1. Introduction
Lung cancer is one of the most frequently diagnosed cancer both in male and female [
1,
2,
3]. Non-small lung cancer (NSCLC) accounts for approximately 27% of all cancer-related deaths worldwide [
4], and accounts for 85% of all lung cancers [
5]. The overall 5-year survival rate of lung cancer is approximately 15-19% [
6,
7]. The most common subtypes of NSCLC are adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma [
8]. Risk factors for lung cancer are cigarette smoking, air pollution, emission fuel combustion, and environmental exposure to radon and asbestos [
9,
10].
Lung adenocarcinoma is one of the most aggressive, rapidly fatal, and was found to be resistant to conventional radiotherapy and chemotherapy [
11]. Identification of cancer stem cells (CSCs) in lung cancer presents a therapeutic challenge [
12]. CSCs are the tumor population that has the potential to self-renew and greatly contribute to lung cancer progression, tumor relapse, and drug resistance [
13]. Lung CSCs are categorized by surface expression of PD-L1, CD133, CD44, CD90, epithelial cell adhesion molecule (EpCAM), and CXCR4 (CXC chemokine receptor type 4) [
14,
15,
16,
17,
18].
Natural killer (NK) cells known for their anticancer function are innate immune cells, that comprise approximately 5 to 20 % of total peripheral blood mononuclear cells (PBMCs) lymphocytes in humans. We have previously shown that NK cells directly kill CSCs or inhibit tumor growth by differentiating tumors via secreted cytokines such as IFN-γ and TNF-α [
19]. Differentiation in CSCs leads to increased expression of MHC-class I in tumors, induced resistance to NK cell-mediated cytotoxicity against these differentiated CSCs, and increased chemotherapy drug-induced killing against these differentiated CSCs [
20,
21]. The diminished function of NK cells is linked to poor prognosis in cancer patients [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32].
To understand the role of tumor differentiation in the chemotherapeutic sensitivity of lung cancer, we induced differentiation in hA549 lung cancer stem-like cell lines before they were exposed to chemotherapeutic drugs. hA549 is the most commonly used human NSCLC line for lung adenocarcinoma research [
33,
34]. The treatment of IL-2 and anti-CD16 mAbs combination induce split anergy in NK cells leading to decreased cytotoxicity but significant induction of IFN-γ and TNF-α, the two cytokines play a crucial role in tumor differentiation. We demonstrate that lung cancer stem cells upon differentiation are resistant to NK cell-mediated cytotoxicity but are susceptible to chemo-drug-mediated cell death.
2. Results
2.1. Enhanced Susceptibility of Chemotherapeutic Drugs Against Differentiated Tumors in Comparison to Their Stem-Like Counterparts
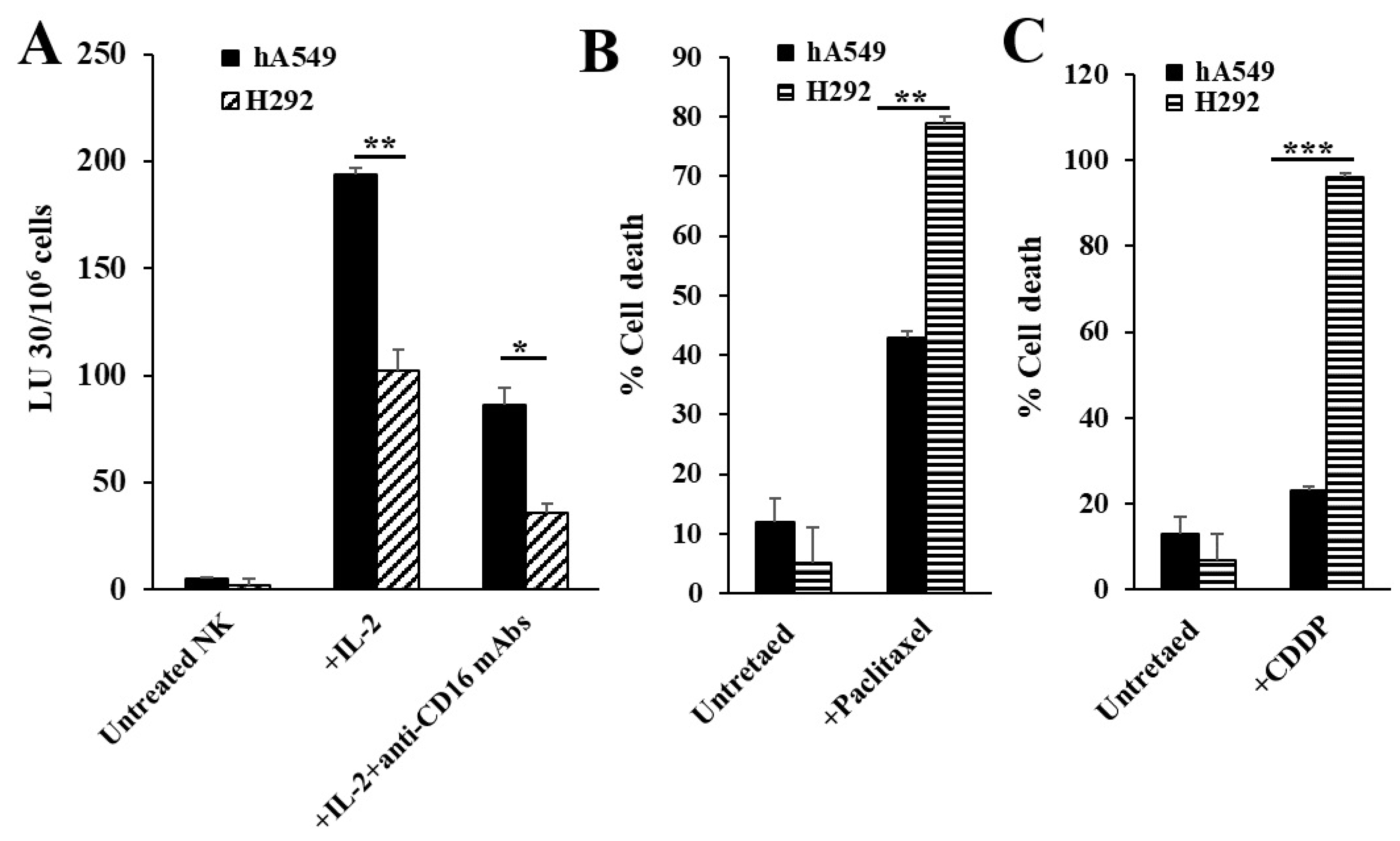
NK cells were left untreated or were treated with IL-2 alone or were treated with a combination of IL-2 and anti-CD16 mAbs for 18-24 hours before they were used as effectors in cytotoxic assay against lung cancer cell lines or to determine the secretion levels of IFN-γ and TNF-α. As shown previously in several publications from our laboratory, IL-2-treated NK cells mediated the highest cytotoxicity (
Figure 1A and
Figure S1A) and decreased cytotoxic activity but increased secretion levels of IFN-γ and TNF-α were seen when NK cells were treated with a combination of IL-2 and anti-CD16 mAbs (
Figure S2). Our previous findings have demonstrated that differentiated tumors were resistant to NK cell-mediated killing but were more sensitive to chemotherapeutics in comparison to CSCs [
21]. Here, we determined the NK cell-mediated cytotoxicity against hA549 (CSCs) and H292 (differentiated lung cancer cell lines) and found higher NK cell-mediated cytotoxicity against hA549 compared to H292 (
Figure 1A). When cell viability of hA549 and H292 with or without the treatments of chemotherapeutic drugs was assessed, the higher cell death was induced by both paclitaxel (
Figure 1B) and CDDP (
Figure 1C) against H292 in comparison to hA549.
