Submitted:
31 October 2024
Posted:
04 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
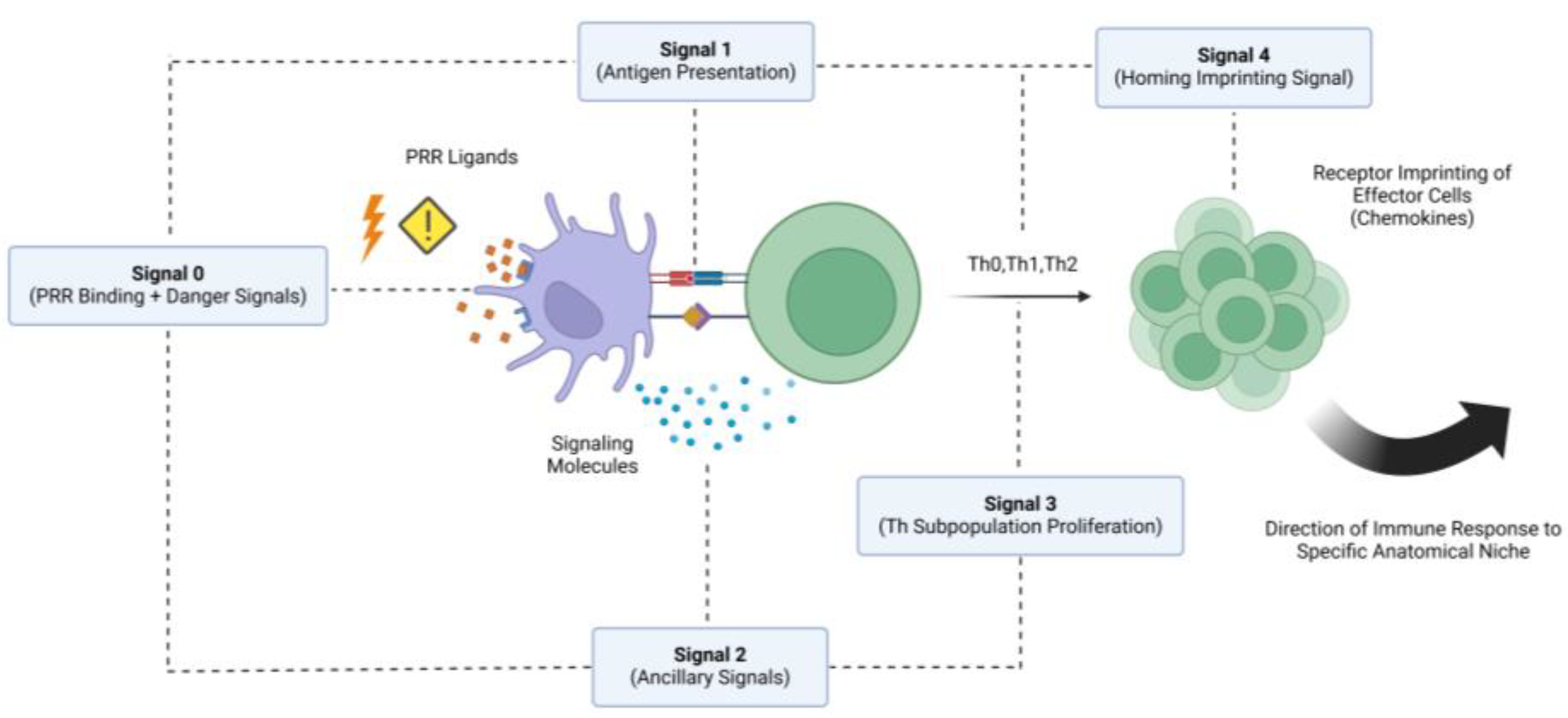
2. Mode of Action: The Two-Signal Model
2.1. Type 1 Signals
2.2. Type 2 Signals
2.3. Further Elaborations on the Two-Signal Model
3. Traditional Adjuvants Used in Aquaculture Vaccinology
3.1. Oil-Based Emulsions
3.2. Aluminum-Based Compounds
3.3. Synthetic Adjuvants and Cytokines
3.4. Structural Microbial Components and Natural Compounds
4. Assessing the Side Effects of Traditional Adjuvants
5. The Promise of Nanoparticle-Based Formulations for the Future
5.1. Polymeric Nanoparticle Formulations
5.2. Lipid-Based Nanoparticle Formulations
5.3. Carbon Nanotubes and Inorganic Nanoparticle Formulations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tammas, I.; Bitchava, K.; Gelasakis, A.I. Transforming Aquaculture through Vaccination: A Review on Recent Developments and Milestones. Vaccines 2024, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci Rep 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Gauthier, J.; Derome, N.; Charette, S.J. The Rise and Fall of Antibiotics in Aquaculture. In Microbial Communities in Aquaculture Ecosystems: Improving Productivity and Sustainability; Derome, N., Ed.; Springer International Publishing: Cham, 2019; pp. 1–19. ISBN 978-3-030-16190-3. [Google Scholar]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Uglem, I.; Mladineo, I. Reared Fish, Farmed Escapees and Wild Fish Stocks—a Triangle of Pathogen Transmission of Concern to Mediterranean Aquaculture Management. Aquaculture Environment Interactions 2013, 3, 153–161. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Miller, K.M.; Bass, A.L.; Bateman, A.W.; Teffer, A.K.; Caleta, J.M.; Di Cicco, E.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; et al. Aquaculture Mediates Global Transmission of a Viral Pathogen to Wild Salmon. Science Advances 2021, 7, eabe2592. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.-H.; Jensen, I.; Mikkelsen, H.; Bjørn, P.-A.; Jansen, P.A.; Bergh, Ø. Disease Interaction and Pathogens Exchange between Wild and Farmed Fish Populations with Special Reference to Norway. Aquaculture 2011, 315, 167–186. [Google Scholar] [CrossRef]
- Dalmo, R.; Bøgwald, J.; Tafalla, C. Adjuvants and Delivery Methods: Current and Novel. In Fish Vaccines; Adams, A., Ed.; Springer: Basel, 2016; pp. 75–103. ISBN 978-3-0348-0980-1. [Google Scholar]
- Mondal, H.; Thomas, J. A Review on the Recent Advances and Application of Vaccines against Fish Pathogens in Aquaculture. Aquacult Int 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Subramani, P.A.; Michael, R.D. Chapter 4 - Prophylactic and Prevention Methods Against Diseases in Aquaculture. In Fish Diseases; Jeney, G., Ed.; Academic Press, 2017; pp. 81–117. ISBN 978-0-12-804564-0. [Google Scholar]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; El-Sayed Ali, T. A Review Article on Nanotechnology in Aquaculture Sustainability as a Novel Tool in Fish Disease Control. Aquacult Int 2021, 29, 1459–1480. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in Nanotechnology for Sustainable Aquaculture and Fisheries. Reviews in Aquaculture 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Sarkar, B.; Mahanty, A.; Gupta, S.K.; Choudhury, A.R.; Daware, A.; Bhattacharjee, S. Nanotechnology: A next-Generation Tool for Sustainable Aquaculture. Aquaculture 2022, 546, 737330. [Google Scholar] [CrossRef]
- Thompson, K.D.; Rodkhum, C.; Bunnoy, A.; Thangsunan, P.; Kitiyodom, S.; Sukkarun, P.; Yostawornkul, J.; Yata, T.; Pirarat, N. Addressing Nanovaccine Strategies for Tilapia. Vaccines 2023, 11, 1356. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Vaseeharan, B.; Ramasamy, P.; Jeyachandran, S. Oral Vaccination for Sustainable Disease Prevention in Aquaculture—an Encapsulation Approach. Aquacult Int 2023, 31, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Turley, J.L.; Lavelle, E.C. Resolving Adjuvant Mode of Action to Enhance Vaccine Efficacy. Current Opinion in Immunology 2022, 77, 102229. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.S.; Schijns, V.E.J.C. Immunology of Vaccine Adjuvants. In Vaccine Adjuvants: Methods and Protocols; Davies, G., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 1–14. ISBN 978-1-60761-585-9. [Google Scholar]
- Schijns, V.E. Immunological Concepts of Vaccine Adjuvant Activity: Commentary. Current Opinion in Immunology 2000, 12, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Belmonte, R. Overview of the Fish Adaptive Immune System. In Fish Vaccines; Adams, A., Ed.; Springer Basel: Basel, 2016; pp. 35–52. ISBN 978-3-0348-0978-8. [Google Scholar]
- Kordon, A.O.; Pinchuk, L.; Karsi, A. Adaptive Immune System in Fish. Turkish Journal of Fisheries and Aquatic Sciences 2021, 22. [Google Scholar] [CrossRef]
- Díaz-Rosales, P.; Muñoz-Atienza, E.; Tafalla, C. Role of Teleost B Cells in Viral Immunity. Fish & Shellfish Immunology 2019, 86, 135–142. [Google Scholar] [CrossRef]
- Johnstone, C.; Chaves-Pozo, E. Antigen Presentation and Autophagy in Teleost Adaptive Immunity. International Journal of Molecular Sciences 2022, 23, 4899. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Ehl, S.; Aichele, P.; Oehen, S.; Kündig, T.; Hengartner, H. Antigen Localisation Regulates Immune Responses in a Dose- and Time-Dependent Fashion: A Geographical View of Immune Reactivity. Immunological Reviews 1997, 156, 199–209. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Kordon, A.O.; Karsi, A.; Pinchuk, L. Innate Immune Responses in Fish: Antigen Presenting Cells and Professional Phagocytes. Turkish Journal of Fisheries and Aquatic Sciences 2018, 18, 1123–1139. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of Fish Toll-like Receptors (TLR) and NOD-like Receptors (NLR). International Journal of Biological Macromolecules 2020, 161, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Su, J. Progresses on Three Pattern Recognition Receptor Families (TLRs, RLRs and NLRs) in Teleost. Developmental & Comparative Immunology 2021, 122, 104131. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like Receptor Recognition of Bacteria in Fish: Ligand Specificity and Signal Pathways. Fish & Shellfish Immunology 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of Fish Toll-like Receptors (TLR) and NOD-like Receptors (NLR). International Journal of Biological Macromolecules 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Peixoto, D.; Losada, A.P.; Quiroga, M.I.; do Vale, A.; Costas, B. Early Innate Immune Responses in European Sea Bass (Dicentrarchus Labrax L.) Following Tenacibaculum Maritimum Infection. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Leiva-Rebollo, R.; Gémez-Mata, J.; Castro, D.; Borrego, J.J.; Labella, A.M. Immune Response of DNA Vaccinated-Gilthead Seabream (Sparus Aurata) against LCDV-Sa Infection: Relevance of the Inflammatory Process. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Franch, R.; Cardazzo, B.; Antonello, J.; Castagnaro, M.; Patarnello, T.; Bargelloni, L. Full-Length Sequence and Expression Analysis of Toll-like Receptor 9 in the Gilthead Seabream (Sparus Aurata L.). Gene 2006, 378, 42–51. [Google Scholar] [CrossRef]
- Miccoli, A.; Buonocore, F.; Picchietti, S.; Scapigliati, G. The Sea Bass Dicentrarchus Labrax as a Marine Model Species in Immunology: Insights from Basic and Applied Research. Aquaculture and Fisheries 2024, 9, 136–143. [Google Scholar] [CrossRef]
- Muñoz, I.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Toll-like Receptor 22 of Gilthead Seabream, Sparus Aurata: Molecular Cloning, Expression Profiles and Post-Transcriptional Regulation. Developmental & Comparative Immunology 2014, 44, 173–179. [Google Scholar] [CrossRef]
- Chen, Z.; Ceballos-Francisco, D.; Guardiola, F.A.; Huang, D.; Esteban, M.Á. The Alleviation of Skin Wound-Induced Intestinal Barrier Dysfunction via Modulation of TLR Signalling Using Arginine in Gilthead Seabream (Sparus Aurata L). Fish & Shellfish Immunology 2020, 107, 519–528. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Zhao, J.; Cao, M.; Yu, Z.; Fu, Q.; Tan, F.; Yang, N.; Li, C. Characterization, Evolution and Expression Analysis of Toll-like Receptor 7 (TLR7) in Turbot (Scophthalmus Maximus L.). Fish & Shellfish Immunology 2022, 125, 9–16. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Liu, D.; Liu, Q.; Hu, G. Cloning and Expression Analysis of a Toll-like Receptor 21 (TLR21) Gene from Turbot, Scophthalmus Maximus. Developmental & Comparative Immunology 2017, 73, 163–168. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, G.; Liu, Q.; Zhang, S. Cloning and Expression Study of a Toll-like Receptor 2 (Tlr2) Gene from Turbot, Scophthalmus Maximus. Fish & Shellfish Immunology 2016, 59, 137–148. [Google Scholar] [CrossRef]
- Dong, X.; Su, B.; Zhou, S.; Shang, M.; Yan, H.; Liu, F.; Gao, C.; Tan, F.; Li, C. Identification and Expression Analysis of Toll-like Receptor Genes (TLR8 and TLR9) in Mucosal Tissues of Turbot (Scophthalmus Maximus L.) Following Bacterial Challenge. Fish & Shellfish Immunology 2016, 58, 309–317. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Yang, N.; Wang, B.; Su, B.; Fu, Q.; Zhang, M.; Tan, F.; Li, C. Characterization of Toll-like Receptor 1 (TLR1) in Turbot (Scophthalmus Maximus L.). Fish & Shellfish Immunology 2021, 115, 27–34. [Google Scholar] [CrossRef]
- Hu, G.-B.; Zhang, S.-F.; Yang, X.; Liu, D.-H.; Liu, Q.-M.; Zhang, S.-C. Cloning and Expression Analysis of a Toll-like Receptor 22 (Tlr22) Gene from Turbot, Scophthalmus Maximus. Fish & Shellfish Immunology 2015, 44, 399–409. [Google Scholar] [CrossRef]
- Liu, F.; Su, B.; Fu, Q.; Shang, M.; Gao, C.; Tan, F.; Li, C. Identification, Characterization and Expression Analysis of TLR5 in the Mucosal Tissues of Turbot (Scophthalmus Maximus L.) Following Bacterial Challenge. Fish & Shellfish Immunology 2017, 68, 272–279. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annual Review of Immunology 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Tanekhy, M. The Role of Toll-like Receptors in Innate Immunity and Infectious Diseases of Teleost. Aquaculture Research 2016, 47, 1369–1391. [Google Scholar] [CrossRef]
- Savelkoul, H.F.J.; Ferro, V.A.; Strioga, M.M.; Schijns, V.E.J.C. Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines 2015, 3, 148–171. [Google Scholar] [CrossRef]
- Salinas, I.; Ding, Y.; Fernández-Montero, Á.; Sunyer, J.O. Mucosal Immunity in Fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, 2022; pp. 387–443. ISBN 978-3-030-85420-1. [Google Scholar]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The Mucosal Immune System of Fish: The Evolution of Tolerating Commensals While Fighting Pathogens. Fish & Shellfish Immunology 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, K.R.; Koppang, E.O.; Christensen, D.; Bojesen, A.M. Alternatives to Mineral Oil Adjuvants in Vaccines against Aeromonas Salmonicida Subsp. Salmonicida in Rainbow Trout Offer Reductions in Adverse Effects. Sci Rep 2017, 7, 5930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Han, Y.; Cui, Y.; Han, X. Study on the Relationships between the Oil HLB Value and Emulsion Stabilization. RSC Advances 2023, 13, 24692–24698. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB Value on Oil-in-Water Emulsions: Droplet Size, Rheological Behavior, Zeta-Potential, and Creaming Index. Journal of Industrial and Engineering Chemistry 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Guldan, A.-C.; Paulus, N.; Runkel, F. Multiple W/O/W Emulsions—Using the Required HLB for Emulsifier Evaluation. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Raman, R.P.; Kumar, S. Adjuvants for Fish Vaccines. In Fish immune system and vaccines; M., M., K.V., R., Eds.; Springer Nature: Singapore, 2022; pp. 231–244. ISBN 978-981-19126-8-9. [Google Scholar]
- Tepparin, S.; Unajak, S.; Hirono, I.; Kondo, H.; Areechon, N. Efficacy of Adjuvanted Streptococcus Agalactiae Vaccine by Montanide ISA 763 A VG in Nile Tilapia (Oreochromis Niloticus Linn.). Journal of Fisheries and Environment 2018, 42, 26–38. [Google Scholar]
- Soltani, M.; Mokhtari, A.; Mirzargar, S.S.; Taherimirghaed, A.; Zargar, A.; Shafiei, S.; Hosseini Shekarabi, S.P. Efficacy and Immune Response of Intraperitoneal Vaccination of Rainbow Trout (Oncorhynchus Mykiss) with a Yersinia Ruckeri Bacterin Formulated with Montanide (TM) ISA 763 AVG Adjuvant; 2016; p. 236.
