Submitted:
04 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. General Characteristics of EVs
3. The Molecular Pathophysiology of IS and the Therapeutic Potential of EVs
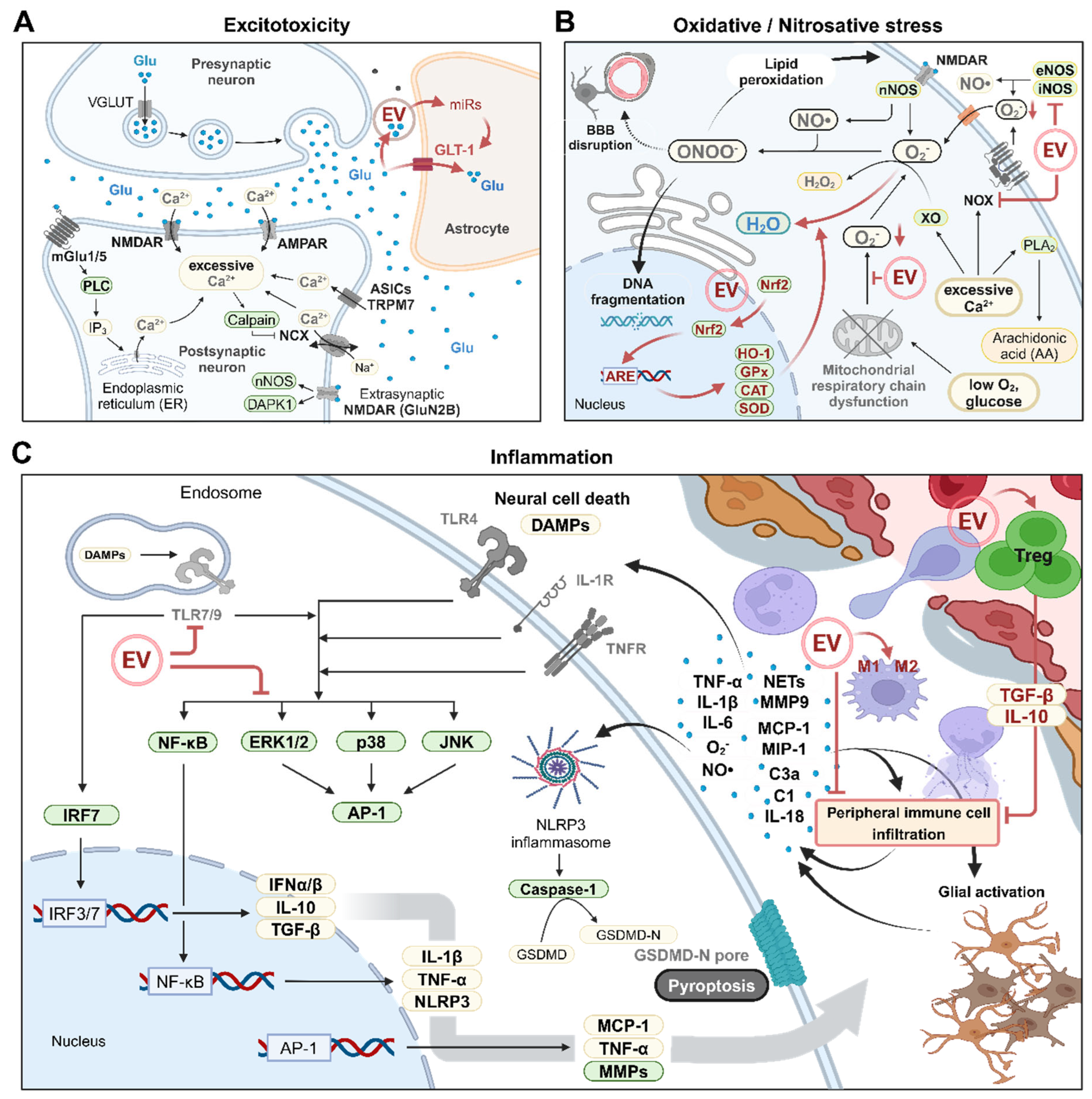
3.1. Excitotoxicity
3.2. Oxidative/Nitrosative Stress
3.3. Inflammation and Ischemia/Reperfusion (I/R) Injury
3.4. Ischemic Brain Cell Death
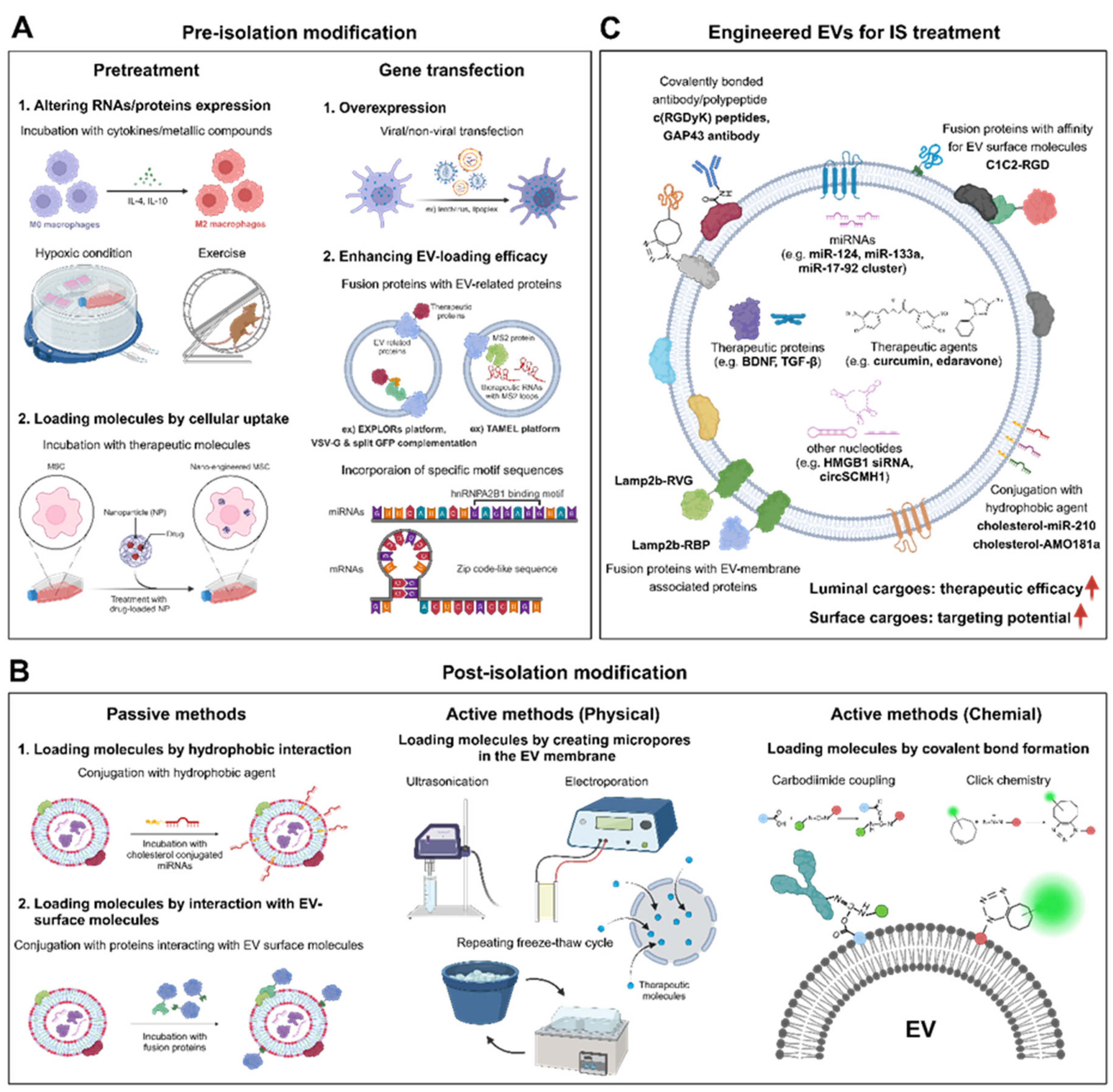
4. EV Engineering Methods
4.1. Pre-Isolation Modification: Pretreatment and Gene Transfection
4.2. Post-Isolation Modification: Passive and Active Methods
4.3. EV Engineering for IS Treatment
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. The Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Mistry, E.A.; Mistry, A.M.; Nakawah, M.O.; Chitale, R.V.; James, R.F.; Volpi, J.J.; Fusco, M.R. Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis. Stroke 2017, 48, 2450–2456. [Google Scholar] [CrossRef]
- Hankey, G.J.; Spiesser, J.; Hakimi, Z.; Bego, G.; Carita, P.; Gabriel, S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007, 68, 1583–1587. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M.; Oxford Vascular, S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol 2019, 18, 248–258. [Google Scholar] [CrossRef]
- Park, J.H.; Ovbiagele, B. Relationship of functional disability after a recent stroke with recurrent stroke risk. Eur J Neurol 2016, 23, 361–367. [Google Scholar] [CrossRef]
- Koton, S.; Pike, J.R.; Johansen, M.; Knopman, D.S.; Lakshminarayan, K.; Mosley, T.; Patole, S.; Rosamond, W.D.; Schneider, A.L.C.; Sharrett, A.R.; et al. Association of Ischemic Stroke Incidence, Severity, and Recurrence With Dementia in the Atherosclerosis Risk in Communities Cohort Study. JAMA Neurol 2022, 79, 271–280. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat Rev Dis Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- George, Paul M.; Steinberg, Gary K. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron 2015, 87, 297–309. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999, 22, 391–397. [Google Scholar] [CrossRef]
- O'Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1,026 Experimental treatments in acute stroke. Annals of Neurology 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Experimental Neurology 2021, 335. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Kondziolka, D.; Steinberg, G.K.; Wechsler, L.; Meltzer, C.C.; Elder, E.; Gebel, J.; Decesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J.; et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg 2005, 103, 38–45. [Google Scholar] [CrossRef]
- Savitz, S.I.; Dinsmore, J.; Wu, J.; Henderson, G.V.; Stieg, P.; Caplan, L.R. Neurotransplantation of Fetal Porcine Cells in Patients with Basal Ganglia Infarcts: A Preliminary Safety and Feasibility Study. Cerebrovascular Diseases 2005, 20, 101–107. [Google Scholar] [CrossRef]
- Chen, J.; Chopp, M. Exosome Therapy for Stroke. Stroke 2018, 49, 1083–1090. [Google Scholar] [CrossRef]
- H. Rashed, M.; Bayraktar, E.; K. Helal, G.; Abd-Ellah, M.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. International Journal of Molecular Sciences 2017, 18. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res Ther 2020, 11, 313. [Google Scholar] [CrossRef]
- Yue, K.-Y.; Zhang, P.-R.; Zheng, M.-H.; Cao, X.-L.; Cao, Y.; Zhang, Y.-Z.; Zhang, Y.-F.; Wu, H.-N.; Lu, Z.-H.; Liang, L.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death & Disease 2019, 10. [Google Scholar] [CrossRef]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 2018, 32, 512–528. [Google Scholar] [CrossRef]
- de Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem 2018, 293, 1957–1975. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: novel biomarkers for neurological disorders. Front Physiol 2012, 3, 63. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Garcia-Manrique, P.; Matos, M.; Gutierrez, G.; Pazos, C.; Blanco-Lopez, M.C. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 2018, 7. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Current Opinion in Cell Biology 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8. [Google Scholar] [CrossRef]
- Hurley, J.H. ESCRTs are everywhere. EMBO J 2015, 34, 2398–2407. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Current Biology 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 2014, 3, 24722. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.; Skog, J.; et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol 2016, 18, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int 2019, 2019, 2402916. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Véron, P.; Segura, E.; Sugano, G.; Amigorena, S.; Théry, C. Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells. Blood Cells, Molecules, and Diseases 2005, 35, 81–88. [Google Scholar] [CrossRef]
- Naslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Obrenovitch, T.P.; Urenjak, J.; Richards, D.A.; Ueda, Y.; Curzon, G.; Symon, L. Extracellular neuroactive amino acids in the rat striatum during ischaemia: comparison between penumbral conditions and ischaemia with sustained anoxic depolarisation. J Neurochem 1993, 61, 178–186. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front Neurosci 2020, 14, 579953. [Google Scholar] [CrossRef]
- Pin, J.P.; Duvoisin, R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Young, K.W.; Guerin, C.J.; Lefeuvre, R.; Rothwell, N.J.; Naldini, L.; Rizzuto, R.; Carafoli, E.; Nicotera, P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 2005, 120, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.G.; Zhu, X.M.; Chu, X.P.; Minami, M.; Hey, J.; Wei, W.L.; MacDonald, J.F.; Wemmie, J.A.; Price, M.P.; Welsh, M.J.; et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 2004, 118, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Iihara, K.; Wei, W.L.; Xiong, Z.G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Progress in Neurobiology 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Serpe, C.; Monaco, L.; Relucenti, M.; Iovino, L.; Familiari, P.; Scavizzi, F.; Raspa, M.; Familiari, G.; Civiero, L.; D’Agnano, I.; et al. Microglia-Derived Small Extracellular Vesicles Reduce Glioma Growth by Modifying Tumor Cell Metabolism and Enhancing Glutamate Clearance through miR-124. Cells 2021, 10. [Google Scholar] [CrossRef]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun 2019, 10, 4136. [Google Scholar] [CrossRef]
- Gosselin, R.D.; Meylan, P.; Decosterd, I. Extracellular microvesicles from astrocytes contain functional glutamate transporters: regulation by protein kinase C and cell activation. Front Cell Neurosci 2013, 7, 251. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Xu, H.-B.; Luo, C.-X.; Wu, H.-Y.; Zhu, M.-M.; Lu, W.; Ji, X.; Zhou, Q.-G.; Zhu, D.-Y. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nature Medicine 2010, 16, 1439–1443. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Hastings, T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 1995, 15, 3318–3327. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Li, R.C.; Zhang, J.; Klaus, J.A.; Kibler, K.K.; Dore, S.; Koehler, R.C.; Sapirstein, A. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J Neuroinflammation 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Urzua, S.; Rojas, I.; Libano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr Pharm Des 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, X.; Kim, Y.; Li, J.; Huang, S.; Saleem, S.; Li, R.C.; Xu, Y.; Dore, S.; Cao, W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med 2012, 52, 928–936. [Google Scholar] [CrossRef]
- Duan, J.; Cui, J.; Yang, Z.; Guo, C.; Cao, J.; Xi, M.; Weng, Y.; Yin, Y.; Wang, Y.; Wei, G.; et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3beta/Nrf2 signaling. J Neuroinflammation 2019, 16, 24. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, Y.; Qu, T.; Zhang, J.; Duan, X.; Xu, H.; Mu, Y.; Ma, H.; Wang, F. Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro. Life Sci 2020, 254, 117772. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther 2021, 6, 354. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.I. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab 2019, 30, 329–342 e325. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Rodríguez-Navarro, J.A.; O’Loghlen, A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metabolism 2020, 32, 71–86.e75. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: from mechanisms to translation. Nat Med 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Therapeutic Advances in Neurological Disorders 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Frijns, C.J.; Kappelle, L.J. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 2002, 33, 2115–2122. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting Neutrophils in Ischemic Stroke: Translational Insights from Experimental Studies. Journal of Cerebral Blood Flow & Metabolism 2015, 35, 888–901. [Google Scholar] [CrossRef]
- McColl, B.W.; Rothwell, N.J.; Allan, S.M. Systemic Inflammation Alters the Kinetics of Cerebrovascular Tight Junction Disruption after Experimental Stroke in Mice. The Journal of Neuroscience 2008, 28, 9451–9462. [Google Scholar] [CrossRef]
- Wu, F.; Zou, Q.; Ding, X.; Shi, D.; Zhu, X.; Hu, W.; Liu, L.; Zhou, H. Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J Neuroinflammation 2016, 13, 23. [Google Scholar] [CrossRef]
- De Simoni, M.G.; Rossi, E.; Storini, C.; Pizzimenti, S.; Echart, C.; Bergamaschini, L. The powerful neuroprotective action of C1-inhibitor on brain ischemia-reperfusion injury does not require C1q. Am J Pathol 2004, 164, 1857–1863. [Google Scholar] [CrossRef]