2.2. Enhanced Susceptibility of Chemotherapeutic Drugs Against NK Cell-Differentiated Tumors in Comparison to Their Stem-Like Counterparts
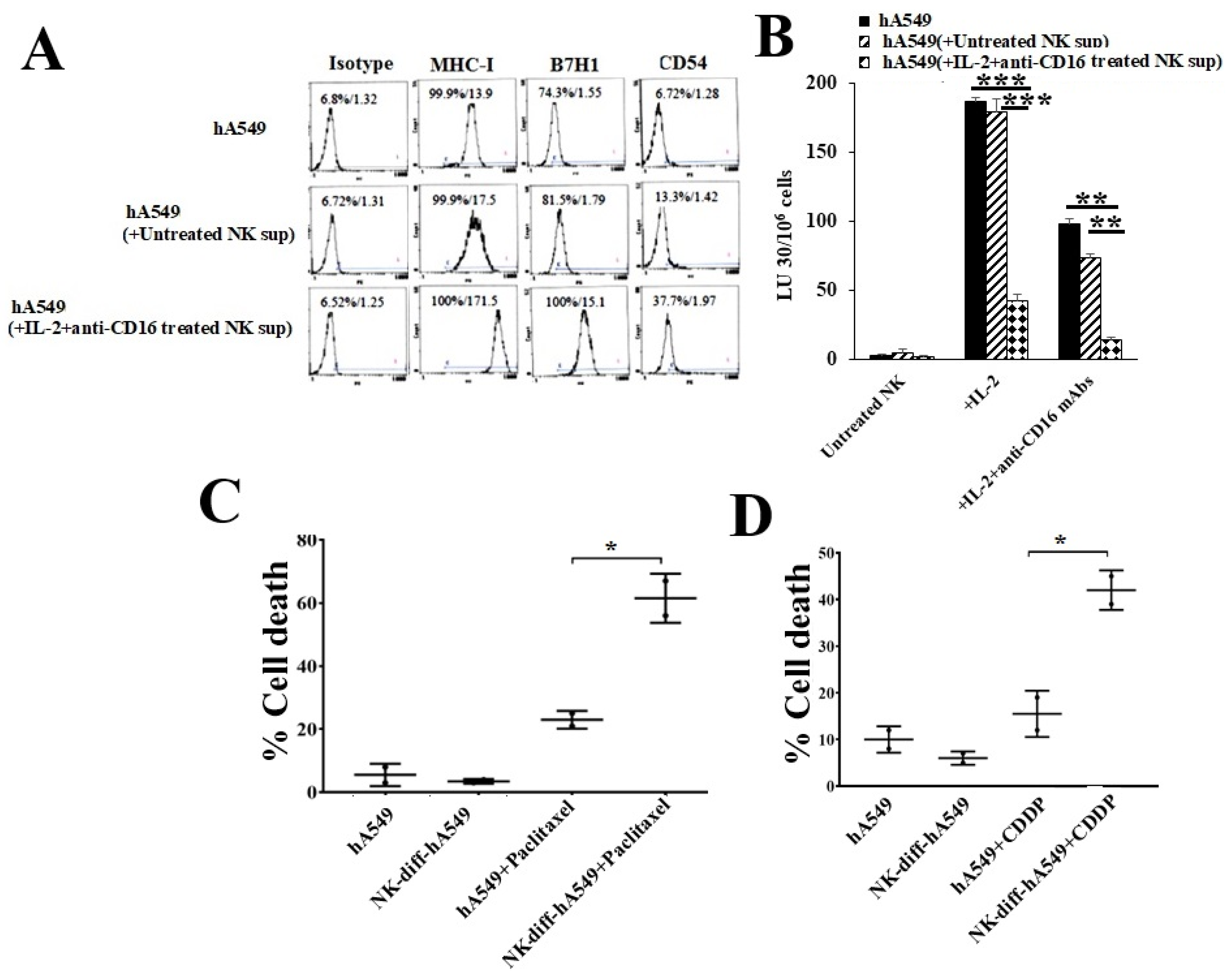
We have previously demonstrated that NK cells become split-anergized (secrete higher cytokines but mediate minimal cytotoxicity) when treated with IL-2 + anti-CD16 mAbs, and the supernatants of these NK cells are best to induce differentiation of stem cells [
19,
21,
35,
36,
37]. CSCs were found to exhibit higher lower surface express levels of MHC-class I, PD-L1 (B7H1), and CD54, increased sensitivity to NK cell-mediated cytotoxicity, and resistance to chemo drug-induced killing compared to differentiated tumors [
19,
21,
35]. In this study, we treated hA549 with either untreated NK cells or IL-2 and anti-CD16 mAbs treated NK cells’ supernatants. hA549 treated with IL-2 and ant-CD16 mAbs treated NK cells’ supernatants expressed a higher increase of MHC-class I, B7H1, and CD54 (
Figure 2A) and increased resistance to NK cell-mediated cytotoxicity (
Figure 2B) in comparison to untreated hA549 or those treated with untreated NK cells’ supernatant. Higher paclitaxel (
Figure 2C) and CDDP (
Figure 2D) induced cell death were seen in IL-2 and anti-CD16 mAbs treated NK cells’ supernatants differentiated hA549 compared to non-differentiated cells.
