Submitted:
01 November 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
Introduction
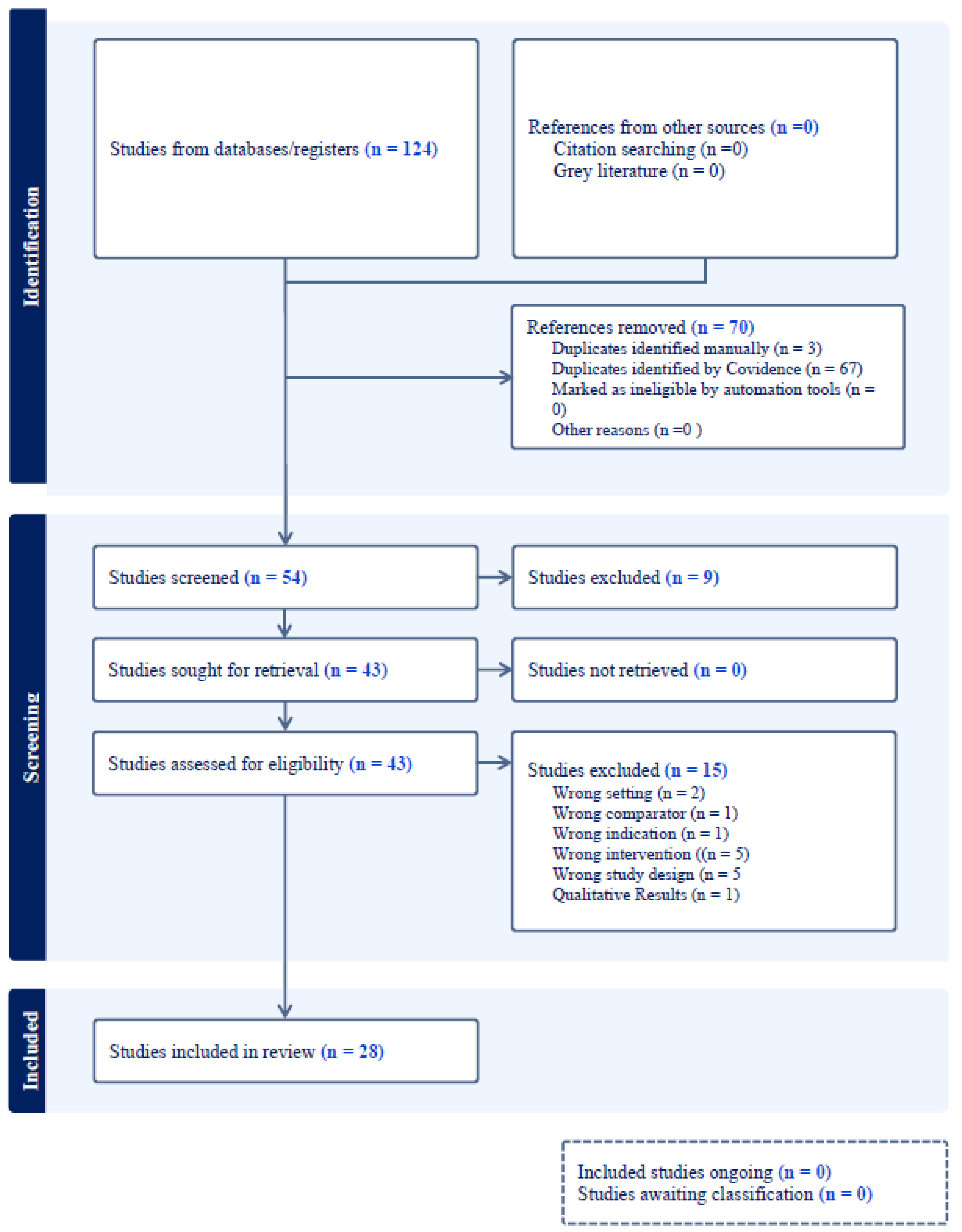
Methods
Exclusion Criteria
Search Strategy
Screening Methods and Data Extraction
Data Synthesis
Results
Healthcare Professional-Led Cognitive Stimulation
Family Led Cognitive Stimulations
Software-Based Cognitive Stimulation
Virtual reality-based cognitive stimulation
Discussion
Limitations
Conclusion
Funding Disclosures
Author Contributions
Funding
Acknowledgments
References
- Leslie, D.L.; Marcantonio, E.R.; Zhang, Y.; Leo-Summers, L.; Inouye, S.K. One-Year Health Care Costs Associated With Delirium in the Elderly Population. Arch. Intern. Med. 2008, 168, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Goldberg TE, Chen C, Wang Y, et al. Association of Delirium With Long-term Cognitive Decline: A Meta-analysis. JAMA Neurology 2020, 77, 1373–81. [Google Scholar] [CrossRef] [PubMed]
- Witlox, J.; Eurelings, L.S.M.; de Jonghe, J.F.M.; Kalisvaart, K.J.; Eikelenboom, P.; van Gool, W.A. Delirium in Elderly Patients and the Risk of Postdischarge Mortality, Institutionalization, and Dementia. JAMA 2010, 304, 443–451. [Google Scholar] [CrossRef]
- Khan, B.A.; Perkins, A.J.; Gao, S.; Hui, S.L.; Campbell, N.L.; Farber, M.O.; Chlan, L.L.; Boustani, M.A. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit. Care Med. 2017, 45, 851–857. [Google Scholar] [CrossRef]
- Collaborative GMR. Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on World Delirium Awareness Day. BMC Medicine 2019;17:229.
- Thein, M.Z.A.; Pereira, J.V.; Nitchingham, A.; Caplan, G.A. A call to action for delirium research: Meta-analysis and regression of delirium associated mortality. BMC Geriatr. 2020, 20, 325. [Google Scholar] [CrossRef]
- Alzheimer's disease facts and figures. Alzheimers Dement 2024;20:3708-821.
- Popp, J.; Kukreja, D.; Günther, U. Delirium in the elderly: Current problems with increasing geriatric age. Indian J. Med Res. 2015, 142, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Kwak, MJ. Delirium in Frail Older Adults. Ann Geriatr Med Res 2021;25:150-9.
