1. Introduction
Motion sensitivity symptoms, like dizziness or unsteadiness, are frequently reported as non-pain symptoms of migraine [
1,
2]. Several possible explanations have been put forward to explain this symptomatology like a malfunctioning of multisensory integration process [
3] and peripheral and central disarrangements [
4,
5], including structures of the inner ear, brainstem [
6], basal ganglia, and cerebellum [
7], leading to a mismatch between proprioceptive cues and vestibular [
8] or visual stimuli [
9]. Balance impairment has also been described in subjects with migraine, with imbalance increasing with the manipulation of sensory inputs while standing [
10]. Using the sensory organization test, which is performed through a dynamic posturography system while standing, differences in postural balance between the subdiagnosis of migraine have been described, with greater instability and falls risk in subjects with vestibular migraine, chronic migraine, and migraine with aura[
10,
11,
12], whereas subjects with episodic migraine showed less pronounced balance alterations, compared with healthy controls and subjects with chronic migraine[
13,
14] However, regardless of the presence of chronic migraine and aura, subjects with migraine may experience abnormalities during mobility tasks and walking, such as reduction of gait speed and increased step width [
15,
16], which are common signs of dynamic imbalance [
17,
18]. This suggests the presence of dynamic instability also in subjects with episodic migraine without referring the experience of aura. In this way, instrumented assessing of balance during gait inertial measurement units (IMUs) may provide insights into the subtle postural instability among the subcategory of subjects with migraine that cannot be found out through the somatosensory orientation test [
19]. Inertial measurement units (IMUs), which extract gait parameters from acceleration and angular velocity data, are widely used for instrumented gait analysis due to their ease of use and ability to retrieve motion data in real-world, non-laboratory settings. Aside from the spatiotemporal and pelvic kinematic gait parameters, trunk acceleration-derived gait quality indexes can be calculated using lower-trunk acceleration signals. Particularly, nonlinear analysis of the trunk acceleration patterns through the short-term maximal Lyapunov’s exponent (sLLE) has proven to accurately characterize gait imbalance in several neurologic conditions [
20,
21,
22,
23,
24]. sLLE quantifies the rate of divergence of the trajectories in a system's state space over a short period and assesses local dynamic stability by measuring the sensitivity of the system to small perturbations in the initial conditions, where a positive exponent indicates divergence (instability), and a negative exponent indicates convergence (stability) of trajectories in the short term [
25,
26].
The aim of this study was to gain new insights into the mechanisms of motion sensitivity symptoms in individuals affected by migraine without aura (MO) studied during the inter-ictal phase. Therefore, using a wearable device, we assessed the ability of the sLLE to detect differences in local dynamic stability between MO and healthy subjects. Greater knowledge of how migraine symptomology and related disability interfere with locomotor demands in patients with MO may facilitate targeted intervention strategies. We hypothesized that the MO may exhibit dynamic imbalance due to abnormalities of the multisensory integration process, and that the sLLE, a measure of the ability to cope with internal perturbations during gait, might reflect local dynamic instability in MO.
2. Materials and Methods
2.1. Participants
This cross – sectional study was conducted at the Traumatic Orthopedic Surgical Institute (ICOT) in Latina, Italy, between March 2022 and April 2024. Fifty-six MO were screened for eligibility and 47 MO were included in this study. Inclusion criteria were: i) the diagnosis of migraine without aura according to the International Classification of Headache Disorders (III edition)[
27]; ii) episodic migraine pattern, defined as 1 to 14 monthly migraine days during the preceding three months; iii) being during the interictal period at the day of the assessment, i.e. at least three days since the last and the next migraine attack; iv) being without any migraine prevention during the preceding 3 months. Exclusion criteria were: i) the presence of other primary or secondary headaches, including migraine with aura; ii) concomitance with the menstrual period for female subjects; iii) orthopedic, neuro-ophthalmologic, and neurologic conditions other than migraine; iv) ongoing pharmacological therapy, including migraine preventives, else than acute migraine attack medications, and contraceptives. The presence of neuro-ophthalmologic disease was verified through examination that included a visual acuity test, an intraocular pressure measurement, and indirect ophthalmoscopy. Subjects were administered the migraine disability assessment (MIDAS) and the headache impact test (HIT-6) scales to assess the impact of migraine on functioning of subjects, the 12-item Allodynia Symptom Checklist (ASC-12), and the numeric pain rating scale (NPRS) to record the perceived pain during migraine attacks.