2.3. Enhanced Susceptibility of Chemotherapeutic Drugs Against rhIFN-γ and rhTNF-α Treated Tumors
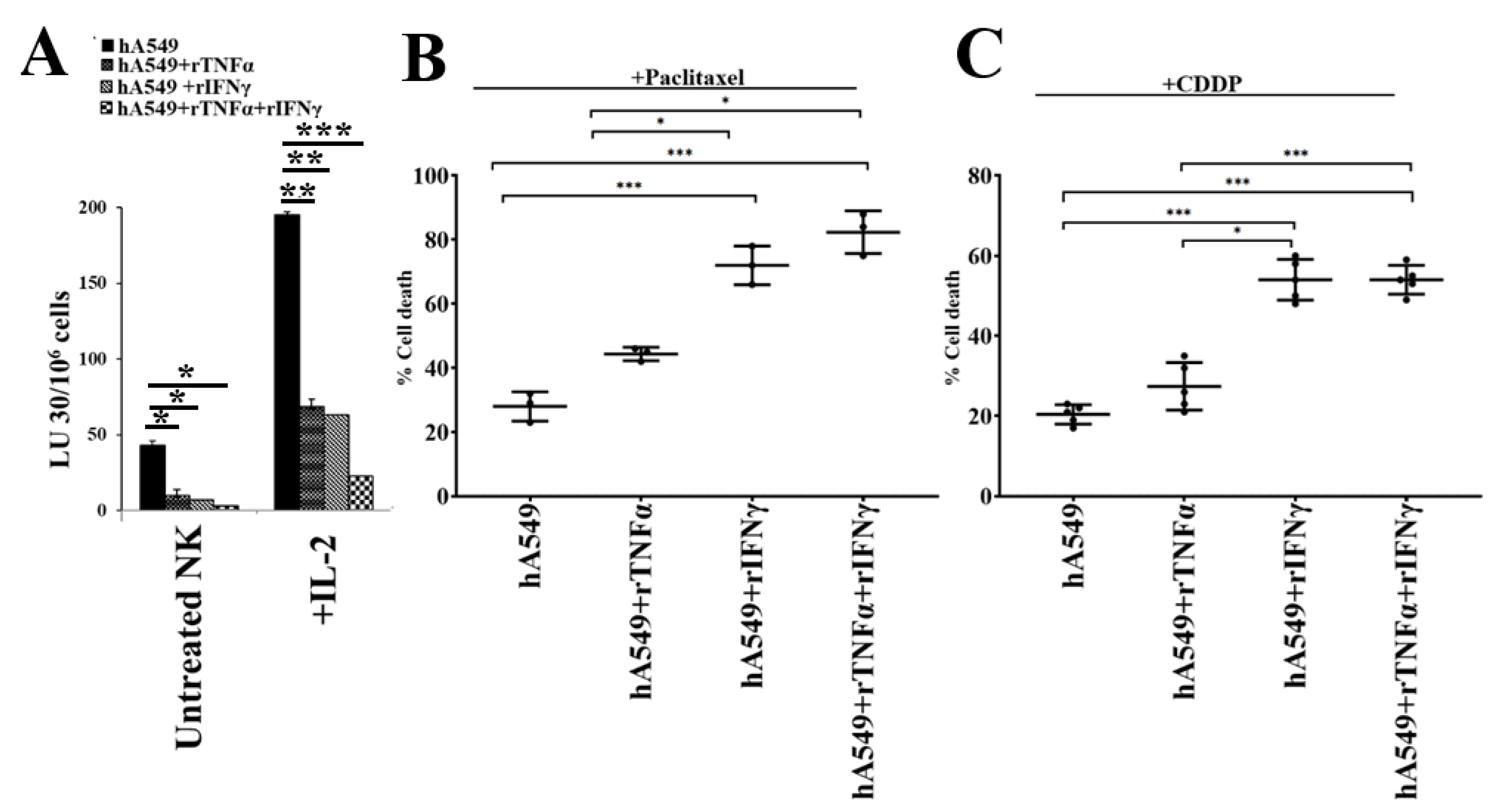
We have previously demonstrated that IFN-γ and TNF-α mediated differentiation of several different CSCs [
36,
37]. Therefore, we used rh-IFN-γ and rh-TNF-α to induce differentiation in hA549 (
Figure 3). We found resistance to NK cell-mediated cytotoxicity in hA549 (
Figure 3A) and increased sensitivity to paclitaxel (
Figure 3B) and CDDP (
Figure 3C) induced cell death with rh-IFN-γ and rh-TNF-α treatments. Increased surface expression of MHC-class I, B7H1, and CD54 were also seen after rh-IFN-γ and rh-TNF-α treatment in hA549 (
Figure S3).
