota in the simulator of the human intestinal microbial ecosystem (SHIME). Food Chem. 2021, 362, 130233. [Google Scholar] [CrossRef]
- Shintani, T.; Sasaki, D.; Matsuki, Y.; Kondo, A. In vitro human colon microbiota culture model for drug research. Med. Drug Discov. 2024, 22, 100184. [Google Scholar] [CrossRef]
- Tabare, E.; Dauchot, T.; Cochez, C.; Glonti, T.; Antoine, C.; Laforêt, F.; Pirnay, J.-P.; Delcenserie, V.; Thiry, D.; Goole, J. Eudragit® FS microparticles containing bacteriophages, prepared by spray-drying for oral administration. Pharmaceutics 2023, 15(6), 1602. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Canett-Romero, R.; Prakash, S.; Rascón-Chu, A.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Micard, V. Arabinoxylans-based oral insulin delivery system targeting the colon: Simulation in a human intestinal microbial ecosystem and evaluation in diabetic rats. Pharmaceuticals 2022, 15(9), 1062. [Google Scholar] [CrossRef]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and testing bioavailability of magnesium supplements. Nutrients 2019, 11(7), 1663. [Google Scholar] [CrossRef]
- Grootaert, C.; Van den Abbeele, P.; Marzorati, M.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2009, 69(2), 231–242. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Gao, Y.; Liu, L.; Duan, Y.; Mao, D.; Luo, Y. Colistin and amoxicillin combinatorial exposure alters the human intestinal microbiota and antibiotic resistome in the simulated human intestinal microbiota. Sci. Total Environ. 2021, 750, 141415. [Google Scholar] [CrossRef]
- McCoubrey, L.E.; Seegobin, N.; Sangfuang, N.; Moens, F.; Duyvejonck, H.; Declerck, E.; Dierick, A.; Marzorati, M.; Basit, A.W. The colon targeting efficacies of mesalazine medications and their impacts on the gut microbiome. J. Control. Release 2024, 369, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Cesar, T.; Salgaço, M.K.; Mesa, V.; Sartoratto, A.; Sivieri, K. Exploring the association between citrus nutraceutical eriocitrin and metformin for improving pre-diabetes in a dynamic microbiome model. Pharmaceuticals 2023, 16(5), 650. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Piche, T.; Barbara, G.; Aubert, P.; Bruley des Varannes, S.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; Neunlist, M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2008, 58(2), 196–201. [Google Scholar] [CrossRef]
- Šuligoj, T.; Vigsnæs, L.K.; Abbeele, P.V. d.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of human milk oligosaccharides on the adult gut microbiota and barrier function. Nutrients 2020, 12(9), 2808. [Google Scholar] [CrossRef] [PubMed]
- Salgaço, M.K.; Perina, N.P.; Tomé, T.M.; Mosquera, E.M.B.; Lazarini, T.; Sartoratto, A.; Sivieri, K. Probiotic infant cereal improves children’s gut microbiota: Insights using the Simulator of Human Intestinal Microbial Ecosystem (SHIME®). Food Res. Int. 2021, 143, 110292. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Van den Abbeele, P.; Bubeck, S.; Bayne, T.; Krishnan, K.; Young, A. Treatment with a spore-based probiotic containing five strains of Bacillus induced changes in the metabolic activity and community composition of the gut microbiota in a SHIME® model of the human gastrointestinal system. Food Res. Int. 2021, 149, 110676. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, E.; Van Coillie, E.; Boon, N.; Heyndrickx, M.; Van de Wiele, T. Transfer of antibiotic resistance plasmid from commensal E. coli towards human intestinal microbiota in the M-SHIME: Effect of E. coli dosis, human individual and antibiotic use. Life 2021, 11(3), 192. [Google Scholar] [CrossRef]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; Van Camp, J. Aronia (Aronia melanocarpa) polyphenols modulate the microbial community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and decrease secretion of proinflammatory markers in a Caco-2/endothelial cell coculture model. Mol. Nutr. Food Res. 2018, 62(22), 1800607. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Liu, S.; Kang, X.; Wang, L.; Zhang, Z.; Liu, D. Study on the effect of Jupi decoction on the structure of intestinal flora based on SHIME. Moden Food Sci. Technol. 2021, 37, 7–15. [Google Scholar]
- Liu, L.; Lu, Y.; Xu, C.; Chen, H.; Wang, X.; Wang, Y.; Cai, B.; Li, B.; Verstrepen, L.; Ghyselinck, J.; Marzorati, M.; Yao, Q. The modulation of Chaihu Shugan formula on microbiota composition in the Simulator of the Human Intestinal Microbial Ecosystem technology platform and its Influence on gut barrier and intestinal immunity in Caco-2/THP1-Blue™ cell co-culture model. Front. Pharmacol. 2022, 13, 820543. [Google Scholar] [CrossRef]
- Falduto, M.; Smedile, F.; Zhang, M.; Zheng, T.; Zhu, J.; Huang, Q.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Anti-obesity effects of Chenpi: an artificial gastrointestinal system study. Microb. Biotechnol. 2022, 15(3), 874–885. [Google Scholar] [CrossRef]
- Macedo, M.H.; Dias Neto, M.; Pastrana, L.; Gonçalves, C.; Xavier, M. Recent advances in cell-based in vitro models to recreate human intestinal inflammation. Adv. Sci. 2023, 10(31), 2301391. [Google Scholar] [CrossRef]
- Bahrami, B.; Child, M.W.; Macfarlane, S.; Macfarlane, G.T. Adherence and cytokine induction in Caco-2 cells by bacterial populations from a three-stage continuous-culture model of the large intestine. Appl. Environ. Microbiol. 2011, 77(9), 2934–2942. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21(10), 571–584. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, M.; Helmrath, M.; Dunn, J.C.; Henning, S.J.; Houchen, C.W.; Kuo, C.; Lynch, J.; Li, L.; Magness, S.T.; Martin, M.G.; Wong, M.H.; Yu, J.; Consortium, N.I.H.I.S.C. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302(12), G1359–G1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Sung, J.H. Recent advances in gut- and gut-organ-axis-on-a-chip models. Adv. Healthc. Mater. 2024, 13(21), e2302777. [Google Scholar] [CrossRef] [PubMed]
- Bubnov, R.; Spivak, M. Pathophysiology-Based Individualized Use of Probiotics and Prebiotics for Metabolic Syndrome: Implementing Predictive, Preventive, and Personalized Medical Approach. In Microbiome in 3P Medicine Strategies: The First Exploitation Guide; Boyko, N., Golubnitschaja, O., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 133–196. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).