The cuddles, loyalty, companionship and unconditional affection they can give make dogs and cats increasingly present in homes all over the world; furthermore, several studies have shown how the presence of a pet also improves the mental and physical health of humans [
1,
2,
3]. Thanks also to a better quality of life, owners treat these animals as real members of the family, paying close attention to their well-being. Following the discovery of the importance and great influence that the intestinal microbiota can have on animal health, nutritional strategies aimed at maintaining a more balanced intestinal microflora are increasingly. It is well known that probiotics can modulate the composition of the canine and feline intestinal microbiota, promoting the intestinal colonization of beneficial bacterial species able to release metabolites that strengthen the integrity of the intestinal barrier, making the environment more hostile to potentially harmful bacteria. However, it is important to remember that when we talk about probiotics, we are referring to live microorganisms that belong not only to different bacterial phyla but also to different bacterial classes, orders, families, genera and species; it is essential to know this because recent studies have highlighted how bacteria belonging to the same species can perform different functions within the organism based on the specific strain to which they belong, the well-known concept of ‘strain-specificity’. Considering what has been illustrated so far, it is better to evaluate the effects of a specific probiotic strain and not of a specific species, in fact, even if bacteria belonging to the same species will have many common characteristics, it is better to analyze the relative strains to have a more accurate and detailed information about their properties. However, among the species of bacteria that, during evolution, have managed to colonize the intestinal tract, adapting to coexist in a mutualistic way with the organism, we find
Lactobacillus reuteri currently known as
Limosilactobacillus reuteri. “Limose” from the latin “
limosus” means slimy and indicates the ability of this bacterial species to produce exopolysaccharides (EPS). The new classification provides a better environmental and functional view and a better understanding of the differences within lactobacilli. Host-adapted bacteria, for example, are more competitive than those that do not have a common evolutionary history with the host, which is interesting for the effectiveness of a product in fighting pathogens. This reclassification of lactobacilli is a unique opportunity to better explain the world of bacteria, to better understand the specificities of new groups and to develop more targeted products and, ultimately, for greater efficacy. EPS are important food and pharmaceutical additives with beneficial antioxidant, antitumor, and immunological effects for human health. However, EPS are difficult to produce due to complex conditions in fermentation. EPS produced by
L. reuteri are important for biofilm formation and adhesion of
L. reuteri to epithelial surfaces; EPS synthesized by
L. reuteri can inhibit the adhesion of
E. coli [
4]. In addition, they can reduce the gene expression of proinflammatory cytokines induced by
Escherichia coli infection (including IL-1b and IL-6). Other
in vivo experiments [
5] showed similar results: EPS produced by
L. reuteri prevent diarrhea in bacterial infection by reducing the adhesion of
E. coli. Furthermore, EPS can act also as a prebiotic. The present study is the last of three research conducted to evaluate the efficacy of a specific strain of
L. reuteri,
L. reuteri NBF 1 DSM 32203, on the intestinal microbiota and fecal parameters of healthy adult dogs. In the two previously published research were evaluated the effects of
L. reuteri NBF 1 DSM 32203 on the intestinal microbiota of medium-large sized dogs (Golden Retriever) and subjects with a predisposition to intestinal diseases like French Bulldogs. We thought it was important to evaluate the effectiveness of this probiotic on small dogs such as Chihuahuas and Dachshunds; in fact, analyzing the growth curves of large and small dogs, those of large dogs show a greater slope underlying how these animals undergo a notable increase in their body weight during the first weeks of life while small breeds show a growth curve with a lesser slope and a moderate increase. These observations suggest that the effort that large breed dogs make during the early stages of growth, when their intestinal microbiota is still unstable, may compromise its integrity in later adulthood by influencing its composition and making it more susceptible to potentially harmful agents; the fact that large dogs live less than small dogs could also be connected to this process. For this reason, it is important to analyze the effect of the same probiotic strain on dogs of different sizes to see if the probiotic can have different effects depending on the intestinal ecosystem it will colonize.
