Submitted:
30 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
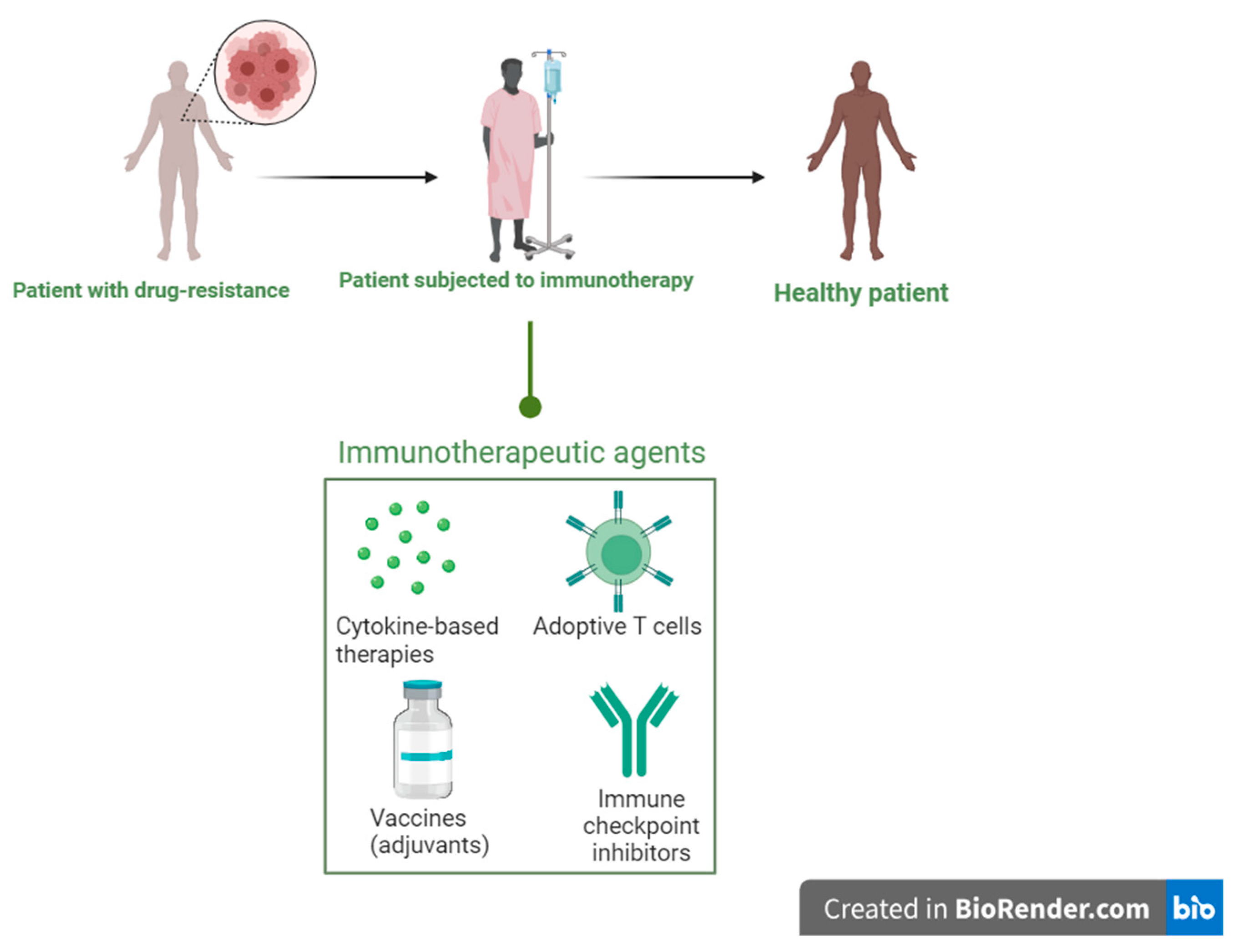
Background
Cytokine-Based Therapies
Adoptive Cell Therapy
Tumour Infiltrating Lymphocytes
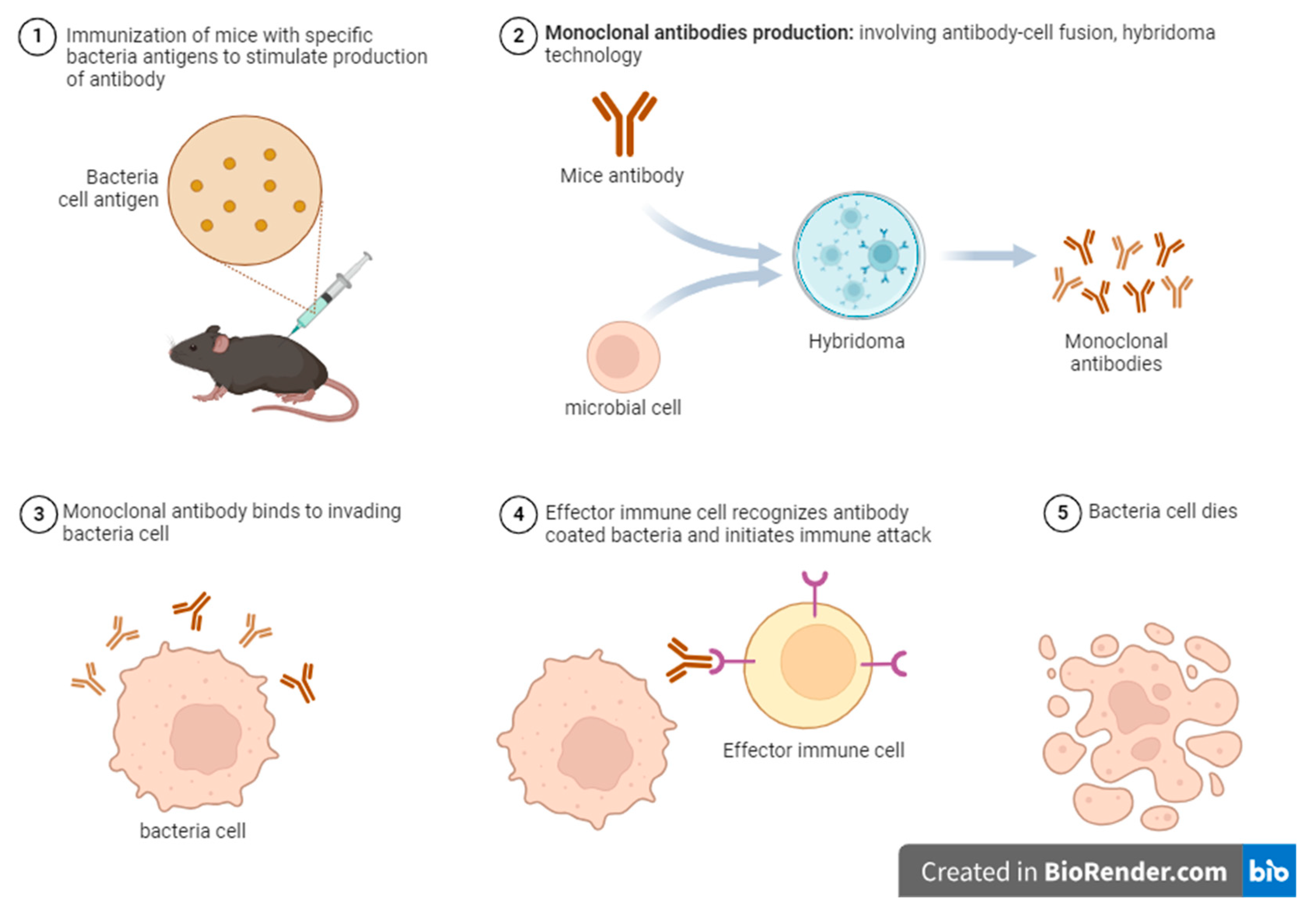
Monoclonal Antibodies
Immune checkpoint inhibitors
Vaccines (Adjuvants)
Invariant Killer T-Cells (INKT)
Mucosal-Associated Invariant T-Cells (MAIT)
Conclusions
Ethical Approval and Consent to Participate
Consent for publication
Availability of Data and Materials
Competing interest
Funding
Authors’ contribution
Acknowledgements
List of Abbreviations
| ACB | Antibody-coated bacteria |
| ACE | Angiotensin-converting enzyme |
| AMR | Antimicrobial resistance |
| CAR | Chimeric antigen receptor |
| CTLA-4 | Cytotoxic T-lymphocytic associated protein -4 |
| DAMP | Damage-associated molecular patterns |
| GM-CSF | Granulocyte Macrophage-stimulating factor |
| HCV | Hepatitis C virus |
| HIV | Human immune deficiency virus |
| ICIs | Immune checkpoint inhibitors |
| ILC | Innate lymphoid cells |
| IL | Interleukin |
| iNKT | Invariant killer T cells |
| ISCOMS | Immune-stimulating complexes |
| LPS | Lipopolysaccharide |
| Mabs | Monoclonal antibodies |
| MAIT | Mucosa-associated invariant T-cells |
| MDP | Muramyl dipeptide |
| MDR-TB | Multi-drug resistant tuberculosis |
| MPL | Monophosphoryl lipid A |
| PAMP | Pathogen-associated molecular patterns |
| PD-L1 | Programmed cell death ligand -1 |
| PRR | Pattern recognition receptors |
| TIL | Tumour infiltrating lymphocytes |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Haldar, J. Confronting the Rising Threat of Antimicrobial Resistance: A Global Health Imperative. ACS Infect. Dis. 2023, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antimicrobial Resistance. 2024 [cited 2024 Jul 20]. Antimicrobial Resistance Threats in the United States, 2021-2022. Available from: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/update-2022.
