Submitted:
31 October 2024
Posted:
04 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables and Data Sources
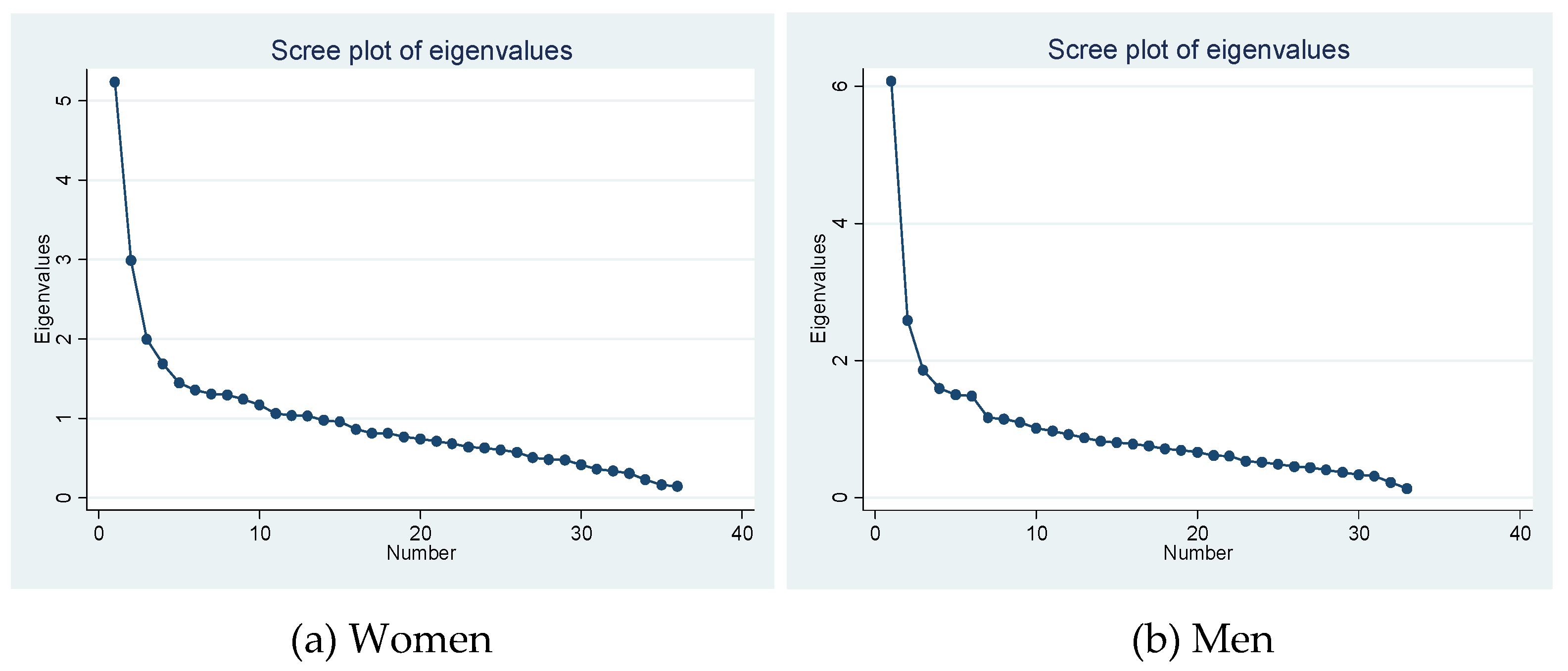
3.3. Statistical Analysis
3. Results
3.1. Most Prevalent Chronic Conditions in Patients with CLD
