Background
Traumatic brain injury (TBI) is a wide umbrella of pathology that refers to neurologic injury resulting from an external mechanical force, including hematomas, contusions, and edema [
10]. It is the most common cause of neurologic disability and has a mortality of 30-50% among patients with severe cases [
1]. Though this range of brain injury has been studied extensively, it is often difficult to do so due to the many mechanisms that can cause TBIs and the multiple disease processes that are involved in them.
In TBI patients, biomarkers, electrolytes, intracranial pressures, and imaging are all utilized to measure the initial severity and progress of injury throughout treatment. These findings, in addition to a patient’s presentation, are critical to the risk stratification of such cases and can help identify cases requiring hospital versus ICU level admissions. One such crucial value is Lactate levels (LLs), something that can be easily obtained and quickly processed during an initial TBI evaluation or anytime thereafter. Lactate is a metabolic byproduct of the glycolysis pathway that increases in quantity when the final product of glycolysis, pyruvate, cannot be adequately shuttled into the pathways of aerobic metabolism. Without sufficient oxygen supply for these secondary pathways, pyruvate is converted to lactate by lactate dehydrogenase [
4]. Thus, a high lactate is often associated with hypoxic injury of any kind.
Among TBI patients specifically, high LLs indicate increased hypoxic brain injury. As described above, mitochondrial dysfunction or lack of available oxygen supply to enter secondary metabolic pathways results in lactate buildup. In TBI patients, often the neuronal activity is actually fueled by lactate, causing an increase in its production. Overall, high lactate levels indicate a more severe injury and can often predict worse outcomes in a patient’s care [
12].
In the trauma bay, each TBI is evaluated by the Glasgow Coma Scale (GCS) to risk stratify and identify the need for specific diagnostic testing or treatment. This scale is divided into the three categories of eye, verbal, and motor response, with scores accumulating to a range from 3 to 15. A severe TBI is classified by a GCS score between 3 and 8. Moderate TBIs range from a GCS of 9 to 12, and mild cases have scores of 13-15 [
9].
The Abbreviated Injury Scale (AIS), last updated in 2005, is an anatomically based scoring system that has been thought of as the gold standard for scoring injury severity. This scoring system grades brain injury on a scale of 1 to 6. 1 and 2 are minor and moderate injuries, respectively. A score of 3 indicates a severe, but not life-threatening injury. Scores of 4, 5, and 6 represent potentially life-threatening injuries with likely survival, critical injuries with uncertain survival, and unsurvivable injuries, respectively. This scale considers scalp injury, skull fractures, intracranial injury, hypoxic or ischemic brain damage, and concussive injury in its calculations [
3]. The injury severity score is an expanded version of the AIS, which considers the six body regions of the head, face, chest, abdomen, extremities (including pelvis), and external. Each of these regions is allocated an AIS score, all of which are combined to provide an ISS from 0 to 75 [
6].
An important feature of this study is its completion in a hospital treating a uniquely diverse population. The center used here is a 545 bed, level 1 trauma center, and a major tertiary care provider within a borough of New York City. It serves an extremely ethnically diverse population of approximately one million people. With 130,042 emergency department visits in the previous year, it has one of the busiest emergency rooms among the five boroughs. It has a 12 bed Surgical Trauma ICU that is operated by surgical critical care, neurocritical care, and surgical departments [
7].
There has been intensive research on TBIs and lactate, including the significance of increased lactate after TBI, the use of lactate in neurocritical care in regards to treatment planning, and the use of lactate as a preferred fuel by the brain following injury [
2,
5]. However, we have yet to thoroughly explore the use of LLs in specific outcome prediction. Additionally, the use of AIS as an indicator of TBI severity is unique to this study, offering a more anatomically specific definition of our inclusion criteria. Through the following paper, we explain the significant correlations we found between lactate levels and multiple values in the categories of demographics, trauma mechanisms, severity scores, and outcomes. While we consider a number of metabolites in the treatment and care of our TBI patients, we found it crucial to understand the significance of each lab value. In this paper, as explained above, we focus on the contributions of lactate.