- Muteeb, G.; Rehman, T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Coico, R. Gram staining. Curr Protoc Microbiol. 2005 Oct;Appendix 3:Appendix 3C.
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Immunotherapies against human bacterial and fungal infectious diseases: A review. Front. Med. 2023, 10, 1135541. [Google Scholar] [CrossRef]
- Mir MA, Hamdani SS, Qadri H. Chapter 6 - Significance of immunotherapy for human bacterial diseases and antibacterial drug discovery. In: Mir MA, editor. Human Pathogenic Microbes [Internet]. Academic Press; 2022 [cited 2024 Jul 20]. p. 129–61. (Developments in Microbiology). Available from: https://www.sciencedirect.com/science/article/pii/B9780323961271000048.
- The Editors of Encyclopaedia Britannica. Cytokines. In 2024. Available from: https://www.britannica.com/science/cytokine.
- Petrina, M.; Martin, J.; Basta, S. Granulocyte macrophage colony-stimulating factor has come of age: From a vaccine adjuvant to antiviral immunotherapy. Cytokine Growth Factor Rev. 2021, 59, 101–110. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; He, L.; Pinn, M.L.; Tsai, Y.-C.; Cheng, M.A.; Farmer, E.; Karakousis, P.C.; Hung, C.-F. Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy against chronic tuberculosis. Cell. Mol. Immunol. 2020, 18, 2393–2401. [Google Scholar] [CrossRef]
- Sheng, L.; Li, X.; Weng, F.; Wu, S.; Chen, Y.; Lou, L. Efficacy and Safety of Adjunctive Recombinant Human Interleukin-2 for Patients with Pulmonary Tuberculosis: A Meta-Analysis. J. Trop. Med. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Karki R, Kanneganti TD. The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol [Internet]. 2021 Aug;42(8):681–705. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1471490621001150.
- Niloufar R. Khanna; Valerie Gerriets. Interferon [Internet]. 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555932/.
- Calabrese, L.H.; Lenfant, T.; Calabrese, C. Interferon therapy for COVID-19 and emerging infections: Prospects and concerns. Clevel. Clin. J. Med. 2020. [Google Scholar] [CrossRef]
- Gunst JD, Goonetilleke N, Rasmussen TA, Søgaard OS. Immunomodulation with IL-7 and IL-15 in HIV-1 infection. J Virus Erad [Internet]. 2023 Sep;9(3):100347. Available from: https://linkinghub.elsevier.com/retrieve/pii/S205566402300033X.
- Morillas RM, Masnou H. Gastroenterología y Hepatología. 2017;40(10).
- Wagoner, J.; Herring, S.; Hsiang, T.-Y.; Ianevski, A.; Biering, S.B.; Xu, S.; Hoffmann, M.; Pöhlmann, S.; Gale, M.; Aittokallio, T.; et al. Combinations of Host- and Virus-Targeting Antiviral Drugs Confer Synergistic Suppression of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0333122. [Google Scholar] [CrossRef]
- Choi, M.H.; Wan, E.Y.F.; Wong, I.C.K.; Chan, E.W.Y.; Chu, W.M.; Tam, A.R.; Yuen, K.Y.; Hung, I.F.N. Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bojkova D, Stack R, Rothenburger T, Kandler JD, Ciesek S, Wass MN, et al. Synergism of interferon-beta with antiviral drugs against SARS-CoV-2 variants. J Infect [Internet]. 2022 Nov;85(5):573–607. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163445322004558.
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Nicholas P McAndrew, MD, MSCE, Noelle Frey, MD, MSCE and David C Fajgenbaum, MD Ms. Cytokine Release Syndrome [Internet]. 2023. Available from: https://emedicine.medscape.com/article/2500111-overview.