2.4. Anti-IFN-γ and anti-TNF-α Blocked NK Cells Supernatant or rhIFN-γ and rhTNF-α Induced Differentiation of hA549 Cells
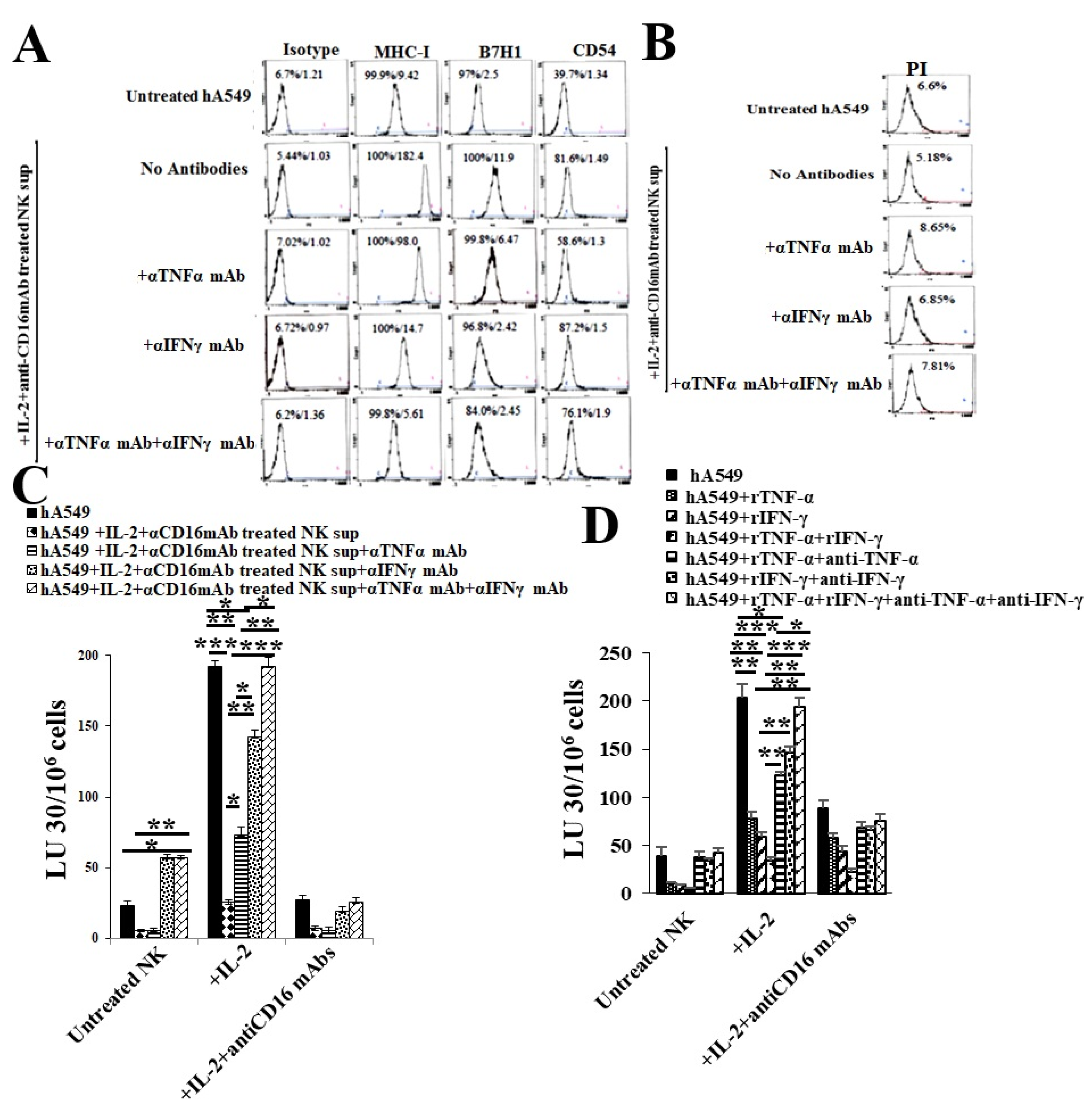
When we used antibodies to IFN-γ and TNF-α to treat NK cells supernatant treated hA549 cells. We found that NK cells induced differentiation in hA549 cells was inhibited by antibodies to IFN-γ and TNF-α as indicated by surface markers (
Figure 4A) and cytotoxicity assay profiles (
Figure 4C), the highest effect was seen with the combination of anti-IFN-γ and anti-TNF-α treatment. No or slight induced hA549 cell death was seen with the treatments of anti-IFN-γ and/or anti-TNF-α (
Figure 4B). To further validate the role of IFN-γ and TNF-α, we treated hA549 cells with rhIFN-γ and rhTNF-α in the absence and presence of antibodies against IFN-γ and TNF-α. We observed that rIFN-γ, rTNF-α or the combination of rIFN-γ and rTNF-α induced differentiation of hA549 which was blocked by anti-TNF-α or anti-IFN-γ or anti-TNF-α+anti-IFN-γ (
Figure 4D). Similar results were seen in SCAP cell lines (
Figures S4 and S5). These results validated the role of IFN-γ and TNF-α in NK cells induced differentiation of CSCs.