Method
This is a level 1 single-center, retrospective study. Data from patients presenting with severe traumatic brain injuries was collected between January 1, 2020 and December 31, 2023. The Abbreviated Injury Scale was utilized as the basis of our inclusion criteria, defining severe TBI as any brain injury with an AIS value of 3 or above. Among these patients, anyone with a COVID-19 infection at the time of injury and death or discharge within 24 hours of initial injury were excluded from the study. These inclusion and exclusion criteria ultimately resulted in a total patient population of 1,125.
The above data was statically analyzed through both excel and R studio. LLs taken at the time of admission, ICU admission, discharge, ICU discharge, and death were utilized in this analysis. Levels taken at initial presentation in the trauma bay were not utilized in this study, as this data is primarily used for initial risk stratification and is not utilized in treatment plans. Both admission and discharge lactate levels were utilized in single factor ANOVA, two tailed T-tests, and linear regression models to identify the correlation between lactate and demographic variables, mechanism of injury, severity scores, hospital outcome variables, and specific diagnoses.
The single factor ANOVA model was utilized to identify possible correlation between LLs and the demographic variables of age and sex. Lactate levels were the dependent variable in both cases. Age was divided into four categories (<18, 18-45, 46-75, >75) and sex was interpreted in the two categories of male and female. Both age and sex were compared to lactate levels at hospital admission, ICU admission, hospital discharge, ICU discharge, and death. Age was also compared to the same five lactate levels via a linear regression model. LLs were again used as a dependent variable when comparing blunt versus penetrating trauma through the two tailed T-test model. Trauma mechanisms were also compared to LLs at hospital admission, ICU admission, hospital discharge, ICU discharge, and death.
LLs and severity score values were initially compared through the single factor ANOVA model. Here, LLs at hospital admission, ICU admission, hospital discharge, ICU discharge, and death were divided into three categories (0-2, 2-4, >4) and utilized as the independent variable. These were analyzed against average Injury Severity Scores and Glasgow Coma Scales. The same five LLs were also compared to ISS and GCS with a linear regression model. Here, LLs were again used as the independent variable.
Both single factor ANOVA and linear regression models were used to study the hospital outcome variables of hospital length of stay, ICU length of stay, days on ventilator, and mortality. For the ANOVA, lactate levels at hospital admission and ICU admission were divided into three categories (0-2, 2-4, >4) and utilized as the independent variable. For the linear regression model, all patients were initially divided into categories based on the number of injuries they had: one, two, three or multiple. Then, within each category, the numerical range of LLs was used as the independent variable and compared to the same four hospital outcome variables.
Each patient in our study had associated ICD codes based on their diagnoses. The various ICD codes were then divided into the following six broad categories: subdural hematoma, subarachnoid hematoma, epidural hematoma, intraparenchymal hemorrhage, concussion, and other. Both concussion and other categories did not have sufficient data to analyze. Within each of the other four injury classification categories, LLs were again used as the independent variable and compared to hospital length of stay, ICU length of stay, days on ventilator, and mortality. Hospital admission and ICU admission LLs were used for the first three outcome variables, and hospital admission, ICU admission, and ICU discharge LLs were used for the analysis of mortality.
Results
For demographic data both age and sex had some statistically significant correlations with LLs (
Table 1). The single-factor ANOVA indicated a significant decrease in LLs with increasing age, at hospital admission (p = 1.13E-16), ICU admission (p = 0.01), hospital discharge (p = 0.02), and death (p = 0.03). It did not show a significant correlation of the same at ICU discharge (p = 0.50). Linear regression also showed a significant decrease in LLs with increasing age at hospital admission (p = 2.65E-09), ICU admission (p = 0.01), and death (p = 0.002). The same correlations were not statistically significant at hospital discharge (p = 0.07) or ICU discharge (p = 0.54). The two tailed T-test indicated a significant increase in LLs among male patients at hospital admission (p = 2.99E-05) and hospital discharge (p = 0.01). The same was not significant at ICU admission (p = 0.06), ICU discharge (p = 0.6), or death (p = 0.44).
In regards to trauma mechanism, the two tailed T-test did not show significant correlation between LLs and blunt versus penetrating trauma (
Table 1). LLs in penetrating trauma were insignificantly higher than blunt trauma at hospital admission (p = 0.46), ICU admission (p = 2.92), and death (p = 0.46). They were lower than blunt trauma at hospital discharge (p = 0.12) and ICU discharge (p = 0.25).