3.2. Chronic Conditions Associated with the Presence of CLD
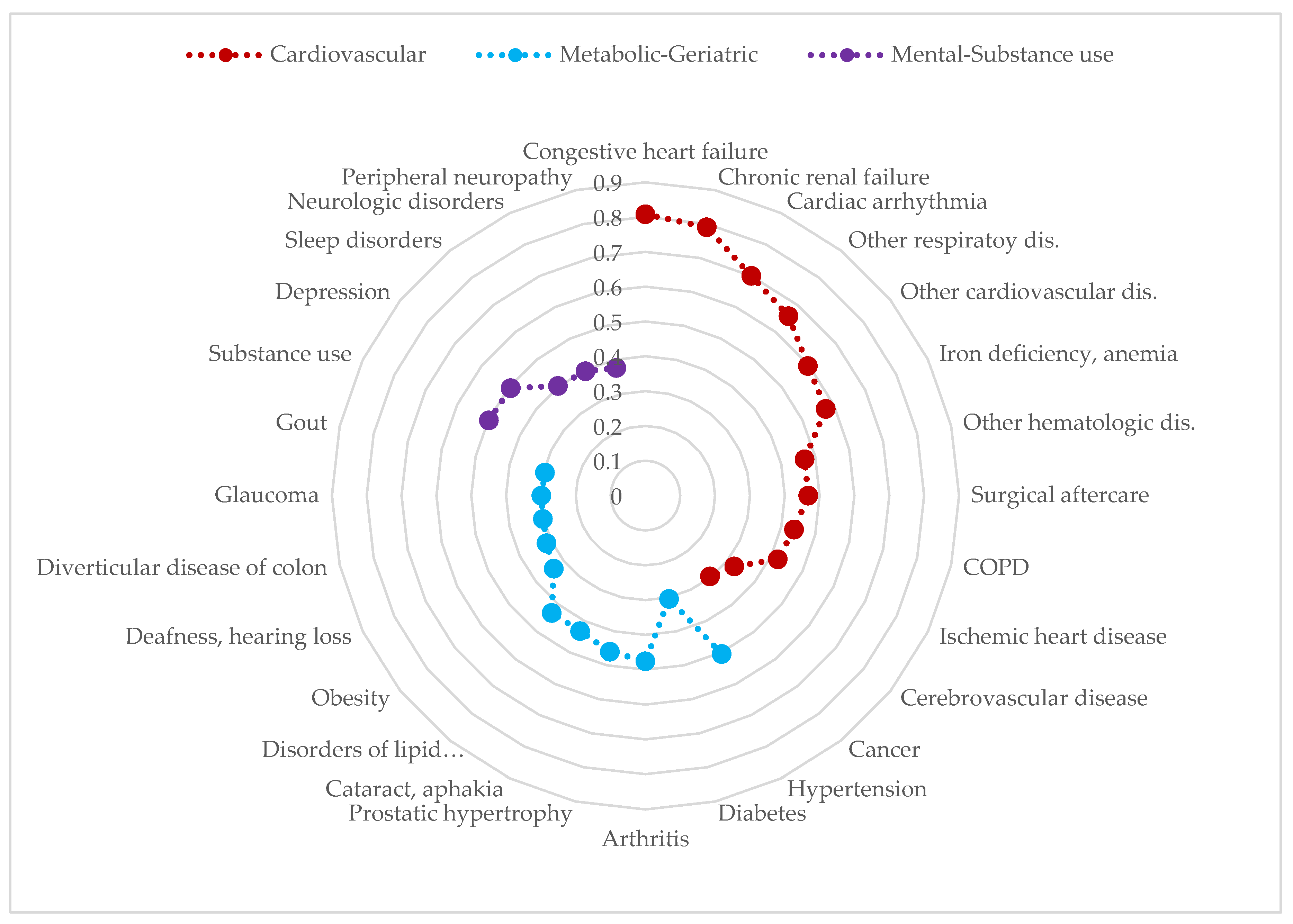
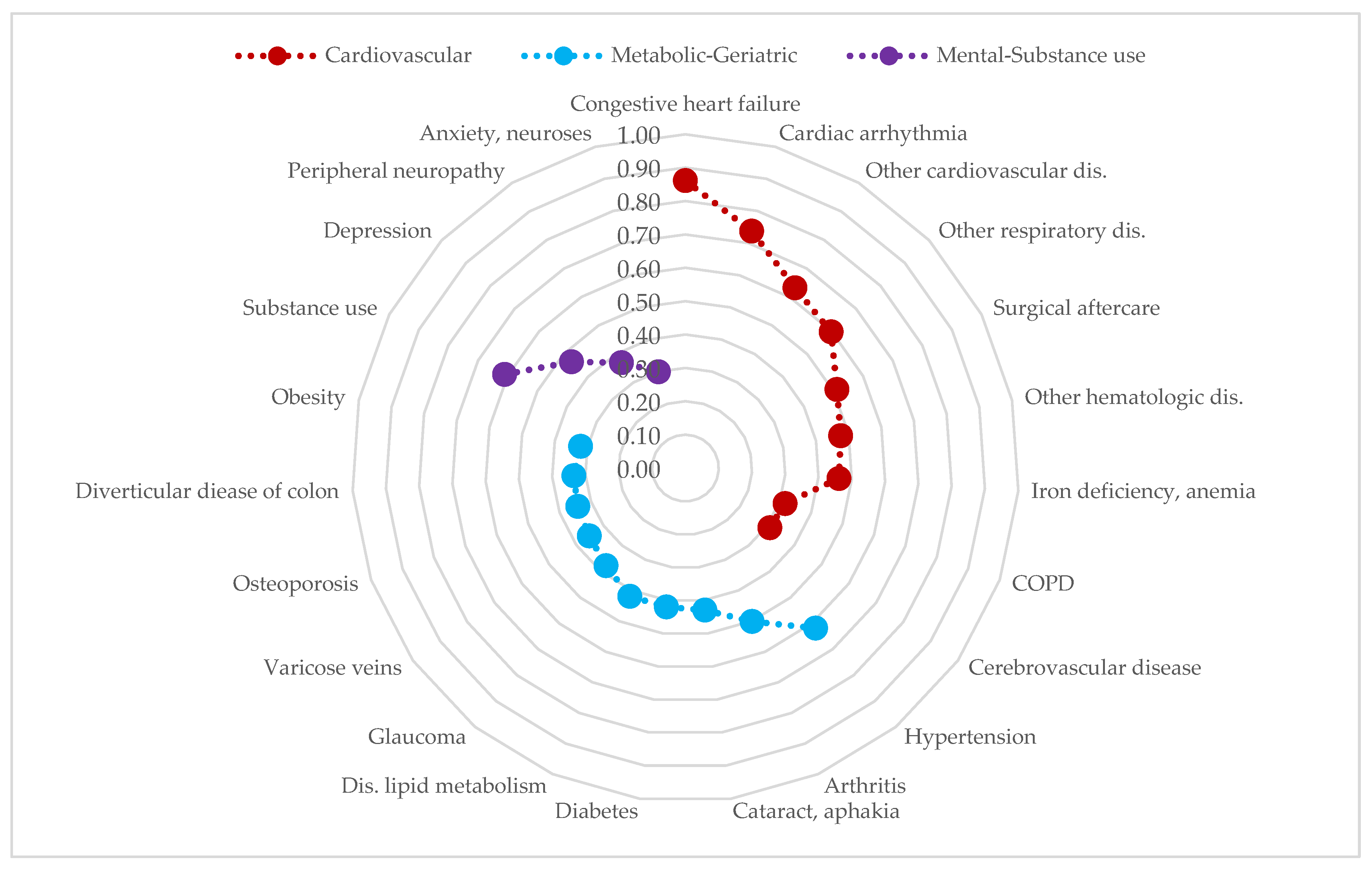
3.2. Multimorbidity Patterns in Patients with CLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EuroStemCell. Enfermedad hepática crónica: ¿Cómo puede ayudar la medicina regenerativa? | Eurostemcell [Internet]. 2020 [cited 2022 Mar 20]. Available from: https://www.eurostemcell.org/es/enfermedad-hepatica-cronica-como-puede-ayudar-la-medicina-regenerativa.