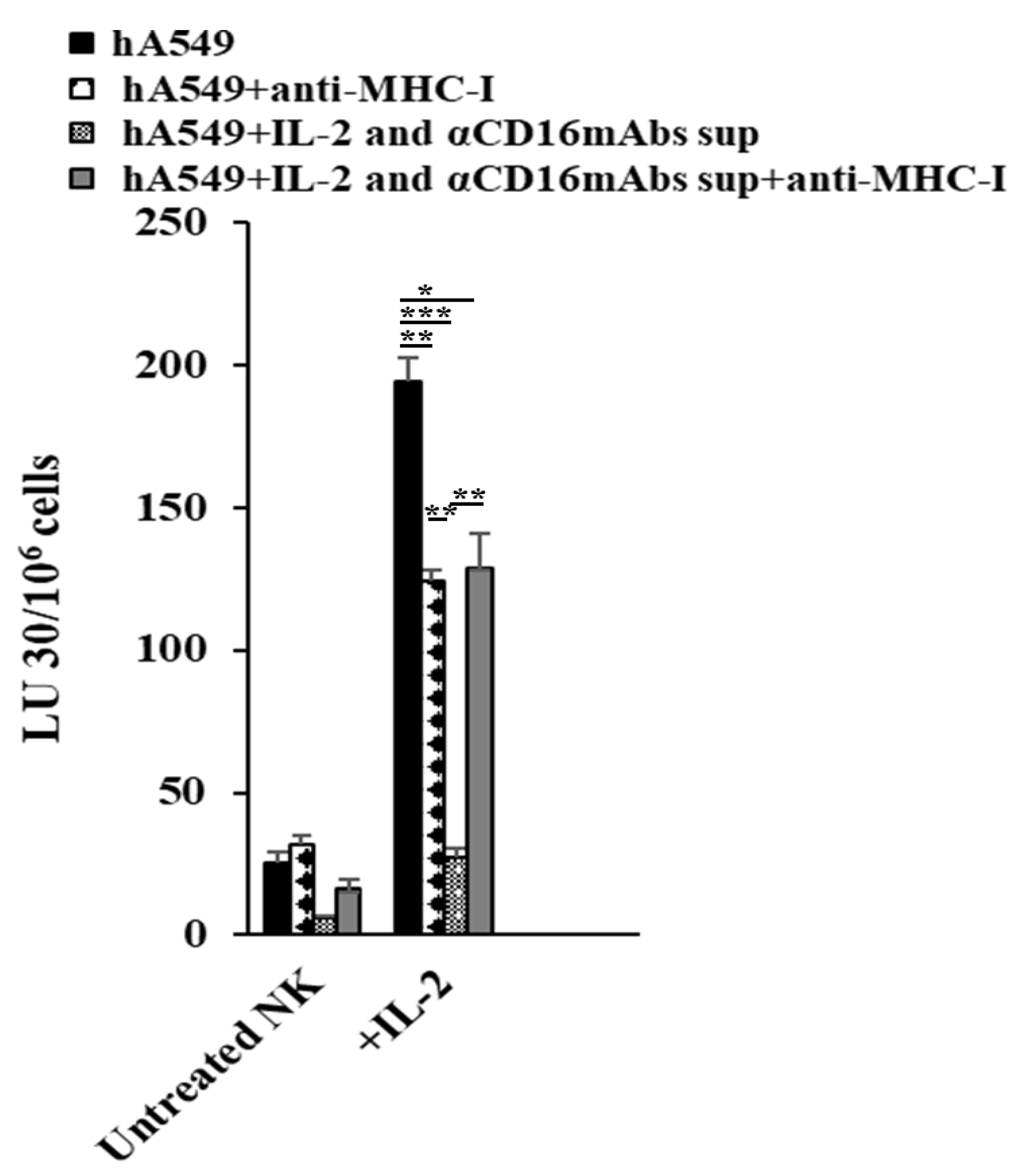
2.5. Anti-MHC-Class I Induces Different Effects in CSCs and Differentiated hA549 Cells
Decreased NK cell-mediated cytotoxicity was seen in hA549 treated with antibodies against MHC-class I. In contrast, increased NK cell-mediated cytotoxicity was seen in NK cell-differentiated hA549 treated with antibodies against MHC-class I (
Figure 5).
3. Discussion
Lung cancer is the leading cause of cancer-related death in men and is the second highest cause of cancer-related death in women after breast cancer [
38]. The focus of this study is to delineate the underlying mechanisms by which lung cancer cells could undergo differentiation to be targeted by chemotherapy. NK cells act as a powerful tool to induce differentiation in tumors [
39]. We have previously introduced the term ‘split anergy’ in NK cells. When treated with a combination of IL-2 and anti-CD16 mAbs, NK cells undergo a split-anergized state that demonstrates reduced NK cell cytotoxicity in the presence of significant secretion of cytokines [
36,
40,
41] (
Figures S1 and S2). The cytokines especially IFN-γ and TNF-α released by split anergized NK cells promote the differentiation of tumors [
42]. Differentiated tumors were found to exhibit lower CD44 and higher MHC-class I, CD54, and PD-L1 (B7H1), and were found to be resistant to the NK cell-mediated cytotoxicity, but susceptible to chemo-drug-induced killing [
19,
21,
35].
hA549 has features of CSCs whereas H292 cell lines represented differentiated cell lines and they were analyzed to assess the key differences. The results indicated a significant correlation between the stage of differentiation of tumors and the level of NK cell-mediated lysis. NK cell-mediated cytotoxicity was higher in hA549 compared to the differentiated H292 cell line. As mentioned earlier, the treatment of NK cells with IL-2 and anti-CD16 mAbs induces split anergy by decreasing NK cell-mediated cytotoxicity and increasing cytokine secretion. In this study, NK cells upon treatment with IL-2 and anti-CD16 mAbs lose cytotoxicity against hA549 cells compared to those treated with IL-2 but were secreting significantly higher levels of IFN-γ and TNF-α secretion. Similar to our previous observation in several other cancer types differentiated lung cancer cell line H292 was found to be more sensitive to chemotherapeutic drugs compared to hA549 [
21]. Similar to differentiated lung cancer cell line H292, NK cell-induced differentiated hA549 tumors were found to be resistant to NK cell-mediated cytotoxicity but were highly susceptible to chemo-drug-induced killing compared to hA549 (
Figure 2).
We have previously demonstrated that NK cells limit tumor growth or expansion by inducing differentiation of the tumor cells by the secretion of IFN-γ and TNF-α [
19]. A great correlation was observed between the differentiation stage of the tumors and their susceptibility to NK cell-mediated cytotoxicity and chemo-drug-mediated killing [
21,
39,
42]. To determine whether treatment with recombinant human IFN-γ and TNF-α is capable of differentiating the hA549 tumor cells, we treated them with rIFN-γ and rTNF-α before we tested the NK cell-mediated cytotoxicity and chemo-drug induced killing of hA549 cells. We found that treatment with rIFN-γ and rTNF-α of hA549 resulted in decreased NK cell-mediated cytotoxicity and increased chemo-drug-induced killing of hA549, and also increased the surface expression levels of CD54, B7H1, and MHC-class I in hA549 cells (
Figure S3). This data indicates that differentiated lung cancer is also more susceptible to chemotherapeutic drug when compared to CSC tumors as we have previously shown this finding in oral and pancreatic tumors [
21].