- Al Farsi, R.S.; Al Alawi, A.M.; Al Huraizi, A.R.; Al-Saadi, T.; Al-Hamadani, N.; Al Zeedy, K.; Al-Maqbali, J.S. Delirium in Medically Hospitalized Patients: Prevalence, Recognition and Risk Factors: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 3897. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.; Du, Y.; Geng, J. Risk factors of delirium after gastrointestinal surgery: A meta-analysis. J. Clin. Nurs. 2023, 32, 3266–3276. [Google Scholar] [CrossRef]
- Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 2015;175:512-20.
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF Bundle in Critical Care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef]
- Tobar, E.; Alvarez, E.; Garrido, M. Cognitive stimulation and occupational therapy for delirium prevention. Rev. Bras. de Ter. Intensiv. 2017, 29, 248–252. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Yang, T.; Gartaganis, S.L.; Yue, J.; Inouye, S.K. Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am. J. Geriatr. Psychiatry 2018, 26, 1015–1033. [Google Scholar] [CrossRef]
- Costa DK, White MR, Ginier E, et al. Identifying Barriers to Delivering the Awakening and Breathing Coordination, Delirium, and Early Exercise/Mobility Bundle to Minimize Adverse Outcomes for Mechanically Ventilated Patients: A Systematic Review. Chest 2017;152:304-11.
- Morandi, A.; Piva, S.; Ely, E.W.; Myatra, S.N.M.; Salluh, J.I.; Amare, D.; Azoulay, E.; Bellelli, G.; Csomos, A.; Fan, E.; et al. Worldwide Survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) Bundle. Crit. Care Med. 2017, 45, e1111–e1122. [Google Scholar] [CrossRef]
- Gibbor, L.; Yates, L.; Volkmer, A.; Spector, A. Cognitive stimulation therapy (CST) for dementia: a systematic review of qualitative research. Aging Ment. Heal. 2021, 25, 980–990. [Google Scholar] [CrossRef]
- Woods, B.; Rai, H.K.; Elliott, E.; Aguirre, E.; Orrell, M.; Spector, A. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst. Rev. 2023, 2023. [Google Scholar] [CrossRef]
- Park, D.C.; Bischof, G.N.; Dc, P. The aging mind: neuroplasticity in response to cognitive training. Dialog- Clin. Neurosci. 2013, 15, 109–119. [Google Scholar] [CrossRef]
- Stern, Y. How Can Cognitive Reserve Promote Cognitive and Neurobehavioral Health? Archives of Clinical Neuropsychology 2021;36:1291-5.
- Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist 2000;40:206-12.
- Taulbee, L.R.; Folsom, J.C. Reality Orientation for Geriatric Patients. Psychiatr. Serv. 1966, 17, 133–135. [Google Scholar] [CrossRef]
- Orrell, M.; Yates, L.; Leung, P.; Kang, S.; Hoare, Z.; Whitaker, C.; Burns, A.; Knapp, M.; Leroi, I.; Moniz-Cook, E.; et al. The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial. PLOS Med. 2017, 14, e1002269. [Google Scholar] [CrossRef]
- Al-Thaqib, A.; Al-Sultan, F.; Al-Zahrani, A.; Al-Kahtani, F.; Al-Regaiey, K.; Iqbal, M.; Bashir, S. Brain Training Games Enhance Cognitive Function in Healthy Subjects. Med Sci. Monit. Basic Res. 2018, 24, 63–69. [Google Scholar] [CrossRef]
- Tapia, J.L.; Puertas, F.J.; Duñabeitia, J.A. Digital Therapeutics for Insomnia: Assessing the Effectiveness of a Computerized Home-Based Cognitive Stimulation Program. J. Integr. Neurosci. 2023, 22, 34. [Google Scholar] [CrossRef]
- Parker, A.M.; Aldabain, L.; Akhlaghi, N.; Glover, M.; Yost, S.; Velaetis, M.; Lavezza, A.; Mantheiy, E.; Albert, K.; Needham, D.M. Cognitive Stimulation in an Intensive Care Unit: A Qualitative Evaluation of Barriers to and Facilitators of Implementation. Crit. Care Nurse 2021, 41, 51–60. [Google Scholar] [CrossRef]
- Kallio, E.-L.; Öhman, H.; Kautiainen, H.; Hietanen, M.; Pitkälä, K. Cognitive Training Interventions for Patients with Alzheimer’s Disease: A Systematic Review. J. Alzheimer's Dis. 2017, 56, 1349–1372. [Google Scholar] [CrossRef]
- Barnes DE, Yaffe K, Belfor N, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord 2009;23:205-10.
- Brugada-Ramentol, V.; Bozorgzadeh, A.; Jalali, H. Enhance VR: A Multisensory Approach to Cognitive Training and Monitoring. Front. Digit. Heal. 2022, 4, 916052. [Google Scholar] [CrossRef]
- Green, C.S.; Bavelier, D. Action video game modifies visual selective attention. Nature 2003, 423, 534–537. [Google Scholar] [CrossRef]
- Lee, H.K.; Kent, J.D.; Wendel, C.; Wolinsky, F.D.; Foster, E.D.; Merzenich, M.M.; Voss, M.W. Home-Based, Adaptive Cognitive Training for Cognitively Normal Older adults: Initial Efficacy Trial. Journals Gerontol. Ser. B 2020, 75, 1144–1154. [Google Scholar] [CrossRef]
- Lumsden, J.; A Edwards, E.; Lawrence, N.S.; Coyle, D.; Munafò, M.R. Gamification of Cognitive Assessment and Cognitive Training: A Systematic Review of Applications and Efficacy. JMIR Serious Games 2016, 4, e11. [Google Scholar] [CrossRef]
- Man, D.W.K.; Poon, W.S.; Lam, C. The effectiveness of artificial intelligent 3-D virtual reality vocational problem-solving training in enhancing employment opportunities for people with traumatic brain injury. Brain Inj. 2013, 27, 1016–1025. [Google Scholar] [CrossRef]
- Deemer, K.; Zjadewicz, K.; Fiest, K.; Oviatt, S.; Parsons, M.; Myhre, B.; Posadas-Calleja, J. Effect of early cognitive interventions on delirium in critically ill patients: a systematic review. Can. J. Anaesth. 2020, 67, 1016–1034. [Google Scholar] [CrossRef]
- Johnson, G.U.; Towell-Barnard, A.; McLean, C.; Ewens, B. Delirium prevention and management in an adult intensive care unit through evidence-based nonpharmacological interventions: A scoping review. Collegian 2024, 31, 232–251. [Google Scholar] [CrossRef]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34.