For group comparison, a group of healthy subjects (HS) matched for age and gait speed was enrolled. A 1:1 optimal data matching procedure using the propensity score difference method was performed to match patients with MO with healthy subjects (HS)[
28]. From a dataset of 96 HS, as a result, 38 HS were included after the matching procedure, whose effectiveness was assessed using an independent sample t-test using age and gait speed as the variables. Every participant in the study was required to complete a headache diary, which was sent to them by mail at least three months prior to their initial visit. The characteristics of the included sample are described in
Table 1. All participants provided informed consent prior to the experimental procedure. The study was approved by the local ethics committee (CE Lazio 2, protocol number 0139696/2021).
2.2. Procedures
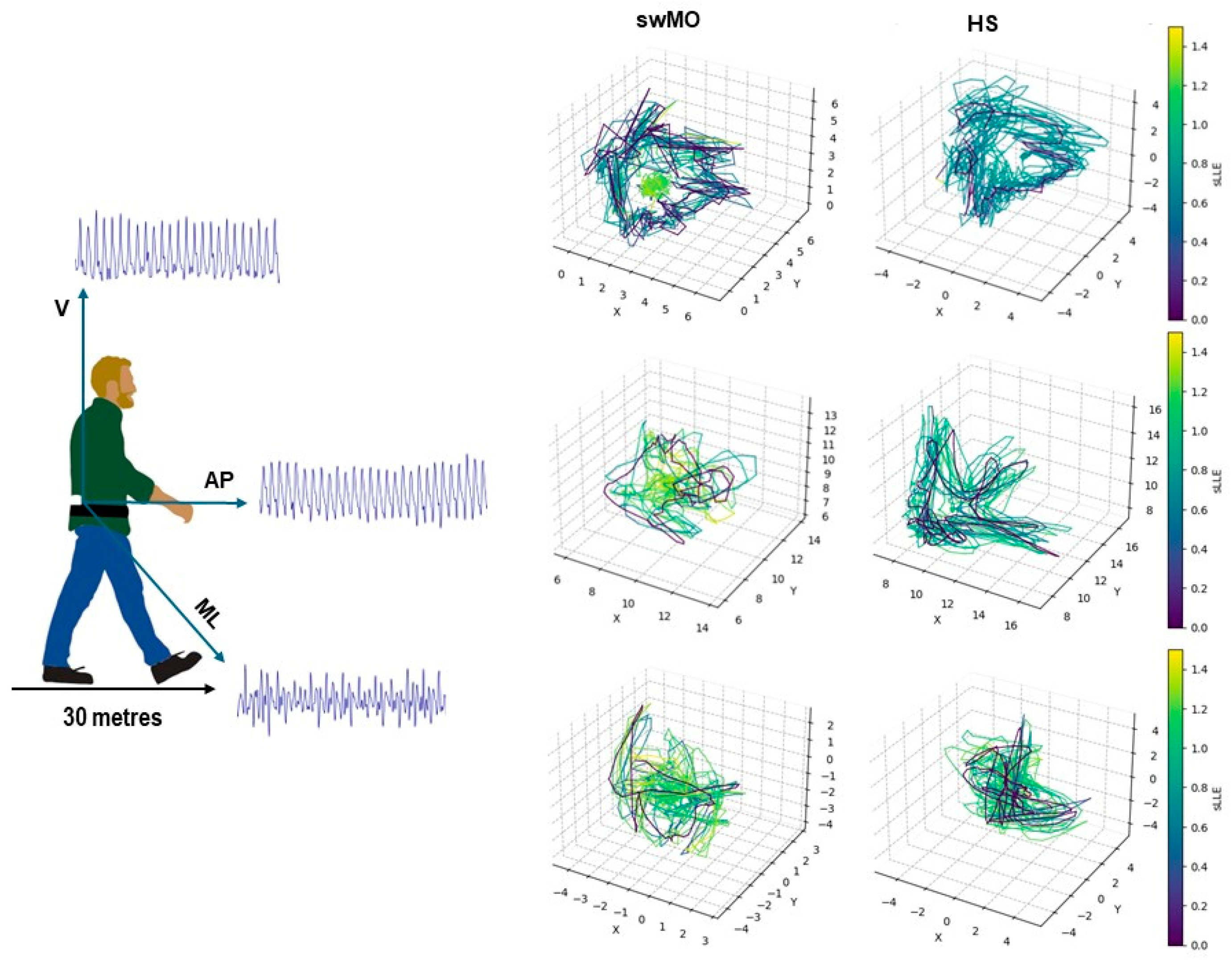
Participants were instructed to walk along a straight path that measured 30 meters in length, at a speed that they personally chose as their favorite walking pace. The corridor floor was linoleum, with no visible pavement joints or demarcation lines, and indirect lighting was evenly distributed along the path. Before the experiment, participants were instructed to walk along the trail to familiarize themselves with the procedure. There were no adverse events recorded during the procedures. There were no external stimuli given during the task. The trunk acceleration signals were recorded at a frequency of 100 Hz using a single magneto-inertial measurement unit (GSensor, BTS, Milano, Italy). The unit was positioned at the L5-S1 level and secured to the pelvis with a velcro belt. Data were acquired through the GStudio software (GStudio, BTS, Milano, Italy) using the “Walk +” embedded tool, and spatio-temporal gait parameters, as well as pelvic kinematics were calculated. To ensure a steady-state walking assessment, we removed the first and last two strides from each 30-meter walk. Any gait trials that had fewer than 20 accurately recorded consecutive strides were not included in the study [
29,
30,
31].