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of MontanideTM ISA 763 A VG as Aquatic Adjuvant Administrated with an Inactivated Vibrio Harveyi Vaccine in Turbot (Scophthalmus Maximus L.). Fish & Shellfish Immunology 2019, 84, 56–61. [Google Scholar] [CrossRef]
- Jaafar, R.M.; Chettri, J.K.; Dalsgaard, I.; Al-Jubury, A.; Kania, P.W.; Skov, J.; Buchmann, K. Effects of Adjuvant MontanideTM ISA 763 A VG in Rainbow Trout Injection Vaccinated against Yersinia Ruckeri. Fish & Shellfish Immunology 2015, 47, 797–806. [Google Scholar] [CrossRef]
- Wangkahart, E.; Thongsrisuk, A.; Vialle, R.; Pholchamat, S.; Sunthamala, P.; Phudkliang, J.; Srisapoome, P.; Wang, T.; Secombes, C.J. Comparative Study of the Effects of MontanideTM ISA 763A VG and ISA 763B VG Adjuvants on the Immune Response against Streptococcus Agalactiae in Nile Tilapia (Oreochromis Niloticus). Fish & Shellfish Immunology 2023, 134, 108563. [Google Scholar] [CrossRef]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune Response and Protective Efficacy of Two New Adjuvants, MontanideTM ISA 763B VG and MontanideTM GEL02, Administered with a Streptococcus Agalactiae Ghost Vaccine in Nile Tilapia (Oreochromis Niloticus). Fish & Shellfish Immunology 2021, 116, 19–29. [Google Scholar] [CrossRef]
- Veenstra, K.A.; Wang, T.; Russell, K.S.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. MontanideTM ISA 763A VG and ISA 761 VG Induce Different Immune Pathway Responses in Rainbow Trout (Oncorhynchus Mykiss) When Used as Adjuvant for an Aeromonas Salmonicida Bacterin. Fish & Shellfish Immunology 2021, 114, 171–183. [Google Scholar] [CrossRef]
- Thim, H.L.; Villoing, S.; McLoughlin, M.; Christie, K.E.; Grove, S.; Frost, P.; Jørgensen, J.B. Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines 2014, 2, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, W. Factors Affecting Alum–Protein Interactions. International Journal of Pharmaceutics 2014, 466, 139–146. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zou, Y.; Hu, Z. Advances in Aluminum Hydroxide-Based Adjuvant Research and Its Mechanism. Human Vaccines & Immunotherapeutics 2015, 11, 477–488. [Google Scholar] [CrossRef]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the Utilization of Aluminum Adjuvants in Vaccines: You Might Just Get What You Want. npj Vaccines 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Angosto, D.; López-Muñoz, A.; García-Alcazar, A.; Meseguer, J.; Sepulcre, M.P.; Mulero, V. Aluminum Is a Powerful Adjuvant in Teleost Fish despite Failing to Induce Interleukin-1β Release. Developmental & Comparative Immunology 2018, 85, 18–24. [Google Scholar] [CrossRef]
- Compan, V.; Baroja-Mazo, A.; López-Castejón, G.; Gomez, A.I.; Martínez, C.M.; Angosto, D.; Montero, M.T.; Herranz, A.S.; Bazán, E.; Reimers, D.; et al. Cell Volume Regulation Modulates NLRP3 Inflammasome Activation. Immunity 2012, 37, 487–500. [Google Scholar] [CrossRef]
- Angosto, D.; López-Castejón, G.; López-Muñoz, A.; Sepulcre, M.P.; Arizcun, M.; Meseguer, J.; Mulero, V. Evolution of Inflammasome Functions in Vertebrates: Inflammasome and Caspase-1 Trigger Fish Macrophage Cell Death but Are Dispensable for the Processing of IL-1β. Innate Immun 2012, 18, 815–824. [Google Scholar] [CrossRef]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of Interleukin-1β. Cytokine & Growth Factor Reviews 2002, 13, 483–502. [Google Scholar] [CrossRef]
- McKee, A.S.; Burchill, M.A.; Munks, M.W.; Jin, L.; Kappler, J.W.; Friedman, R.S.; Jacobelli, J.; Marrack, P. Host DNA Released in Response to Aluminum Adjuvant Enhances MHC Class II-Mediated Antigen Presentation and Prolongs CD4 T-Cell Interactions with Dendritic Cells. Proceedings of the National Academy of Sciences of the United States of America 2013, 110, E1122–E1131. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA Released from Dying Host Cells Mediates Aluminum Adjuvant Activity. Nat Med 2011, 17, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Pétrilli, V.; De Smedt, T.; Rolaz, A.; Hammad, H.; van Nimwegen, M.; Bergen, I.M.; Castillo, R.; Lambrecht, B.N.; Tschopp, J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. The Journal of Immunology 2008, 181, 3755–3759. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Villegas, J.; García-Alcazar, A.; Meseguer, J.; Mulero, V. Aluminum Adjuvant Potentiates Gilthead Seabream Immune Responses but Induces Toxicity in Splenic Melanomacrophage Centers. Fish & Shellfish Immunology 2019, 85, 31–43. [Google Scholar] [CrossRef]
- Guo, M.; Li, C. An Overview of Cytokine Used as Adjuvants in Fish: Current State and Future Trends. Reviews in Aquaculture 2021, 13, 996–1014. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current Use and Development of Fish Vaccines in China. Fish & Shellfish Immunology 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Thirumalaikumar, E.; Vimal, S.; Sathishkumar, R.; Ravi, M.; Karthick, V.; Ramya, S.; Thomas, J.; Kumar, V.; Kamaraj, C.; Citarasu, T. DNA Vaccine Incorporated Poly (Lactic-Co-Glycolic) Acid (PLGA) Microspheres Offer Enhanced Protection against Aeromonas Hydrophila Infection. International Journal of Biological Macromolecules 2023, 253, 127182. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. PLGA Encapsulated Inactivated-Viral Vaccine: Formulation and Evaluation of Its Protective Efficacy against Viral Haemorrhagic Septicaemia Virus (VHSV) Infection in Olive Flounder (Paralichthys Olivaceus) Vaccinated by Mucosal Delivery Routes. Vaccine 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. PLGA Encapsulated Inactivated-Viral Vaccine: Formulation and Evaluation of Its Protective Efficacy against Viral Haemorrhagic Septicaemia Virus (VHSV) Infection in Olive Flounder (Paralichthys Olivaceus) Vaccinated by Mucosal Delivery Routes. Vaccine 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. PLGA Encapsulated Inactivated-Viral Vaccine: Formulation and Evaluation of Its Protective Efficacy against Viral Haemorrhagic Septicaemia Virus (VHSV) Infection in Olive Flounder (Paralichthys Olivaceus) Vaccinated by Mucosal Delivery Routes. Vaccine 2019, 37, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Huo, X.; Tang, L.; Hu, M.; Yang, C.; Luo, D.; Su, J. Oral PLGA-Based DNA Vaccines Using Interferons as Adjuvants Remarkably Promote the Immune Protection of Grass Carp (Ctenopharyngodon Idella) against GCRV Infection. Water Biology and Security 2023, 2, 100143. [Google Scholar] [CrossRef]
- Yogeshwari, G. Poly D, L-Lactide-Co-Glycolic Acid (PLGA)-Encapsulated CpG-Oligonucleotide (ODN) on Immune Response in Cyprinus Carpio against Aeromonas Hydrophila. Journal of Aquaculture Research & Development 2015, 06. [Google Scholar] [CrossRef]
- Diao, J.; Ye, H.; Yu, X.; Fan, Y.; Xu, L.; Li, T.; Wang, Y. Adjuvant and Immunostimulatory Effects of LPS and β-Glucan on Immune Response in Japanese Flounder, Paralichthys Olivaceus. Veterinary Immunology and Immunopathology 2013, 156, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wangkahart, E.; Secombes, C.J.; Wang, T. Studies on the Use of Flagellin as an Immunostimulant and Vaccine Adjuvant in Fish Aquaculture. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef]
- Sahoo, L.; Parhi, J.; Debnath, C.; Prasad, K.P. Effect of Feeding Lipopolysaccharide as an Immunostimulant on Immune Response and Immune Gene Expression of Labeo Bata. Veterinary Immunology and Immunopathology 2017, 188, 48–58. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Salinas, I.; Tafalla, C.; Dalmo, R.A. Editorial: Vaccines and Immunostimulants for Finfish. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The Response of Fish to Immunostimulant Diets. Fish & Shellfish Immunology 2016, 56, 34–69. [Google Scholar] [CrossRef]
- Giri, S.S.; Chi, C.; Jun, J.W.; Park, S.C. Use of Bacterial Subcellular Components as Immunostimulants in Fish Aquaculture. Reviews in Aquaculture 2018, 10, 474–492. [Google Scholar] [CrossRef]
- Hoel, K.; Lillehaug, A. Adjuvant Activity of Polar Glycopeptidolipids fromMycobacterium Chelonaein Experimental Vaccines againstAeromonas Salmonicidain Salmonid Fish. Fish & Shellfish Immunology 1997, 7, 365–376. [Google Scholar] [CrossRef]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunology 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vinay, T.-N.; Park, C.-S.; Kim, H.-Y.; Jung, S.-J. Toxicity and Dose Determination of Quillaja Saponin, Aluminum Hydroxide and Squalene in Olive Flounder (Paralichthys Olivaceus). Veterinary Immunology and Immunopathology 2014, 158, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Huang, J.; Li, J. Adjuvant Effect of Quillaja Saponaria Saponin (QSS) on Protective Efficacy and IgM Generation in Turbot (Scophthalmus Maximus) upon Immersion Vaccination. International Journal of Molecular Sciences 2016, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H.; Jung, S.-J.; Kim, T. Saponin and Chitosan-Based Oral Vaccine against Viral Haemorrhagic Septicaemia Virus (VHSV) Provides Protective Immunity in Olive Flounder (Paralichthys Olivaceus). Fish & Shellfish Immunology 2022, 126, 336–346. [Google Scholar] [CrossRef]