- Sharma A, Nagalli S. Chronic Liver Disease. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
- Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical Gastroenterology and Hepatology. 2020;18:2650–66. [CrossRef]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–858. [CrossRef]
- Cheemerla S, Balakrishnan M. Global Epidemiology of Chronic Liver Disease. Clinical Liver Disease. 2021;17:365–70. [CrossRef]
- Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. Journal of Hepatology. 2018;69:718–35. [CrossRef]
- Abbas Z, Shazi L. Pattern and profile of chronic liver disease in acute on chronic liver failure. Hepatol Int. 2015;9:366–72. [CrossRef]
- Van Den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. European Journal of General Practice. 1996;2:65–70. [CrossRef]
- Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ. 2015;350:h1059–h1059. [CrossRef]
- Grissa D, Nytoft Rasmussen D, Krag A, Brunak S, Juhl Jensen L. Alcoholic liver disease: A registry view on comorbidities and disease prediction. Rzhetsky A, editor. PLoS Comput Biol. 2020;16:e1008244. [CrossRef]
- Maev IV, Samsonov AA, Palgova LK, Pavlov CS, Shirokova E, Starostin KM. Real-world comorbidities and treatment patterns among patients with non-alcoholic fatty liver disease receiving phosphatidylcholine as adjunctive therapy in Russia. BMJ Open Gastroenterol. 2019;6:e000307. [CrossRef]
- Gu W, Hortlik H, Erasmus H-P, Schaaf L, Zeleke Y, Uschner FE, et al. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population-based study (2005 to 2018). The Lancet Regional Health - Europe. 2022;12:100240. [CrossRef]
- Mu X-M, Wang W, Jiang Y-Y, Feng J. Patterns of Comorbidity in Hepatocellular Carcinoma: A Network Perspective. IJERPH. 2020;17:3108. [CrossRef]
- Prados-Torres A, Poblador-Plou B, Gimeno-Miguel A, Calderón-Larrañaga A, Poncel-Falcó A, Gimeno-Feliú LA, et al. Cohort Profile: The Epidemiology of Chronic Diseases and Multimorbidity. The EpiChron Cohort Study. International Journal of Epidemiology. 2018;47:382–384e. [CrossRef]
- The Johns Hopkins University. Johns Hopkins ACG® System [Internet]. 2022 [cited 2022 Jul 25]. Available from: https://www.hopkinsacg.org/.
- Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61:e12–21. [CrossRef]
- Dziuban CD, Shirkey EC. When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychological Bulletin. 1974;81:358–61. [CrossRef]
- Cooperman AW, Waller NG. Heywood you go away! Examining causes, effects, and treatments for Heywood cases in exploratory factor analysis. Psychological Methods. 2022;27:156–76. [CrossRef]
- Mäkelä P, Gmel G, Grittner U, Kuendig H, Kuntsche S, Bloomfield K, et al. Drinking patterns and their gender differences in Europe. Alcohol and Alcoholism. 2006;41:i8–18. [CrossRef]
- Patel PJ, Hayward KL, Rudra R, Horsfall LU, Hossain F, Williams S, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: Implications for disease severity and management. Medicine. 2017;96:e6761. [CrossRef]
- Uhanova J, O’Brien M, Minuk G, Tate R. Chronic Liver Disease and Metabolic Comorbidities in Healthy Young Males Followed for 65 Years: The Manitoba Follow-up Study. Clinical Gastroenterology and Hepatology. 2021;19:2417-2424.e2. [CrossRef]
- 22. Jepsen P. Comorbidity in cirrhosis. WJG. 2014;20:7223. [CrossRef]
- Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, Van Den Akker M. Multimorbidity patterns: a systematic review. Journal of Clinical Epidemiology. 2014;67:254–66. [CrossRef]
- Ioakeim-Skoufa I, Poblador-Plou B, Carmona-Pírez J, Díez-Manglano J, Navickas R, Gimeno-Feliu LA, et al. Multimorbidity Patterns in the General Population: Results from the EpiChron Cohort Study. IJERPH. 2020;17:4242. [CrossRef]
- Colaci C, Gambardella ML, Maria Scarlata GG, Boccuto L, Colica C, Luzza F, et al. Dysmetabolic comorbidities and non-alcoholic fatty liver disease: a stairway to metabolic dysfunction-associated steatotic liver disease. Hepatoma Res. 2024;10:16. [CrossRef]
- Pang Y, Han Y, Yu C, Kartsonaki C, Guo Y, Chen Y, et al. The role of lifestyle factors on comorbidity of chronic liver disease and cardiometabolic disease in Chinese population: A prospective cohort study. The Lancet Regional Health - Western Pacific. 2022;28:100564. [CrossRef]
- Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. Singal A, editor. PLoS Med. 2020;17:e1003100. [CrossRef]
- Morgan M. The prognosis and outcome of alcoholic liver disease. 1994;2:335–43.