2.3. sLLE Calculations
The short-term maximum finite-time Lyapunov’s exponents (sLLE) were calculated based on the acceleration patterns for antero -posterior (sLLEAP), medio-lateral (sLLEML), and vertical (sLLEV) directions according to the Rosenstein’s algorithm using the Lyaprosen toolbox for nonlinear time series analysis in MATLAB environment (MATLAB 7.4.0, MathWorks, Natick, MA, USA). Twenty consecutive strides were considered for the calculations, and the acceleration signals were time-normalized to obtain 100 datapoints per stride [
32]. The embedding dimension was calculated using the false nearest neighbor method, and time delay was calculated as the first minimum of the average mutual information (AMI) function [
32]. Consequently, in this study an embedding dimension = 5 and time delay = 10 were used for multidimensional state - space reconstruction from the recorded one-dimensional time-series data by juxtaposing the original data and delayed copies (
Figure 1). Higher sLLE values reflects higher local dynamic instability.
2.4. Statistical Analysis
A sample size of at least 48 subjects, 24 MO, and 24 controls, was calculated to identify a good ability (AUC > 0.70) to discriminate between MO and controls at a 95% significance level and 80% power.
Statistical analysis was conducted using JASP software (Version 0.17.2.1), and NCSS 2023 software. Correlation analysis was conducted using the “Pingouin” Python package [
33], vers. 0.5.3.
After verifying the homogeneity of the variances and the normality of the distributions using the Levene’s test and Shapiro-Wilk test, an independent sample t-test or Mann-Whitney test was implemented to assess the differences in gait parameters between MO subjects and HS. Cohen’s effect size (d) was also calculated to assess the magnitude of the differences.
Receiver operating characteristics (ROC) were plotted for the significant gait variables, and the area under the ROC curve (AUC) was calculated to assess the overall ability of the significant gait variables to discriminate between the groups. AUC values ≥ 0.70 were considered as reflecting good discriminative ability. The optimal cutoff point (OCP) was calculated as the value that maximizes the sum of sensitivity and specificity, and positive and negative likelihood ratios (LR+ and LR-, respectively) at the OCP were calculated. Positive and negative post-test probabilities (PTP+ and PTP-) were calculated by transforming the likelihood ratios through a Fagan's nomogram to estimate the likelihood of correctly classifying at the OCP. To improve the generalizability of the results, the 12% [
34] prevalence of episodic migraine was used as the prior probability in the post-test probabilities calculations.
Spearman’s partial correlation coefficients (ρ) excluding the effects of gait speed were conducted to assess the correlation between the gait variables with good discriminative ability and the clinical and gait variables. To account for tied scores, the tie correction factor was applied to the correlation coefficients using the formula:
where
di is the difference between the ranks of the values corresponding to the two variables,
n is the number of observations, and
ti is the number of tied ranks for each tie group. The term
is the sum of the cubes of the number of ties minus the number of ties for each distinct number of tied values, summed over all sets of ties.
3. Results
Forty - seven MO subjects, aged 34.13 ± 13.89 years, of whom 38 (81.25%) were females, and walking at an average speed of 1.16 ± 0.17 m/s were included. Subjects had been diagnosed with MO since 19.82 ± 12.21 months, with an average of 5.64 ± 4.63 days of migraine per month, lasting 43.85 ± 40.38 hours on average. NSAIDs were the most common type of symptomatic medication, with an average of 6.79 ± 6.30 doses per month. Subjects with MO were assessed 10.64 ± 15.15 days since the last migraine attack. The included 38 healthy participants were 38.27 ± 12.46 years old, 27 of whom were females, and walking at an average gait speed of 1.21 ± 0.15 m/s. As a result of the matching procedure, no significant differences in age and gait speed were found between subjects with MO and HS (age: p = 0.15, Cohen’s d = 0.31; gait speed: p = 0.11, Cohen’s d = 0.36).