- Jung, M.-H.; Kole, S.; Jung, S.-J. Efficacy of Saponin-Based Inactivated Rock Bream Iridovirus (RBIV) Vaccine in Rock Bream (Oplegnathus Fasciatus). Fish & Shellfish Immunology 2022, 121, 12–22. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Zhang, Y.; Liu, Q.; Wang, Q.; Liu, X. A Compound Ginseng Stem Leaf Saponins and Aluminium Adjuvant Enhances the Potency of Inactivated Aeromonas Salmonicida Vaccine in Turbot. Fish & Shellfish Immunology 2022, 128, 60–66. [Google Scholar] [CrossRef]
- Sun, F.; Wu, Y.; Zhang, Y.; Liu, Q.; Wang, Q.; Liu, X. An Aluminium Adjuvant Compound with Ginseng Stem Leaf Saponins Enhances the Potency of Inactivated Pseudomonas Plecoglossicida Vaccine in Large Yellow Croaker (Larimichthys Crocea). Fish & Shellfish Immunology 2024, 144, 109243. [Google Scholar] [CrossRef]
- Soltani, M.; Lymbery, A.; Song, S.K.; Hosseini Shekarabi, P. Adjuvant Effects of Medicinal Herbs and Probiotics for Fish Vaccines. Reviews in Aquaculture 2019, 11, 1325–1341. [Google Scholar] [CrossRef]
- Beck, B.R.; Lee, S.H.; Kim, D.; Park, J.H.; Lee, H.K.; Kwon, S.-S.; Lee, K.H.; Lee, J.I.; Song, S.K. A Lactococcus Lactis BFE920 Feed Vaccine Expressing a Fusion Protein Composed of the OmpA and FlgD Antigens from Edwardsiella Tarda Was Significantly Better at Protecting Olive Flounder (Paralichthys Olivaceus) from Edwardsiellosis than Single Antigen Vaccines. Fish & Shellfish Immunology 2017, 68, 19–28. [Google Scholar] [CrossRef]
- Lee, S.H.; Beck, B.R.; Hwang, S.-H.; Song, S.K. Feeding Olive Flounder (Paralichthys Olivaceus) with Lactococcus Lactis BFE920 Expressing the Fusion Antigen of Vibrio OmpK and FlaB Provides Protection against Multiple Vibrio Pathogens: A Universal Vaccine Effect. Fish & Shellfish Immunology 2021, 114, 253–262. [Google Scholar] [CrossRef]
- Kim, D.; Beck, B.R.; Lee, S.M.; Jeon, J.; Lee, D.W.; Lee, J.I.; Song, S.K. Pellet Feed Adsorbed with the Recombinant Lactococcus Lactis BFE920 Expressing SiMA Antigen Induced Strong Recall Vaccine Effects against Streptococcus Iniae Infection in Olive Flounder (Paralichthys Olivaceus). Fish & Shellfish Immunology 2016, 55, 374–383. [Google Scholar] [CrossRef]
- Anuradha, K.; Foo, H.L.; Mariana, N.S.; Loh, T.C.; Yusoff, K.; Hassan, M.D.; Sasan, H.; Raha, A.R. Live Recombinant Lactococcus Lactis Vaccine Expressing Aerolysin Genes D1 and D4 for Protection against Aeromonas Hydrophila in Tilapia (Oreochromis Niloticus). Journal of Applied Microbiology 2010, 109, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Samani, M.; Soltani, M.; Dadar, M.; Taheri-Mirghaed, A.; Zargar, A.; Ahmadivand, S.; Hassanzadeh, R.; Goudarzi, L.M. Oral Immunization of Trout Fry with Recombinant Lactococcus Lactis NZ3900 Expressing G Gene of Viral Hemorrhagic Septicaemia Virus (VHSV). Fish & Shellfish Immunology 2020, 105, 62–70. [Google Scholar] [CrossRef]
- Docando, F.; Nuñez-Ortiz, N.; Gonçalves, G.; Serra, C.R.; Gomez-Casado, E.; Martín, D.; Abós, B.; Oliva-Teles, A.; Tafalla, C.; Díaz-Rosales, P. Bacillus Subtilis Expressing the Infectious Pancreatic Necrosis Virus VP2 Protein Retains Its Immunostimulatory Properties and Induces a Specific Antibody Response. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-D.; Yao, Y.-Y.; Cui, Z.-W.; Zhang, X.-Y.; Guo, X.; Zhou, Y.-Y.; Zhang, Y.-A. Comparative Study of the Immunoprotective Effect of Two Grass Carp-Sourced Bacillus Subtilis Spore-Based Vaccines against Grass Carp Reovirus. Aquaculture 2019, 504, 88–95. [Google Scholar] [CrossRef]
- Gonçalves, G.; Santos, R.A.; Coutinho, F.; Pedrosa, N.; Curado, M.; Machado, M.; Costas, B.; Bonneville, L.; Serrano, M.; Carvalho, A.P.; et al. Oral Vaccination of Fish against Vibriosis Using Spore-Display Technology. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Ning, Y.; Liu, S.; Liang, Z.; Zhang, Z.; Chen, Y.; Cao, J.; Wang, F.; Lan, L.; et al. Surface Display of Major Capsid Protein on Bacillus Subtilis Spores against Largemouth Bass Virus (LMBV) for Oral Administration. Fish & Shellfish Immunology 2023, 135, 108627. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Wang, Y.; Niu, C.; Liu, Z.; Yao, X.; Jiang, X.; Pan, R.; Jia, S.; Li, D.; et al. Oral Probiotic Vaccine Expressing Koi Herpesvirus (KHV) ORF81 Protein Delivered by Chitosan-Alginate Capsules Is a Promising Strategy for Mass Oral Vaccination of Carps against KHV Infection. Journal of Virology 2021, 95. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Ganesan, A.R.; Divya, D.; Johansen, J.; Zhang, S. Chitin, Chitosan and Chitooligosaccharides as Potential Growth Promoters and Immunostimulants in Aquaculture: A Comprehensive Review. International Journal of Biological Macromolecules 2023, 251, 126285. [Google Scholar] [CrossRef]
- Collado-González, M.; Esteban, M.Á. Chitosan-Nanoparticles Effects on Mucosal Immunity: A Systematic Review. Fish & Shellfish Immunology 2022, 130, 1–8. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J.; Sun, X. Chitosan Microspheres as Candidate Plasmid Vaccine Carrier for Oral Immunisation of Japanese Flounder (Paralichthys Olivaceus). Veterinary Immunology and Immunopathology 2008, 126, 220–229. [Google Scholar] [CrossRef] [PubMed]
- León-Rodríguez, L.; Luzardo-Álvarez, A.; Blanco-Méndez, J.; Lamas, J.; Leiro, J. A Vaccine Based on Biodegradable Microspheres Induces Protective Immunity against Scuticociliatosis without Producing Side Effects in Turbot. Fish & Shellfish Immunology 2012, 33, 21–27. [Google Scholar] [CrossRef]
- Jin, P.; Sun, F.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. An Oral Vaccine Based on Chitosan/Aluminum Adjuvant Induces Both Local and Systemic Immune Responses in Turbot (Scophthalmus Maximus). Vaccine 2021, 39, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-F.; Jiang, F.-Y.; Zhou, G.-Q.; Xia, J.-Y.; Yang, F.; Zhu, B. The Recombinant Subunit Vaccine Encapsulated by Alginate-Chitosan Microsphere Enhances the Immune Effect against Micropterus Salmoides Rhabdovirus. Journal of Fish Diseases 2022, 45, 1757–1765. [Google Scholar] [CrossRef]
- Wang, E.; Wang, X.; Wang, K.; He, J.; Zhu, L.; He, Y.; Chen, D.; Ouyang, P.; Geng, Y.; Huang, X.; et al. Preparation, Characterization and Evaluation of the Immune Effect of Alginate/Chitosan Composite Microspheres Encapsulating Recombinant Protein of Streptococcus Iniae Designed for Fish Oral Vaccination. Fish & Shellfish Immunology 2018, 73, 262–271. [Google Scholar] [CrossRef]
- Behera, T.; Swain, P. Antigen Encapsulated Alginate-Coated Chitosan Microspheres Stimulate Both Innate and Adaptive Immune Responses in Fish through Oral Immunization. Aquacult Int 2014, 22, 673–688. [Google Scholar] [CrossRef]
- Behera, T.; Swain, P. Alginate–Chitosan–PLGA Composite Microspheres Induce Both Innate and Adaptive Immune Response through Parenteral Immunization in Fish. Fish & Shellfish Immunology 2013, 35, 785–791. [Google Scholar] [CrossRef]
- Dezfuly, Z.T.; Alishahi, M.; Ghorbanpoor, M.; Tabandeh, M.R.; Mesbah, M. Immunogenicity and Protective Efficacy of Yersinia Ruckeri Lipopolysaccharide (LPS), Encapsulated by Alginate-Chitosan Micro/Nanoparticles in Rainbow Trout (Oncorhyncus Mykiss). Fish & Shellfish Immunology 2020, 104, 25–35. [Google Scholar] [CrossRef]
- González-Chavarría, I.; Roa, F.J.; Sandoval, F.; Muñoz-Flores, C.; Kappes, T.; Acosta, J.; Bertinat, R.; Altamirano, C.; Valenzuela, A.; Sánchez, O.; et al. Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus Mykiss. International Journal of Molecular Sciences 2023, 24, 14685. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and Future Perspectives of Vaccines for Industrialised Fin-Fish Farming. Fish & Shellfish Immunology 2013, 35, 1759–1768. [Google Scholar] [CrossRef]
- Hoare, R.; Jung, S.-J.; Ngo, T.P.H.; Bartie, K.; Bailey, J.; Thompson, K.D.; Adams, A. Efficacy and Safety of a Non-Mineral Oil Adjuvanted Injectable Vaccine for the Protection of Atlantic Salmon (Salmo Salar L.) against Flavobacterium Psychrophilum. Fish & Shellfish Immunology 2019, 85, 44–51. [Google Scholar] [CrossRef]
- Poppe, T.T.; Koppang, E.O. Side-Effects of Vaccination. In Fish Vaccination; John Wiley & Sons, Ltd., 2014; pp. 153–161. ISBN 978-1-118-80691-3. [Google Scholar]