To further validate the role of NK cells and/or IFN-γ and TNF-α in inducing differentiation in lung cancer, we added antibodies against both IFN-γ and TNF-α to determine if we can block NK cell or rIFN-γ and rTNF-α induced differentiation of hA549. The antibodies against IFN-γ were found to induce greater inhibition of hA549 tumor differentiation compared to antibodies against TNF-α. The combination of both antibodies against TNF-α and IFN-γ induced a complete blocking of differentiation suggesting that it is the synergistic function of TNF-α and IFN-γ secreted by split anergized NK cells that induce differentiation of lung tumors. Upon treatment with antibodies against both TNF-α and IFN-γ in NK cells or rIFN-γ and rTNF-α differentiated hA549, they regained NK cell-mediated cytotoxicity similar to untreated hA549. Together these results confirmed that it is the synergistic effect of TNF-α and IFN-γ secreted by anergized NK cells that induce lung tumors to differentiate thereby becoming resistant to NK cell-mediated cytotoxicity. Similar results were seen when SCAP cells were treated with anergized NK supernatants and recombinant IFN-γ and TNF-α, and NK cells or IFN-γ and TNF-α induced differentiation was blocked by antibodies against IFN-γ and TNF-α (
Figures S4 and S5).
We observed that the levels of pJNK and pAKT were increased when hA549 were differentiated with NK cells or rIFN-γ and rTNF-α. The JNK and AKT pathways have been found to regulate cell death and an increase in JNK and AKT correlates with increased cell survival [
43,
44]. The treatment of hA549 cells with IFN-γ or TNF-α or IFN-γ+rTNF-α did not increase STAT3 but it was increased when hA549 cells were treated with anergized NK cells’ supernatants suggesting increased levels of STAT3 in lung tumor is specific to NK cells induced differentiation. Interestingly, the addition of antibodies against TNF-α and IFN-γ to hA549 cells treated with anergized NK supernatants decreased STAT3, which suggests TNF-α and IFN-γ are involved in NK cells mediated increase of STAT3. TNF-α and IFN-γ may be working synergistically with other NK cells secreted cytokines, which awaits future investigation.
We have previously demonstrated that CSC tumors exhibit lower MHC-class I, whereas well-differentiated tumors exhibit increased MHC-class I surface expression [
19,
21,
35]. In the current study, we observed the highest surface expression levels of MHC-class I in NK cells or IFN-γ and TNF-α-induced differentiated hA549. Increased surface expression levels of MHC-class I correlated with resistance of hA549 to NK cell-mediated cytotoxicity. We have shown similar results with the healthy stem cells SCAP and several other tumors [
36], suggesting that this phenomenon is not exclusive to lung tumors. To explore more on the significance or role of MHC-class I in inducing resistance of tumors to NK cell-mediated cytotoxicity, we added antibodies against MHC-class I in untreated and NK supernatant-treated hA549s. Increased resistance to NK cell-mediated cytotoxicity was observed when untreated hA549 were treated with antibodies against MHC-class I, whereas the opposite effect was seen in NK cell-differentiated hA549 (
Figure 5). Since, hA549 express low levels of MHC-class I, thus, it may be that adding anti-MHC-class I didn’t have any significant effect in the tumors [
45,
46]. It has been shown that adding anti-MHC to activated T cells suppresses their function [
47]. On the other hand, when NK cell-differentiated hA549 were treated with anti-MHC-class I, we observed exacerbation of lysis by NK. Differentiation in tumors increases MHC-class I and blocks NK cell-mediated cytotoxicity. When MHC class I is blocked in differentiated tumors the effect of an increase in NK cell-mediated cytotoxicity is highly elevated.
Overall, this study showed that NK cells secreted cytokines IFN-γ and TNF-α induced differentiation and increased MHC-class I surface expression is one of the hallmarks of differentiation. Differentiated lung cancer was shown to be resistant to NK cell-mediated cytotoxicity but sensitive to chemo-drug-induced killing.
4. Materials and Methods
4.1. Cell Lines, Reagents, and Antibodies
Human NK cells were cultured in RPMI 1640 (Invitrogen by Life Technologies, CA), supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, CA). hA549 (Catalog# CCL-185), the human acinar adenocarcinoma cell line with gland formation was purchased from ATCC (Manassas, VA, USA), and was cultured in DMEM media. Recombinant IL-2 was obtained from NIH-BRB. Antibodies to CD16, recombinant IFN-γ, and recombinant TNF-α were from Biolegend (San Diego, CA). Antibodies to MHC-class I, IFN-γ and TNF-α were prepared in our laboratory. Antibodies used for flow cytometry—IgG2, MHC-class I, B7H1, and CD54 were purchased from Biolegend (San Diego, CA). Human NK cells purification kits were obtained from Stem Cell Technologies (Vancouver, BC, Canada). Propidium iodide (PI) and Chromium-51 was purchased from PeproTech (Cranbury, NJ, USA). Cisplatin and Paclitaxel were purchased from Ronald Reagan Pharmacy at UCLA. ELISA kits for IFN-γ were purchased from Biolegend (San Diego, CA).
4.2. Purification of Human NK Cells and Monocytes
Written informed consents, approved by UCLA Institutional Review Board (IRB), were obtained from healthy individuals, and all procedures were approved by the UCLA-IRB. Peripheral blood was separated using ficoll-hypaque centrifugation, after which the white, cloudy layer, containing peripheral blood mononuclear cells (PBMCs) was harvested. NK cells were negatively selected from PBMCs using the EasySep® Human NK cell enrichment purchased from Stem Cell Technologies (Vancouver, BC, Canada). Purified NK cells were stained with anti-CD16 to measure purity using flow cytometric analysis. Samples showing greater than 95% purity were used for study.
4.3. NK Cells’ Supernatant Collection and Stem Cell Differentiation
Purified NK cells were left untreated or activated with rh-IL-2 (1000 U/ml) and anti-CD16 mAb (3 µg/ml) for 18-20 hours before the supernatant was harvested, and was used in differentiation of hA549 cells. The supernatant volume was determined based on IFN-γ required, and was accessed with ELISA specific to IFN-γ. Differentiation of hA549 was conducted with an average total of 6000 pg over the course of 5 days. On day 0, 1 X 106 tumor cells were cultured, on day 1 unattached tumor cells were removed and attached tumor cells were treated with NK cells’ supernatants on days 1, 2, 3 and 4. On day 5, tumor cells were rinsed with 1 X PBS, detached and used for experiments.