- Grønkjær LL, Lauridsen MM. Quality of life and unmet needs in patients with chronic liver disease: A mixed-method systematic review. JHEP Reports. 2021;3:100370. [CrossRef]
- Lenti MV, Ballesio A, Croce G, Brera AS, Padovini L, Bertolino G, et al. Comorbidity and multimorbidity in patients with cirrhosis, hospitalised in an internal medicine ward: a monocentric, cross-sectional study. BMJ Open. 2024;14:e077576. [CrossRef]
| Sex (n, %) | Women (2563; 37.5%) | Men (4273; 62.5%) | Total (6836; 100%) |
| Age (n, %) | |||
| 18-39 years | 142 (5.54) | 236 (5.52) | 378 (5.53) |
| 40-64 years | 1170 (45.7) | 2375 (55.6) | 3545 (51.9) |
| ≥65 years | 1251 (48.8) | 1662 (38.9) | 2913 (42.6) |
| Mean age (s.e1), years | 63.3 (0.29) | 60.6 (0.21) | 61.6 (0.17) |
| Multimorbidity2 (%) | 99.6 | 99.1 | 99.3 |
| Number of chronic diseases, mean (s.d.3) | 14.1 (7.31) | 12.5 (7.21) | 13.1 (7.29) |
| Most prevalent chronic diseases (EDC4, %) | Arterial hypertension (49.9) | Arterial hypertension (49.9) | Arterial hypertension (48.0) |
| Disorders of lipid metabolism (42.3) | Disorders of lipid metabolism (41.3) | Disorders of lipid metabolism (41.3) | |
| Arthritis (28.2) | Diabetes (28.8) | Diabetes (26.9) | |
| Varicose veins (26.9) | Substance abuse (24.3) | Obesity (19.7) | |
| Depression (24.7) | Obesity (17.6) | Arthritis (19.1) | |
| Diabetes (24.1) | Cancer (17.5) | Substance abuse (17.6) | |
| Obesity (23.4) | COPD5 (14.6) | Depression (16.5) | |
| Osteoporosis (19.0) | Arthritis (13.7) | Cancer (16.0) | |
| Iron deficiency, anemia (17.4) | Cardiac arrhythmia (13.3) | Varicose veins (15.2) | |
| Hypothyroidism (16.3) | Iron deficiency, anemia (12.7) | Iron deficiency, anemia (14.4) |
| Women aged 18-39 years (n=142) | Men aged 18-39 years (n=236) | |||||
| Mean number of chronic diseases: 9.2 | Mean number of chronic diseases: 7.3 | |||||
| EDC1 | Comorbidity | N (%) | EDC | Comorbidity | N (%) | |
| CAR11 | Dis.2 of lipid metabolism | 27 (19.0) | CAR11 | Dis. of lipid metabolism | 61 (25.7) | |
| NUT03 | Obesity | 21 (14.8) | PSY02 | Substance use | 38 (16.0) | |
| SKN02 | Dermatitis and eczema | 21 (14.8) | NUT03 | Obesity | 37 (15.6) | |
| HEM02 | Iron deficiency, anemia | 20 (14.1) | ALL03 | Allergic rhinitis | 34 (14.3) | |
| ALL03 | Allergic rhinitis | 18 (12.7) | SKN02 | Dermatitis and eczema | 30 (12.6) | |
| END05 | Other endocrine dis. | 18 (12.7) | ASMA | Asthma | 24 (10.1) | |
| PSY01 | Anxiety, neuroses | 17 (12.0) | DIAB | Diabetes | 20 (8.44) | |
| SKN13 | Disease of hair | 15 (10.6) | HTA | Arterial hypertension | 18 (7.59) | |
| END04 | Hypothyroidism | 14 (9.86) | MUS14 | Low back pain | 18 (7.59) | |
| MUS14 | Low back pain | 14 (9.86) | PSY09 | Depression | 13 (5.48) | |
| Women aged 40-64 years (n=1170) | Men aged 40-64 years (n=2375) | |||||
| Mean number of chronic diseases: 12.2 | Mean number of chronic diseases: 11.1 | |||||