- Miranda F, Gonzalez F, Plana MN, Zamora J, Quinn TJ, Seron P. Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) for the diagnosis of delirium in adults in critical care settings. Cochrane Database Syst Rev 2023;11:Cd013126.
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
- Chen, C.-Y.; Ding, H.; Wang, S.-S. Effectiveness of Roy Adaptation Model-Based Cognitive Stimulation Therapy in Elderly Patients with Non-Small Cell Lung Cancer Undergoing Curative Resection. Tohoku J. Exp. Med. 2024, 263, 27–34. [Google Scholar] [CrossRef]
- Gaudreau, J.-D.; Gagnon, P.; Harel, F.; Roy, M.-A. Impact on delirium detection of using a sensitive instrument integrated into clinical practice. Gen. Hosp. Psychiatry 2005, 27, 194–199. [Google Scholar] [CrossRef]
- Faustino, T.N.; Suzart, N.A.; Rabelo, R.N.d.S.; Santos, J.L.; Batista, G.S.; de Freitas, Y.S.; Saback, D.A.; Sales, N.M.M.D.; Barreto, B.B.; Gusmao-Flores, D. Effectiveness of combined non-pharmacological interventions in the prevention of delirium in critically ill patients: A randomized clinical trial. J. Crit. Care 2022, 68, 114–120. [Google Scholar] [CrossRef]
- Martínez, F.; Donoso, A.M.; Marquez, C.; Labarca, E. Implementing a Multicomponent Intervention to Prevent Delirium Among Critically Ill Patients. Crit. Care Nurse 2017, 37, 36–46. [Google Scholar] [CrossRef]
- Mudge, A.M.; Giebel, A.J.; Cutler, A.J. Exercising Body and Mind: An Integrated Approach to Functional Independence in Hospitalized Older People. J. Am. Geriatr. Soc. 2008, 56, 630–635. [Google Scholar] [CrossRef]
- Inouye, S.K.; Leo-Summers, L.; Zhang, Y.; Bogardus, S.T.; Leslie, D.L.; Agostini, J.V. A Chart-Based Method for Identification of Delirium: Validation Compared with Interviewer Ratings Using the Confusion Assessment Method. J. Am. Geriatr. Soc. 2005, 53, 312–318. [Google Scholar] [CrossRef]
- Álvarez, E.A.; Garrido, M.A.; Tobar, E.A.; Prieto, S.A.; Vergara, S.O.; Briceño, C.D.; González, F.J. Occupational therapy for delirium management in elderly patients without mechanical ventilation in an intensive care unit: A pilot randomized clinical trial. J. Crit. Care 2017, 37, 85–90. [Google Scholar] [CrossRef]
- Rivosecchi, R.M.; Kane-Gill, S.L.; Svec, S.; Campbell, S.; Smithburger, P.L. The implementation of a nonpharmacologic protocol to prevent intensive care delirium. J. Crit. Care 2016, 31, 206–211. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Bergeron, N.; Dubois, M.-J.; Dumont, M.; Dial, S.; Skrobik, Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensiv. Care Med. 2001, 27, 859–864. [Google Scholar] [CrossRef]
- Colombo, R.; Corona, A.; Praga, F.; Minari, C.; Giannotti, C.; Castelli, A.; Raimondi, F. A reorientation strategy for reducing delirium in the critically ill. Results of an interventional study.. 2012, 78, 1026–1033. [Google Scholar]
- Şanlıtürk, D.; Kaplan, V.; Dörtkardeş, N. Preventive Effect of Cognitive Stimulation and Sleep Hygiene on Delirium in COVID-19 Intensive Care Patients. J. Turk. Sleep Med. 2023, 10, 206–215. [Google Scholar] [CrossRef]
- Mitchell, M.L.; Kean, S.; Rattray, J.E.; Hull, A.M.; Davis, C.; Murfield, J.E.; Aitken, L.M. A family intervention to reduce delirium in hospitalised ICU patients: A feasibility randomised controlled trial. Intensiv. Crit. Care Nurs. 2017, 40, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.L.; Cairns, P.; Ji, M.; Calero, K.; Anderson, W.M.; Liang, Z. Delirium prevention in critically ill adults through an automated reorientation intervention – A pilot randomized controlled trial. Hear. Lung 2017, 46, 234–238. [Google Scholar] [CrossRef] [PubMed]
- A Alvarez, E.; Garrido, M.; Ponce, D.P.; Pizarro, G.; A Córdova, A.; Vera, F.; Ruiz, R.; Fernández, R.; Velásquez, J.D.; Tobar, E.; et al. A software to prevent delirium in hospitalised older adults: development and feasibility assessment. Age and Ageing 2020, 49, 239–245. [Google Scholar] [CrossRef]