4.4. Surface Staining and Cell Death Analysis
Staining was performed by labeling the cells with antibodies as described previously [
48,
49,
50]. The percentage of dead cells was determined by propidium iodine (PI) (100 μg/ml) staining using flow cytometric analysis. Flow cytometric analysis was performed using Beckman Coulter Epics XL cytometer (Brea, CA), and results were analyzed in the FlowJo vX software (Ashland, OR).
4.5. Enzyme-Linked Immunosorbent Assays (ELISAs)
Single ELISAs were performed as previously described [
50]. To analyze and obtain the cytokine and chemokine concentration, a standard curve was generated by either two- or three-fold dilutions of recombinant cytokines provided by the manufacturer.
4.6. 51Cr Release Cytotoxicity Assay
The
51Cr release cytotoxicity assay was performed as previously described [
51]. Briefly, different ratios of NK cells and
51Cr–labeled ovarian cell lines were incubated for four hours. After this, the supernatants were harvested from each sample, and the released radioactivity was counted using the gamma counter. The percentage-specific cytotoxicity was calculated as follows:
LU 30/106 is calculated by using the inverse of the number of NK cells needed to lyse 30% of ovarian cell lines ×100.
4.7. Statistical Analyses
Prism-9 software was used for statistical analysis. An unpaired or paired, two-tailed Student’s t-test was performed for experiments with two groups. One-way ANOVA with a Bonferroni post-test was used to compare different groups for experiments with more than two groups. Duplicate or triplicate samples were used for assessment. The following symbols represent the levels of statistical significance within each analysis: ***(p value <0.001), **(p value 0.001-0.01), *(p value 0.01-0.05).
Abbreviations
| NK cells |
Natural Killer cells |
| MHC-Class I |
Major histocompatibility complex molecule class I |
| IFN-γ |
Interferon-gamma |
| TNF-α |
Tumor necrosis factor-α |
| CSCs |
Cancer stem cells |
| rhIL-2 |
Recombinant human IL-2 |
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
AJ was the principal investigator, obtained the funding, designed the study, and wrote the manuscript along with KK. KK and ASC performed all the experiments. KK analyzed the results and wrote the manuscript.
Funding
NIH-NIDCR RO1-DE022552, RO1 DE12880, UCLA Academic senate grant and School of Dentistry Seed grant.
Institutional Review Board Statement
This study was conducted according to UCLA Institutional Review Board (IRB). Protocol ID: IRB#11-000781. Approval Date: 2 December 2020.
Informed Consent Statement
Written informed consents approved by the UCLA Institutional Review Board (IRB) were obtained from healthy donors and cancer patients, and all procedures were approved by the UCLA-IRB.
Data Availability Statement
Data generated or analyzed during the study are included in this submitted article.
Conflicts of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner, H. ; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo G, Imbimbo M, Garassino MC. Is the chemotherapy era in advanced non-small cell lung cancer really over? Maybe not yet. Tumori. 2016, 2016, 223–225. [Google Scholar]
- Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi, T. , Awad MM; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Icard P, Damotte D, Alifano, M. New Therapeutic Strategies for Lung Cancer. Cancers (Basel). 2021, 13. [Google Scholar]
- Khajuria, O. , Sharma, N. Epigenetic targeting for lung cancer treatment via CRISPR/Cas9 technology. Advances in Cancer Biology—Metastasis. 2021, 3, 100012. [Google Scholar] [CrossRef]
- Siegel RL, Miller KD, Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. 2020, 70, 7–30.
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: Epidemiology, etiology, and prevention. Clin Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Duffy MJ, O’Byrne, K. Tissue and Blood Biomarkers in Lung Cancer: A Review. Adv Clin Chem. 2018, 86, 1–21. [Google Scholar]
- Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci Rep. 2017, 7, 14300. [Google Scholar] [CrossRef]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia, M. , Weinmayr, G.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Denisenko TV, Budkevich IN, Zhivotovsky, B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Testa U, Castelli G, Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers (Basel). 2018; 10, https://doi.org/10.3390/cancers10080248.
- Gao Q, Zhan Y, Sun L, Zhu, W. Cancer Stem Cells and the Tumor Microenvironment in Tumor Drug Resistance. Stem Cell Rev Rep. 2023, 19, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar, S. , Ma, S.; et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012, 148, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares A, de-Maya-Girones JD, Calabuig-Fariñas S, Lucas R, Martínez A, Pardo-Sánchez JM; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Raniszewska A, Polubiec-Kownacka M, Rutkowska, E. , Domagala-Kulawik, J. PD-L1 Expression on Lung Cancer Stem Cells in Metastatic Lymph Nodes Aspirates. Stem Cell Rev Rep. 2019, 15, 324–330. [Google Scholar] [CrossRef]
- Raniszewska A, Vroman H, Dumoulin D, Cornelissen R, Aerts, J. , Domagała-Kulawik, J. PD-L1(+) lung cancer stem cells modify the metastatic lymph-node immunomicroenvironment in nsclc patients. Cancer Immunol Immunother. 2021, 70, 453–461. [Google Scholar] [CrossRef]
- Raniszewska A, Kwiecień I, Sokołowski R, Rutkowska E, Domagała-Kulawik, J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers (Basel). 2020, 12. [Google Scholar]
- Bui VT, Tseng HC, Kozlowska A, Maung PO, Kaur K, Topchyan, P. ; et al. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front Immunol. 2015, 6, 576. [Google Scholar]
- Lanier, LL. NK cell recognition. Annu Rev Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Kozlowska AK, Topchyan P, Kaur K, Tseng HC, Teruel, A. , Hiraga, T.; et al. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer. 2017, 8, 537–554. [Google Scholar] [CrossRef]
- Hersey P, Edwards A, Honeyman, M. , McCarthy WH. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br J Cancer. 1979, 40, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kuss I, Saito T, Johnson JT, Whiteside TL. Clinical significance of decreased zeta chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res. 1999, 5, 329–334. [Google Scholar]
- Guillerey, C. NK Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020, 1273, 69–90. [Google Scholar] [PubMed]
- Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Imai K, Matsuyama S, Miyake, S, Suga, K., Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet (London, England). 2000, 356, 1795–1799, https://doi.org/10.1016/s0140-6736(00)03231-1.