| EDC | Comorbidity | N (%) | EDC | Comorbidity | N (%) | |
| CAR11 | Dis. of lipid metabolism | 482 (41.2) | CAR11 | Dis. of lipid metabolism | 989 (41.6) | |
| HTA | Arterial hypertension | 366 (31.3) | HTA | Arterial hypertension | 934 (39.3) | |
| NUT03 | Obesity | 282 (24.1) | PSY02 | Substance use | 722 (30.4) | |
| PSY09 | Depression | 280 (23.9) | DIAB | Diabetes | 561 (23.6) | |
| GSU08 | Varicose veins | 275 (23.5) | NUT03 | Obesity | 419 (17.6) | |
| MUS03 | Arthritis | 208 (17.8) | PSY09 | Depression | 315 (13.3) | |
| DIAB | Diabetes | 190 (16.2) | CANCER | Cancer | 274 (11.5) | |
| END04 | Hypothyroidism | 188 (16.1) | SKN02 | Dermatitis and eczema | 259 (10.9) | |
| PSY19 | Sleep disorders | 164 (14.0) | PSY19 | Sleep disorders | 258 (10.8) | |
| END05 | Other endocrine dis. | 158 (13.5) | RES04 | COPD3 | 248 (10.4) | |
| Women aged ≥65 years (n=1251) | Men aged ≥65 years (n=1662) | |||||
| Mean number of chronic diseases: 16.5 | Mean number of chronic diseases: 15.4 | |||||
| EDC | Comorbidity | N (%) | EDC | Comorbidity | N (%) | |
| HTA | Arterial hypertension | 908 (72.5) | HTA | Arterial hypertension | 1059 (63.7) | |
| CAR11 | Dis. of lipid metabolism | 574 (45.8) | CAR11 | Dis. of lipid metabolism | 714 (43.0) | |
| MUS03 | Arthritis | 512 (40.9) | DIAB | Diabetes | 649 (39.0) | |
| DIAB | Diabetes | 417 (33.3) | CANCER | Cancer | 470 (28.3) | |
| GSU08 | Varicose veins | 402 (32.1) | GUR04 | Prostatic hypertrophy | 414 (24.9) | |
| END02 | Osteoporosis | 362 (28.9) | CAR09 | Cardiac arrhythmia | 405 (24.4) | |
| PSY09 | Depression | 339 (27.1) | MUS03 | Arthritis | 374 (22.5) | |
| NUT03 | Obesity | 294 (23.5) | RES04 | COPD | 374 (22.5) | |
| HEM02 | Iron deficiency, anemia | 285 (22.8) | HEM02 | Iron deficiency, anemia | 326 (19.6) | |
| EYE06 | Cataract, aphakia | 266 (21.2) | ADM02 | Surgical aftercare | 315 (19.0) | |
| EDC1 | Comorbidity | Crude OR2 (95% CI)3 |
p value | Adjusted OR4 (95% CI) |
p value |
|---|---|---|---|---|---|
| GAS12 | Chronic pancreatitis | 66.1 (53.9-81.0) | <0.001 | 41.2 (33.5-50.6) | <0.00001 |
| GTC02 | Inherited metabolic disorders | 22.9 (18.2-28.8) | <0.001 | 14.9 (11.8-18.8) | <0.00001 |
| HEM07 | Hemophilia, coagulation disorder | 21.6 (17.0-27.5) | <0.001 | 14.0 (11.0-17.9) | <0.00001 |
| HEM06 | Deep vein thrombosis | 21.4 (16.8-27.3) | <0.001 | 13.4 (10.5-17.1) | <0.00001 |
| INF04 | HIV5 | 14.4 (12.4-16.7) | <0.001 | 13.3 (11.4-15.4) | <0.00001 |
| CAR10 | Generalized atherosclerosis | 20.1 (17.1-23.6) | <0.001 | 12.2 (10.3-14.4) | <0.00001 |
| PSY13 | Adjustment disorder | 14.0 (10.4-18.9) | <0.001 | 11.0 (8.14-14.9) | <0.00001 |
| PSY02 | Substance use | 13.9 (13.1-14.8) | <0.001 | 10.6 (9.93-11.3) | <0.00001 |
| GAS08 | Gastroesophageal reflux | 15.1 (13.4-17.1) | <0.001 | 10.0 (8.85-11.3) | <0.00001 |