- Mutoloki, S.; Cooper, G.A.; Marjara, I.S.; Koop, B.F.; Evensen, Ø. High Gene Expression of Inflammatory Markers and IL-17A Correlates with Severity of Injection Site Reactions of Atlantic Salmon Vaccinated with Oil-Adjuvanted Vaccines. BMC Genomics 2010, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Cheng, S.; Hu, Y.; Sun, L. Comparative Study of the Effects of Aluminum Adjuvants and Freund’s Incomplete Adjuvant on the Immune Response to an Edwardsiella Tarda Major Antigen. Vaccine 2010, 28, 1832–1837. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A Sequential Study of Incomplete Freund’s Adjuvant-Induced Peritonitis in Atlantic Cod. Fish & Shellfish Immunology 2012, 32, 141–150. [Google Scholar] [CrossRef]
- Spinos, E.; Kokkoris, G.D.; Bakopoulos, V. Prevention of Sea Bass (Dicentrarchus Labrax, L. 1758) Photobacteriosis and Vibriosis. Long Term Efficacy Study of Intraperitoneally Administered Bivalent Commercial Vaccines. Aquaculture 2017, 471, 172–184. [Google Scholar] [CrossRef]
- Veenstra, K.A.; Wang, T.; Alnabulsi, A.; Douglas, A.; Russell, K.S.; Tubbs, L.; Arous, J.B.; Secombes, C.J. Analysis of Adipose Tissue Immune Gene Expression after Vaccination of Rainbow Trout with Adjuvanted Bacterins Reveals an Association with Side Effects. Molecular Immunology 2017, 88, 89–98. [Google Scholar] [CrossRef]
- Li, J.; Tang, L.; Li, S.; Li, G.; Mo, Z. The Efficacy and Side-Effects of Oil-Based Adjuvants Emulsified Vibrio Anguillarum Bivalent Inactivated Vaccine in Turbot (Scophthalmus Maximus) under Production Mode. Aquaculture 2020, 524, 735259. [Google Scholar] [CrossRef]
- Tziouvas, H.; Varvarigos, P. Intensity Scale of Side Effects in European Sea Bass (Dicentrarchus Labrax) Post Intraperitoneal Injection with Commercial Oil-Adjuvanted Vaccines. Bulletin of the EAFP 2021, 41, 103–110. [Google Scholar] [CrossRef]
- Midtlyng, P.J.; Reitan, L.J.; Speilberg, L. Experimental Studies on the Efficacy and Side-Effects of Intraperitoneal Vaccination of Atlantic Salmon (Salmo salarL.) against Furunculosis. Fish & Shellfish Immunology 1996, 6, 335–350. [Google Scholar] [CrossRef]
- Harshitha, M.; Nayak, A.; Disha, S.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; Karunasagar, I.; Maiti, B. Nanovaccines to Combat Aeromonas Hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines 2023, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Grip, J. PLGA/PLA Micro- and Nanoparticle Formulations Serve as Antigen Depots and Induce Elevated Humoral Responses after Immunization of Atlantic Salmon (Salmo Salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Sævareid, K.; McAuley, L.; Lane, M.E.; Bøgwald, J.; Dalmo, R.A. Early Immune Responses in Atlantic Salmon (Salmo Salar L.) after Immunization with PLGA Nanoparticles Loaded with a Model Antigen and β-Glucan. Vaccine 2011, 29, 8338–8349. [Google Scholar] [CrossRef]
- Adomako, M.; St-Hilaire, S.; Zheng, Y.; Eley, J.; Marcum, R.D.; Sealey, W.; Donahower, B.C.; LaPatra, S.; Sheridan, P.P. Oral DNA Vaccination of Rainbow Trout, Oncorhynchus Mykiss (Walbaum), against Infectious Haematopoietic Necrosis Virus Using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] Nanoparticles. Journal of Fish Diseases 2012, 35, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yu, J. Poly(Lactic-Co-Glycolic Acid) Nanoparticles as Candidate DNA Vaccine Carrier for Oral Immunization of Japanese Flounder (Paralichthys Olivaceus) against Lymphocystis Disease Virus. Fish & Shellfish Immunology 2011, 30, 109–117. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Paul, J.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; et al. Aeromonas Hydrophila OmpW PLGA Nanoparticle Oral Vaccine Shows a Dose-Dependent Protective Immunity in Rohu (Labeo Rohita). Vaccines 2016, 4, 21. [Google Scholar] [CrossRef]
- Harshitha, M.; D’souza, R.; Akshay, S.D.; Nayak, A.; Disha, S.; Aditya, V.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; et al. Oral Administration of Recombinant Outer Membrane Protein A-Based Nanovaccine Affords Protection against Aeromonas Hydrophila in Zebrafish. World J Microbiol Biotechnol 2024, 40, 250. [Google Scholar] [CrossRef]
- Alishahi, M.; Vaseghi, M.; Tabandeh, M.R.; Khosravi, M. Immunogenic and Protective Effects of an Oral Polylactic-Co-Glycolic Acid Nano Encapsulated DNA Vaccine Encoding aopB Gene of Aeromonas Hydrophila in Common Carp. Aquacult Int 2024, 32, 1169–1190. [Google Scholar] [CrossRef]
- Alishahi, M.; Lababian, H.; Heidari, H.; Tabandeh, M.R.; Khosravi, M. Development of an Injectable DNA Vaccine Against Aeromonas Hydrophila Infection Nanoencapsulated With Poly(Lactic-Co-Glycolic) Acid (PLGA) in Common Carp. Aquaculture Research 2024, 2024, 7270489. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. PLGA Encapsulated Inactivated-Viral Vaccine: Formulation and Evaluation of Its Protective Efficacy against Viral Haemorrhagic Septicaemia Virus (VHSV) Infection in Olive Flounder (Paralichthys Olivaceus) Vaccinated by Mucosal Delivery Routes. Vaccine 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, P.-Q.; Guo, S.; Zhao, Z.; Wang, G.-X.; Zhu, B. Dual-Targeting Polymer Nanoparticles Efficiently Deliver DNA Vaccine and Induce Robust Prophylactic Immunity against Spring Viremia of Carp Virus Infection. Microbiology Spectrum 2022, 10, e03085-. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent Progress in Biomedical Applications of Chitosan and Its Nanocomposites in Aquaculture: A Review. Research in Veterinary Science 2019, 126, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; et al. Applications of Chitosan and Chitosan Nanoparticles in Fish Aquaculture. Egyptian Journal of Aquatic Biology and Fisheries 2022, 26, 23–43. [Google Scholar] [CrossRef]
- Kole, S.; Dar, S.A.; Shin, S.-M.; Jeong, H.-J.; Jung, S.-J. Potential Efficacy of Chitosan-Poly (Lactide-Co-Glycolide)-Encapsulated Trivalent Immersion Vaccine in Olive Flounder (Paralichthys Olivaceus) Against Viral Hemorrhagic Septicemia Virus, Streptococcus Parauberis Serotype I, and Miamiensis Avidus (Scuticociliate). Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Hassanzadeh, R.; Soltani, E. Oral DNA Vaccines Based on CS-TPP Nanoparticles and Alginate Microparticles Confer High Protection against Infectious Pancreatic Necrosis Virus (IPNV) Infection in Trout. Developmental & Comparative Immunology 2017, 74, 178–189. [Google Scholar] [CrossRef]
- Rao, B.M.; Kole, S.; Gireesh-Babu, P.; Sharma, R.; Tripathi, G.; Bedekar, M.K. Evaluation of Persistence, Bio-Distribution and Environmental Transmission of Chitosan/PLGA/pDNA Vaccine Complex against Edwardsiella Tarda in Labeo Rohita. Aquaculture 2019, 500, 385–392. [Google Scholar] [CrossRef]
- Halimi, M.; Alishahi, M.; Abbaspour, M.R.; Ghorbanpoor, M.; Tabandeh, M.R. Valuable Method for Production of Oral Vaccine by Using Alginate and Chitosan against Lactococcus Garvieae/Streptococcus Iniae in Rainbow Trout (Oncorhynchus Mykiss). Fish & Shellfish Immunology 2019, 90, 431–439. [Google Scholar] [CrossRef]
- Huo, X.; Tang, L.; Liu, Q.; Zhu, W.; Zhang, J.; Hu, M.; Zhao, F.; Wang, P.; Yuan, G.; Yang, C.; et al. Oral pcDNA3.1-VP4/VP56-FlaC DNA Vaccine Encapsulated by Chitosan/Sodium Alginate Nanoparticles Confers Remarkable Protection against GCRV Infection in Grass Carp. Aquaculture 2023, 577, 739996. [Google Scholar] [CrossRef]
- Leya, T.; Ahmad, I.; Sharma, R.; Tripathi, G.; Kurcheti, P.P.; Rajendran, K.V.; Bedekar, M.K. Bicistronic DNA Vaccine Macromolecule Complexed with Poly Lactic-Co-Glycolic Acid-Chitosan Nanoparticles Enhanced the Mucosal Immunity of Labeo Rohita against Edwardsiella Tarda Infection. International Journal of Biological Macromolecules 2020, 156, 928–937. [Google Scholar] [CrossRef]
- Li, L.; Lin, S.-L.; Deng, L.; Liu, Z.-G. Potential Use of Chitosan Nanoparticles for Oral Delivery of DNA Vaccine in Black Seabream Acanthopagrus Schlegelii Bleeker to Protect from Vibrio Parahaemolyticus. Journal of Fish Diseases 2013, 36, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Kumar, S.; Ishaq Ahmed, V.P.; Parameswaran, V.; Sudhakaran, R.; Sarath Babu, V.; Sahul Hameed, A.S. Potential Use of Chitosan Nanoparticles for Oral Delivery of DNA Vaccine in Asian Sea Bass (Lates Calcarifer) to Protect from Vibrio (Listonella) Anguillarum. Fish & Shellfish Immunology 2008, 25, 47–56. [Google Scholar] [CrossRef]