- Faisal, H.; Masud, F.N.; Junhyoung, K.; Podell, K.; Xu, J.; Boncyk, C.; Taffet, G.E.; Boustani, M.A. Virtual reality-based cognitive exercise games in geriatric surgical patients: A pilot trial. J. Am. Geriatr. Soc. 2024. [Google Scholar] [CrossRef]
- Faisal, H.; Lim, W.; Dattagupta, A.; Lin, P.; Gupta, R.; Lai, E.C.; Xu, J.; Wong, S.T.; Masud, F.N. Usability and Tolerability of Virtual Reality-Based Cognitive Stimulation in Healthy Elderly Volunteers—A Feasibility Clinical Trial. Games Heal. J. 2024. [Google Scholar] [CrossRef]
| Study | Purpose/intervention | Design, age, sample size | Outcomes and outcome measures | Results | Conclusion |
|---|---|---|---|---|---|
| Healthcare Professional-led cognitive stimulation | |||||
|
Chen et al. (2024) [40] |
To investigate the effects of a Royal Adaptation Model (RAM)-based cognitive stimulation therapy (CST) on older patients with primary non-small cell lung cancer (NSCLC) undergoing curative resection | Single-center Randomized controlled trial (RCT) Age > 65 years n=280 |
Delirium prevalence/incidence using the Nursing Delirium Screening Scale. [41] |
Incidence of delirium: 20.71 % in the control group Vs. 10.71% in the RAM-based CST group (P=0.032) | RAM-based CST in elderly NSCLC patients undergoing curative resection yielded reduced delirium incidence. |
| Faustino et al. (2022) [42] |
To evaluate the effectiveness of combined non-pharmacological interventions (periodic reorientation, cognitive stimulation, correction of sensory deficits) in preventing delirium in critically ill patients | Single-center RCT Age >18 years n= 144 |
Delirium incidence density using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) tool. [38] | Incidence density of delirium: (2.3 × 10−2 person-days) in control group Vs. (1.3 × 10−2 person-days) in the intervention group. | Combined non-pharmacological interventions reduced delirium in critically ill patients compared to standard care. |
| Martinez et al. (2017) [43] |
To assess the effectiveness of a tailored multicomponent intervention (early mobilization for preventing the incidence of delirium among critically ill patients. | A before-and-after study Age> 18 years n=227 |
Delirium incidence using CAM-ICU tool. [38] | Incidence of delirium: Reduced from 38% to 24% (relative risk, 0.62; 95% CI, 0.40-0.94; P = .02) | Multicomponent strategy successfully reduced delirium. Early participation of the whole team, shared leadership, and the provision of concrete tasks were key to the intervention's success. |
| Mudge et al. (2008) [44] | To evaluate the effect of a structured, multi-component, early rehabilitation program on delirium of older acute medical inpatients. |
Prospective controlled trial Age ≥ 65 years n=124 |
Incidence of delirium. Delirium was identified according to chart review using validated methodology. [45] | Incidence of delirium: 35.5% in control group Vs. 19.4% in the intervention group (P=0.19) | In the intervention group, there was a reduction in delirium. |
| Alvarez et al. (2017) [46] |
To determine the impact of occupational therapy (OT) -led cognitive intervention protocol on the incidence, duration, and severity of delirium in older ICU patients | Pilot study, RCT Age>60 years n=140 |
Delirium incidence and duration using the CAM ICU tool.[38] | Incidence of delirium: 20% in the control group Vs 3 % in the treatment group (P=0.01) Duration of delirium: lower in the treatment group (IRR, 0.15; 95% CI, 0.12 to 0.19; P<0.001): Control group (IRR, 6.7; 95% CI, 5.2 to 8.3; P<0.001). |
A combination of early OT and cognitive intervention strategies decreases the incidence and duration of delirium. |
| Rivosecchi et al. (2016) [47] |
To assess whether an evidence-based non-pharmacologic protocol could further decrease the duration of delirium in patients in a medical ICU that already implements a sedation and mobility protocol. [47] | Prospective, pre-post intervention QI project. (n=483). Phase I: baseline data collection before protocol implementation (n=230). Phase II: development and implementation of non-pharmacologic protocol |
