1. Introduction
Dental implants have become a reliable alternative solution for replacing either some or all missing teeth [
1].
In recent decades, the survival rate of dental implants has notably increased. Most studies have reported survival rates between 90% and 95% in the first 10 years and between 51.97 and 75.8% between 16 and 20 years [
2]. Despite these findings, numerous factors can affect implantation success, from patient-related aspects to surgical and prosthetic protocols [
1]. An implantation is considered successful when there is no pain, infection or mobility at the site of the implant or radiolucency around it. If the implant must be removed due to any of these factors, it is considered a failure [
3].
Time-wise, failure can be classified as early or late depending on whether it occurs before loading or after loading. The rate of early failure is estimated at 5% and has been related to local and systemic factors, as well as surgical factors that lead to osseointegration failure. Late failure (accounting for approximately 95% of cases) is related to the presence of bacteria, the mechanical factors of the prosthesis and patient habits [
1].
Dental implant placement requires an incision in the gums, except in those cases where a flapless intervention is performed [
4].
When an incision has been made, after surgery, the wound margins are brought together and sutured, initiating the healing process. This process has been described as a two-stage sequence in which homeostasis and immune mechanisms are interconnected. Classically, healing is divided into 3 periods: inflammation, proliferation and maturation [
5]. Some authors additionally distinguish coagulation from the inflammatory phase, thus dividing healing into 4 periods (coagulation, inflammation, proliferation and maturation) [
6].
These phases overlap and involve a number of synchronized processes, as outlined below:
-
Inflammatory phase: 3–5 days
-
Immune response: 6 h to 3 or 5 days [Vasodilation]
-
Proliferative phase: Day 3 up to 3 weeks: (fibroblasts, keratinocytes and endothelium)
Angiogenesis
Regeneration
Shrinkage
Epithelialization
Maturation phase: 6-week (can last up to 2 years) substitution of type III to type I cross-linked collagen.
This cascade of events can be affected by soft tissue dehiscence, jeopardizing healing. For this reason, it is very important for the surgeon to manage the flap appropriately and select the ideal type of suture to provide the best stability to the flap without excessive tension [
5].
As mentioned above, various factors can affect the healing process, including patient factors (age, nutritional status), local factors (scarcity of tissues, wound movement, early infection), interventional factors (extension, duration, tension, delicateness) and acquired factors (medication, habits) [
5].
The early detection, and, if possible, early correction of any deviations in tissue healing and, could increase the rate of dental implant success [
7].
To monitor healing, different types of indices have been developed that can help detect these initial changes. However, there are no clear indications for their use, and their correlation with the degree of well-being of the patient have not been clarified [
7].
The objective of this study was to assess whether there is a correlation between the Wachtel healing index and early dental implant success or failure at the time of loading.
As secondary objectives, we assessed whether there is any relationship between other variables studied and early success or failure; these variables include:
2. Materials and Methods
2.1. Patients
Patients who had undergone tooth extraction (ideally with teeth remaining on either side of the extraction) and required the placement of an implant for tooth restoration were selected. All patients were treated at the University Dental Clinic. All of them were informed of the purpose of the study, both orally and in writing. Specifically, the patients were informed that participation was voluntary and that they were able to withdraw from the study at any time without the need to provide any explanation. All data were anonymized. The study was authorized by the ethics committee (ID: 3544/2021) and was conducted in accordance with the Helsinki guidelines of 1964 and their periodic updates.
The sample was balanced for sex, and 25 men and 25 women were recruited.
A total of 50 patients who received 78 implants were selected. For patients who received more than one implant, the first implant to be placed was selected for follow-up, and the remaining implants were ignored. A value of p <0.05 indicated statistical significance.
2.1.1. Inclusion Criteria
Age above 18 years
Agreement to participate in the study
Loss of at least one tooth
Teeth adjacent to the edentulous area
A smoking history of fewer than 10 cigarettes a day (including no smoking history)
No known history of illegal substance abuse
Plaque index <2
2.1.2. Exclusion Criteria
Pregnancy
Lactation
Inability to comply with the study needs
Administration of analgesics or antibiotics before surgery (at lest 2 weeks prior)
Treatment with anticoagulants
Treatment with corticosteroids
Poorly controlled diabetes (Hb1Ac> 7%)
Immunosuppression
Refusal to continue with the study
2.2. Technique
The surgical technique was performed by submerging the implants and performing primary closure of the wound. This is known as a two-stage technique, as it requires a second stage to expose the implants to the oral cavity. This technique allows improvement in healing by isolating the wound from bacteria in the oral cavity [
3].
All patients received a postoperative drug regimen consisting of 500 mg of amoxicillin every 8 hours for 5 days and 650 mg of paracetamol every 8 hours for the first 24 hours. For patients with allergies to these drugs, 500 mg azithromycin was used every 24 hours for 5 days, and/or 600 mg ibuprofen was used every 8 hours during the first 24 hours. In the case of persistent pain, patients were allowed to continue to take analgesics as necessary and asked to report it to the clinic.
2.3. Implants
Commercially pure titanium implants were used, with a surface blasted with absorbable particles (RBM), an altered platform and an internal conical connection (Ticare®, Mozo Grau, Valladolid, Spain). The implants were placed following the manufacturer’s indications in terms of both the preparation of the bed and the final position of the implant in the alveolus. The implants have been approved by the Spanish medicine agency and bear the CE marking. The manufacturer is under official directive 93/42/EEC and ISO accreditation 13485.2016.