- Deckers, J.; Anbergen, T.; Hokke, A.M.; de Dreu, A.; Schrijver, D.P.; de Bruin, K.; Toner, Y.C.; Beldman, T.J.; Spangler, J.B.; de Greef, T.F.A.; et al. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 2023, 1, 286–303. [Google Scholar] [CrossRef]
- Pires, I.S.; Hammond, P.T.; Irvine, D.J. Engineering Strategies for Immunomodulatory Cytokine Therapies: Challenges and Clinical Progress. Adv. Ther. 2021, 4, 2100035. [Google Scholar] [CrossRef]
- Walti, C.S.; Stuehler, C.; Palianina, D.; Khanna, N. Immunocompromised host section: Adoptive T-cell therapy for dsDNA viruses in allogeneic hematopoietic cell transplant recipients. Curr. Opin. Infect. Dis. 2022, 35, 302–311. [Google Scholar] [CrossRef]
- Karsten, H.; Matrisch, L.; Cichutek, S.; Fiedler, W.; Alsdorf, W.; Block, A. Broadening the horizon: potential applications of CAR-T cells beyond current indications. Front. Immunol. 2023, 14, 1285406. [Google Scholar] [CrossRef]
- Morte-Romea, E.; Pesini, C.; Pellejero-Sagastizábal, G.; Letona-Giménez, S.; Martínez-Lostao, L.; Aranda, S.L.; Toyas, C.; Redrado, S.; Dolader-Ballesteros, E.; Arias, M.; et al. CAR Immunotherapy for the treatment of infectious diseases: a systematic review. Front. Immunol. 2024, 15, 1289303. [Google Scholar] [CrossRef]
- Motta, C.M.; Keller, M.D.; Bollard, C.M. Applications of virus-specific T cell therapies post-BMT. Semin. Hematol. 2023, 60, 10–19. [Google Scholar] [CrossRef]
- Keller, M.D.; Hanley, P.J.; Chi, Y.-Y.; Aguayo-Hiraldo, P.; Dvorak, C.C.; Verneris, M.R.; Kohn, D.B.; Pai, S.-Y.; Saldaña, B.J.D.; Hanisch, B.; et al. Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment. Nat. Commun. 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Tzannou, I.; Wu, M.; Ramos, C.; Sasa, G.; Martinez, C.; Lulla, P.; Krance, R.A.; Scherer, L.; Ruderfer, D.; et al. Posoleucel, an Allogeneic, Off-the-Shelf Multivirus-Specific T-Cell Therapy, for the Treatment of Refractory Viral Infections in the Post-HCT Setting. Clin. Cancer Res. 2023, 29, 324–330. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; Baiocchi, R.; Beitinjaneh, A.; Chaganti, S.; Choquet, S.; Dierickx, D.; Dinavahi, R.; Duan, X.; Gamelin, L.; Ghobadi, A.; et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein–Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol. 2024, 25, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Einsele, H.; Löffler, J. CAR T Cells Beyond Cancer: Hope for Immunomodulatory Therapy of Infectious Diseases. Front. Immunol. 2019, 10, 2711. [Google Scholar] [CrossRef] [PubMed]
- Rothemejer, F.H.; Lauritsen, N.P.; Søgaard, O.S.; Tolstrup, M. Strategies for enhancing CAR T cell expansion and persistence in HIV infection. Front. Immunol. 2023, 14, 1253395. [Google Scholar] [CrossRef]
- Mazzi, M.T.; Hajdu, K.L.; Ribeiro, P.R.; Bonamino, M.H. CAR-T cells leave the comfort zone: current and future applications beyond cancer. Immunother. Adv. 2020, 1, ltaa006. [Google Scholar] [CrossRef] [PubMed]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T Cell Therapy beyond Oncology: Autoimmune Diseases and Viral Infections. Biomedicines 2021, 9, 59. [Google Scholar] [CrossRef]
- Maldini, C.R.; Ellis, G.I.; Riley, J.L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 2018, 18, 605–616. [Google Scholar] [CrossRef]
- Schreiber, S.; Dressler, L.S.; Loffredo-Verde, E.; Asen, T.; Färber, S.; Wang, W.; Groll, T.; Chakraborty, A.; Kolbe, F.; Kreer, C.; et al. CARs derived from broadly neutralizing, human monoclonal antibodies identified by single B cell sorting target hepatitis B virus-positive cells. Front. Immunol. 2024, 15, 1340619. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Nesterenko, L.N.; Bruter, A.V.; Balunets, D.V.; Chudakov, D.M.; Izraelson, M.; Britanova, O.V.; Khromykh, L.M.; Kazansky, D.B. Adoptive Immunotherapy Based on Chain-Centric TCRs in Treatment of Infectious Diseases. iScience 2020, 23, 101854. [Google Scholar] [CrossRef]
- Tacke, R.; Sun, J.; Uchiyama, S.; Polovina, A.; Nguyen, D.G.; Nizet, V. Protection Against Lethal Multidrug-Resistant Bacterial Infections Using Macrophage Cell Therapy. Infect. Microbes Dis. 2019, 1, 61–69. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Cheng, W.; Li, Y.; Li, D.; Wang, L.; Zhang, X.; Xiao, Y. Adoptive macrophage directed photodynamic therapy of multidrug-resistant bacterial infection. Nat. Commun. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Chung, Y.R.; Dangi, T.; Palacio, N.; Sanchez, S.; Penaloza-MacMaster, P. Adoptive B cell therapy for chronic viral infection. Front. Immunol. 2022, 13, 908707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Yang, Y.; Cui, J.; Sun, J.; Liu, Y. The prognostic and biology of tumour-infiltrating lymphocytes in the immunotherapy of cancer. Br. J. Cancer 2023, 129, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we?. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2020 Dec 1;1874(2):188441.