Single-factor ANOVA indicated significant correlations between LL ranges and ISS and GCS (
Table 2). ISS increased significantly with increasing LLs at hospital admission (p = 5.22E-06), ICU admission (p = 1.92E-05), and hospital discharge (p = 4.62E-04). The same correlation was not significant at ICU discharge (p = 0.19) and death (p = 0.08). Similarly, GCS scores increased significantly with increasing LLs at hospital admission (p = 3.42E-08), ICU admission (p = 1.73E-09), and ICU discharge (p = 5.91E-07). The same correlation was not significant at hospital discharge (p = 0.44) or death (p = 0.17).
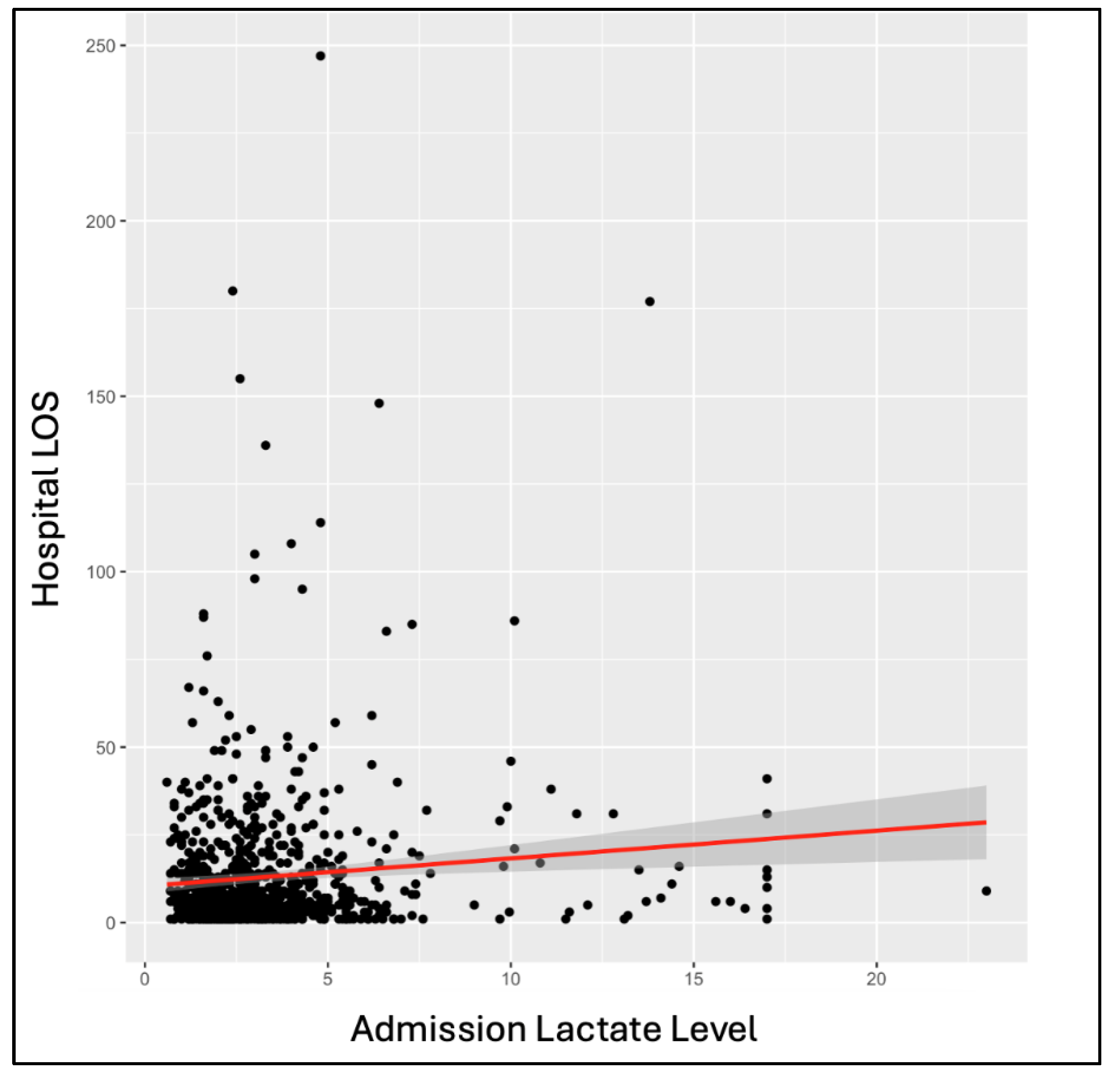
Among the overall patient population, outcome variables showed significant correlations with LLs. Single-factor ANOVA showed a significant increase in hospital length of stay with increasing hospital admission (p = 0.008) and ICU admission (p = 1.79E-04) LLs. The same significant correlation was seen through linear regression at hospital admission (p = 0.003) and ICU admission (p = 0.02) (