- Sukkarun, P.; Kitiyodom, S.; Yostawornkul, J.; Chaiin, P.; Yata, T.; Rodkhum, C.; Boonrungsiman, S.; Pirarat, N. Chitosan-Polymer Based Nanovaccine as Promising Immersion Vaccine against Aeromonas Veronii Challenge in Red Tilapia (Oreochromis Sp.). Fish & Shellfish Immunology 2022, 129, 30–35. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Yostawornkul, J.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Enhanced Efficacy of Immersion Vaccination in Tilapia against Columnaris Disease by Chitosan-Coated “Pathogen-like” Mucoadhesive Nanovaccines. Fish & Shellfish Immunology 2019, 95, 213–219. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; Karunasagar, I.; et al. Edwardsiella Tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo Fimbriatus). Vaccines 2016, 4, 40. [Google Scholar] [CrossRef]
- Thirumalaikumar, E.; Lelin, C.; Sathishkumar, R.; Vimal, S.; Anand, S.B.; Babu, M.M.; Citarasu, T. Oral Delivery of pVAX-OMP and pVAX-Hly DNA Vaccine Using Chitosan-Tripolyphosphate (Cs-TPP) Nanoparticles in Rohu, (Labeo Rohita) for Protection against Aeromonas Hydrophila Infection. Fish & Shellfish Immunology 2021, 115, 189–197. [Google Scholar] [CrossRef]
- Kole, S.; Kumari, R.; Anand, D.; Kumar, S.; Sharma, R.; Tripathi, G.; Makesh, M.; Rajendran, K.V.; Bedekar, M.K. Nanoconjugation of Bicistronic DNA Vaccine against Edwardsiella Tarda Using Chitosan Nanoparticles: Evaluation of Its Protective Efficacy and Immune Modulatory Effects in Labeo Rohita Vaccinated by Different Delivery Routes. Vaccine 2018, 36, 2155–2165. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.; Wang, G.; Ling, F. Mannosylated Chitosan Nanoparticles Loaded with ABP Antigen Server as a Novel Nucleic Acid Vaccine against Nocardia Seriolae Infection in Micropterus Salmoides. Aquaculture 2023, 574, 739635. [Google Scholar] [CrossRef]
- Ponce, M.; Zuasti, E.; Anguís, V.; Fernández-Díaz, C. Anti-Bacterial and Immunostimulatory Properties of Ulvan-Loaded Chitosan Nanoparticles for Use in Aquaculture. Mar Biotechnol 2024, 26, 19–27. [Google Scholar] [CrossRef]
- Sukkarun, P.; Kitiyodom, S.; Kamble, M.T.; Bunnoy, A.; Boonanuntanasarn, S.; Yata, T.; Boonrungsiman, S.; Thompson, K.D.; Rodkhum, C.; Pirarat, N. Systemic and Mucosal Immune Responses in Red Tilapia (Oreochromis Sp.) Following Immersion Vaccination with a Chitosan Polymer-Based Nanovaccine against Aeromonas Veronii. Fish & Shellfish Immunology 2024, 146, 109383. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the Mucosal Immune Response of Red Tilapia (Oreochromis Sp.) against Columnaris Disease Using a Biomimetic-Mucoadhesive Nanovaccine. Fish & Shellfish Immunology 2021, 112, 81–91. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Thompson, K.D.; Costa, J.; Elumalai, P.; Katagiri, T.; Temisak, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis Sp.) against Flavobacterium Columnare Challenge. Vaccines 2021, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Z.; Zhou, J.; Wang, W.; Su, J.; Yuan, G. Carboxymethyl Chitosan Nanoparticles Loaded with Ctenopharyngodon Idella Interferon-Γ2 (CiIFN-Γ2) Enhance Protective Efficacy against Bacterial Infection in Grass Carp. Aquaculture 2023, 572, 739554. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Kitiyodom, S.; Yata, T.; Jantharadej, K.; Adamek, M.; Surachetpong, W. Chitosan Nanoparticle Immersion Vaccine Offers Protection against Tilapia Lake Virus in Laboratory and Field Studies. Fish & Shellfish Immunology 2022, 131, 972–979. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, H.; Sun, X.; Zhang, Y.; Zhang, B.; Teng, Z.; Hou, Y.; Wang, B. Development of Oral DNA Vaccine Based on Chitosan Nanoparticles for the Immunization against Reddish Body Iridovirus in Turbots (Scophthalmus Maximus). Aquaculture 2016, 452, 263–271. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. Nanoencapsulation of Inactivated-Viral Vaccine Using Chitosan Nanoparticles: Evaluation of Its Protective Efficacy and Immune Modulatory Effects in Olive Flounder (Paralichthys Olivaceus) against Viral Haemorrhagic Septicaemia Virus (VHSV) Infection. Fish & Shellfish Immunology 2019, 91, 136–147. [Google Scholar] [CrossRef]
- Vimal, S.; Abdul Majeed, S.; Nambi, K.S.N.; Madan, N.; Farook, M.A.; Venkatesan, C.; Taju, G.; Venu, S.; Subburaj, R.; Thirunavukkarasu, A.R.; et al. Delivery of DNA Vaccine Using Chitosan–Tripolyphosphate (CS/TPP) Nanoparticles in Asian Sea Bass, Lates Calcarifer (Bloch, 1790) for Protection against Nodavirus Infection. Aquaculture 2014, 420–421, 240–246. [Google Scholar] [CrossRef]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.Á.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An Oral Chitosan DNA Vaccine against Nodavirus Improves Transcription of Cell-Mediated Cytotoxicity and Interferon Genes in the European Sea Bass Juveniles Gut and Survival upon Infection. Developmental & Comparative Immunology 2016, 65, 64–72. [Google Scholar] [CrossRef]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.Á.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An Oral Chitosan DNA Vaccine against Nodavirus Improves Transcription of Cell-Mediated Cytotoxicity and Interferon Genes in the European Sea Bass Juveniles Gut and Survival upon Infection. Developmental & Comparative Immunology 2016, 65, 64–72. [Google Scholar] [CrossRef]
- Thu Lan, N.G.; Dong, H.T.; Vinh, N.T.; Salin, K.R.; Senapin, S.; Pimsannil, K.; St-Hilaire, S.; Shinn, A.P.; Rodkhum, C. A Novel Vaccination Strategy against Vibrio Harveyi Infection in Asian Seabass (Lates Calcarifer) with the Aid of Oxygen Nanobubbles and Chitosan. Fish & Shellfish Immunology 2024, 149, 109557. [Google Scholar] [CrossRef]
- Andresen, A.M.S.; Gjøen, T. Chitosan Nanoparticle Formulation Attenuates Poly (I:C) Induced Innate Immune Responses against Inactivated Virus Vaccine in Atlantic Salmon (Salmo Salar). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 2021, 40, 100915. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; Esteban, M.Á. Chitosan-Nanoparticles Effects on Mucosal Immunity: A Systematic Review. Fish & Shellfish Immunology 2022, 130, 1–8. [Google Scholar] [CrossRef]
- Ji, J.; Merino, S.; Tomás, J.M.; Roher, N. Nanoliposomes Encapsulating Immunostimulants Modulate the Innate Immune System and Elicit Protection in Zebrafish Larvae. Fish & Shellfish Immunology 2019, 92, 421–429. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Kitiyodom, S.; Srisapoome, P.; Rodkhum, C. Mucoadhesive Cationic Lipid-Based Flavobacterium Oreochromis Nanoencapsulation Enhanced the Efficacy of Mucoadhesive Immersion Vaccination against Columnaris Disease and Strengthened Immunity in Asian Sea Bass (Lates Calcarifer). Fish & Shellfish Immunology 2022, 127, 633–646. [Google Scholar] [CrossRef]
- Thangsunan, P.; Kitiyodom, S.; Srisapoome, P.; Pirarat, N.; Yata, T.; Thangsunan, P.; Boonrungsiman, S.; Bunnoy, A.; Rodkhum, C. Novel Development of Cationic Surfactant-Based Mucoadhesive Nanovaccine for Direct Immersion Vaccination against Francisella Noatunensis Subsp. Orientalis in Red Tilapia (Oreochromis Sp.). Fish & Shellfish Immunology 2022, 127, 1051–1060. [Google Scholar] [CrossRef]
- Ruyra, A.; Cano-Sarabia, M.; MacKenzie, S.A.; Maspoch, D.; Roher, N. A Novel Liposome-Based Nanocarrier Loaded with an LPS-dsRNA Cocktail for Fish Innate Immune System Stimulation. PLOS ONE 2013, 8, e76338. [Google Scholar] [CrossRef]
- Ruyra, A.; Cano-Sarabia, M.; García-Valtanen, P.; Yero, D.; Gibert, I.; Mackenzie, S.A.; Estepa, A.; Maspoch, D.; Roher, N. Targeting and Stimulation of the Zebrafish (Danio Rerio) Innate Immune System with LPS/dsRNA-Loaded Nanoliposomes. Vaccine 2014, 32, 3955–3962. [Google Scholar] [CrossRef]
- Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo Salar). Vaccines 2024, 12, 788. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Kwon, J.; Lee, S.B.; Jung, W.J.; Park, S.C. Applications of Carbon Nanotubes and Polymeric Micro-/Nanoparticles in Fish Vaccine Delivery: Progress and Future Perspectives. Reviews in Aquaculture 2021, 13, 1844–1863. [Google Scholar] [CrossRef]
- Giri, S.S.; Park, S.C. Application of Carbon Nanotubes in the Advancement of Fish Vaccine. In Biotechnological Advances in Aquaculture Health Management; Gupta, S.K., Giri, S.S., Eds.; Springer Nature: Singapore, 2021; ISBN 9789811651953. [Google Scholar]