Trunk Acceleration-Derived Gait Indexes
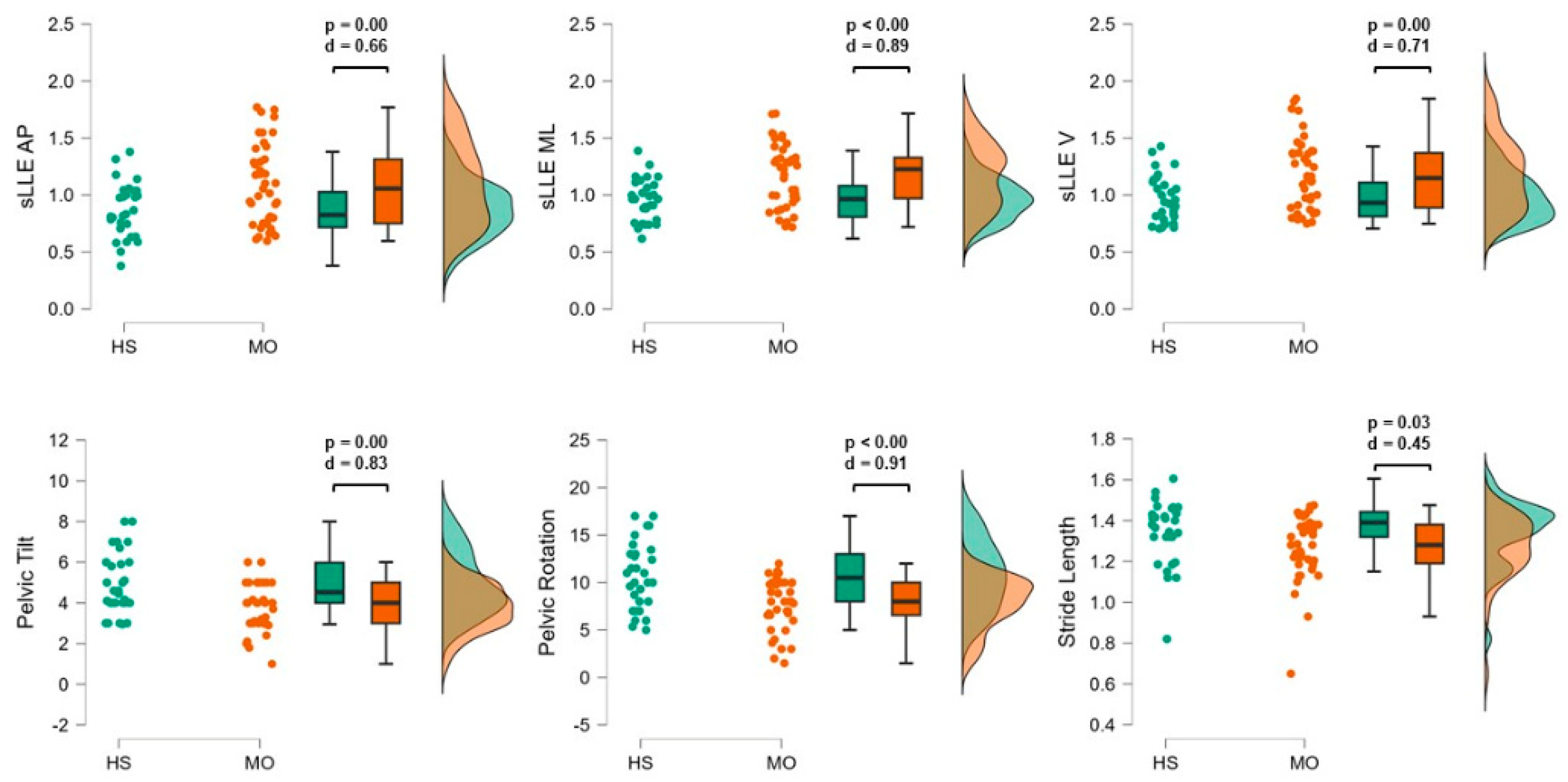
Compared with HS, subjects with MO resulted in higher sLLEAP (p < 0.01; d = 0.66), sLLEML (p < 0.01; d = 0.89), and sLLEV (p < 0.01; d = 0.71) and reduced pelvic rotation (p <0.01; d = 0.91), pelvic tilt (p < 0.01; d = 0.83), and stride length values (p = 0.03; d = 0.45) (
Figure 2).
sLLEML and pelvic rotation showed good ability to discriminate between MO and HS (
Table 2). After adjusting the post-test probabilities based on the 12% prevalence of episodic migraine in the general population, sLLEML values higher than 1.18 and pelvic rotation values lower than 11.50° showed 73% and 70% probability to correctly classify patients with MO (
Table 2).