2.4. Pain Scale
A VAS was used to evaluate the pain perceived by the patients one week after implantation. This scale is depicted as a 100 mm line on which the total absence of pain corresponds to one end and the maximum pain that the patient could perceive corresponds to the other.
Pain was evaluated by measuring the distance from the mark made by the patient on the pain line from the end of the line.
For convenience, the pain scale score was separated into three categories. A measurement of less than 30 mm was considered discomfort, a measurement of 40–60 mm was considered moderate pain, and a measurement greater than 70 mm was considered intense pain.
2.5. Evaluation Criteria
The patients were contacted 24 hours after the intervention by telephone, then returned one week later for stitch removal and every month until 2 months for general assessment of the healing process.
2.6. Final Appointment
At 2 months and 1 week after surgery, the patients were evaluated to assess the implants and the final result in terms of success or failure according to the Albrektson criteria [
9].
2.7. Methods
Once signed consent and radiographic studies were carried out, the implants were placed according to the manufacturer’s instructions. The alveoli were milled at 800 rpm and a torque of less than 20 N/C*m. The drilling sequence began with a marking drill (1.8 mm), a pilot drill (2.2 mm) and a series of drills until the size corresponding to the selected implant was reached.
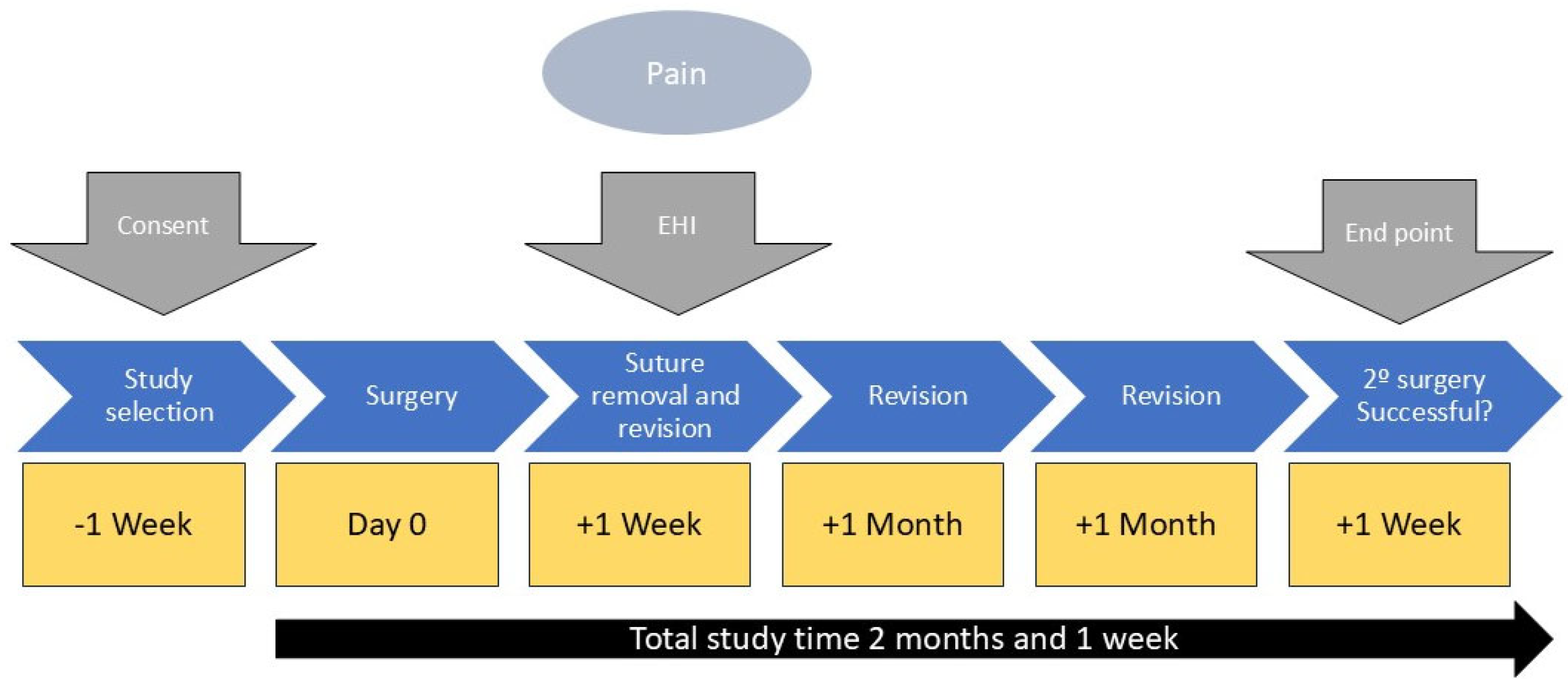
2.7.1. Data Recording
The study data regarding pain and healing were recorded one week after surgery. The data implant success or failure were evaluated at 2 months and 1 week during implant assessment. The study protocol is shown in
Figure 1.
2.8. Approval by the Ethics Committee of the University of Murcia
The research protocol and study design were approved by the Ethics Committee of the University of Murcia (ID: 3544/2021). This study was conducted under the World Medical Association Declaration of Helsinki adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964 and amended by the 52nd General Assembly, Edinburgh, Scotland, October 2000, current data protection law. The study was conducted following the guidelines established for observational studies (STROBE guide) available through the EQUATOR network.