- Jewett A, Teruel A, Romero M, Head, C, Cacalano, N. Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab’)2 fragment of anti-CD16 antibody. Cancer immunology, immunotherapy : CII. 2008, 57, 1053–1066, https://doi.org/10.1007/s00262-007-0437-6.
- Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto, A. ; et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer research. 2012, 72, 1407–1415. [Google Scholar] [CrossRef]
- Hershey P, Edwards A, Milton GW, McCarthy WH. Relationship of cell-mediated cytotoxicity against melanoma cells to prognosis in melanoma patients. Br J Cancer. 1978, 37, 505–513. [Google Scholar] [CrossRef]
- Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani, G. , Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996, 2, 161–173. [Google Scholar]
- Burke S, Lakshmikanth T, Colucci, F. , Carbone, E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends in immunology. 2010, 31, 339–345. [Google Scholar] [CrossRef]
- Brittenden J, Heys SD, Ross J, Eremin, O. Natural killer cells and cancer. Cancer. 1996, 77, 1226–1243. [Google Scholar] [CrossRef]
- Chary, A. Culturing Human Lung Adenocarcinoma Cells in a Serum-Free Environment. Methods Mol Biol. 2023, 2645, 165–172. [Google Scholar] [PubMed]
- Amin A, Koul AM, Wani UM, Farooq F, Amin B, Wani Z; et al. Dissection of paracrine/autocrine interplay in lung tumor microenvironment mimicking cancer cell-monocyte co-culture models reveals proteins that promote inflammation and metastasis. BMC Cancer. 2023, 23, 926, https://doi.org/10.1186/s12885-023-11428-7. [Google Scholar]
- Kaur K, Kozlowska AK, Topchyan P, Ko MW, Ohanian N, Chiang, J.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers (Basel). 2019, 12. https://doi.org/10.3390/cancers12010063.
- Tseng HC, Bui V, Man YG, Cacalano N, Jewett, A. Induction of Split Anergy Conditions Natural Killer Cells to Promote Differentiation of Stem Cells through Cell-Cell Contact and Secreted Factors. Front Immunol. 2014, 5, 269, https://doi.org/10.3389/fimmu.2014.00269. [Google Scholar]
- Bui VT, Tseng H-C, Maung PO, Kozlowska A, Mann K, Topchyan P; et al. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Frontiers in immunology. 2015, 6. https://doi.org/10.3389/fimmu.2015.00576.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal, A. ; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kaur K, Nanut MP, Ko M-W, Safaie, T. , Kos, J., Jewett, A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: Strategies to optimize their growth and expansion for effective cancer immunotherapy. Current Opinion in Immunology. 2018, 51, 170–180. [Google Scholar] [CrossRef]
- Magister S, Obermajer N, Mirkovic B, Svajger U, Renko M, Softic, A. ; et al. Regulation of cathepsins S and L by cystatin F during maturation of dendritic cells. Eur J Cell Biol. 2012, 91, 391–401. [Google Scholar] [CrossRef]
- Tseng HC, Cacalano, N. , Jewett, A. Split anergized Natural Killer cells halt inflammation by inducing stem cell differentiation, resistance to NK cell cytotoxicity and prevention of cytokine and chemokine secretion. Oncotarget. 2015, 6, 8947–8959. [Google Scholar] [CrossRef]
- Bui VT, Tseng H-C, Kozlowska A, Maung PO, Kaur, K., Topchyan, P.; et al. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Frontiers in Immunology. 2015, 6. https://doi.org/10.3389/fimmu.2015.00576.
- Shacka JJ, Garner MA, Gonzalez JD, Ye YZ, D’Alessandro TL, Estévez AG. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death & Differentiation. 2006, 13, 1506–1514, https://doi.org/10.1038/sj.cdd.4401831. [Google Scholar]
- Abdelrahman KS, Hassan HA, Abdel-Aziz SA, Marzouk AA, Narumi, A. , Konno, H.; et al. JNK signaling as a target for anticancer therapy. Pharmacological Reports. 2021, 73, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Thielens A, Vivier E, Romagné, F. NK cell MHC class I specific receptors (KIR): From biology to clinical intervention. Current Opinion in Immunology. 2012, 24, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chang Y-T, Prompsy P, Kimeswenger S, Tsai Y-C, Ignatova, D. , Pavlova, O.; et al. MHC-I upregulation safeguards neoplastic T cells in the skin against NK cell-mediated eradication in mycosis fungoides. Nat Commun. 2024, 15, 752. [Google Scholar] [CrossRef]
- Joetham, A. TK, Miyahara, N., Matsubara, S., Ohnishi, H., Koya, T., Dakhama, A., Gelfand, E.W. Activation of naturally occurring lung CD54+CD25+ regulatory T cells requires CD8 and MHC 1 interaction. PNAS. 2007, 104, 1507–1516. [Google Scholar] [CrossRef]
- Jewett A, Cavalcanti M, Bonavida, B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol. 1997, 159, 4815–4822. [Google Scholar] [CrossRef]
- Jewett, A. , Bonavida, B. Interferon-alpha activates cytotoxic function but inhibits interleukin-2-mediated proliferation and tumor necrosis factor-alpha secretion by immature human natural killer cells. J Clin Immunol. 1995, 15, 35–44. [Google Scholar] [CrossRef]
- Jewett, A. , Bonavida, B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996, 156, 907–915. [Google Scholar] [CrossRef]
- Jewett A, Wang MY, Teruel A, Poupak Z, Bostanian Z, Park NH. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: Effect on the function of natural killer cells. Hum Immunol. 2003, 64, 505–520. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).