- Yeo, D.; Giardina, C.; Saxena, P.; Rasko, J.E. The next wave of cellular immunotherapies in pancreatic cancer. Mol. Ther. - Oncolytics 2022, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Du, L.; Li, H.; Zhu, X.; Cui, L.; Li, X. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully. Biomed. Pharmacother. 2020, 132, 110873. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, F.; Zhang, Y.; Yu, X.; Li, J. Metabolic diversity of tumor-infiltrating T cells as target for anti-immune therapeutics. Cancer Immunol. Immunother. 2023, 72, 3453–3460. [Google Scholar] [CrossRef]
- Fan, J.-X.; Niu, M.-T.; Qin, Y.-T.; Sun, Y.-X.; Zhang, X.-Z. Progress of engineered bacteria for tumor therapy. Adv. Drug Deliv. Rev. 2022, 185, 114296. [Google Scholar] [CrossRef]
- Xu, W.; Ren, D.; Yu, Z.; Hou, J.; Huang, F.; Gan, T.; Ji, P.; Zhang, C.; Ma, L.; Hu, Y. Bacteria-mediated tumor immunotherapy via photothermally-programmed PD1 expression. Nanoscale Adv. 2022, 4, 1577–1586. [Google Scholar] [CrossRef]
- Jia, X.; Guo, J.; Guo, S.; Zhao, T.; Liu, X.; Cheng, C.; Wang, L.; Zhang, B.; Meng, C.; Jia, H.; et al. Antitumor effects and mechanisms of CpG ODN combined with attenuated Salmonella-delivered siRNAs against PD-1. Int. Immunopharmacol. 2020, 90, 107052. [Google Scholar] [CrossRef]
- Sieow, B.F.-L.; Wun, K.S.; Yong, W.P.; Hwang, I.Y.; Chang, M.W. Tweak to Treat: Reprograming Bacteria for Cancer Treatment. Trends Cancer 2020, 7, 447–464. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.; Zhang, C.; Hu, L.; Yang, K. Engineered bacteria in tumor immunotherapy. Cancer Lett. 2024, 589, 216817. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef] [PubMed]
- El-Rebey, H.S.; Abdou, A.G.; Sultan, M.M.; Ibrahim, S.H.M.; Holah, N.S. The Profile and Role of Tumor-infiltrating Lymphocytes in Hepatocellular Carcinoma: An Immunohistochemical Study. Appl. Immunohistochem. Mol. Morphol. 2020, 29, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Atkins, M.B.; Koon, H.B.; Dummer, R.; Ascierto, P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer 2020, 8, e000948. [Google Scholar] [CrossRef] [PubMed]
- Zippel, D.; Friedman-Eldar, O.; Rayman, S.; Hazzan, D.; Nissan, A.; Schtrechman, G.; Markel, G.; Schachter, J.; Itzhaki, O.; Besser, M.J. Tissue Harvesting for Adoptive Tumor Infiltrating Lymphocyte Therapy in Metastatic Melanoma. Anticancer. Res. 2019, 39, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Gastman, B.; Agarwal, P.K.; Berger, A.; Boland, G.; Broderick, S.; Butterfield, L.H.; Byrd, D.; E Fecci, P.; Ferris, R.L.; Fong, Y.; et al. Defining best practices for tissue procurement in immuno-oncology clinical trials: consensus statement from the Society for Immunotherapy of Cancer Surgery Committee. J. Immunother. Cancer 2020, 8, e001583. [Google Scholar] [CrossRef]
- Khushalani, N.I.; Truong, T.-G.; Thompson, J.F. Current Challenges in Access to Melanoma Care: A Multidisciplinary Perspective. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e295–e303. [Google Scholar] [CrossRef]
- Cancer Research UK. Monoclonal antibodies (MABs). Immunotherapy 2022. https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/monoclonal-antibodies#:~:text=Examples%20of%20MABS%20that%20work,and%20head%20and%20neck%20cancer.