2.9. Statistical Analyses
Statistical tests were performed for the quantitative and qualitative variables. Measurement data were evaluated between groups by Student’s t test. Qualitative variables were evaluated between groups with the chi-square test or Fisher’s exact test when the number of cells was less than 5. Differences between groups were identified using one-way ANOVA tests (p< 0.05). Normality tests were performed via the Kolmogorov‒Smirnov method since the sample size reached 50 implants. P values less than 0.05 were considered to indicate statistical significance.
2.10. P Statistical Arameters
The following parameters were used:
Number of tails = 2
Significance level = 95% (α = 0.05).
Effect size (d) = 10 mm VAS
Variance = 1.77 (previous study DOI: 10.1563/aaid-joi-D-17-00185)
Calculated β value = 0.10
Obtained power = 1—β = 1—0.10 = 0.90.
Sample size (n) = 37
Sample size adjusted for a potential 15% dropout = 44
Final size included in the study = 50 patients
2.11. Index
The soft tissues were evaluated with the Wachtel index, an early healing index (EHI) developed in 2003 with the aim of evaluating the condition of the wound one and two weeks after surgery. An EHI of 0-2 indicates complete flap closure, whereas a value of 3-4 indicates incomplete closure [
3,
4] (
Table 1) [
5,
10,
11].
3. Results
Given the low presence of severe pain, patients with moderate or severe pain (VAS mark 40 mm or greater) were grouped as having pain (40 mm or greater), whereas patients with VAS marks less than 40 mm were considered to have discomfort.
3.1. Sample Description
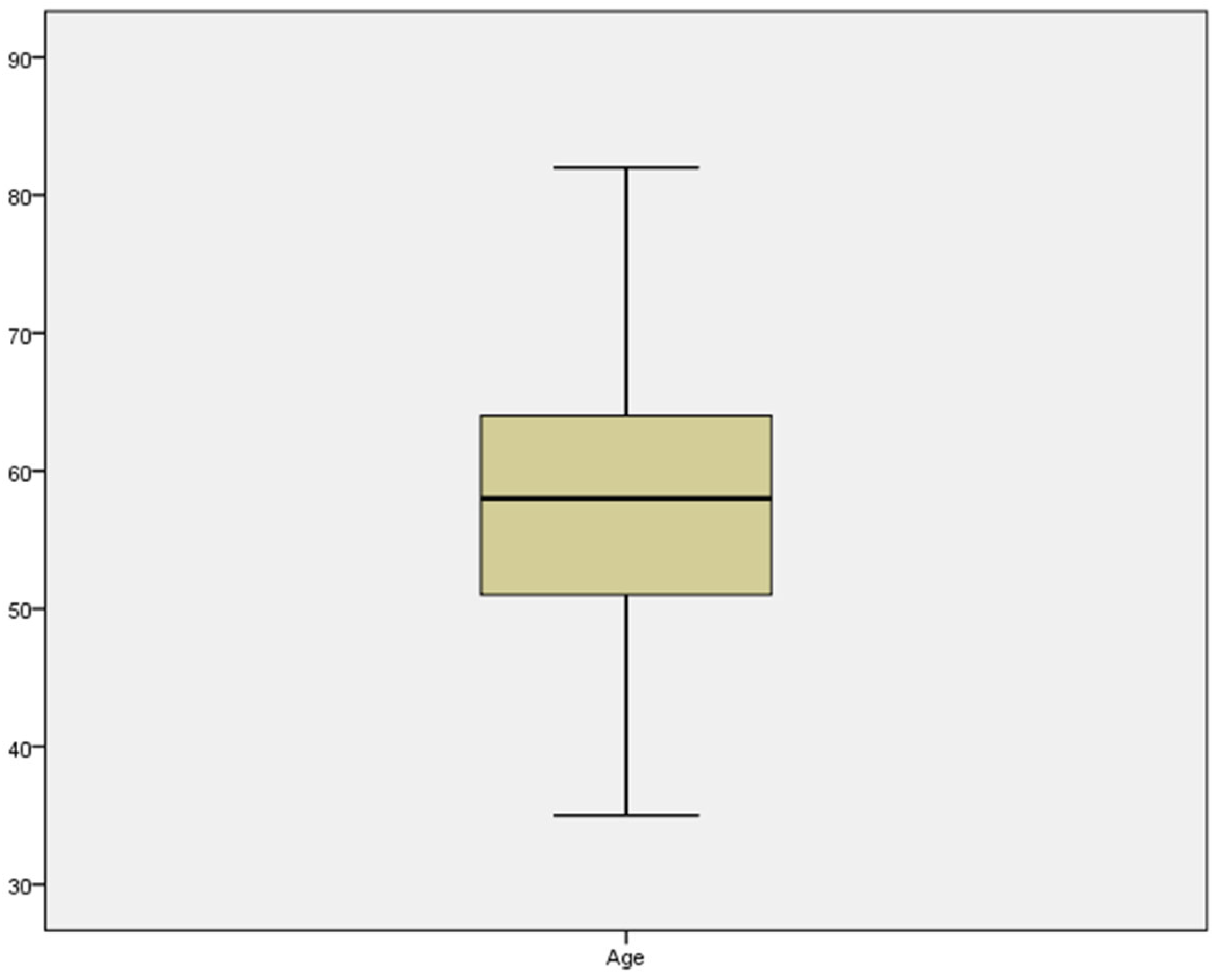
A total of 50 patients met the inclusion criteria; among them, 25 were men, and 25 were women. The mean age of the entire sample was 57.76 years (55.00-60.52) (
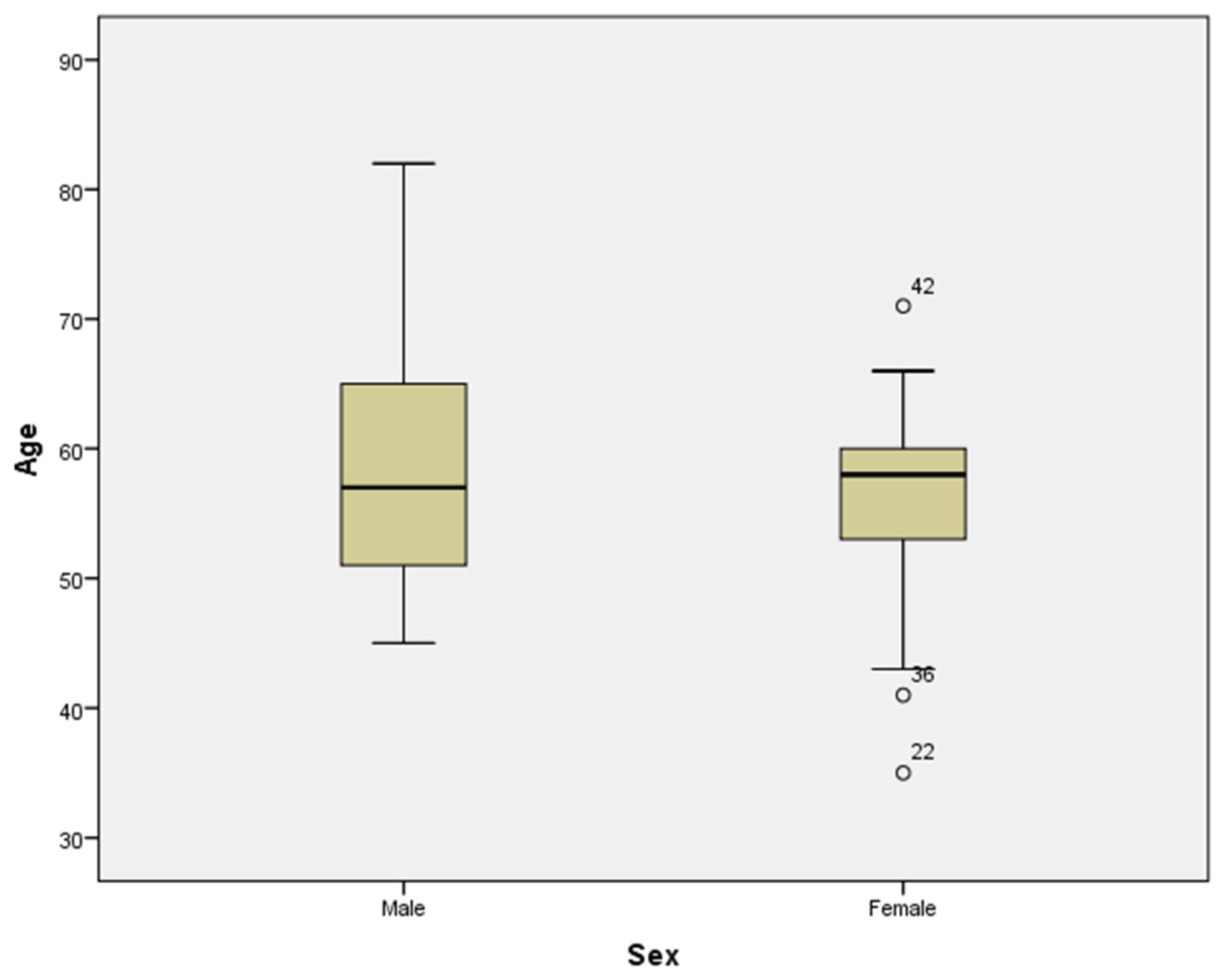
Figure 2). The mean age for men was 59.20 years (54.73-63.67), whereas for women, it was 56.32 years (52.86–59.78). The difference was not statistically significant (Student’s t test) (
Figure 3).
Among these 50 patients, 4 had well-controlled diabetes (glycosylated haemoglobin <7%), and 5 had other systemic diseases. Furthermore, 10 patients were smokers.
Forty-six patients reported discomfort 24 hours after surgery, whereas 4 patients reported moderate to severe pain. In terms of inflammation, 9 patients experienced inflammation according to their telephone follow-up.
A total of 10 patients required GBR via autologous bone obtained during the alveolus drilling.
Of the 50 implants placed, 4 failed prior to placement of the prosthesis; in other words, the early success rate was 92%.