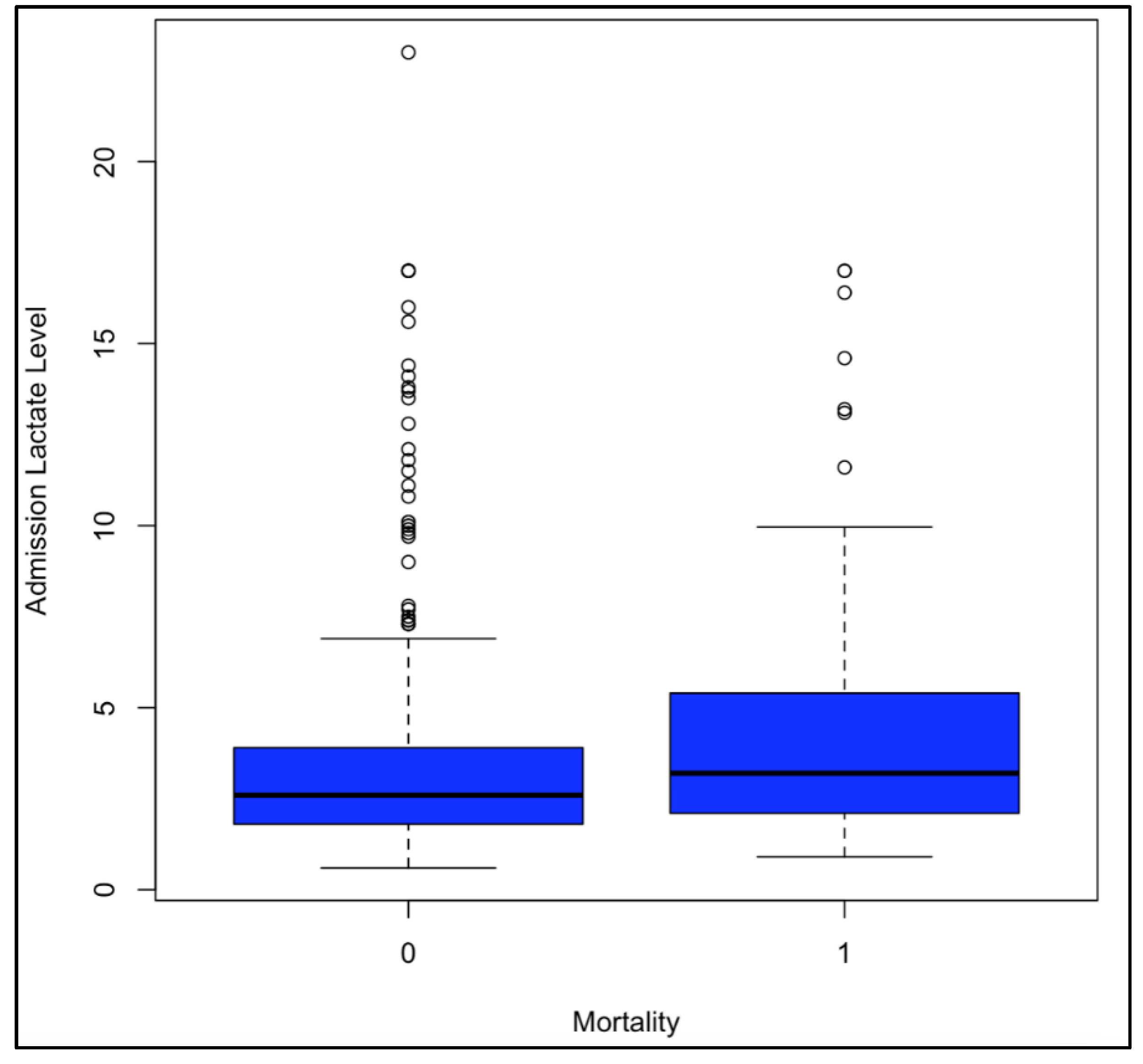
Figure 2). Single-factor ANOVA indicated a significant increase in ICU length of stay with increased LLs at hospital admission (p = 0.046) and ICU admission (p = 3.25E-04). The same was seen through linear regression with p-values of 0.01 at hospital admission and 0.02 at ICU admission. Single-factor ANOVA showed a significant increase in days on a ventilator with increased LLs at hospital admission (p = 0.007) and ICU admission (p = 4.06E-04). The same was seen with linear regression, calculating p-values of 0.002 at hospital admission and 8.23E-05 at ICU admission. Single-factor ANOVA only showed significantly increased likelihood of mortality with increased LLs at ICU admission (p = 0.008), not hospital admission (p = 0.07). Linear regressions indicated an increased likelihood of mortality with increased LLs at hospital admission (p = 8.13E-05), ICU admission (p = 3.93E-5), and ICU discharge (p = 2.37E-08) (
Figure 3).