- Iqbal, T.; Choudhary, P.; Raza, K. Monoclonal Antibodies in Cancer Therapy. Int. J. Multidiscip. Res. 2023, 5. [Google Scholar] [CrossRef]
- American Cancer Society. Monoclonal Antibodies and Their Side Effects. 2022. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html.
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Otsubo, R.; Yasui, T. Monoclonal antibody therapeutics for infectious diseases: Beyond normal human immunoglobulin. Pharmacol. Ther. 2022, 240, 108233–108233. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Forte, D.N.; Forte, A.C.; Mimica, I.; Forte, W.C.N. The value of antibody-coated bacteria in tracheal aspirates for the diagnosis of ventilator-associated pneumonia: a case-control study. J. Bras. de Pneumol. 2016, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.E. Human Antibodies for Viral Infections. Annu. Rev. Immunol. 2022, 40, 349–386. [Google Scholar] [CrossRef] [PubMed]
- Qadri, H.; Shah, A.H.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Immunotherapies against human bacterial and fungal infectious diseases: A review. Front. Med. 2023, 10, 1135541. [Google Scholar] [CrossRef] [PubMed]
- Gubser, C.; Chiu, C.; Lewin, S.R.; Rasmussen, T.A. Immune checkpoint blockade in HIV. EBioMedicine 2022, 76, 103840. [Google Scholar] [CrossRef]
- Shah, N.J.; Della Pia, A.; Wu, T.; Williams, A.; Weber, M.; Sinclaire, B.; Paleoudis, E.G.; Alaoui, A.; Lev-Ari, S.; Adams, S.; et al. Clinical Outcomes of Immune Checkpoint Inhibitors in Unique Cohorts Underrepresented in Clinical Trials. Cancers 2024, 16, 2223. [Google Scholar] [CrossRef]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2017, 18, 91–104. [Google Scholar] [CrossRef]
- Vance, R.E.; Eichberg, M.J.; Portnoy, D.A.; Raulet, D.H. Listening to each other: Infectious disease and cancer immunology. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Saeidi, A.; Zandi, K.; Cheok, Y.Y.; Saeidi, H.; Wong, W.F.; Lee, C.Y.Q.; Cheong, H.C.; Yong, Y.K.; Larsson, M.; Shankar, E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018, 9, 2569. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, R.; Zhang, W. CTLA-4 interferes with the HBV-specific T�cell immune response (Review). Int. J. Mol. Med. 2018, 42, 703–712. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, R.M.; Kumar, N.A.; Pascoe, R.D.; Zerbato, J.M.; Evans, V.A.; Dantanarayana, A.I.; Anderson, J.L.; Sékaly, R.P.; Fromentin, R.; Chomont, N.; et al. Combination Immune Checkpoint Blockade to Reverse HIV Latency. J. Immunol. 2020, 204, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.E.; Poteet, E.C.; Liu, D.; Chen, C.; LaBranche, C.C.; Stanfield-Oakley, S.A.; Montefiori, D.C.; Ferrari, G.; Yao, Q. CTLA-4 Blockade, during HIV Virus-Like Particles Immunization, Alters HIV-Specific B-Cell Responses. Vaccines 2020, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, S.C.; Vanshylla, K.; Dahlke, C.; Addo, M.M.; Cornely, O.A.; Klein, F.; Persigehl, T.; Rybniker, J.; Gruell, H.; Bröckelmann, P.J. Case Report: Clinical Management of a Patient With Metastatic Non-Small Cell Lung Cancer Newly Receiving Immune Checkpoint Inhibition During Symptomatic COVID-19. Front. Immunol. 2021, 12, 798276. [Google Scholar] [CrossRef]
- Pan, Y.; Tan, J.; Li, J.; Li, T.; Li, J.; Cao, Y.; Yang, L.; Lin, X.; Li, M.; Liang, X. Immune checkpoint inhibitors in cancer patients with COVID-19. Open Life Sci. 2023, 18, 20220641. [Google Scholar] [CrossRef]
- Tezera, L.B.; Bielecka, M.K.; Ogongo, P.; Walker, N.F.; Ellis, M.; Garay-Baquero, D.J.; Thomas, K.; Reichmann, M.T.; A Johnston, D.; Wilkinson, K.A.; et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife 2020, 9. [Google Scholar] [CrossRef]
- Ogishi, M.; Yang, R.; Aytekin, C.; Langlais, D.; Bourgey, M.; Khan, T.; Al Ali, F.; Rahman, M.; Delmonte, O.M.; Chrabieh, M.; et al. Inherited PD-1 deficiency underlies tuberculosis and autoimmunity in a child. Nat. Med. 2021, 27, 1646–1654. [Google Scholar] [CrossRef]