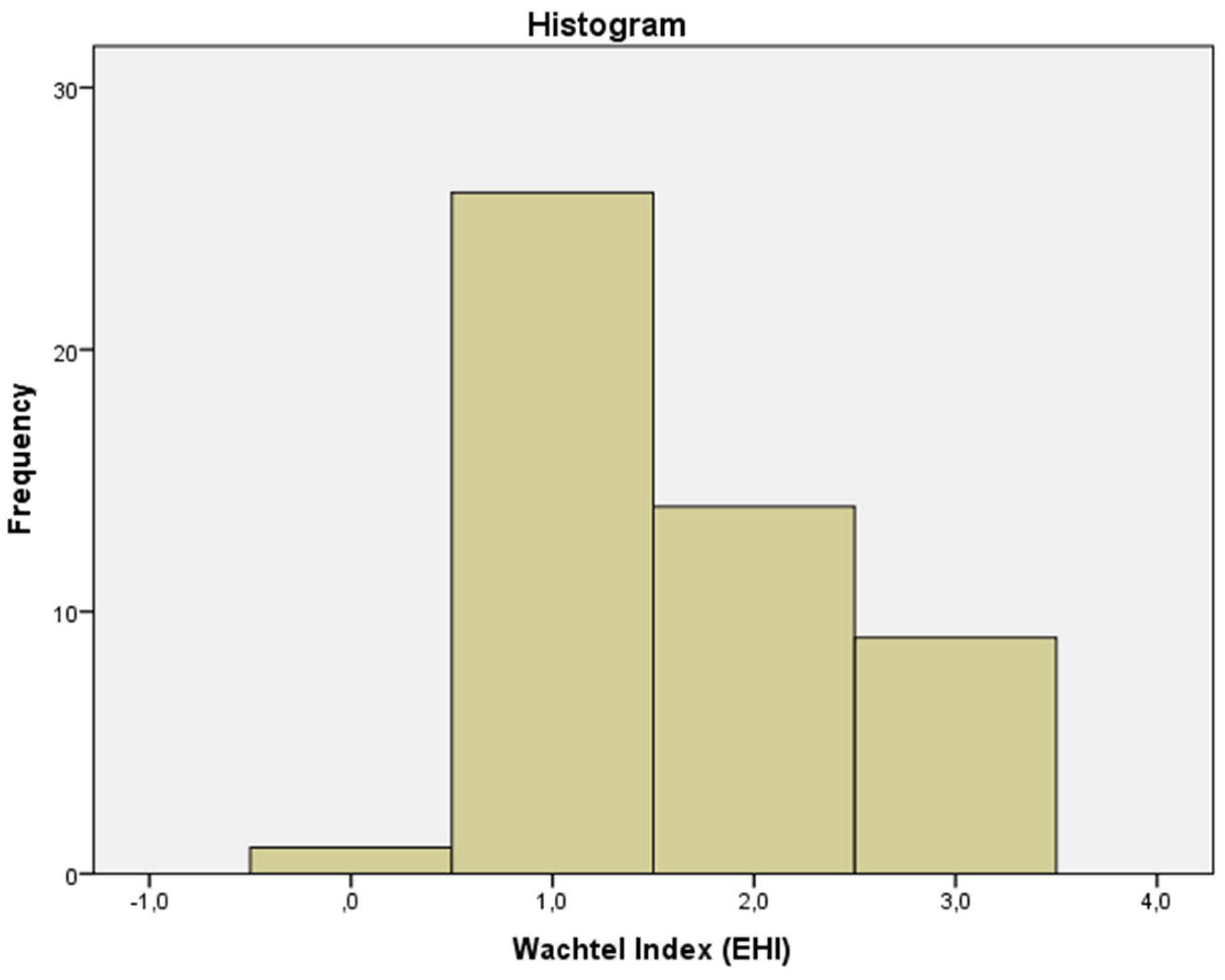
The mean Wachtel healing index was 1.5 (most patients had a fine line between the edges of the incision with a small amount of fibrin interposed) (
Figure 4 and
Figure 5).
No qualitative variable was significantly associated with implant success.
According to ANOVA, no statistically significant differences were found between short-term implant success and the healing variables recorded.
4. Discussion
Our study sought to predict early implant success with variables frequently associated with this outcome.
In our study, most of our patients had good healing (mean Wachtel index 1.5). Notably, no patient had a score greater than 3 (incomplete closure), indicating that the surgery was performed without serious incidents. Perhaps because of this, we did not find a significant association between the index and success (Fisher’s exact test).
4.1. Healing Indices
Several indices have been used in various studies to monitor wound healing. Some of them are variations of other indices adapted for a specific study.
As a review, we cite some of the indices that have appeared in different studies.
4.1.1. Landry Index
The Landry index was one of the first indices used to track wound healing. Five variables are assessed: tissue colour, bleeding on palpation, the presence of granulation tissue, the incision margin and suppuration. It ranges from 1 to 5, with 1 indicating poor healing and 5 indicating excellent healing [
12].
4.1.2. Huang et al. [13] Index
This soft tissue healing index considers parameters such as oedema, erythema, suppuration, discomfort and dehiscence. It has a score of 1 to 3.
A low score indicates proper healing, and a high score indicates poor healing [
13].
The evaluation is carried out at two and three weeks.
4.1.3. Marini Index
Owing to the limitations of the indices previously described, in 2018, the “Early Wound Healing Score (EWHS)” was developed, the intention of which was to assess tissue healing 24 hours after surgery [
7].
This index assesses three parameters: clinical signs of reepithelialisation, haemostasis, and inflammation.