- Maddahi, A.; Edvinsson, L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation 2010, 7, 14. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury. Front Pharmacol 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.C.; Irving, E.A.; Ray, A.M.; Lee, J.C.; Kassis, S.; Kumar, S.; Badger, A.M.; Legos, J.J.; Erhardt, J.A.; Ohlstein, E.H.; et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev 2001, 21, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Ridder, D.A.; Schwaninger, M. NF-κB signaling in cerebral ischemia. Neuroscience 2009, 158, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.; Lim, Y.A.; Cheng, Y.L.; Lok, K.Z.; Chunduri, P.; Baik, S.H.; Drummond, G.R.; Dheen, S.T.; Sobey, C.G.; Jo, D.G.; et al. Evidence that NF-kappaB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol Neurobiol 2018, 55, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Marsh, B.J.; Williams-Karnesky, R.L.; Stenzel-Poore, M.P. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 2009, 158, 1007–1020. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, Y.; Xu, G.; Yin, G.; Liu, D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem Res 2020, 45, 1180–1190. [Google Scholar] [CrossRef]
- Wang, C.; Borger, V.; Sardari, M.; Murke, F.; Skuljec, J.; Pul, R.; Hagemann, N.; Dzyubenko, E.; Dittrich, R.; Gregorius, J.; et al. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke 2020, 51, 1825–1834. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-kappaB/MAPK pathways. Brain Res Bull 2020, 163, 84–94. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, G.; Chen, Y.; Yuan, J.; Zhang, J.; Wang, S.; Li, Q.; Wang, Y.; Deng, Z. Embryonic Stem Cell Derived Small Extracellular Vesicles Modulate Regulatory T Cells to Protect against Ischemic Stroke. ACS Nano 2021, 15, 7370–7385. [Google Scholar] [CrossRef]
- Han, B.H.; Holtzman, D.M. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci 2000, 20, 5775–5781. [Google Scholar] [CrossRef]
- Zhao, B.-Q.; Wang, S.; Kim, H.-Y.; Storrie, H.; Rosen, B.R.; Mooney, D.J.; Wang, X.; Lo, E.H. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature Medicine 2006, 12, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hebert, M.; Gowing, G.; Simard, A.; Weng, Y.C.; Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 2007, 27, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Q.Z.; Zhang, S.T.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev 2022, 42, 259–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, H.; Zhan, S.; Krajewski, S.; Reed, J.C.; Gottlieb, R.A. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 2001, 276, 30724–30728. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xing, J.; Xiao, X.; Liou, A.K.; Gao, Y.; Yin, X.M.; Clark, R.S.; Graham, S.H.; Chen, J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci 2007, 27, 9278–9293. [Google Scholar] [CrossRef]
- Schinzel, A.C.; Takeuchi, O.; Huang, Z.; Fisher, J.K.; Zhou, Z.; Rubens, J.; Hetz, C.; Danial, N.N.; Moskowitz, M.A.; Korsmeyer, S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A 2005, 102, 12005–12010. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Translational Stroke Research 2020, 11, 1185–1202. [Google Scholar] [CrossRef]
- Nijboer, C.H.; Heijnen, C.J.; van der Kooij, M.A.; Zijlstra, J.; van Velthoven, C.T.J.; Culmsee, C.; van Bel, F.; Hagberg, H.; Kavelaars, A. Targeting the p53 pathway to protect the neonatal ischemic brain. Annals of Neurology 2011, 70, 255–264. [Google Scholar] [CrossRef]
- Niizuma, K.; Endo, H.; Nito, C.; Myer, D.J.; Chan, P.H. Potential Role of PUMA in Delayed Death of Hippocampal CA1 Neurons After Transient Global Cerebral Ischemia. Stroke 2009, 40, 618–625. [Google Scholar] [CrossRef]
- Endo, H.; Kamada, H.; Nito, C.; Nishi, T.; Chan, P.H. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J Neurosci 2006, 26, 7974–7983. [Google Scholar] [CrossRef] [PubMed]
- Badiola, N.; Malagelada, C.; Llecha, N.; Hidalgo, J.; Comella, J.X.; Sabriá, J.; Rodríguez-Alvarez, J. Activation of caspase-8 by tumour necrosis factor receptor 1 is necessary for caspase-3 activation and apoptosis in oxygen–glucose deprived cultured cortical cells. Neurobiology of Disease 2009, 35, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wu, Z.; Xu, J.; Xu, Y. Calreticulin Binds to Fas Ligand and Inhibits Neuronal Cell Apoptosis Induced by Ischemia-Reperfusion Injury. BioMed Research International 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villalba, A.; Herr, I.; Jeremias, I.; Hahne, M.; Brandt, R.; Vogel, J.; Schenkel, J.; Herdegen, T.; Debatin, K.M. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 1999, 19, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Pignataro, G.; Di Benedetto, G.; Anzilotti, S.; Vinciguerra, A.; Cuomo, O.; Di Renzo, G.F.; Parenti, C.; Annunziato, L.; Bernardini, R. Ischemic tolerance modulates TRAIL expression and its receptors and generates a neuroprotected phenotype. Cell Death Dis 2014, 5, e1331. [Google Scholar] [CrossRef]