Incidence and duration of delirium in phase 1 vs 2, using the Intensive Care Delirium Screening Checklist (ICDSC). [49] |
Phase I Vs. Phase II delirium incidence (15.7% Vs. 9.4%; P=0.04). Median duration of delirium in Phase I (20 hours) and Phase II (16 hours), (50.6% reduction; P<0.001) |
Nonpharmacologic strategies reduce risk and duration of delirium in the ICU, even if a mobilization protocol and sedation algorithm are already in place. |
| Colombo et al. (2012)50 | To assess the efficacy of the cognitive stimulation protocol (orientation, environmental, acoustic, and visual interventions) on delirium in medical and surgical ICU patients | Two-stage prospective- observational study. Age > 18 years Phase 1: observational (n=170) phase II interventional (n=144) |
Delirium occurrence using the CAM-ICU tool.[38] |
Delirium occurrence was lower (36% in phase I vs 22% in phase II, P=0.02). |
A reorientation strategy was associated with a reduced incidence of delirium. |
| Şanlıtürk et al. (2023)51 | To evaluate the effect of two-stage intervention (sensory stimulation and sleep hygiene) on delirium in Coronavirus disease-2019 (COVID-19) patients | Pre-test/post-test control group and trial model. Age>18 years n=92 |
Delirium incidence using CAM-ICU tool.[38] | Incidence of delirium: 80% in control group Vs. 56% in the intervention group (P<0.05) |
The sensory stimulation and sleep hygiene intervention based on the nursing model effectively reduced the incidence of delirium in critically ill COVID-19 patients. |
| Family-led cognitive stimulations | |||||
| Mitchell et al. (2017) [52] | To evaluate the feasibility and acceptability of a family-delivered intervention (orientation or memory clues, sensory checks, and therapeutic or cognitive stimulation) to reduce delirium in hospitalized ICU patients. | Single-center Feasibility RCT Age ≥16 years n=61 |
Retention of family members, feasibility, and acceptability of the intervention |
No family member withdrew from the intervention group, and one withdrew from the control group. Low recruitment rate (28%) | The feasibility of recruiting and retaining family members participants; nurse supportive of interventions |
| Munro et al. (2017) [53] | To determine if recorded audio-orienting messages (automated orientation messages in a family member's voice) reduce the risk of delirium in critically ill adults. | Prospective RCT Age> 18 years n=30 |
Delirium-free days evaluated by CAM-ICU. [38] | Mean delirium-free days: 1.9 in the family voice group, 1.6unknownvogroup, and 1.6 in the control group (P =0.04) | Participants exposed to recorded voice messages from family members had more delirium-free days. |
| Software-based & Virtual-Reality (VR)-based cognitive stimulation | |||||
| E A. Alvarez et al. (2020) [54] | To determine the clinical feasibility assessment of software by older adults | Feasibility study Age> 75 years n=30 |
Delirium incidence using the CAM -ICU tool. [38] |
Software use was associated with a decrease in delirium incidence of 5 of 32 (15.6%) at baseline to 2 of 30 (6.6%) after its implementation. |
Use of software to improve the delivery of non-pharmacological interventions may prevent delirium. |
| Faisal et al. (2024) [55] | To determine VR-based cognitive stimulation games' safety, feasibility, and acceptability for preventing delirium in older surgical patients. | Pilot trial Age ≥ 60 years n=30 |
Safety, feasibility, and acceptability. Delirium incidence using the CAM tool. [38] |
ReCognitionVR-based cognitive games were safe, feasible, and mean Mean System Usability Scale (SUS) score of 92 (SD = 8) |
The study did not observe any differences in delirium occurrence due to the small sample size. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