- Cimbaluk, G.V.; Ramsdorf, W.A.; Perussolo, M.C.; Santos, H.K.F.; Da Silva De Assis, H.C.; Schnitzler, M.C.; Schnitzler, D.C.; Carneiro, P.G.; Cestari, M.M. Evaluation of Multiwalled Carbon Nanotubes Toxicity in Two Fish Species. Ecotoxicology and Environmental Safety 2018, 150, 215–223. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of Functionalized Multiwalled Carbon Nanotubes and Comparison of Their Cellular Toxicity between HEK 293 Cells and Zebra Fish in Vivo. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yan, X.; Pu, Y.; Xiao, F.; Wang, D.; Yang, M. Risks of Single-Walled Carbon Nanotubes Acting as Contaminants-Carriers: Potential Release of Phenanthrene in Japanese Medaka (Oryzias Latipes). Environ. Sci. Technol. 2013, 47, 4704–4710. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zheng, X.; Gao, S.; Huang, Y.; Xiong, J.; Ren, H. Toxicity of Amine-Functionalized Single-Carbon Nanotube (NH2 f-SWCNT) to Channel Catfish (Ietalurus Punetaus): Organ Pathologies, Oxidative Stress, Inflammation, and Apoptosis. Chemosphere 2021, 282, 131133. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Shaw, B.J.; Handy, R.D. Toxicity of Single Walled Carbon Nanotubes to Rainbow Trout, (Oncorhynchus Mykiss): Respiratory Toxicity, Organ Pathologies, and Other Physiological Effects. Aquatic Toxicology 2007, 82, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, G.-L.; Ling, F.; Song, L.-S.; Wang, G.-X. Development Toxicity of Functionalized Single-Walled Carbon Nanotubes on Rare Minnow Embryos and Larvae. Nanotoxicology 2015, 9, 579–590. [Google Scholar] [CrossRef]
- Li, Y.; Men, B.; He, Y.; Xu, H.; Liu, M.; Wang, D. Effect of Single-Wall Carbon Nanotubes on Bioconcentration and Toxicity of Perfluorooctane Sulfonate in Zebrafish (Danio Rerio). Science of The Total Environment 2017, 607–608, 509–518. [Google Scholar] [CrossRef]
- Bisesi, J.H.; Ngo, T.; Ponnavolu, S.; Liu, K.; Lavelle, C.M.; Afrooz, A.R.M.N.; Saleh, N.B.; Ferguson, P.L.; Denslow, N.D.; Sabo-Attwood, T. Examination of Single-Walled Carbon Nanotubes Uptake and Toxicity from Dietary Exposure: Tracking Movement and Impacts in the Gastrointestinal System. Nanomaterials 2015, 5, 1066–1086. [Google Scholar] [CrossRef]
- Sohn, E.K.; Chung, Y.S.; Johari, S.A.; Kim, T.G.; Kim, J.K.; Lee, J.H.; Lee, Y.H.; Kang, S.W.; Yu, I.J. Acute Toxicity Comparison of Single-Walled Carbon Nanotubes in Various Freshwater Organisms. BioMed Research International 2015, 2015, 323090. [Google Scholar] [CrossRef]
- Jiang, T.; Alberto Amadei, C.; Gou, N.; Lin, Y.; Lan, J.; D. Vecitis, C.; Z. Gu, A. Toxicity of Single-Walled Carbon Nanotubes (SWCNTs): Effect of Lengths, Functional Groups and Electronic Structures Revealed by a Quantitative Toxicogenomics Assay. Environmental Science: Nano 2020, 7, 1348–1364. [Google Scholar] [CrossRef]
- Wisdom, K.S.; Bhat, I.A.; Chanu, T.I.; Kumar, P.; Pathakota, G.-B.; Nayak, S.K.; Walke, P.; Sharma, R. Chitosan Grafting onto Single-Walled Carbon Nanotubes Increased Their Stability and Reduced the Toxicity in Vivo (Catfish) Model. International Journal of Biological Macromolecules 2020, 155, 697–707. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; González-Ortega, O. Carbon Nanotubes-Based Mucosal Vaccines. In Nanovaccines: An Innovative Technology to Fight Human and Animal Diseases; Rosales-Mendoza, S., González-Ortega, O., Eds.; Springer International Publishing: Cham, 2019; pp. 159–179. ISBN 978-3-030-31668-6. [Google Scholar]
- Gong, Y.-X.; Zhu, B.; Liu, G.-L.; Liu, L.; Ling, F.; Wang, G.-X.; Xu, X.-G. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effects of a Recombinant Vaccine against Aeromonas Hydrophila. Fish & Shellfish Immunology 2015, 42, 213–220. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Y.-X.; Liu, G.-L.; Zhu, B.; Wang, G.-X. Protective Immunity of Grass Carp Immunized with DNA Vaccine against Aeromonas Hydrophila by Using Carbon Nanotubes as a Carrier Molecule. Fish & Shellfish Immunology 2016, 55, 516–522. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Ma, R.; Qi, X.; Wang, G.; Zhu, B.; Ling, F. The Immunoprotective Effect of Whole-Cell Lysed Inactivated Vaccine with SWCNT as a Carrier against Aeromonas Hydrophila Infection in Grass Carp. Fish & Shellfish Immunology 2020, 97, 336–343. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Liu, G.; Du, H.; Liu, T.; Liu, T.; Li, P.; Yu, Q.; Wang, G.; Wang, E. A Nanocarrier Immersion Vaccine Encoding Surface Immunogenic Protein Confers Cross-Immunoprotection against Streptococcus Agalactiae and Streptococcus Iniae Infection in Tilapia. Fish & Shellfish Immunology 2024, 144, 109267. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, C.; Zhao, Z.; Wang, G.-X. Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease. Vaccines 2020, 8, 87. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Gong, Y.-X.; Ling, F.; Song, L.-S.; Wang, G.-X. Single-Walled Carbon Nanotubes as Candidate Recombinant Subunit Vaccine Carrier for Immunization of Grass Carp against Grass Carp Reovirus. Fish & Shellfish Immunology 2014, 41, 279–293. [Google Scholar] [CrossRef]
- Qiu, D.-K.; Jia, Y.-J.; Gong, Y.-M.; Zheng, Y.-Y.; Wang, G.-X.; Zhu, B. Optimizing the Immunization Procedure of Single-Walled Carbon Nanotubes Based Vaccine against Grass Carp Reovirus for Grass Carp. Aquaculture 2021, 533, 736152. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.-L.; Li, D.-L.; Ling, F.; Zhu, B.; Wang, G.-X. The Protective Immunity against Grass Carp Reovirus in Grass Carp Induced by a DNA Vaccination Using Single-Walled Carbon Nanotubes as Delivery Vehicles. Fish & Shellfish Immunology 2015, 47, 732–742. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Gong, Y.-X.; Ling, F.; Wang, G.-X. Protective Immunity of Grass Carp Immunized with DNA Vaccine Encoding the Vp7 Gene of Grass Carp Reovirus Using Carbon Nanotubes as a Carrier Molecule. Fish & Shellfish Immunology 2015, 42, 325–334. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Qiu, D.-K.; Guo, Z.-R.; Gong, Y.-M.; Wang, G.-X.; Zhu, B. Evaluation of SWCNTs-Loaded DNA Vaccine Encoding Predominant Antigen Epitope VP4-3 against Type II GCRV. Aquaculture 2021, 534, 736197. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Liu, G.-Y.; Li, J.; Wang, G.-X.; Zhu, B. Immune Response and Protective Effect against Spring Viremia of Carp Virus Induced by Intramuscular Vaccination with a SWCNTs-DNA Vaccine Encoding Matrix Protein. Fish & Shellfish Immunology 2018, 79, 256–264. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.-H.; Wang, J.; Zhao, Z.; Li, J.; Tu, X.; Huang, A.-G.; Wang, G.-X.; Zhu, B. Enhanced Protective Immunity against Spring Viremia of Carp Virus Infection Can Be Induced by Recombinant Subunit Vaccine Conjugated to Single-Walled Carbon Nanotubes. Vaccine 2018, 36, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Zha, J.-W.; Wang, G.-X.; Zhu, B. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effect of a DNA Vaccine against Spring Viremia of Carp Virus in Common Carp. Fish & Shellfish Immunology 2017, 71, 191–201. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.-Y.; Gong, Y.-M.; Zhao, Z.; Guo, Z.-R.; Jia, Y.-J.; Wang, G.-X.; Zhu, B. Evaluation of Immune Response and Protection against Spring Viremia of Carp Virus Induced by a Single-Walled Carbon Nanotubes-Based Immersion DNA Vaccine. Virology 2019, 537, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-M.; Zhang, C.; Li, Y.; Chen, G.; Wang, G.-X.; Zhu, B. Optimization of Immunization Procedure for SWCNTs-Based Subunit Vaccine with Mannose Modification against Spring Viraemia of Carp Virus in Common Carp. Journal of Fish Diseases 2021, 44, 1925–1936. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Lin, Q.; Li, N.-Q.; Huang, Z.-B.; Fu, X.-Z.; Wang, G.-X.; Zhu, B. Immersion Vaccination of Mandarin Fish Siniperca Chuatsi against Infectious Spleen and Kidney Necrosis Virus with a SWCNTs-Based Subunit Vaccine. Fish & Shellfish Immunology 2019, 92, 133–140. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiong, Y.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Wang, G.-X.; Zhu, B. Optimization of the Efficacy of a SWCNTs-Based Subunit Vaccine against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish. Fish & Shellfish Immunology 2020, 106, 190–196. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Lin, Q.; Li, N.; Huang, Z.-B.; Zhao, M.; Fu, X.-Z.; Wang, G.-X.; Zhu, B. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effect of an Immersion DNA Vaccine against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish. Fish & Shellfish Immunology 2020, 97, 432–439. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Chen, G.; Zhang, C.; Wang, G.-X.; Zhu, B. Protective Immunity against Infectious Spleen and Kidney Necrosis Virus Induced by Mannose Modified Subunit Vaccine with Carbon Nanotubes in Mandarin Fish. Aquaculture Research 2022, 53, 2175–2184. [Google Scholar] [CrossRef]