- Benchoua, A.; Guegan, C.; Couriaud, C.; Hosseini, H.; Sampaio, N.; Morin, D.; Onteniente, B. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci 2001, 21, 7127–7134. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Ferrari, D.; Stepczynska, A.; Los, M.; Wesselborg, S.; Schulze-Osthoff, K. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J Exp Med 1998, 188, 979–984. [Google Scholar] [CrossRef]
- Vieira, M.; Fernandes, J.; Carreto, L.; Anuncibay-Soto, B.; Santos, M.; Han, J.; Fernandez-Lopez, A.; Duarte, C.B.; Carvalho, A.L.; Santos, A.E. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis 2014, 68, 26–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, X.; Zhang, H.; Wang, L.; Wu, X.; Zhang, H.; Luo, Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis 2020, 11, 565. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, D.C.; Gorman, L.G.; Traystman, R.J.; Hurn, P.D. Low molecular weight iron in cerebral ischemic acidosis in vivo. Stroke 1998, 29, 487–492, discussion 493. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, G.; Chen, B.; Xu, L.; Ye, Y.; He, J.; Bao, Z.; Zhao, P.; Miao, Z.; Zhao, L.; et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res 2021, 174, 105933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Li, Q.; Sun, H.; Wang, H. Pharmacological Inhibition of Ferroptosis as a Therapeutic Target for Neurodegenerative Diseases and Strokes. Advanced Science 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Huang, Z.; Taheri, M.R.; O'Leary, E.; Li, E.; Moskowitz, M.A.; Sapirstein, A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 1997, 390, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Ratan, R.R. The Chemical Biology of Ferroptosis in the Central Nervous System. Cell Chem Biol 2020, 27, 479–498. [Google Scholar] [CrossRef]
- Liu, S.; Luo, W.; Wang, Y. Emerging role of PARP-1 and PARthanatos in ischemic stroke. J Neurochem 2022, 160, 74–87. [Google Scholar] [CrossRef]
- Poh, L.; Kang, S.W.; Baik, S.H.; Ng, G.Y.Q.; She, D.T.; Balaganapathy, P.; Dheen, S.T.; Magnus, T.; Gelderblom, M.; Sobey, C.G.; et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun 2019, 75, 34–47. [Google Scholar] [CrossRef]
- Ye, X.; Shen, T.; Hu, J.; Zhang, L.; Zhang, Y.; Bao, L.; Cui, C.; Jin, G.; Zan, K.; Zhang, Z.; et al. Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp Neurol 2017, 292, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Emmrich, J.V.; Fricker, M.; Mander, P.K.; Thery, C.; Brown, G.C. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 2013, 110, E4098–4107. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol Ther 2021, 225, 107848. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhang, M.; Feng, Y.S.; Xing, Y.; Tan, Z.X.; Li, W.B.; Dong, F.; Zhang, F. Electroacupuncture Inhibits Neuronal Autophagy and Apoptosis via the PI3K/AKT Pathway Following Ischemic Stroke. Front Cell Neurosci 2020, 14, 134. [Google Scholar] [CrossRef]
- Xu, D.; Kong, T.; Zhang, S.; Cheng, B.; Chen, J.; Wang, C. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cell Signal 2021, 79, 109839. [Google Scholar] [CrossRef]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007, 9, 218–224. [Google Scholar] [CrossRef]
- Nowosad, A.; Jeannot, P.; Callot, C.; Creff, J.; Perchey, R.T.; Joffre, C.; Codogno, P.; Manenti, S.; Besson, A. p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy-lysosomal pathway and coordinate cell cycle and cell growth. Nat Cell Biol 2020, 22, 1076–1090. [Google Scholar] [CrossRef]
- Sun, B.; Ou, H.; Ren, F.; Huan, Y.; Zhong, T.; Gao, M.; Cai, H. Propofol inhibited autophagy through Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Molecular Medicine 2018, 24. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Q.; Yan, J.; Yang, X.; Shi, X.; Lei, J.; Chen, L.; Huang, H.; Han, J.; Zhang, J.H.; et al. Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis 2009, 33, 509–517. [Google Scholar] [CrossRef]
- Barteczek, P.; Li, L.; Ernst, A.S.; Bohler, L.I.; Marti, H.H.; Kunze, R. Neuronal HIF-1alpha and HIF-2alpha deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke. J Cereb Blood Flow Metab 2017, 37, 291–306. [Google Scholar] [CrossRef]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains. Molecular and Cellular Biology 2023, 29, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Kim, E.; Beemiller, P.; Wang, C.Y.; Swanson, J.; You, M.; Guan, K.L. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem 2007, 282, 35803–35813. [Google Scholar] [CrossRef] [PubMed]
- Crighton, D.; Wilkinson, S.; O'Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yu, M.; Bu, Z.; He, P.; Feng, J. FKBP5 Exacerbates Impairments in Cerebral Ischemic Stroke by Inducing Autophagy via the AKT/FOXO3 Pathway. Front Cell Neurosci 2020, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yu, M.; He, X.; Wen, L.; Bu, Z.; Feng, J. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 2019, 18, e12940. [Google Scholar] [CrossRef]