Regardless of gait speed, sLLEML positively correlated with MIDAS score (ρ = 0.43, p = 0.01), and negatively correlated with pelvic rotation (ρ = ─ 0.47, p = 0.00), and NRPS (ρ = ─ 0.33, p = 0.03). Pelvic rotation values also negatively correlated with NPRS (ρ = ─ 0.35, p = 0.00) and MIDAS score (ρ = ─ 0.35, p = 0.03).
4. Discussion
The objective of this study was to investigate gait indexes reflecting mechanisms of motion sensitivity symptoms in a well-characterized group of people suffering from episodic migraine without aura between attacks by the evaluation of the ability of the sLLE to detect gait instability. We found that, regardless of age and gait speed, patients with MO exhibit greater sLLE values in all spatial directions compared with healthy subjects (
Figure 2). Particularly, sLLE values in the ML direction ≥ 1.18 characterized MO with 73% probability (
Table 2). Moreover, correlation analysis revealed that the sLLEML reflects the level of pain and disability caused by migraine, with greater gait local instability correlating with greater disability and severity of headache, as measured by the MIDAS questionnaire and NRPS scale, respectively. No correlations between sLLE and disease duration, the duration of the attacks, the monthly migraine days, nor the days passing since the last migraine attack at the moment of the gait assessment were found, suggesting that subjects with MO present local dynamic instability regardless of the global disease activity.
Postural balance impairment has been described in subjects with vestibular migraine and chronic migraine, with subjects with episodic migraine reporting similar postural balance findings to healthy controls [
11,
35,
36,
37]. However, these observations are based on the somatosensory orientation test, which does not assess the dynamic behavior during natural gait. There is many compelling evidence that subjects with episodic migraine without aura do exhibit significant cortical dysexcitability, abnormalities in executive functions, and in integrative pain processing [
38,
39], which could influence sensorimotor activity. Consequently, effective balance function and motion, i.e. dynamic stability may result impaired. Furthermore, data from functional neuroimaging studies showed altered brain network patterns committed to multisensory integration, including sensorimotor and executive control, in migraine without aura [
40,
41]. These functional changes affect key brain regions related to pain perception, autonomic responses, gait control, and cognitive processing, suggesting that migraine might hinder the ability of the brain to process and integrate sensory information effectively [
41]. We argue that this interictal disrupted sensory processing, in conjunction with the well-known abnormalities in the visual processing [
3,
42,
43] may contribute to dynamic instability by reducing individuals' ability to respond to environmental changes and maintain balance. Therefore, the findings of our study suggest that assessing local dynamic stability during gait through the sLLEML may detect the subtle abnormalities in dynamic balance control also in subjects with episodic migraine without aura, as a result of the impairment of multisensory integration process [
3].
Furthermore, we found that participants with MO exhibited lower pelvic rotation range during gait (
Figure 2), compared with HS, and that pelvic rotation values lower than 11.50° characterized MO with 70% probability (
Table 2). Reduction in pelvic rotation was also correlated with higher sLLEML values, MIDAS questionnaire and NPRS. Subjects with migraine have been described to present decreased mobility of cervical spine and increased stiffness of neck muscles [
44]. Moreover, the results are consistent with other studies reporting the sLLEML to correlate with pelvic mobility [
45,
46]. Therefore, another possible explanation of our results is that patients with MO stiffen their axial movements, in the context of cautious gait pattern due to perceived pain and instability due to multisensory integration impairment, thus increasing local instability of the trunk acceleration patterns.
This study presents several limitations. Although inclusion criteria satisfied the definition of migraine without aura according to the International Classification of Headache Disorders, a complete vestibular screening was not conducted. Furthermore, although we used the actual prevalence of episodic migraine in the general population as the pre-test probability in the post-test probability calculations, our sample was relatively small and did not reflect the prevalence of migraine. Therefore, further studies with broader populations are needed to confirm our findings.