4.1.4. Hamzani and Chaushu [4] Index
This index, also called “IPR” (I, inflammatory, P, proliferative and R, remodelling) arose from the need to evaluate healing at each phase to better understand the healing process and determine when the clinician needs to intervene in cases of deviation.
During the inflammatory phase, bleeding, the presence of granulation tissue, haematomas, margins, suppuration, oedema and pain are evaluated via a VAS table. This phase is the most important and is scored from 0 to 9 points, in which a score between 5 and 8 is considered satisfactory.
In the proliferative phase, re-epithelialization, colour, eschar, suppuration and pain are evaluated via the VAS table. The maximum score is 5, and a score between 3 and 5 is considered satisfactory.
For remodelling phase, which carries the least weight, variables such as irregular eschar, colour and pain are assess with the VAS table. If two of the three scores are good, the healing is considered satisfactory [
4].
Each index has a series of advantages and disadvantages compared with the index used in this study. A summary of the characteristics of the indices is shown in the following table (
Table 2).
All these indices can be used effectively according to the needs of each study or evaluation, but it should be noted that the Wachtel index presents several limitations in terms of its use, since, in this case, the patients are only evaluated in the first week after implant placement. Although it may offer a more complete evaluation than the Landry or Marini indices, it is unable to detect significant deviations and relate them to implant success or failure in the short term.
Other indices that evaluate more parameters and for a longer time, such as the Hamzani index (e.g., the IPR index), could be more effective in detecting these deviations and allow more opportunities for intervention, as indicated by the study carried out by Yahya et al. [
14], who evaluated wound healing after surgical extraction. This index is able to differentiate between the different healing phases, although this means more chair time for the patient and for the dentist.
In our study, we preferred to use the Wachtel index because of its ease of use and simplicity. In addition, it allowed us to evaluate the patient at just 7 days, during suture removal.
4.2. Factors that May Influence Early Healing
As mentioned earlier, different factors have been linked to surgical wound healing and early implant success or failure. Among them, we describe below those that have achieved a high degree of consensus.
4.2.1. Antiinflammatory Use
Numerous drugs are used for treating postoperative pain, such as glucocorticoids or nonsteroidal anti-inflammatory drugs (NSAIDs). Some authors have noted that the use of these drugs can reduce healing/cointegration, such as Winnett et al. [
15], who found that 44% of the failures involved patients with a history of NSAID use. Additionally, Etikala et al. [
16] reported that the use of NSAIDs, more specifically COX2 inhibitors, could prevent osseointegration of the implant.
However, Alissa et al. [
17], who analysed the data from two groups of 30 patients to evaluate the effect of ibuprofen on alveolar bone, found that at three and six months after implant placement, there was no significant difference in the amount of marginal bone between the group that received ibuprofen and the group that did not.
Other authors have focused on the effects of NSAIDs and their associations with the growth of the alveolar bone crest. One example is Urdaneta et al. [
18], who evaluated 326 implants in 81 patients and reported a relationship between the use of NSAIDs and the growth of the alveolar bone crest. However, these patients also took other medications apart from NSAIDs, so this bone growth may have be due to the combination of several drugs, and thus the study results are not conclusive.
Various animal studies have also been conducted on this topic. This includes the study Krischak et al. [
19], who analysed the effect of diclofenac on wounds caused by incisions in 20 rats and found a decrease in fibroblasts and reduced wound contraction, demonstrating the antiproliferative effect of diclofenac.
In the study by Pablos et al. [
20], the authors examined the effect of diclofenac and meloxicam on bone healing around the implant in 30 rats. The group that was administered diclofenac demonstrated delayed healing, whereas meloxicam had no effect on healing around the implant.
All these studies may have be influenced by different biases and circumstances, however, and so more studies in humans are necessary to conclude that NSAIDs have an inhibitory effect on implant healing.
In our study, all patients took paracetamol as an analgesic. We did not find a relationship between implant success at 2 months and the control of postoperative pain or discomfort. Only 4 patients reported pain equal to or greater than a 40 mm mark on the VAS line, but none of them experienced early implant failure.
4.2.2. Diabetes
An increasing number of patients with diabetes are receiving dental implants and therefore are becoming even more susceptible to infection and poor wound healing than they already are. Thus, as reported in the work of Mellado et al. [
21] on the effects of diabetes on soft tissue healing, hyperglycaemia will have a negative effect on both implant osseointegration and soft tissue healing. Poor glycaemic control seems to be a predisposing factor for infections, and microangiopathy can compromise the vascularization of the flap.
Romero et al. [
22] conducted a 7-year comparative study of implant treatment in 48 patients, who were divided into two groups of 24, one consisting of patients with diabetes and one with relatively healthy patients. A total of 96 implants were placed, two of which were lost in two patients. There were no significant differences in implant success between the two groups.
In several studies, such as the one carried out by MCCraken et al. [
23], and the one made by Le et al. [
24], animal models of diabetes demonstrated a greater alteration in the bone remodelling process and a decrease in the osseointegration of the implant.
In our study, we did not find a relationship between the healing of the surgical wound at 1 week and implant success at 2 months with respect to diabetes status. A possible explanation is that only 4 patients had diabetes, but it was controlled in all cases, with glycosylated haemoglobin levels lower than 7%, and none of these patients experienced implant failure. We believe that this lack of a relationship is due to the small number of diabetic patients—particularly those with poor diabetes control—included in the study.