- Mei, Z.G.; Huang, Y.G.; Feng, Z.T.; Luo, Y.N.; Yang, S.B.; Du, L.P.; Jiang, K.; Liu, X.L.; Fu, X.Y.; Deng, Y.H.; et al. Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging (Albany NY) 2020, 12, 13187–13205. [Google Scholar] [CrossRef]
- Xu, S.; Huang, P.; Yang, J.; Du, H.; Wan, H.; He, Y. Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomedicine 2023, 115, 154845. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Zhang, S.; Sun, X. Protective effects of Gypenoside XVII against cerebral ischemia/reperfusion injury via SIRT1-FOXO3A- and Hif1a-BNIP3-mediated mitochondrial autophagy. Journal of Translational Medicine 2022, 20. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.-C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef]
- Xiao, Y.; Geng, F.; Wang, G.; Li, X.; Zhu, J.; Zhu, W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem 2019, 120, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Zhao, T.; He, W.; Xia, J.; Huang, Q.; Yang, J.; Gu, W.; Chen, C.; Zhang, N.; Liu, Y.; et al. Exosomal circBBS2 inhibits ferroptosis by targeting miR-494 to activate SLC7A11 signaling in ischemic stroke. FASEB J 2023, 37, e23152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. Journal of Nanobiotechnology 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Liu, H.; Zhu, R.; He, H.; Zhou, Y.; Zhang, Y.; Li, C.; Liang, D.; Zeng, Q.; et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Experimental Neurology 2021, 341. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, L.L.; Wang, X.P.; Guo, W.; Guo, R.B.; Sun, Y.Q.; Xue, T.F.; Cai, Z.Y.; Ji, J.; Cheng, H.; et al. Neuronal extracellular vesicle derived miR-98 prevents salvageable neurons from microglial phagocytosis in acute ischemic stroke. Cell Death Dis 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Venkataramani, V.; Kilic, E.; Hermann, D.M.; Majid, A.; Bahr, M.; Doeppner, T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles 2020, 10, e12024. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Zhang, L.; Ferguson, C.M.; Song, T.; Jiang, K.; Conley, S.M.; Krier, J.D.; Tang, H.; Saadiq, I.; et al. Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem Cells Dev 2020, 29, 1190–1200. [Google Scholar] [CrossRef]
- Xie, W.; Luo, T.; Ma, Z.; Xue, S.; Jia, X.; Yang, T.; Song, Z. Tumor Necrosis Factor Alpha Preconditioned Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Enhance the Inhibition of Necroptosis of Acinar cells in Severe Acute Pancreatitis. Tissue Eng Part A 2023, 29, 607–619. [Google Scholar] [CrossRef]
- Chen, J.; Ma, S.; Luo, B.; Hao, H.; Li, Y.; Yang, H.; Zhu, F.; Zhang, P.; Niu, R.; Pan, P. Human umbilical cord mesenchymal stromal cell small extracellular vesicle transfer of microRNA-223-3p to lung epithelial cells attenuates inflammation in acute lung injury in mice. J Nanobiotechnology 2023, 21, 295. [Google Scholar] [CrossRef]
- Khan, H.; Pan, J.-J.; Li, Y.; Zhang, Z.; Yang, G.-Y. Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Z.; Wang, H.; Yu, Y.; Chen, W.; Waqas, A.; Wang, Y.; Chen, L. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J Adv Res 2020, 24, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Zheng, X.; Kuang, Y.; Lieschke, S.; Janssen, L.; Bosche, B.; Jin, F.; Hein, K.; Kilic, E.; Venkataramani, V.; et al. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med 2021, 10, 357–373. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.W.; Ko, S.B.; et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, L.; Shi, Y.; Liang, J. Edaravone-Loaded Macrophage-Derived Exosomes Enhance Neuroprotection in the Rat Permanent Middle Cerebral Artery Occlusion Model of Stroke. Mol Pharm 2020, 17, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl 2020, 117, 111314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bahr, M.; Doeppner, T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-beta/Smad2/3 pathway. Cell Death Dis 2021, 12, 1068. [Google Scholar] [CrossRef]
- Wang, K.; Ru, J.; Zhang, H.; Chen, J.; Lin, X.; Lin, Z.; Wen, M.; Huang, L.; Ni, H.; Zhuge, Q.; et al. Melatonin Enhances the Therapeutic Effect of Plasma Exosomes Against Cerebral Ischemia-Induced Pyroptosis Through the TLR4/NF-kappaB Pathway. Front Neurosci 2020, 14, 848. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Chen, S.; Zhang, W.; Chen, Y.; Yang, Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Experimental Neurology 2020, 330. [Google Scholar] [CrossRef]