Linear regression models were also utilized to compare change in lactate over the course of admission to the above mentioned four outcome variables. First off, the change in LLs from admission to ICU admission were used as the independent variable against hospital length of stay, ICU length of stay, days on ventilator, and mortality. This analysis did not show any significant relationship. Then, the change in LLs from ICU admission to ICU discharge was utilized as an independent variable against the same four outcomes. Here we saw an increased hospital length of stay (p = 3.04E-04), ICU length of stay (5.72E-04), and number of days on a ventilator (p = 5.00E-06) with decreased change in LLs. The change in lactate admissions over ICU stay did not significantly affect mortality (
Table 3).
Analyzing the same four outcome values within groups of patients based on number of diagnoses also produced some significant data points (
Table 3). Among patients with only one severe TBI diagnosis, linear regressions indicated that both ICU length of stay (p = 0.01) and days on ventilator (p = 0.048) were significantly increased with higher lactate levels at hospital admission. In the same group, analysis showed a significantly increased likelihood of mortality with higher LLs at hospital admission (p = 0.018), ICU admission (p = 0.001), and ICU discharge (7.92E-04). Within the group of patients who had two diagnoses, hospital length of stay (p = 2.80E-05), ICU length of stay (p = 0.001), and days on the ventilator (p = 0.002) all increased significantly with higher ICU admission lactate levels (p = 2.80E-05). Among the same patients, likelihood of mortality was significantly higher among patients who had higher LLs at hospital admission (p = 0.04) and ICU discharge (p = 2.01E-04).
Among patients with three TBI diagnoses, linear regression indicated a significant increase in days on ventilator with higher LLs at ICU admission (p = 0.046) and increased likelihood of mortality with higher LLs on ICU discharge (0.018) (
Table 3). Within the group of patients with more than 3 diagnoses, the likelihood of mortality was significantly increased with higher LLs at hospital admission (p = 0.008), ICU admission (p = 0.046), and ICU discharge (p = 0.007) (
Table 4).
The same linear regression analysis completed for data grouped by specific diagnosis also showed some important results. Among patients with a single subdural hematoma, ICU length of stay (p = 0.02) and days on the ventilator (p = 0.045) were both significantly increased with higher lactate levels at hospital admission. In this group, the likelihood of mortality was significantly higher with higher LLs at ICU admission (p = 0.02) and ICU discharge (p = 0.03). In patients with a single subarachnoid hematoma, the likelihood of mortality was significantly increase with higher LLs at ICU admission (p = 0.049). There were no significant findings through linear regression when comparing the same outcome values with LLs in patients with a single epidural hematoma. Among patients with a single intraparenchymal hemorrhage, hospital length of stay was significantly higher with increased LLs at hospital admission (p = 0.03) and ICU admission (p = 0.01). In the same group, ICU length of stay was also significantly longer with higher lactate levels at hospital admission (p = 0.01) and ICU admission (p = 0.03). Days on the ventilator were significantly increased with higher LLs at hospital admission (p = 3.25E-11). Additionally, in this group, the likelihood of mortality was significantly higher with increasing LLs at hospital admission (p = 0.04) and ICU discharge (p = 2.66E-04).