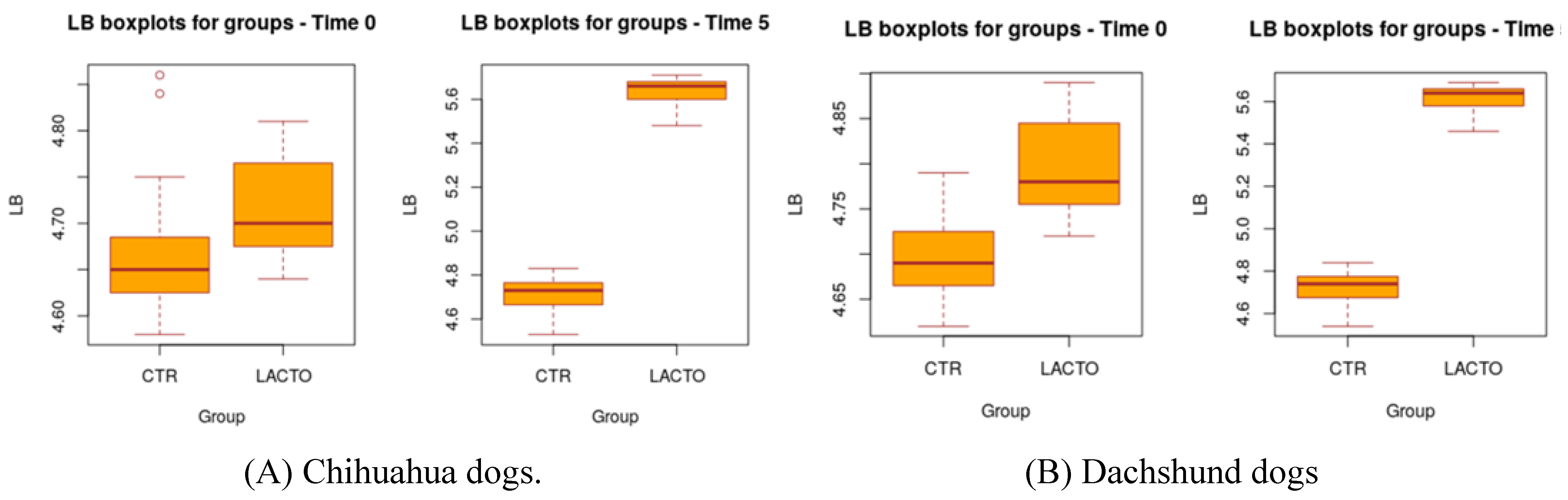
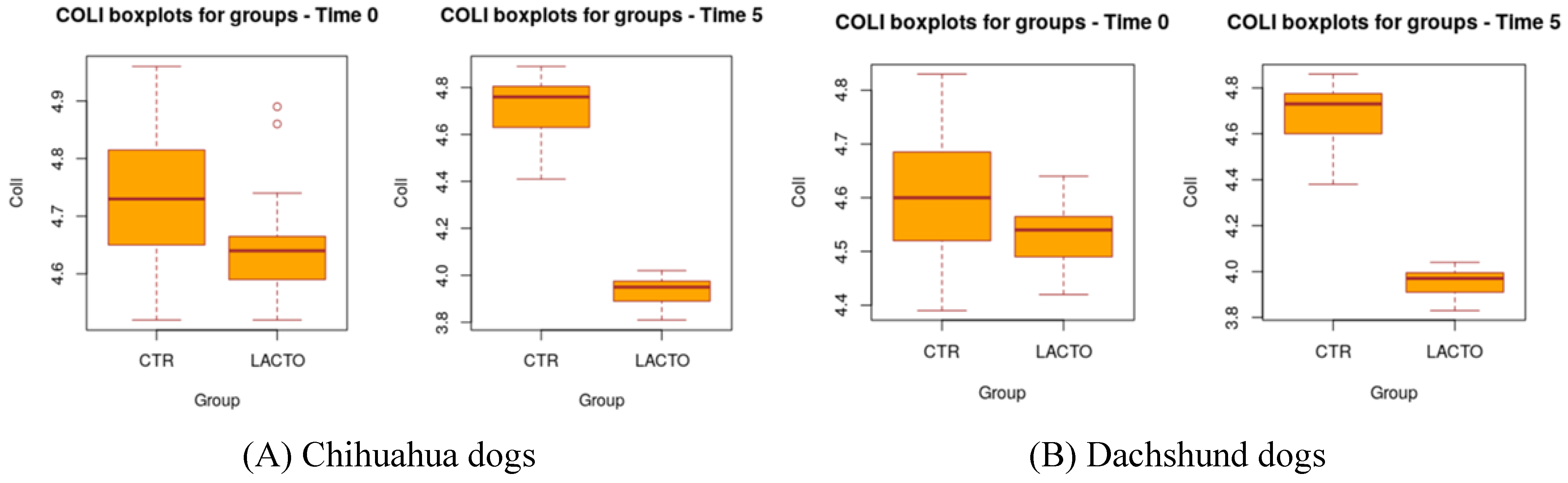
L. reuteri NBF 1 DSM 32203 can improve fecal quality parameters in healthy Golden Retriever dogs promoting an increase in the amount of
Lactobacillus spp., a beneficial bacterial group able to favor a balanced intestinal ecosystem [
7]. Similar results were reported by another study that analyzed the effects of the same probiotic strain on the intestinal microbiota of French bulldogs [
8], also in this case,
L. reuteri NBF 1 DSM 32203 was able to improve the Fecal Score decreasing the fecal humidity with a production of more consistent and well-formed feces indicator of an adequate intestinal function, in addition, the probiotic enhanced the amount of lactobacilli causing a small reduction in the total coliforms. Except for these two studies, in literature, there are no publications on the effects of
L. reuteri NBF 1 DSM 32203 on the intestinal microbiota and fecal parameters of adult dogs. Zhao
et al. (2023) [
6] investigated the effects of another strain of
L. reuteri, specifically
Limosilactobacillus reuteri ZJF036, on growth performance and gut microbiota of young Beagle dogs showing that this specific probiotic strain is not able to influence the daily weight gain, but it is able to decrease Chao1 index and ACE index increasing the relative abundance of
Firmicutes and
Fusobacteria. Obviously, this study is not comparable with those conducted on adult animals because, in young dogs the intestinal microbiota has yet to fully stabilize.