- Huang, M.; Cheng, S.; Li, Z.; Chen, J.; Wang, C.; Li, J.; Zheng, H. Preconditioning Exercise Inhibits Neuron Ferroptosis and Ameliorates Brain Ischemia Damage by Skeletal Muscle–Derived Exosomes via Regulating miR-484/ACSL4 Axis. Antioxidants & Redox Signaling 2024. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, X.; Liu, M.; He, M.; Long, W.; Xu, Z.; Zhang, K.; Liu, T.; So, K.F.; Fu, Q.L.; et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J Control Release 2023, 357, 1–19. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, Z.; Buller, B.; Li, Y.; Golembieski, W.; Gan, X.; Wang, F.; Lu, M.; Ali, M.M.; Zhang, Z.G.; et al. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab 2021, 41, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 2016, 7, 12277. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Q.; Zi, Z.; Liu, Z.; Wan, C.; Crisman, L.; Shen, J.; Liu, X. Programmable Extracellular Vesicles for Macromolecule Delivery and Genome Modifications. Dev Cell 2020, 55, 784–801 e789. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutierrez-Vazquez, C.; Sanchez-Cabo, F.; Perez-Hernandez, D.; Vazquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sanchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013, 4, 2980. [Google Scholar] [CrossRef]
- Bolukbasi, M.F.; Mizrak, A.; Ozdener, G.B.; Madlener, S.; Strobel, T.; Erkan, E.P.; Fan, J.B.; Breakefield, X.O.; Saydam, O. miR-1289 and "Zipcode"-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids 2012, 1, e10. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles 2016, 5, 31027. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology 2019, 17, 29. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, Z.; Huang, L.; Liang, J.; Wang, P.; Zhao, L.; Shi, Y. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnology 2021, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. Journal of Controlled Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface functionalization of exosomes using click chemistry. Bioconjug Chem 2014, 25, 1777–1784. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, L.; Huang, L.; Shi, Y.; Liang, J.; Zhao, L. Baicalin-loaded macrophage-derived exosomes ameliorate ischemic brain injury via the antioxidative pathway. Mater Sci Eng C Mater Biol Appl 2021, 126, 112123. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.; Hwang, D.W.; Lee, M. Delivery of High Mobility Group Box-1 siRNA Using Brain-Targeting Exosomes for Ischemic Stroke Therapy. J Biomed Nanotechnol 2019, 15, 2401–2412. [Google Scholar] [CrossRef]
- Guo, L.; Pan, J.; Li, F.; Zhao, L.; Shi, Y. A novel brain targeted plasma exosomes enhance the neuroprotective efficacy of edaravone in ischemic stroke. IET Nanobiotechnology 2021, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 2016, 79, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, Y.; Lee, M. Hypoxia-specific anti-RAGE exosomes for nose-to-brain delivery of anti-miR-181a oligonucleotide in an ischemic stroke model. Nanoscale 2021, 13, 14166–14178. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Cao, L.; He, C.; Ye, Q.; Liang, R.; You, W.; Zhang, H.; Wu, J.; Ye, J.; Tannous, B.A.; et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 2021, 11, 6507–6521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, R.; Wang, C.; Guo, Y.; Xu, T.; Zhang, Z.; Yang, G.Y.; Xu, H.; Tang, Y. Brain Microenvironment Responsive and Pro-Angiogenic Extracellular Vesicle-Hydrogel for Promoting Neurobehavioral Recovery in Type 2 Diabetic Mice After Stroke. Adv Healthc Mater 2022, 11, e2201150. [Google Scholar] [CrossRef]
- Bang, O.Y.; Kim, E.H. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front Neurol 2019, 10, 211. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med 2015, 4, 1131–1143. [Google Scholar] [CrossRef]
- Rocha, M.; Jovin, T.G. Fast Versus Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke: Clinical and Research Implications. Stroke 2017, 48, 2621–2627. [Google Scholar] [CrossRef]
- Ollen-Bittle, N.; Roseborough, A.D.; Wang, W.; Wu, J.-l.D.; Whitehead, S.N. Mechanisms and Biomarker Potential of Extracellular Vesicles in Stroke. Biology 2022, 11. [Google Scholar] [CrossRef]
- van Kralingen, J.C.; McFall, A.; Ord, E.N.J.; Coyle, T.F.; Bissett, M.; McClure, J.D.; McCabe, C.; Macrae, I.M.; Dawson, J.; Work, L.M. Altered Extracellular Vesicle MicroRNA Expression in Ischemic Stroke and Small Vessel Disease. Translational Stroke Research 2019, 10, 495–508. [Google Scholar] [CrossRef]