4.2.3. Tobacco Use
Tobacco has a strong negative influence on the success of any dental treatment, including implant surgery. The results of the review by Hadadi and Mezied [
25] on the effect of smoking on the outcome of osseointegrated implants indicated that nonsmokers were more likely to experience implant success than smokers. Likewise, in the study carried out by Sánchez et al. [
26], it was concluded that the consumption of more than 20 cigarettes a day increases the risk of implant loss by 30.8%.
Consistent with these studies, Twito and Sade [
27] concluded that smoking patients had a higher failure rate, which increased further for those who more than 30 cigarettes per day. Similarly, smoking 100 cigarettes a year (5 packs) was shown t increase the possibility of implant failure increases.
In the present study, we did not find a relationship between smoking and early failure. We believe that this could be because the inclusion criteria did not allow patients who smoked more than 10 cigarettes a day. As has been proven in other studies, the deleterious effects of tobacco increase with the number of cigarettes smoked per day, plateauing once the habit reaches more than a pack a day, especially in terms of late implant failure.
4.2.4. Age and Sex
Age and sex have also been considered to be associated with implant success. Although in our study, no significant associations were found in terms of age or sex, some studies have related both variables to implant success or failure, as in the study carried out by Padron et al. [
28], who identified a higher percentage (65.6%) of failure in men than in women (34.4%). In addition, a greater percentage of failure occurred in young people, i.e., those aged 33 to 50 years (53.1%) than in people aged 51 to 81 years (31.2%).
4.2.5. GBR
GBR is used not only for immediate implants placed in postextraction sockets but also for narrow alveoli and/or bone defects incidentally identified that are accidental findings during surgery and that require GBR to continue with the implantation.
In all situations in which GBR is necessary, primary wound closure must be achieved, and special care must be taken to maintain tension-free wound closure [
10,
29,
30,
31].
In our study, all implants that required GBR involved primary closure, avoiding exposure of the material or the implant. Perhaps for this reason, we did not identify implant failure among the patients who required GBR treatment.
In summary, the Wachtel healing index is an effective tool for early wound assessment. However, it is not a predictor of early implant success or failure. Similarly, none of the other variables analysed (pain, NSAID intake, GBR, low tobacco consumption, controlled diabetes, age or sex) predicted early implant success or failure at 2 months and 1 week.
4.3. Limitations of the Present Study
Regarding the limitations of this study, this was a short-term evaluation, as the index was calculated only at the one-week follow-up, and so we could not assess deviations beyond that period. The evaluation only assess a specific moment in the healing process, similar to a still photograph of an instant of a tremendously dynamic process.
Another drawback is the size of the sample studied (50 patients). For this reason, it was not possible to establish subgroups of an adequate size, despite the statistical power achieved. Future studies should involve larger sample sizes and longer follow-up times to better determine the effects in different subgroups (e.g., based on pain, NSAID intake, GBR, tobacco consumption, degree of diabetes control, age and sex).
5. Conclusions
The Wachtel healing indices of the patients revealed that initial healing after implant placement occurs with few alterations.
The Wachtel index does not predict osseointegration at two months and one week.
None of the surrogate variables significantly predicted the outcome of early failure.
Author Contributions
Conceptualisation: N. M.-L. and A. S.-P. Methodology: A. S.-P., A. J.-G. and J.M.M-C. Validation: J.M.M-C and M.J.M-V Formal analysis: A.S.-P. Investigation: N. M-L. Data curation: N. M-L and A.S.-P. Writing: N. M-L and A.S.-P. Review and editing: M.J.M-V., J.M.M-C, A.J.-G. Visualisation: M.J.M-V and A.J.-G. Supervision: A.S.-P. All the authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data for this study were obtained from the analysis of published articles.