5. Conclusions
Subjects with episodic migraine without aura exhibit abnormalities in trunk local dynamic stability during gait, as measured by the sLLE in the medio – lateral direction. This parameter correlated with disease-related disability levels, severity of pain during the attacks, and reduction in pelvic rotation during gait. The sLLE in the ML direction can be used as an index to quantify gait instability and the ability to face small perturbations during gait in subjects with episodic migraine.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, G.C., S.F.C., and M.S.; Data curation, D.T., S.F.C., G.S., C.A., F.C., C.D.L., R.D.I.; Formal analysis, D.T., S.F.C., A.D.R.; Investigation, G.C., C.D.L., G.S., C.A., F.C., R.D.I., L.Z., V.P.; Methodology, D.T., S.F.C., A.D.R.; Resources, G.C. and M.S.; Supervision, G.C.; Visualization, D.T. and S.F.C.; Writing – original draft, D.T. and S.F.C.; Writing – review & editing, C.T., V.P., M.S. and G.C.. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee Lazio 2 of Rome (protocol number 0139696/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The contribution of the G.B. Bietti Foundation to this paper was supported by the Italian Ministry of Health and Fondazione Roma.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Furman, J.M.; Marcus, D.A. Migraine and Motion Sensitivity. Continuum (Minneap Minn) 2012, 18, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Bisdorff, A. Migraine and Dizziness. Curr Opin Neurol 2014, 27, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sebastianelli, G.; Abagnale, C.; Casillo, F.; Cioffi, E.; Parisi, V.; Di Lorenzo, C.; Serrao, M.; Porcaro, C.; Schoenen, J.; Coppola, G. Bimodal Sensory Integration in Migraine: A Study of the Effect of Visual Stimulation on Somatosensory Evoked Cortical Responses. Cephalalgia 2022, 42, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Puledda, F.; Viganò, A.; Sebastianelli, G.; Parisi, V.; Hsiao, F.J.; Wang, S.J.; Chen, W.T.; Massimini, M.; Coppola, G. Electrophysiological Findings in Migraine May Reflect Abnormal Synaptic Plasticity Mechanisms: A Narrative Review. Cephalalgia 2023, 43, 03331024231195780. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Straumann, D. Moving in a Moving World: A Review on Vestibular Motion Sickness. Front Neurol 2016, 7, 14. [Google Scholar] [CrossRef]
- Hong, S.M.; Kim, S.K.; Park, C.H.; Lee, J.H. Vestibular-Evoked Myogenic Potentials in Migrainous Vertigo. Otolaryngol Head Neck Surg 2011, 144, 284–287. [Google Scholar] [CrossRef]
- Mehnert, J.; May, A. Functional and Structural Alterations in the Migraine Cerebellum. Journal of Cerebral Blood Flow and Metabolism 2019, 39, 730–739. [Google Scholar] [CrossRef]
- Murdin, L.; Chamberlain, F.; Cheema, S.; Arshad, Q.; Gresty, M.A.; Golding, J.F.; Bronstein, A. Motion Sickness in Migraine and Vestibular Disorders. J Neurol Neurosurg Psychiatry 2015, 86, 585–587. [Google Scholar] [CrossRef]
- Cronin, T.; Arshad, Q.; Seemungal, B.M. Vestibular Deficits in Neurodegenerative Disorders: Balance, Dizziness, and Spatial Disorientation. Front Neurol 2017, 8, 538. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Becnel, A.R.; Miske, C.; Szikszay, T.M.; Adamczyk, W.M.; Luedtke, K. Postural Control Impairment in Patients with Headaches-A Systematic Review and Meta-Analysis. Headache 2022, 62, 241–270. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Bonato, P.; Florencio, L.L.; Pinheiro, C.F.; Dach, F.; Bigal, M.E.; Bevilaqua-Grossi, D. Balance Impairments in Different Subgroups of Patients With Migraine. Headache 2017, 57, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.F.; Chaves, T.C.; Dach, F.; Pinheiro, C.F.; Gonçalves, M.C.; Florencio, L.L.; Ferreira, K.S.; Bigal, M.E.; Bevilaqua-Grossi, D. Influence of Migraine and of Migraine Aura on Balance and Mobility--a Controlled Study. Headache 2013, 53, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Dumanlidag, S.; Milanlioglu, A. Comparison of Static and Dynamic Balance Measurements among Chronic and Episodic Migraine Patients. Arq Neuropsiquiatr 2021, 79, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.F.; Becnel, A.R.; Miske, C.; Szikszay, T.M.; Adamczyk, W.M.; Luedtke, K. Postural Control Impairment in Patients with Headaches-A Systematic Review and Meta-Analysis. Headache 2022, 62, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.F.; Florencio, L.L.; Pinheiro, C.F.; Dach, F.; Bigal, M.E.; Bevilaqua-Grossi, D. Functional Balance Deterioration on Daily Activities in Patients With Migraine: A Controlled Study. Am J Phys Med Rehabil 2018, 97, 90–95. [Google Scholar] [CrossRef]