- Couch, Y.; Akbar, N.; Davis, S.; Fischer, R.; Dickens, A.M.; Neuhaus, A.A.; Burgess, A.I.; Rothwell, P.M.; Buchan, A.M. Inflammatory Stroke Extracellular Vesicles Induce Macrophage Activation. Stroke 2017, 48, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.Y.S.; Alsop, E.; Meechoovet, B.; Beecroft, T.; Agrawal, K.; Whitsett, T.G.; Huentelman, M.J.; Spetzler, R.F.; Nakaji, P.; Kim, S.; et al. Extracellular microRNAs in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J Extracell Vesicles 2020, 9, 1713540. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, E.; Ortega, L.; Hernandez-Guillamon, M.; Penalba, A.; Fernandez-Cadenas, I.; Rosell, A.; Montaner, J. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol 2008, 84, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dhir, N.; Medhi, B.; Prakash, A.; Goyal, M.K.; Modi, M.; Mohindra, S. Pre-clinical to Clinical Translational Failures and Current Status of Clinical Trials in Stroke Therapy: A Brief Review. Curr Neuropharmacol 2020, 18, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, L.; Khojasteh, A.; Soleimani, M.; Oraee-Yazdani, S.; Keshel, S.H.; Saadatnia, M.; Saboori, M.; Zali, A.; Hashemi, S.M.; Soleimani, R. Safety of Intraparenchymal Injection of Allogenic Placenta Mesenchymal Stem Cells Derived Exosome in Patients Undergoing Decompressive Craniectomy Following Malignant Middle Cerebral Artery Infarct, A Pilot Randomized Clinical Trial. Int J Prev Med 2022, 13, 7. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]

| EV source | Major cargo molecules | In vitro stroke model | In vivo stroke model | Major targeted molecules/pathway | Outcome | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| animal model | administration route | dosage | Time point of administration | ||||||
| rat BM-MSCs | - | - | rat tMCAO model (2h) | tail vein | 100μg | 24h | - | enhanced neurite remodeling enhanced neurogenesis & angiogenesis |
[23] |
| human iPSC-derived MSCs | - | OGD/R-HUVECs (8h) | rat tMCAO model (2h) | tail vein | 1 x 10^11 particles | 4h | STAT3 | enhanced angiogenesis reduced autophagy |
[24] |
| HUVECs | miR-1290 | OGD/R-neurons (1.5h) | mouse tMACO model (1h) | Intracranial (AP: 2.0 mm, ML: 1.7 mm, DV: 1.35 mm) |
5μg | Immediately (0h) |
- | reduced apoptosis | [25] |
| human NSCs | - | glucose-free H/R model (1.5h) | - | - | - | - | - | reduced apoptosis and oxidative stress enhanced axonal elongation enhanced angiogenesis |
[67] |
| rat BM-MSCs | - | OGD/R-microglia (1 - 5h) | rat tMCAO model (1.5h) | tail vein | 120μg | 2h | cysLT2R ERK1/2 |
mitigated microglia M1 polarization | [86] |
| human BM-MSCs | - | - | mouse tMACO model (0.5h) | tail vein | released by 2×10^6 MSCs | Immediately (0h) |
- | reducing apoptosis reduced peripheral immune cell inflitration |
[87] |
| astrocytes | miR-34c | OGD/R-N2a cells (-) | rat tMACO model (-) | tail vein | - | - | TLR7 NF-κB/MAPK pathway |
reduced apoptosis and inflammation | [88] |
| human ESCs | TGF-β & Smad2 & Smad4 |
- | mouse tMACO model (1h) | tail vein | 1 x 10^9 particles | 2h and day 1, 2 (3 times) |
TGF-β/Smad pathway | reduced apoptosis and inflammation reduced peripheral immune cell inflitration |
[89] |
| UC-MSCs | circBBS2 | H/R model of SH-SY5Y cells (4h) | rat tMCAO model (2h) | tail vein | 50μg | 4h and day 1, 2 (3 times) |
miR-494 | reduced ferroptosis by upregulation of SLC7A11 | [141] |
| mouse AD-MSCs | miR-760-3p | OGD/R-N2a cells (4h) | mouse tMACO model (1h) | intranasal | 10μg | day 1, 3, 5 (3 times) |
CHAC1 | reduced ferroptosis | [142] |
| rat BM-MSCs | - | OGD/R-BV2 & PC12 cells (6h) | rat tMCAO model (2h) | tail vein | 80μg (low) 100μg (medium) 120μg (high) |
2h | - | shift of microglial polarization state toward M2 phenotype reduced pyroptosis and inflammation |
[143] |
| mouse AD-MSCs | miR-25-3p | OGD/R-neurons (10h) | mouse tMACO model (1h) | femoral vein | 10μg | Immediately (0h) or 12h |
p53-BNIP3 signaling | reduced autophagy | [145] |
| human BM-MSCs | - | - | mouse tMACO model (0.5h) | femoral vein | EVs released by 2 x 10^6 MSCs | day 1, 3, 5 (3 times) |
- | neuroprotection enhanced neurogenesis & angiogenesis modulated peripheral immune response |
[187] |
| EV source | Modification | Major cargo molecules | In vitro stroke model | In vivo stroke model | Major targeted molecules/pathway | Outcome | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| animal model | administration route | dosage | Time point of administration | |||||||
| BV2 cells | IL-4 pretreatment | miR-124 | OGD/R-neurons (45min) | mouse tMACO model (1h) | tail vein | 100μg | 0h and day 1, 2 (3 times) |
USP14 | reduced apoptosis | [150] |
| human NSCs | INF-γ pretreatment | miR-206 & miR-133a-3p & miR-3656 |
- | rat tMACO model (-) |
intracranially (striatum) | 4 x 10^9 particles | 24h | - | reduced apoptosis and oxidative stress | [151] |
| mouse BM-MSCs | lithium pretreatment | miR-1906 | OGD/R-neurons (1h) OGD/R-microglia (8h) OGD/R-astrocytes (12h) |
mouse tMACO model (1h) | femoral vein (day 1) retro-orbital vein (day 3, 5) |
13.5μg | day 1, 3, 5 (3 times) |
TLR4/NF-κB pathway | reduced apoptosis enhanced neurogenesis & angiogenesis reduced peripheral immune cell inflitration |
[152] |
| human BM-MSCs | iron oxide nanoparticle (IONP) pretreatment | IONP & various growth factors |
LPS treated hypoxia-PC12 or rBMDM cells (24h) | rat tMACO model (1h) |
tail vein | 200μg | Immediately (0h) |