- Barber, D.L.; Sakai, S.; Kudchadkar, R.R.; Fling, S.P.; Day, T.A.; Vergara, J.A.; Ashkin, D.; Cheng, J.H.; Lundgren, L.M.; Raabe, V.N.; et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Anand, K.; Sahu, G.; Burns, E.; Ensor, A.; Ensor, J.; Pingali, S.R.; Subbiah, V.; Iyer, S.P. Mycobacterial infections due to PD-1 and PD-L1 checkpoint inhibitors. ESMO Open 2020, 5, e000866. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chang, J.J.; Dantanarayana, A.I.; Solomon, A.; Evans, V.A.; Pascoe, R.; Gubser, C.; Trautman, L.; Fromentin, R.; Chomont, N.; et al. Combination Immune Checkpoint Blockade Enhances IL-2 and CD107a Production from HIV-Specific T Cells Ex Vivo in People Living with HIV on Antiretroviral Therapy. J. Immunol. 2021, 208, 54–62. [Google Scholar] [CrossRef]
- Gay, C.L.; Bosch, R.J.; McKhann, A.; Moseley, K.F.; Wimbish, C.L.; Hendrickx, S.M.; Messer, M.; Furlong, M.; Campbell, D.M.; Jennings, C.; et al. Suspected Immune-Related Adverse Events With an Anti-PD-1 Inhibitor in Otherwise Healthy People With HIV. Am. J. Ther. 2021, 87, e234–e236. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhan, H.; Ye, Y.; Yang, J.; Zhang, M.; Li, J.; Zhuang, Y. Current Progress and Future Perspectives of Immune Checkpoint in Cancer and Infectious Diseases. Front. Genet. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.M.; Stephenson, R.J. Advances in Infectious Disease Vaccine Adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 1–24. [Google Scholar] [CrossRef]
- Manriquez, G.G.G.; Tuero, I. Adjuvants: friends in vaccine formulations against infectious diseases. Hum. Vaccines Immunother. 2021, 17, 3539–3550. [Google Scholar] [CrossRef]
- Crothers, J.W.; Norton, E.B. Recent advances in enterotoxin vaccine adjuvants. Curr. Opin. Immunol. 2023, 85, 102398–102398. [Google Scholar] [CrossRef]
- Centres for Disease Control and Prevention. Vaccine safety: adjuvants and vaccines. Available from: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html.
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. TLR agonists as vaccine adjuvants in the prevention of viral infections: an overview. Front. Microbiol. 2023, 14, 1249718. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015, 15, 51–7. [Google Scholar] [CrossRef]
- Creative Biolabs. Vaccine Analytics and Qualifications: Bacteria-derived Adjuvant. Available from: https://www.creative-biolabs.com/vaccine/bacteria-derived-adjuvant.htm.
- Creative Biogene. Bacteria-Derived Adjuvant. Available from: https://www.creative-biogene.com/products/bacteria-derived-adjuvant.html.
- Dhanda, G.; Acharya, Y.; Haldar, J. Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance. ACS Omega 2023, 8, 10757–10783. [Google Scholar] [CrossRef]
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. In brief: The innate and adaptive immune systems. [Updated 2023 Aug 14]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279396/.
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2010, 343, 43–55. [Google Scholar] [CrossRef]
- Jeong, D.; Woo, Y.D.; Chung, D.H. Invariant natural killer T cells in lung diseases. Exp. Mol. Med. 2023, 55, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Van Kaer, L. Natural killer T cells in health and disease. Front. Biosci. 2011, S3, 236–251. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tan, Y.; Wu, J.; Ren, Z. Lipid droplets: a cellular organelle vital in cancer cells. Cell Death Discov. 2023, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lee, J.S.; Gartlan, K.H.; Schuster, I.S.; Comerford, I.; Varelias, A.; Ullah, M.A.; Vuckovic, S.; Koyama, M.; Kuns, R.D.; et al. Eomesodermin promotes the development of type 1 regulatory T (TR1) cells. Sci. Immunol. 2017, 2, eaah7152. [Google Scholar] [CrossRef]
- Batista, I.A.; Quintas, S.T.; Melo, S.A. The Interplay of Exosomes and NK Cells in Cancer Biology. Cancers 2021, 13, 473. [Google Scholar] [CrossRef]
- Razizadeh, M.H.; Zafarani, A.; Taghavi-Farahabadi, M.; Khorramdelazad, H.; Minaeian, S.; Mahmoudi, M. Natural killer cells and their exosomes in viral infections and related therapeutic approaches: where are we? Cell Commun. Signal. 2023, 21, 1–22. [Google Scholar] [CrossRef]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef]
- Sada-Ovalle, I., Chiba, A., Gonzales, A., Brenner, M. B. & Behar, S. M. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 4, e1000239 (2008).