| RHU01 | Autoimmune, connective tissue dis. | 12.5 (10.0-15.6) | <0.001 | 8.55 (6.84-10.7) | <0.00001 |
| REN01 | Chronic renal failure | 10.8 (9.68-12.0) | <0.001 | 7.74 (6.91-8.67) | <0.00001 |
| PSY20 | Major depression | 7.38 (5.14-10.6) | <0.001 | 7.01 (4.87-10.1) | <0.00001 |
| CAR07 | Cardiomyopathy | 11.7 (9.46-14.5) | <0.001 | 6.66 (5.37-8.26) | <0.00001 |
| RES13 | Chronic respiratory failure | 10.3 (8.09-13.1) | <0.001 | 6.40 (5.02-8.15) | <0.00001 |
| RES06 | Sleep apnea | 10.3 (8.79-12.1) | <0.001 | 6.17 (5.25-7.25) | <0.00001 |
| ADM02 | Surgical aftercare | 8.97 (8.34-9.66) | <0.001 | 6.02 (5.58-6.49) | <0.00001 |
| REN06 | End stage renal disease | 9.78 (6.80-14.1) | <0.001 | 5.67 (3.94-8.16) | <0.00001 |
| RES11 | Respiratory disorders, other | 7.22 (6.65-7.84) | <0.001 | 4.55 (4.19-4.95) | <0.00001 |
| ALL06 | Disorders of the immune system | 6.02 (5.22-6.94) | <0.001 | 4.36 (3.78-5.03) | <0.00001 |
| NUR17 | Paralytic syndromes, other | 6.60 (5.13-8.48) | <0.001 | 4.10 (3.18-5.27) | <0.00001 |
| CAR05 | Congestive heart failure | 5.24 (4.80-5.72) | <0.001 | 3.86 (3.52-4.25) | <0.00001 |
| GSU15 | Alimentary, excretory surgic. openings | 6.44 (4.59-9.03) | <0.001 | 3.77 (2.69-5.30) | <0.00001 |
| REN04 | Nephritis, nephrosis | 5.18 (4.09-6.56) | <0.001 | 3.74 (2.95-4.74) | <0.00001 |
| RHU03 | Arthropathy | 5.74 (3.68-8.98) | <0.001 | 3.71 (2.37-5.80) | <0.00001 |
| REN05 | Renal disorders, other | 5.08 (4.22-6.11) | <0.001 | 3.66 (3.04-4.40) | <0.00001 |
| PSY08 | Personality disorders | 3.42 (2.81-4.16) | <0.001 | 3.34 (2.75-4.07) | <0.00001 |
| CAR16 | Cardiovascular disorders, other | 5.06 (4.69-5.47) | <0.001 | 3.22 (2.98-3.48) | <0.00001 |
| GAS10 | Diverticular disease of colon | 4.72 (4.27-5.22) | <0.001 | 3.18 (2.87-3.52) | <0.00001 |
| HEM02 | Iron deficiency, anemias | 3.68 (3.44-3.94) | <0.001 | 3.08 (2.87-3.30) | <0.00001 |
| HEM08 | Hematologic disorders, other | 4.54 (4.20-4.90) | <0.001 | 2.93 (2.70-3.17) | <0.00001 |
| CAR06 | Cardiac valve disorders | 4.42 (3.94-4.97) | <0.001 | 2.87 (2.55-3.24) | <0.00001 |
| INF01 | Tuberculosis infection | 3.09 (2.56-3.73) | <0.001 | 2.83 (2.34-3.42) | <0.00001 |
| DIAB | Diabetes | 4.31 (4.08-4.55) | <0.001 | 2.72 (2.57-2.88) | <0.00001 |
| EYE13 | Diabetic retinopathy | 4.24 (3.58-5.01) | <0.001 | 2.69 (2.27-3.18) | <0.00001 |
| GSU11 | Peripheral vascular disease | 4.50 (3.80-5.32) | <0.001 | 2.62 (2.21-3.10) | <0.00001 |
| RES04 | COPD6 | 4.34 (4.03-4.68) | <0.001 | 2.54 (2.34-2.74) | <0.00001 |
| CAR09 | Cardiac arrhythmia | 3.75 (3.49-4.04) | <0.001 | 2.38 (2.21-2.57) | <0.00001 |
| REC03 | Chronic ulcer of the skin | 3.37 (2.99-3.79) | <0.001 | 2.31 (2.04-2.61) | <0.00001 |
| NUR07 | Seizure disorder | 2.64 (2.27-3.07) | <0.001 | 2.28 (1.96-2.65) | <0.00001 |
| EYE03 | Retinal disorders (excl. diabetic retinopathy) | 3.20 (2.08-4.93) | <0.001 | 2.21 (1.43-3.41) | 0.00033 |