Acknowledgements
The authors would like to thank the support staff of the University Dental Clinic.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Schwarz, F.; Ramanauskaite, A. It is all about peri-implant tissue health. Periodontol 2000 2022, 88, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kullar, A.S.; Miller, C.S. Are there contraindications for placing dental implants? Dent. Clin. North Am. 2019, 63, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, J.C.; Landinez, N.S.; Garzón-Alvarado, D.A. Generalidades de la interfase hueso-implante dental. Rev. Cuba. Investig. Bioméd. 2009, 28, 130–146. [Google Scholar]
- Hamzani, Y.; Chaushu, G. Evaluation of early wound healing scales/indexes in oral surgery: A literature review. Clin. Implant Dent. Relat. Res. 2018, 20, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Simonelli, A.; Rizzi, A.; Pramstraller, M.; Cucchi, A.; Trombelli, L. Early postoperative healing following buccal single flap approach to access intraosseous periodontal defects. Clin. Oral Investig. 2013, 17, 1573–1583. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Marini, L.; Rojas, M.A.; Sahrmann, P.; Aghazada, R.; Pilloni, A. Early wound healing score: a system to evaluate the early healing of periodontal soft tissue wounds. J. Periodontal Implant Sci. 2018, 48, 274–283. [Google Scholar] [CrossRef]
- Löe, H. The gingival index, the plaque index and the retention index systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Wachtel, H.; Schenk, G.; Böhm, S.; Weng, D.; Zuhr, O.; Hürzeler, M.B. Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: A controlled clinical study. J. Clin. Periodontol. 2003, 30, 496–504. [Google Scholar] [CrossRef]
- Wachtel, H.C.; Langford, A.; Bernimoulin, J.P.; Reichart, P. Guided bone regeneration next to osseointegrated implants in humans. Int. J. Oral Maxillofac. Implants 1991, 6, 127–135. [Google Scholar] [PubMed]
- Landry, R.; Turnbull, R.; Howley, T. Effectiveness of benzydamine HC1 in the treatment of periodontal post-surgical patients. Res. Clin. Forums 1988, 10, 105–18. [Google Scholar]
- Huang, L.H.; Neiva, R.E.; Wang, H.L. Factors affecting the outcomes of coronally advanced flap root coverage procedure. J. Periodontol. 2005, 76, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Yahya, B.H.; Chaushu, G.; Hamzani, Y. Evaluation of wound healing following surgical extractions using the IPR Scale. Int. Dent. J. 2021, 71, 133–139. [Google Scholar] [CrossRef]
- Winnett, B.; Tenenbaum, H.C.; Ganss, B.; Jokstad, A. Perioperative use of non-steroidal anti-inflammatory drugs might impair dental implant osseointegration. Clin. Oral Implants Res. 2016, 27, e1–e7. [Google Scholar] [CrossRef]
- Etikala, A.; Tattan, M.; Askar, H.; Wang, H.L. Effects of NSAIDs on periodontal and dental implant therapy. Compend. Contin. Educ. Dent. 2019, 40, e1–e9. [Google Scholar]
- Alissa, R.; Sakka, S.; Oliver, R.; Horner, K.; Esposito, M.; Worthington, H.V.; Coulthard, P. Influence of ibuprofen on bone healing around dental implants: a randomised double-blind placebo-controlled clinical study. Eur. J. Oral Implantol. 2009, 2, 185–199. [Google Scholar]
- Urdaneta, R.A.; Daher, S.; Lery, J.; Emanuel, K.; Chuang, S.K. Factors associated with crestal bone gain on single-tooth locking-taper implants: the effect of nonsteroidal anti-inflammatory drugs. Int. J. Oral Maxillofac. Implants 2011, 26, 1063–1078. [Google Scholar]
- Krischak, G.D.; Augat, P.; Claes, L.; Kinzl, L.; Beck, A. The effects of non-steroidal anti-inflammatory drug application on incisional wound healing in rats. J. Wound Care 2007, 16, 76–78. [Google Scholar] [CrossRef]
- Pablos, A.B.; Ramalho, S.A.; König, B., Jr.; Furuse, C.; de Araújo, V.C.; Cury, P.R. Effect of meloxicam and diclofenac sodium on peri-implant bone healing in rats. J. Periodontol. 2008, 79, 300–306. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; García, J.C.F.; Ballester, A.H.; Rueda, C.L. Effects of diabetes on the osseointegration of dental implants. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E38–E43. [Google Scholar] [PubMed]
- Romero, J.L.R.; García, I.O.; Guerra, A.J.; Garrido, N.M.; López, A.E.; Guil, L.M.; Ortega, E.V. Tratamiento de implantes en pacientes con diabetes. Un estudio comparativo a los 7 años. Av. Odontoestomatol. 2020, 36, 81–88. [Google Scholar] [CrossRef]
- McCracken, M.; Lemons, J.E.; Rahemtulla, F.; Prince, C.W.; Feldman, D. Bone response to titanium alloy implants placed in diabetic rats. Int. J. Oral Maxillofac. Implants 2000, 15, 345–354. [Google Scholar] [PubMed]
- Le, N.N.; Rose, M.B.; Levinson, H.; Klitzman, B. Implant healing in experimental animal models of diabetes. J. Diabetes Sci. Technol. 2011, 5, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.A.; Mezied, M.S. Evidence-based analysis of the effect of smoking on osseointegrated implant outcome. Natl. J. Maxillofac. Surg. 2021, 12, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, A.; Moya-Villaescusa, M.J.; Caffesse, R.G. Tobacco as a risk factor for survival of dental implants. J. Periodontol. 2007, 78, 351–359. [Google Scholar] [CrossRef]
- Twito, D.; Sade, P. The effect of cigarette smoking habits on the outcome of dental implant treatment. PeerJ 2014, 2, e546. [Google Scholar] [CrossRef]
- Padrón, A.P.; Quiñones, J.A.P.; Rodriguez, M.d.C.C.; Martell, Y.D.; Carvajal, T.S.; Martí, C.D.G. Causes and consequences of failures of the dental implantology. Matanzas. Rev. Méd. Electrón. 2018, 40, 1023–1031. [Google Scholar]
- Becker, W.; Becker, B.E.; Handlesman, M.; Celletti, R.; Ochsenbein, C.; Hardwick, R.; Langer, B. Bone formation at dehisced dental implant sites treated with implant augmentation material: a pilot study in dogs. Int. J. Periodontics Restorative Dent. 1990, 10, 92–101. [Google Scholar]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int. J. Oral Maxillofac. Implants 1989, 4, 19–25. [Google Scholar]
- Warrer, L.; Gotfredsen, K.; Hjørting-Hansen, E.; Karring, T. Guided tissue regeneration ensures osseointegration of dental implants placed into extraction sockets. An experimental study in monkeys. Clin. Oral Implants Res. 1991, 2, 166–171. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).