- Akdal, G.; Balci, B.D.; Angin, S.; Öztürk, V.; Halmagyi, G.M. A Longitudinal Study of Balance in Migraineurs. Acta Otolaryngol 2012, 132, 27–32. [Google Scholar] [CrossRef]
- Manto, M.; Serrao, M.; Filippo Castiglia, S.; Timmann, D.; Tzvi-Minker, E.; Pan, M.K.; Kuo, S.H.; Ugawa, Y. Neurophysiology of Cerebellar Ataxias and Gait Disorders. Clin Neurophysiol Pract 2023, 8, 143–160. [Google Scholar] [CrossRef]
- Serrao, M.; Ranavolo, A.; Casali, C. Neurophysiology of Gait. Handb Clin Neurol 2018, 154, 299–303. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. The Relevance of Clinical Balance Assessment Tools to Differentiate Balance Deficits. Eur J Phys Rehabil Med 2010, 46, 239. [Google Scholar]
- Castiglia, S.F.; Tatarelli, A.; Trabassi, D.; De Icco, R.; Grillo, V.; Ranavolo, A.; Varrecchia, T.; Magnifica, F.; Di Lenola, D.; Coppola, G.; et al. Ability of a Set of Trunk Inertial Indexes of Gait to Identify Gait Instability and Recurrent Fallers in Parkinson’s Disease. Sensors 2021, Vol. 21, Page 3449 2021, 21, 3449. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; Tatarelli, A.; Ranavolo, A.; Varrecchia, T.; Fiori, L.; Di Lenola, D.; Cioffi, E.; Raju, M.; Coppola, G.; et al. Identification of Gait Unbalance and Fallers Among Subjects with Cerebellar Ataxia by a Set of Trunk Acceleration-Derived Indices of Gait. Cerebellum 2023, 22, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Strik, M.; Van der Walt, A.; Kilpatrick, T.J.; Kolbe, S.C.; Galea, M.P. Gait Stability Reflects Motor Tracts Damage at Early Stages of Multiple Sclerosis. Mult Scler 2022, 28, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Caronni, A.; Gervasoni, E.; Ferrarin, M.; Anastasi, D.; Brichetto, G.; Confalonieri, P.; Giovanni, R.D.I.; Prosperini, L.; Tacchino, A.; Solaro, C.; et al. Local Dynamic Stability of Gait in People With Early Multiple Sclerosis and No-to-Mild Neurological Impairment. IEEE Trans Neural Syst Rehabil Eng 2020, 28, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Chini, G.; Ranavolo, A.; Draicchio, F.; Casali, C.; Conte, C.; Martino, G.; Leonardi, L.; Padua, L.; Coppola, G.; Pierelli, F.; et al. Local Stability of the Trunk in Patients with Degenerative Cerebellar Ataxia During Walking. Cerebellum 2017, 16, 26–33. [Google Scholar] [CrossRef]
- Amirpourabasi, A.; Lamb, S.E.; Chow, J.Y.; Williams, G.K.R. Nonlinear Dynamic Measures of Walking in Healthy Older Adults: A Systematic Scoping Review. Sensors 2022, 22, 4408. [Google Scholar] [CrossRef]
- Mehdizadeh, S. The Largest Lyapunov Exponent of Gait in Young and Elderly Individuals: A Systematic Review. Gait Posture 2018, 60, 241–250. [Google Scholar] [CrossRef]
- Olesen, J.; Bes, A.; Kunkel, R.; Lance, J.W.; Nappi, G.; Pfaffenrath, V.; Rose, F.C.; Schoenberg, B.S.; Soyka, D.; Tfelt-Hansen, P.; et al. The International Classification of Headache Disorders, 3rd Edition (Beta Version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst 2017, 109, djw323. [Google Scholar] [CrossRef]
- Kroneberg, D.; Elshehabi, M.; Meyer, A.C.; Otte, K.; Doss, S.; Paul, F.; Nussbaum, S.; Berg, D.; Kühn, A.A.; Maetzler, W.; et al. Less Is More - Estimation of the Number of Strides Required to Assess Gait Variability in Spatially Confined Settings. Front Aging Neurosci 2019, 10, 435. [Google Scholar] [CrossRef]