- | enhanced neurogenesis & angiogenesisreduced apoptosis and inflammationshift of macrophage polarization state toward M2 phenotype | [153] |
| RAW264.7 cells | edaravone pretreatment | edaravone | - | rat pMACO model | tail vein | - | day 1-7 (7 times) |
- | Neuroprotection shift of microglial polarization state toward M2 phenotype |
[154] |
| RAW264.7 cells | curcumin pretreatment | curcumin | - | rat tMACO model (2h) |
tail vein | - | Immediately (0h) |
- | reduced oxidative stress and apoptosisneuroprotection attenuated BBB damage |
[155] |
| mouse microglia | OGD/R preconditioning | TGF-β | OGD/R-neurons (6h) OGD/R-microglia (4h) OGD/R-bEnd.3 (16h) |
mouse tMACO model (1h) | femoral vein | 10μg | 0h, 6h (2 times) |
TGF-β/Smad2/3 pathway | promotion of endothelial cell survival and migration reduced neuronal apoptosis enhanced angiogenesis shift of microglial polarization state toward M2 phenotype |
[156] |
| rat plasma | Melatonin pretreatment | various miRNAs | - | rat pMACO model | tail vein | 100μg | 1h, 12h, 36h (3 times) |
NLRP3-mediated pathway & TLR4/NF-κB pathway |
reduced pyroptosis and inflammation | [157] |
| circulating endothelial progenitor cells | treadmill exercise | miR-126 | H/R-N2a cells (-) | mouse pMACO model | - | - | - | BDNF & PI3k/Akt pathway |
reduced apoptosis enhanced neurogenesis & angiogenesis |
[158] |
| rat skeletal muscle | treadmill exercise | miR-484 | OGD/R-PC12 cells (4h) | rat tMACO model (1h) |
tail vein | - | 2h before operation | ACSL4 | reduced ferroptosis | [159] |
| human iPSC-derived MSCs | transfection (BDNF) | BDNF | - | mouse tMACO model (45min) | intranasally | 1 x 10^10 particles | 2h, 24h, 48h (3 times) |
BDNF/TrkB signaling | reduced apoptosis and inflammation enhanced neurogenesis & angiogenesisneuroprotection |
[160] |
| rat BM-MSCs | transfection (miR-17-92 cluster) | miR-17-92 cluster | - | rat tMACO model (2h) |
intravenously | 100μg (3 x 10^11 particles) |
24h | PTEN & PI3k/Akt/mTOR pathway |
enhanced neurite remodeling and neuronal plasticity enhanced neurogenesis & oligodendrogenesis enhanced cortico-spinal tract axonal remodeling |
[161] |
| HEK293T cells | transfection (RGV-Lamp2b, circSCMH1) | RGV-Lamp2b & circSCMH1 |
OGD/R-neurons (3h) | mouse photothrombosis (PT) model mouse dMCAO/tMCAO (1h) model rhesus monkey PT stroke model |
mouse : tail veinrhesus monkey : hind limb vein | mouse : 12mg/kg rhesus monkey : 3mg/kg |
mouse : 24h rhesus monkey : 24h, 48h (2 times) |
MeCP2 | enhanced neuronal plasticity reduced glial activation reduced peripheral immune cell infiltration |
[163] |
| mouse BM-MSCs | passive loading (miR-210-cholesterol) click chemistry (c(RGDyK) peptides) |
miR-210 & c(RGDyK) peptides |
- | mouse tMACO model (0.5h or 1h) | tail vein | 100μg | 24h | VEGF | enhanced angiogenesis | [170] |
| rat blood | active loading-ultrasonication (quercetin) carbodiimide coupling (GAP43 antibody) |
Quercetin & GAP43 antibody |
OGD/R-SH-SY5Y cells (1h) | rat tMACO model (2h) |
tail vein | - | 24h | GAP43 & Nrf2/HO-1 pathway |
reduced apoptosis and oxidative stress | [172] |
| mouse BM-MSCs | passive loading (curcumin) click chemistry (c(RGDyK) peptides) |
Curcumin & c(RGDyK) peptides |
- | mouse tMACO model (1h) | tail vein | 300μg | 12h | NF-κB | reduced apoptosis and inflammation | [177] |
| RAW264.7 cells | active loading-ultrasonication (baicalin) | baicalin | OGD/R-SH-SY5Y cells (1h) | rat pMCAO/tMACO (2h) model |
tail vein | 1.6mg baicalin | Immediately (0h) |
Nrf2/HO-1 pathway | reduced apoptosis and oxidative stress | [178] |
| mouse BM-MSCs | transfection (RVG-Lamp2b)active loading-electroporation (miR-124) | miR-124 | - | mouse PT stroke model | tail vein | - | day 1 | Gli3 &Stat3 |
enhanced neurogenesis | [179] |
| HEK293T cells | transfection (RVG-Lamp2b)active loading-electroporation (HMGB1 siRNA) | HMGB1 siRNA | - | rat tMACO model (1h) |
tail vein | 30μg siRNAs | 18h before operation | HMGB1 | reduced apoptosis and inflammation | [180] |
| rat plasma | active loading-ultrasonication (edaravone) | edaravone | - | rat pMACO model | tail vein | 10mg/kg edaravone | day 1-7 (7 times) |
- | neuroprotection | [181] |
| mouse ESCs | active loading-freeze-thawing (curcumin) | curcumin | - | mouse tMACO model (40min) | intranasally | - | day 0-7 (twice a day) |
- | reduced oxidative stress and inflammationreduced glial activation and loss of vascular integrity | [182] |
| HEK293T cells | transfection (RBP-Lamp2b)passive loading (AMO181a-cholesterol) | RBP-Lamp2b &AMO181a |
hypoxia-Neuro2A cells (24h) | rat tMACO model (1h) |
intranasally | 75μg | 1h | RAGE &miR-181a |
reduced apoptosis and inflammation | [183] |
| human NPCs (ReN cells) | passive loading (RGD-C1C2) | RGD-C1C2 &various miRNAs |
- | mouse tMACO model (1h) | tail vein | 300μg | 12h | p38 MAPK pathway | reduced inflammation | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