Discussion
Lactate is a commonly used and essential biomarker in the risk stratification and following treatment of TBIs, especially in severe cases. The significance of an elevated lactate level in this patient population has long been understood and documented [
2]. As such, many resources have already been dedicated to further understanding the significance of lactate in the overall treatment of TBIs. In this field of research, much attention has been dedicated to the formation and use of lactate as a possible source of energy after brain injury on a biochemical level [
5]. Those studies that do focus on the use of LLs in outcome prediction either look at a different variety of outcome variables among all TBI patients or categorize TBI severity using GCS scores [
8,
11].
Though the GCS score is a widely used scale for defining severe trauma diagnoses like TBIs, the AIS score used in our analysis offers a more specific, anatomically based approach by looking at identifiers of injury as opposed to symptomatic presentations seen secondary to a variety of traumatic injury. Especially because it is not commonplace to define the severity of TBIs in this way, our work offers a unique and possibly more accurate analysis of outcome variables in TBI patients. Additionally, it allows us to consider utilizing the AIS score more often when evaluating patients in the trauma bay [
6].
The results we described above have a variety of significant findings indicating multiple important learning points from our study. Our analysis of demographic data showed an inverse correlation between age and LLs, with LLs decreasing with increasing age. Though it is not exactly clear why this is the case, it is possible that the atrophy of the brain and its decreased mass at older ages means that there are fewer cells to undergo metabolic pathways and thus fewer opportunities to build up the byproducts of these pathways [
13]. Interestingly, LLs were shown to be higher in men at their hospital admission and hospital discharge. The reasoning behind this is again unclear, but may have to do with brain mass or metabolism rates.
Among the many analyses described above, we would like to highlight those identifying the possible effects of LLs on severity scores and outcome variables. In our data, the analysis comparing LLs at hospital admission, ICU admission, and ICU discharge against ISS and GCS scores indicated a significant correlation between LLs and these two severity scores. Not only does this show that LLs during these specific time markers in a patient’s admission can be used as predictors of the severity of injury, but it also confirms the validity of both ISS and GCS scoring systems as both have a similar response. Though we were purposeful in using the abbreviated ISS in our categorization of TBI severity in hopes to be more accurate, it is clear the there is a strong foundation behind the widespread use of GCS to achieve the same.
Similar to this, our analysis of hospital admission and ICU admission LLs against hospital length of stay, ICU length of stay, days on the ventilator, and morality was crucial to this study. Here we saw that higher LLs at hospital and ICU admission caused longer hospital admissions, longer ICU admissions, and more days of mechanical ventilation. These findings are bolstered by the similar results via single-factor ANOVA and linear regressions. Mortality was found to be more likely with higher LLs at ICU admission through single-factor ANOVA. However, the correlation was seen between worse mortality and higher LLs at hospital admission, ICU admission, and ICU discharge through linear regression.
While the above analysis shows that lactate levels, especially at hospital and ICU admission can indicate worse overall outcomes, the change in LLs over ICU admission is also very important. As mentioned above, we found that a decreased change in lactate over the course of a patient’s ICU admission would indicate longer hospital admissions, ICU admissions, and ventilator courses. It is certainly important that we can use LLs taken on admission to predict hospital outcomes, but this analysis shows the importance of trending LLs throughout a patient’s course. It has long been known that higher LLs often indicate increased severity of brain injury, based on the idea that severe TBI’s will have more hypoxic brain injury than other, less critical cases. However, our work through this project has allowed us to clearly indicate the role of these lactate levels in predicting tangible outcomes that are often discussed with patients and their families.
It is important to note that the analysis of LLs and outcome variables was most fruitful when looking at the overall data base. Dividing our patients into groups based on specific diagnoses or number of diagnoses did not provide any clear indications that lactate levels could be more useful to predict outcomes in any specific group of patients. Similar to the analysis of the overall population, the outcome variables of hospital length of stay, ICU length of stay, days on ventilator, and mortality were generally worse off with higher LLs among patients with one, two, three, or more than three diagnoses. Among each of these subsets, LLs at different time points in the hospital course seemed to have a stronger effect. Patients with one diagnosis seemed to have more of a dependance on the LLs at initial hospital admission, those with two, three, or more than diagnoses had a stronger correlation with LLs at ICU admission. The variation in this could have been secondary to the small number of patients within each category, or the pattern could be due to the fact that those with more injuries are more likely to have a significant course in the ICU.