- Trabassi, D.; Castiglia, S.F.; Bini, F.; Marinozzi, F.; Ajoudani, A.; Lorenzini, M.; Chini, G.; Varrecchia, T.; Ranavolo, A.; De Icco, R.; et al. Optimizing Rare Disease Gait Classification through Data Balancing and Generative AI: Insights from Hereditary Cerebellar Ataxia. Sensors 2024, 24, 3613. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait Variability and Stability Measures: Minimum Number of Strides and within-Session Reliability. Comput Biol Med 2014, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Raffalt, P.C.; Kent, J.A.; Wurdeman, S.R.; Stergiou, N. Selection Procedures for the Largest Lyapunov Exponent in Gait Biomechanics. Ann Biomed Eng 2019, 47, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Vallat, R. Pingouin: Statistics in Python. J Open Source Softw 2018, 3, 1026. [Google Scholar] [CrossRef]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol Clin 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Chaves, T.C.; Dach, F.; Pinheiro, C.F.; Gonçalves, M.C.; Florencio, L.L.; Ferreira, K.S.; Bigal, M.E.; Bevilaqua-Grossi, D. Influence of Migraine and of Migraine Aura on Balance and Mobility--a Controlled Study. Headache 2013, 53, 1116–1122. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Luedtke, K.; Pinheiro, C.F.; Moraes, R.; Lemos, T.W.; Carneiro, C.G.; Bigal, M.E.; Dach, F.; Bevilaqua-Grossi, D. Migraine and Balance Impairment: Influence of Subdiagnosis, Otoneurological Function, Falls, and Psychosocial Factors. Headache 2022, 62, 548–557. [Google Scholar] [CrossRef]
- Zorzin, L.; Carvalho, G.F.; Kreitewolf, J.; Teggi, R.; Pinheiro, C.F.; Moreira, J.R.; Dach, F.; Bevilaqua-Grossi, D. Subdiagnosis, but Not Presence of Vestibular Symptoms, Predicts Balance Impairment in Migraine Patients - a Cross Sectional Study. J Headache Pain 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Coppola, G.; Parisi, V.; Di Renzo, A.; Pierelli, F. Cortical Pain Processing in Migraine. J Neural Transm 2020, 127, 551–566. [Google Scholar] [CrossRef]
- Deodato, M.; Granato, A.; Martini, M.; Buoite Stella, A.; Galmonte, A.; Murena, L.; Manganotti, P. Neurophysiological and Clinical Outcomes in Episodic Migraine Without Aura: A Cross-Sectional Study. J Clin Neurophysiol 2024, 41, 388–395. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, D.; Guo, Y.; Yan, W.; Liu, X.; Yang, Z.; Deng, J.; Wang, H. Altered Large-Scale Internetwork Functional Connectivity in Patients with Vestibular Migraine and Migraine without Aura. Neurosci Lett 2023, 800, 137123. [Google Scholar] [CrossRef]
- Rao, Y.; Liu, W.; Zhu, Y.; Lin, Q.; Kuang, C.; Huang, H.; Jiao, B.; Ma, L.; Lin, J. Altered Functional Brain Network Patterns in Patients with Migraine without Aura after Transcutaneous Auricular Vagus Nerve Stimulation. Sci Rep 2023, 13, 9604. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, C.; Ranieri, F.; Di Renzo, A.; Parisi, V.; Serrao, M.; Di Lazzaro, V.; Lisicki, M.; Coppola, G.; Pierelli, F. Impaired Short-Term Visual Paired Associative Plasticity in Patients with Migraine between Attacks. Pain 2021, 162, 803–810. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Ambrosini, A.; Brighina, F.; Coppola, G.; Perrotta, A.; Pierelli, F.; Sandrini, G.; Valeriani, M.; Marinazzo, D.; Stramaglia, S.; et al. Altered Processing of Sensory Stimuli in Patients with Migraine. Nat Rev Neurol 2014, 10, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Anarte-Lazo, E.; Carvalho, G.F.; Schwarz, A.; Luedtke, K.; Falla, D. Differentiating Migraine, Cervicogenic Headache and Asymptomatic Individuals Based on Physical Examination Findings: A Systematic Review and Meta-Analysis. BMC Musculoskelet Disord 2021, 22, 1–18. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; Conte, C.; Gioiosa, V.; Sebastianelli, G.; Abagnale, C.; Ranavolo, A.; Di Lorenzo, C.; Coppola, G.; Casali, C.; et al. Local Dynamic Stability of Trunk During Gait Is Responsive to Rehabilitation in Subjects with Primary Degenerative Cerebellar Ataxia. Cerebellum 2024. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; Tatarelli, A.; Ranavolo, A.; Varrecchia, T.; Fiori, L.; Di Lenola, D.; Cioffi, E.; Raju, M.; Coppola, G.; et al. Identification of Gait Unbalance and Fallers Among Subjects with Cerebellar Ataxia by a Set of Trunk Acceleration-Derived Indices of Gait. Cerebellum 2023, 22, 46–58. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).