- Chaudhari, P.; Ghate, V.; Nampoothiri, M.; Lewis, S. Multifunctional role of exosomes in viral diseases: From transmission to diagnosis and therapy. Cell. Signal. 2022, 94, 110325–110325. [Google Scholar] [CrossRef]
- Fiore, P.F.; Di Pace, A.L.; Conti, L.A.; Tumino, N.; Besi, F.; Scaglione, S.; Munari, E.; Moretta, L.; Vacca, P. Different effects of NK cells and NK-derived soluble factors on cell lines derived from primary or metastatic pancreatic cancers. Cancer Immunol. Immunother. 2022, 72, 1417–1428. [Google Scholar] [CrossRef]
- Hatami, Z.; Hashemi, Z.S.; Eftekhary, M.; Amiri, A.; Karpisheh, V.; Nasrollahi, K.; Jafari, R. Natural killer cell-derived exosomes for cancer immunotherapy: innovative therapeutics art. Cancer Cell Int. 2023, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Prokopeva, A.E.; Emene, C.C.; Gomzikova, M.O. Antitumor Immunity: Role of NK Cells and Extracellular Vesicles in Cancer Immunotherapy. Curr. Issues Mol. Biol. 2023, 46, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: key mechanisms, prognostication and new therapeutic opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Chai, D.; Dang, Y.; Zheng, J.; Li, H. iNKT: A new avenue for CAR-based cancer immunotherapy. Transl. Oncol. 2022, 17, 101342. [Google Scholar] [CrossRef]
- Tognarelli, E.I.; Gutiérrez-Vera, C.; Palacios, P.A.; Pasten-Ferrada, I.A.; Aguirre-Muñoz, F.; Cornejo, D.A.; González, P.A.; Carreño, L.J. Natural Killer T Cell Diversity and Immunotherapy. Cancers 2023, 15, 5737. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Q.; Li, Y.; Lu, L.; Xiang, Z.; Yin, Z.; Kabelitz, D.; Wu, Y. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 1–38. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Chen, H.; Zhang, J.; He, W. Novel insights based on the plasticity of γδ T cells in the tumor microenvironment. Explor. Immunol. 2022, 2, 98–132. [Google Scholar] [CrossRef]
- A Revesz, I.; Joyce, P.; Ebert, L.M.; A Prestidge, C. Effective γδ T-cell clinical therapies: current limitations and future perspectives for cancer immunotherapy. Clin. Transl. Immunol. 2024, 13, e1492. [Google Scholar] [CrossRef]
- Mak, J.Y.W.; Liu, L.; Fairlie, D.P. Chemical Modulators of Mucosal Associated Invariant T Cells. Accounts Chem. Res. 2021, 54, 3462–3475. [Google Scholar] [CrossRef]
- Hagel J, Garner L, Bilton M, Mehta H, Leng T, Hackstein C, Phalora P, Amini A, Akther H, Provine N, Edmans M, Willberg C, Klenerman P. Human MAIT Cell Activation. Methods in Moelcular Biology. 2020;2098:97–124.
- Rha, M.-S.; Han, J.W.; Kim, J.H.; Koh, J.-Y.; Park, H.J.; Kim, S.I.; Kim, M.S.; Lee, J.G.; Lee, H.W.; Lee, D.H.; et al. Human liver CD8+ MAIT cells exert TCR/MR1-independent innate-like cytotoxicity in response to IL-15. J. Hepatol. 2020, 73, 640–650. [Google Scholar] [CrossRef]
- Ioannidis, M.; Cerundolo, V.; Salio, M. The Immune Modulating Properties of Mucosal-Associated Invariant T Cells. Front. Immunol. 2020, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, M.; Wang, J.; Fan, H.; Yang, D.; Zhang, L.; Gu, X.; Nie, J.; Chen, Z.; Corbett, A.J.; et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 2020, 13, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Horigane-Konakai, Y.; Ishii, Y.; Sugimoto, C.; Wakao, H. Mucosal-associated invariant T cells repress group 2 innate lymphoid cells in Alternaria alternata-induced model of allergic airway inflammation. Front. Immunol. 2022, 13, 1005226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