- Zhao, Z.; Meng, Q.; Sun, T.-Z.; Zhu, B. Mannose Modified Targeted Immersion Vaccine Delivery System Improves Protective Immunity against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish (Siniperca Chuatsi). Vaccine 2024, 42, 2886–2894. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Wang, E.-L.; Qu, X.-Y.; Yang, K.-C.; Zhang, Z.-Y.; Liu, J.-Y.; Zhang, C.; Zhu, B.; Wang, G.-X. Single-Walled Carbon Nanotubes Enhance the Immune Protective Effect of a Bath Subunit Vaccine for Pearl Gentian Grouper against Iridovirus of Taiwan. Fish & Shellfish Immunology 2020, 106, 510–517. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Liu, J.; Zhu, B.; Wang, G.; Ling, F. Epitope Screening of the Major Capsid Protein within Grouper Iridovirus of Taiwan and the Immunoprotective Effect with SWCNTs as the Vaccine Carrier. Fish & Shellfish Immunology 2021, 117, 17–23. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Wang, G.; Zhu, B.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Carbon Nanotube-Based DNA Vaccine against Koi Herpesvirus given by Intramuscular Injection. Fish & Shellfish Immunology 2020, 98, 810–818. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Zhu, B.; Wu, S.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Immersion Immunization of Koi (Cyprinus Carpio) against Cyprinid Herpesvirus 3 (CyHV-3) with Carbon Nanotube-Loaded DNA Vaccine. Aquaculture 2021, 539, 736644. [Google Scholar] [CrossRef]
- Guo, Z.-R.; Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Zhu, B.; Wang, G.-X. Carbon Nanotubes-Loaded Subunit Vaccine Can Increase Protective Immunity against Rhabdovirus Infections of Largemouth Bass (Micropterus Salmoides). Fish & Shellfish Immunology 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Li, J.; Zhang, Z.-Y.; Liu, J.-Y.; Zhang, C.; Zhu, B.; Wang, G.-X. An Immersion Subunit Vaccine Loaded by Single-Walled Carbon Nanotube Protects Pearl Gentian Grouper from Viral Nervous Necrosis Virus. Aquaculture 2021, 541, 736813. [Google Scholar] [CrossRef]
- Jia, Y.-J.; Guo, Z.-R.; Ma, R.; Qiu, D.-K.; Zhao, Z.; Wang, G.-X.; Zhu, B. Immune Efficacy of Carbon Nanotubes Recombinant Subunit Vaccine against Largemouth Bass Ulcerative Syndrome Virus. Fish & Shellfish Immunology 2020, 100, 317–323. [Google Scholar] [CrossRef]
- Vijayaram, S.; Tsigkou, K.; Zuorro, A.; Sun, Y.-Z.; Rabetafika, H.; Razafindralambo, H. Inorganic Nanoparticles for Use in Aquaculture. Reviews in Aquaculture 2023, 15, 1600–1617. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered Composite Based on Halloysite and Natural Polymers: A Carrier for the pH Controlled Release of Drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Pumchan, A.; Sae-Ueng, U.; Prasittichai, C.; Sirisuay, S.; Areechon, N.; Unajak, S. A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis Sp.). Vaccines 2022, 10, 1180. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Xiao, F.; Liu, X.; Xie, A.; Chen, F.; Dong, P.; Lin, P.; Zheng, C.; Zhang, H.; et al. pH-Controlled Release of Antigens Using Mesoporous Silica Nanoparticles Delivery System for Developing a Fish Oral Vaccine. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]


| Toll-like Receptors (TLRs) | ||
|---|---|---|
| Receptor | Associated Ligands | Indicative Teleost Fish Species |
| TLR1 | Lipopeptides | Rainbow Trout, Large Yellow Croaker, Carp, Pufferfish, Orange-Spotted Grouper, European Sea Bass, Turbot |
| TLR2 | Lipopeptides, PGN, LTA, Pam3CSK4 | Carp, Catfish, Orange-Spotted Grouper, European Sea Bass, Turbot, Gilthead Seabream |
| TLR3 | dsRNA, poly(I:C) | Carp, Pufferfish, Zebrafish |
| TLR4 | Unknown | Carp, Catfish, Rare Minnow, Zebrafish |
| TLR5 | Flagellin | Atlantic Salmon, Japanese Flounder, Channel Catfish, Gilthead Seabream, Rainbow Trout, Pufferfish, Zebrafish, Turbot |
| TLR7 | ssRNA | Channel Catfish, Grass Carp, Pufferfish, Rainbow Trout, Zebrafish, Turbot |
| TLR8 | ssRNA | Atlantic Salmon, Channel Catfish, Pufferfish, Rainbow Trout, Turbot |
| TLR9 | CpG motifs | Atlantic Salmon, Cobia, Japanese Flounder, Rainbow Trout, Zebrafish, European Seabass, Gilthead Seabream, Turbot |
| TLR13 | rRNA | Atlantic Salmon, Channel Catfish, Orange-Spotted Grouper |
| TLR14 | Unknown | Japanese Flounder, Orange-Spotted Grouper, Pufferfish |
| TLR18 | Unknown | Channel Catfish, Grass Carp, Zebrafish |
| TLR19 | dsRNA | Channel Catfish, Grass Carp, Zebrafish |
| TLR20 | Unknown | Carp, Channel Catfish, Zebrafish |
| TLR21 | CpG motifs | Channel Catfish, Grass Carp, Orange-Spotted Grouper, Zebrafish, Turbot |
| TLR22 | dsRNA, poly(I:C) | Atlantic Cod, Channel Catfish, Grass Carp, Pufferfish, Zebrafish, European Seabass, Turbot, Gilthead Seabream |
| TLR23 | Unknown | Atlantic Cod, Pufferfish |
| TLR25 | Unknown | Channel Catfish, Fathead Minnow, Nile Tilapia |
| TLR26 | LPS, poly(I:C) | Channel Catfish, Yellow Catfish |
| TLR28 | LPS, poly(I:C) | Brown Croaker |
| NOD-like Receptor (NLR) Superfamilies | ||
| NLR-A | LPS, PGN (iE-DAP, MDP), Poly (I:C) | Grass Carp, Nile Tilapia, Rainbow Trout, Channel Catfish, Zebrafish |
| NLR-B | Unknown | Grass Carp |
| NLR-C | Unknown | Grass Carp, Brown Croaker |
| RIG-1-like Receptors (RLRs) | ||
| RIG-1 | dsRNA | Zebrafish |
| MDA5 | dsRNA | Grass Carp |
| LGP2 | dsRNA | Grass Carp |
| Currently Available MontanideTM Adjuvants for Use in Aquaculture Vaccinology | ||
|---|---|---|
| Series Name | Technology | Route |
| MontanideTM ISA 61 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 50 V2 | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 70 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 71 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 71 R VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 761 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 78 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 763B VG | Non-Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 660 VG | Non-Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM GR | Oil base for W/O Emulsions containing a Protective Matrix | Oral |
| MontanideTM IMS 1312 VG | Combination of Micro-Emulsions with an Immunostimulating Compound | Immersion |
| Score (0-6) | Spielberg Scale [129] (Atlantic Salmon) |
Tziouvas & Varvarigos Scale [128] (European Sea Bass) |
|---|---|---|
| 0 |
|
|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
| CNT Type | Vaccine Technology | Delivery Route | Pathogen (Antigen) | Teleost Species | Source |
|---|---|---|---|---|---|
| Single-Walled | Recombinant Subunit |
Intramuscular Injection & Bath Immersion |
Aeromonas hydrophila (aerA) |
Grass Carp | [192] |
| Single-Walled | DNA Vaccine | Intramuscular Injection |
Aeromonas hydrophila (aerA) |
Grass Carp | [193] |
| Single-Walled | Whole-cell Inactivated Vaccine |
Intraperitoneal Injection & Bath Immersion |
Aeromonas hydrophila (Bacterial Lysate) |
Grass Carp | [194] |
| Single-Walled | Recombinant Subunit |
Bath Immersion |
Streptococcus sp. (rSip) |
Tilapia | [195] |
| Single-Walled (Mannose Modified) |
Recombinant Subunit |
Bath Immersion | GCRV (VP7) | Grass Carp | [196] |
| Single-Walled | Recombinant Subunit |
Bath Immersion | GCRV (VP7) | Grass Carp | [197] |
| Single-Walled | Recombinant Subunit |
Bath Immersion | GCRV (VP4-3) | Grass Carp | [198] |
| Single-Walled | DNA Vaccine | Intramuscular Injection & Bath Immersion |
GCRV (VP4-3, VP5, VP7) | Grass Carp | [199,200,201] |
| Single-Walled | DNA Vaccine | Intramuscular Injection | SVCV (M) | Common Carp | [202] |
| Single-Walled | Recombinant Subunit |
Intramuscular Injection & Bath Immersion |
SVCV (G) | Common Carp | [203] |
| Single-Walled | DNA Vaccine | Intramuscular Injection & Bath Immersion |
SVCV (G) | Common Carp | [204] |
| Single-Walled | DNA Vaccine | Bath Immersion | SVCV (M) | Common Carp | [205] |
| Single-Walled (Mannose Modified) |
Recombinant Subunit |
Bath Immersion | SVCV (G) | Common Carp | [206] |
| Single-Walled | Recombinant Subunit | Bath Immersion | ISKNV (MCP) | Mandarin Fish | [207,208] |
| Single-Walled | DNA Vaccine | Bath Immersion | ISKNV (MCP) | Mandarin Fish | [209] |
| Single-Walled (Mannose Modified) |
Recombinant Subunit | Intramuscular Injection & Bath Immersion |
ISKNV (MCP) | Mandarin Fish | [210,211] |
| Single-Walled | Recombinant Subunit | Bath Immersion | TGIV (MCP, P2) | Pearl Gentian Grouper | [212,213] |
| Single-Walled | DNA Vaccine | Intramuscular Injection & Bath Immersion |
KHV (ORF 149) | Koi Fish | [214,215] |
| Single-Walled | Recombinant Subunit | Bath Immersion | MSRV (G) | Largemouth Bass | [216] |
| Single-Walled | Recombinant Subunit | Bath Immersion | NNV (MCP) | Pearl Gentian Grouper | [217] |
| Single-Walled | Recombinant Subunit | Bath Immersion | LBUSV (MCP) | Largemouth Bass | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