Similar to these categories, data within groups dedicated to the specific diagnoses of subdural hematoma, subarachnoid hematoma, epidural hematoma, intraparenchymal hemorrhage varied, but mostly showed worse outcomes with higher LLs. There was no clear pattern among the different diagnoses, again possibly due to the limited number of data points within each diagnostic group.
A vital aspect to our study’s importance is the setting in which our data has been collected. As we highlighted earlier, the center used in this study serves an ethnically diverse and economically disadvantaged patient population. Many times, this population is overlooked in research due to issues with funding and patient outreach. Not only is it important to focus our work on this population and further explore its needs, studying a group of patients with this level of diversity confirms that our findings are not confounded by the utilization of one specific demographic.
Strengths
As we have highlighted above, we believe the strengths of this study lie in our unique utilization of the AIS score to define TBI severity. This study also looks at a large variety of data points, analyzed in various combinations. The simultaneous use of single-factor ANOVA and linear regression analysis has allowed us to bolster the multiple significant findings we have described above. Additionally, the use of this analysis both in the overall population and within specific categories based on type of diagnosis or number of diagnoses identifies that lactate levels are very useful in predicting outcomes, but must be utilized within the correct context.
Limitations
As a single-center study with 1,125 patients, it is important to note that the size of our study causes an important limitation on our findings. While the above findings are significant, they have not been confirmed over a wide base of hospitals and patients. In the future, we aim to expand our data collection to more sites across the country. Including both academic and community centers with varying settings in urban, suburban, and rural areas.
Additionally, this data was collected through the COVID-19 pandemic. Though patients with COVID-19 infections were excluded from the study, it is important to note that limitations in resources caused significant changes to patient care during this time. In many hospitals within epicenters of the pandemic, like Elmhurst, it was challenging to manage severely increase volumes of patients. While lab results and trauma evaluations were conducted by protocol, it is possible that treatment modules had to change to accommodate for limited resources. We hope to expand data collection in order to include enough data that falls outside of the critical months of the pandemic.
Future Directions
As discussed above, this study has helped us clarify the use of LLs in predicting outcomes for patients with severe TBIs. In the future, we hope to bolster the significant findings established through our work and expand upon the existing dataset. First off, it will be crucial to continue collecting data from patients with TBIs. While we will certainly continue data collection at the initial center, we also hope to expand this to multiple centers around the country. Involving multiple centers will not only increase the amount of data available for analysis, but also involve a variety of patient populations and hospital settings. This will inevitably create variation in TBI injury mechanisms and TBI risk stratification and treatment protocols. This will strengthen our current findings and make our data more applicable to the general public.
Additionally, it will be helpful to expand upon the specific points we have chosen to analyze. To decrease any possible confounding factors, we could consider standardizing exact timings that LLs are collected on hospital or ICU admission. In order to better understand the change in lactate, we may consider collecting LLs at more time points in patients’ hospital courses. This would allow us to further clarify the importance of acting upon a patients lactate at certain parts of a hospital course. Collecting lactate after the first 24 or 48 hours for example, may help identify specific periods of time during which a significant change in a LL can impact overall outcome more.
Due to the prevalence of ICU admissions for patients with severe TBIs, it may be beneficial to compare data between LLs during floor or step-down courses against those during ICU admissions. As we note above, higher LLs are often associated with more severe TBI cases. Because of this, it is very possible that TBIs that result in long ICU courses may be impacted by LLs differently than those cases that are stable enough to spend longer courses on non-ICU units.
This study is part of a larger effort to understand the role of specific metabolites in the risk stratification, treatment, and recovery associated with severe TBI injuries. After we have expanded upon our understanding of LLs in this space, we will also look at other metabolites and important lab values. In the long run, it may also be helpful to combine the analysis of LLs with that of other important values like pH on blood gas.