| CANCER | Cancer | 3.26 (3.06-3.48) | <0.001 | 2.09 (1.96-2.24) | <0.00001 |
| GUR09 | Renal calculi | 2.91 (2.58-3.29) | <0.001 | 1.99 (1.76-2.25) | <0.00001 |
| NUT03 | Obesity | 2.62 (2.46-2.78) | <0.001 | 1.99 (1.87-2.11) | <0.00001 |
| HEM01 | Hemolytic anemia | 1.82 (1.32-2.52) | 0.00030 | 1.90 (1.37-2.63) | 0.00012 |
| NUR21 | Neurologic disorders, other | 2.47 (2.27-2.68) | <0.001 | 1.86 (1.71-2.03) | <0.00001 |
| NUR03 | Peripheral neuropathy, neuritis | 2.14 (1.94-2.36) | <0.001 | 1.85 (1.68-2.04) | <0.00001 |
| NUR19 | Developmental disorder | 0.85 (0.64-1.13) | 0.272 | 1.85 (1.38-2.47) | 0.00003 |
| GAS02 | Inflammatory bowel disease | 2.25 (1.78-2.85) | <0.001 | 1.80 (1.42-2.28) | <0.00001 |
| HTA | Hypertension | 3.27 (3.11-3.43) | <0.001 | 1.79 (1.70-1.89) | <0.00001 |
| RHU02 | Gout | 3.38 (3.05-3.75) | <0.001 | 1.77 (1.59-1.97) | <0.00001 |
| RES08 | Pulmonary embolism | 2.71 (2.04-3.60) | <0.001 | 1.76 (1.32-2.33) | 0.00010 |
| GAS09 | Irritable bowel syndrome | 2.05 (1.77-2.37) | <0.001 | 1.69 (1.46-1.96) | <0.00001 |
| MUS14 | Low back pain | 2.11 (1.95-2.29) | <0.001 | 1.68 (1.55-1.83) | <0.00001 |
| HEM03 | Thrombophlebitis | 2.33 (2.06-2.64) | <0.001 | 1.66 (1.47-1.89) | <0.00001 |
| PSY01 | Anxiety, neuroses | 1.73 (1.57-1.91) | <0.001 | 1.64 (1.48-1.82) | <0.00001 |
| PSY09 | Depression | 2.02 (1.90-2.16) | <0.001 | 1.64 (1.53-1.75) | <0.00001 |
| NUR24 | Dementia | 2.00 (1.73-2.32) | <0.001 | 1.58 (1.36-1.85) | <0.00001 |
| IHD | Ischemic heart disease | 2.74 (2.50-3.00) | <0.001 | 1.55 (1.41-1.70) | <0.00001 |
| END04 | Hypothyroidism | 1.63 (1.50-1.77) | <0.001 | 1.54 (1.41-1.68) | <0.00001 |
| END02 | Osteoporosis | 1.74 (1.60-1.89) | <0.001 | 1.50 (1.37-1.65) | <0.00001 |
| END05 | Other endocrine disorders | 1.57 (1.44-1.72) | <0.001 | 1.50 (1.38-1.64) | <0.00001 |
| PSY07 | Schizophrenia, affective psychosis | 1.90 (1.59-2.25) | <0.001 | 1.50 (1.26-1.78) | <0.00001 |
| NUR05 | Cerebrovascular disease | 2.24 (2.02-2.48) | <0.001 | 1.39 (1.25-1.54) | <0.00001 |
| MUS13 | Cervical pain syndromes | 1.67 (1.45-1.92) | <0.001 | 1.35 (1.17-1.56) | 0.00003 |
| PSY19 | Sleep disorders | 1.88 (1.75-2.02) | <0.001 | 1.35 (1.26-1.45) | <0.00001 |
| SKN12 | Psoriasis | 1.71 (1.51-1.94) | <0.001 | 1.34 (1.18-1.52) | <0.00001 |
| GSU08 | Varicose veins of lower extremities | 1.58 (1.48-1.68) | <0.001 | 1.30 (1.21-1.40) | <0.00001 |
| CAR11 | Disorders of lipid metabolism | 2.25 (2.14-2.36) | <0.001 | 1.27 (1.21-1.34) | <0.00001 |
| SKN02 | Dermatitis and eczema | 0.89 (0.82-0.96) | 0.00168 | 1.23 (1.14-1.33) | <0.00001 |
| EYE06 | Cataract, aphakia | 1.84 (1.70-1.99) | <0.001 | 1.17 (1.07-1.27) | 0.00029 |
| MUS03 | Arthritis | 1.82 (1.71-1.93) | <0.001 | 1.17 (1.10-1.25) | <0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