Conclusions
While there is more work to be done, it is certainly clear through our study that lactate levels have a significant effect, not only on initial risk stratification but also on multiple complications throughout treatment. For many years, we have been using whole number scoring systems in the trauma bay for patients with TBIs. While this is effective in providing fast risk stratification in an emergency setting, it may be beneficial to expand our approach to include metabolites like lactate. This will help to provide a more accurate understanding of the severity of our patients’ injuries and possible complications that they can cause down the line.
Author Contributions
Conceptualization- BS; writing—original draft preparation- YD; writing—review and editing- BS, KT, NDB, GA, ZS, and JW; figures and table, WJ; supervision- BS; project administration- BS
Funding
There is no grant support or financial relationship for this manuscript.
Institutional Review Board (IRB) Statement
This retrospective study was approved by the IRB at Elmhurst Facility on July 5, 2024, with IRB number 24-12-092-05G
Acknowledgment
Not Applicable
Conflicts of Interest
The authors have no competing interests to declare.
References
- Banoei, M.M., Lee, C.H., Hutchison, J. et al. Using metabolomics to predict severe traumatic brain injury outcome (GOSE) at 3 and 12 months. Crit Care 27, 295 (2023). [CrossRef]
- Carpenter KL, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci. 2015 Apr 8;9:112. https://doi.org/10.3389/fnins.2015.00112. PMID: 25904838; PMCID: PMC4389375.
- Carroll CP, Cochran JA, Price JP, Guse CE, Wang MC. The AIS-2005 Revision in Severe Traumatic Brain Injury: Mission Accomplished or Problems for Future Research? Ann Adv Automot Med. 2010;54:233-8. PMCID: PMC3242550. [PubMed]
- Chaudhry R, Varacallo M. Biochemistry, Glycolysis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482303/.
- Dienel, GA. Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab. 2014 Nov;34(11):1736-48. https://doi.org/10.1038/jcbfm.2014.153. Epub 2014 Sep 10. PMID: 25204393; PMCID: PMC4269761.
- Eleni, Th. Petridou, Constantine N. Antonopoulos, Injury Epidemiology, Editor(s): Stella R. Quah, International Encyclopedia of Public Health (Second Edition), Academic Press, 2017, Pages 258-274, ISBN 9780128037089. [CrossRef]
- ELMHURST HOSPITAL CENTER. (n.d.). https://www.nyc.gov/html/hhc/qhn/text_only/ehc.html.
- Hu F, Zhu J, Zhang S, Wang C, Zhang L, Zhou H, Shi H. A predictive model for the risk of sepsis within 30 days of admission in patients with traumatic brain injury in the intensive care unit: a retrospective analysis based on MIMIC-IV database. Eur J Med Res. 2023 Aug 18;28(1):290. https://doi.org/10.1186/s40001-023-01255-8. PMID: 37596695; PMCID: PMC10436454.
- Jain S, Iverson LM. Glasgow Coma Scale. [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513298/.
- Lefevre-Dognin C, Cogné M, Perdrieau V, Granger A, Heslot C, Azouvi P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie. 2021 May;67(3):218-221. https://doi.org/10.1016/j.neuchi.2020.02.002. Epub 2020 May 6. PMID: 32387427.
- Martin-Rodriguez F, Sanz-Garcia A, Lopez-Izquierdo R, Delgado Benito JF, Martínez Fernández FT, Otero de la Torre S, Del Pozo Vegas C. Prehospital Lactate Levels Obtained in the Ambulance and Prediction of 2-Day In-Hospital Mortality in Patients With Traumatic Brain Injury. Neurology. 2024 Aug 27;103(4):e209692. https://doi.org/10.1212/WNL.0000000000209692. Epub 2024 Aug 1. PMID: 39088773.
- Stefani MA, Modkovski R, Hansel G, Zimmer ER, Kopczynski A, Muller AP, Strogulski NR, Rodolphi MS, Carteri RK, Schmidt AP, Oses JP, Smith DH, Portela LV. Elevated glutamate and lactate predict brain death after severe head trauma. Ann Clin Transl Neurol. 2017 May 4;4(6):392-402. https://doi.org/10.1002/acn3.416. PMID: 28589166; PMCID: PMC5454398.
- Tzankoff, S.P. , Norris, A.H. Age-related differences in lactate distribution kinetics following maximal exercise. Europ. J. Appl. Physiol. 1979, 42, 35–40. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).