Submitted:
03 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Global population projected to reach 10 billion by 2050 emphasizing the need for sustainable food production. Traditional protein sources present environmental and scalability challenges, demanding the diversification of protein sources. Edible insects, such as house crickets (Acheta domesticus), emerged as a promising solution due to their high nutritional value, efficient feed conversion rates, and lower environmental impact compared to conventional protein sources. Incorporating insect powders into new food products can improve consumer acceptance, but often lead to poor functionality and/or undesirable organoleptic characteristics. However, protein isolates were revealed to be effective in enhancing functionality and consumer acceptance. This study aimed to develop and compare the yield of three different protein extraction methods, using sodium hydroxide, ascorbic acid or alcalase, from house crickets (Acheta domesticus) for food applications. Protein extraction was performed on cricket powder with a mean protein content of 57.95 g/100 g, and the results were evaluated. The enzymatic method showed the highest protein extraction rate of 85.97% with a mean protein content of 74.03 g/100 g, while extraction with NaOH or ascorbic acid resulted in rates of 74.32 and 56.99%, respectively. Further studies on the functionality and sensorial evaluation of products developed with this protein extract are recommended.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Crickets
2.2.1. Crickets rearing and harvesting
2.2.2. Processing cricket into powder
2.3. Study design
2.4. Protein extraction
2.4.1. Method 1: Protein extraction with NaOH
2.4.2. Method 2: Protein extraction with ascorbic acid
2.4.3. Method 3: protein extraction with enzyme
2.4.4. Extraction yield and rates
2.5. Nutrient composition
2.6. Statistical analysis
3. Results
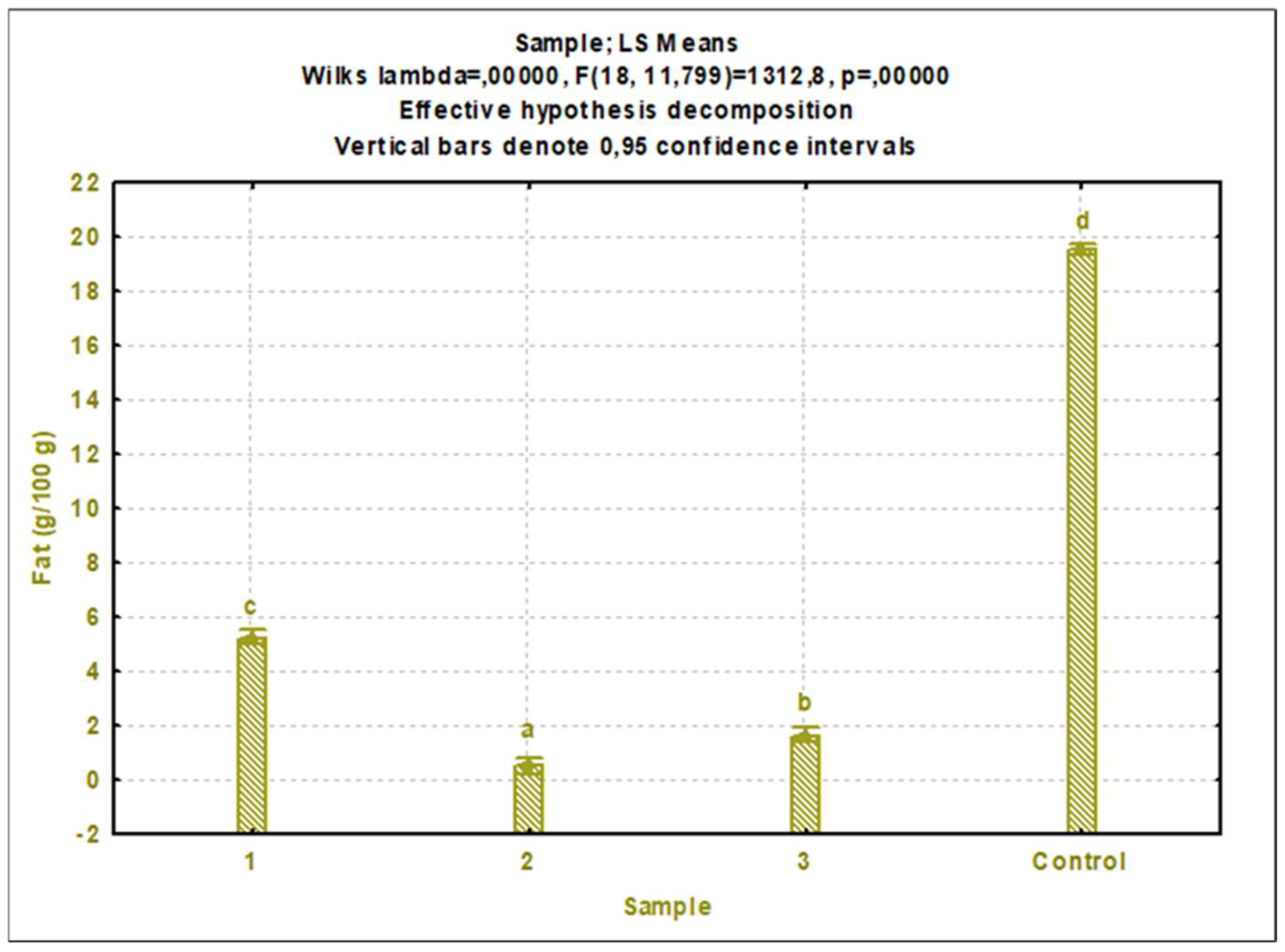
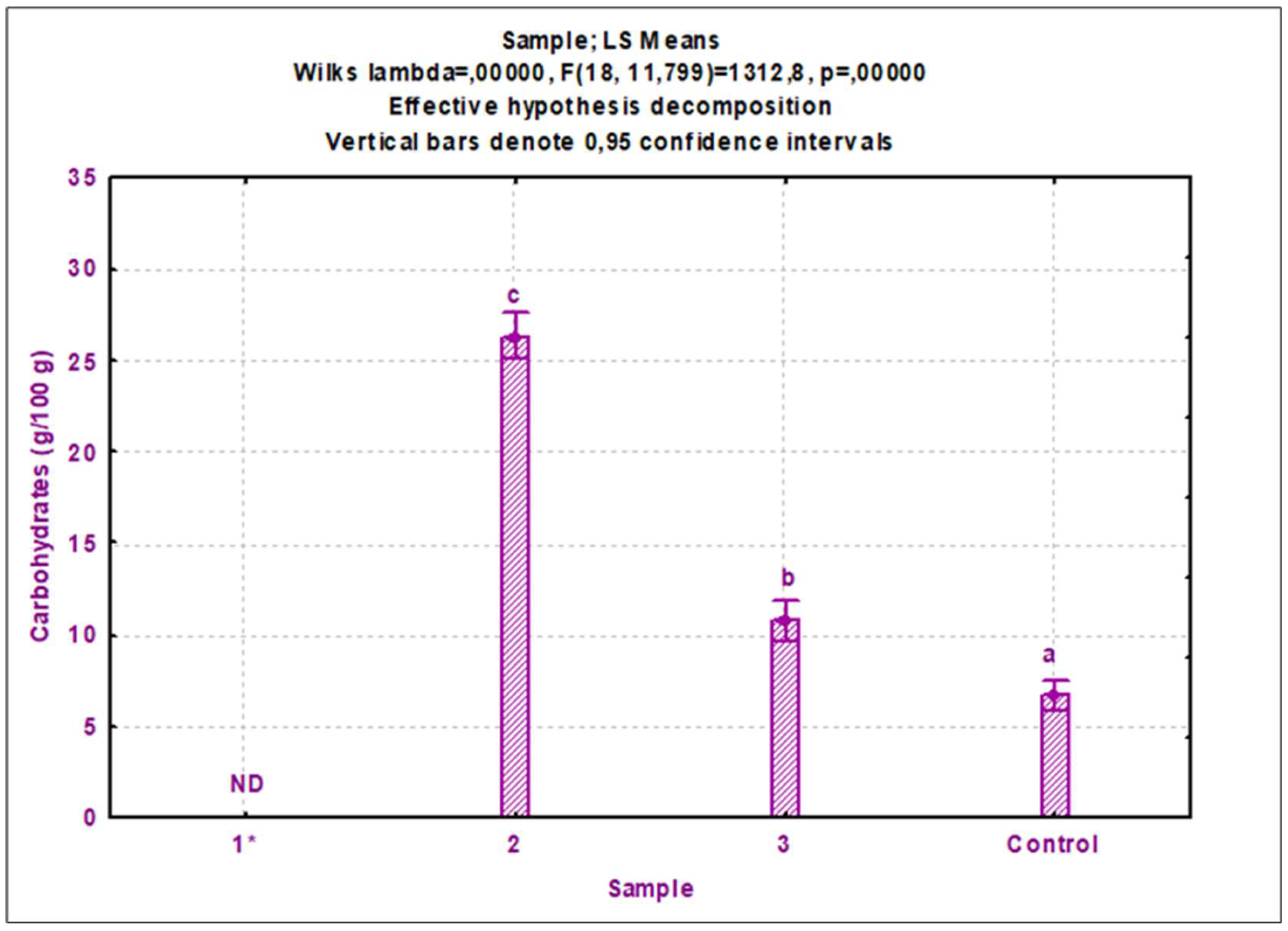
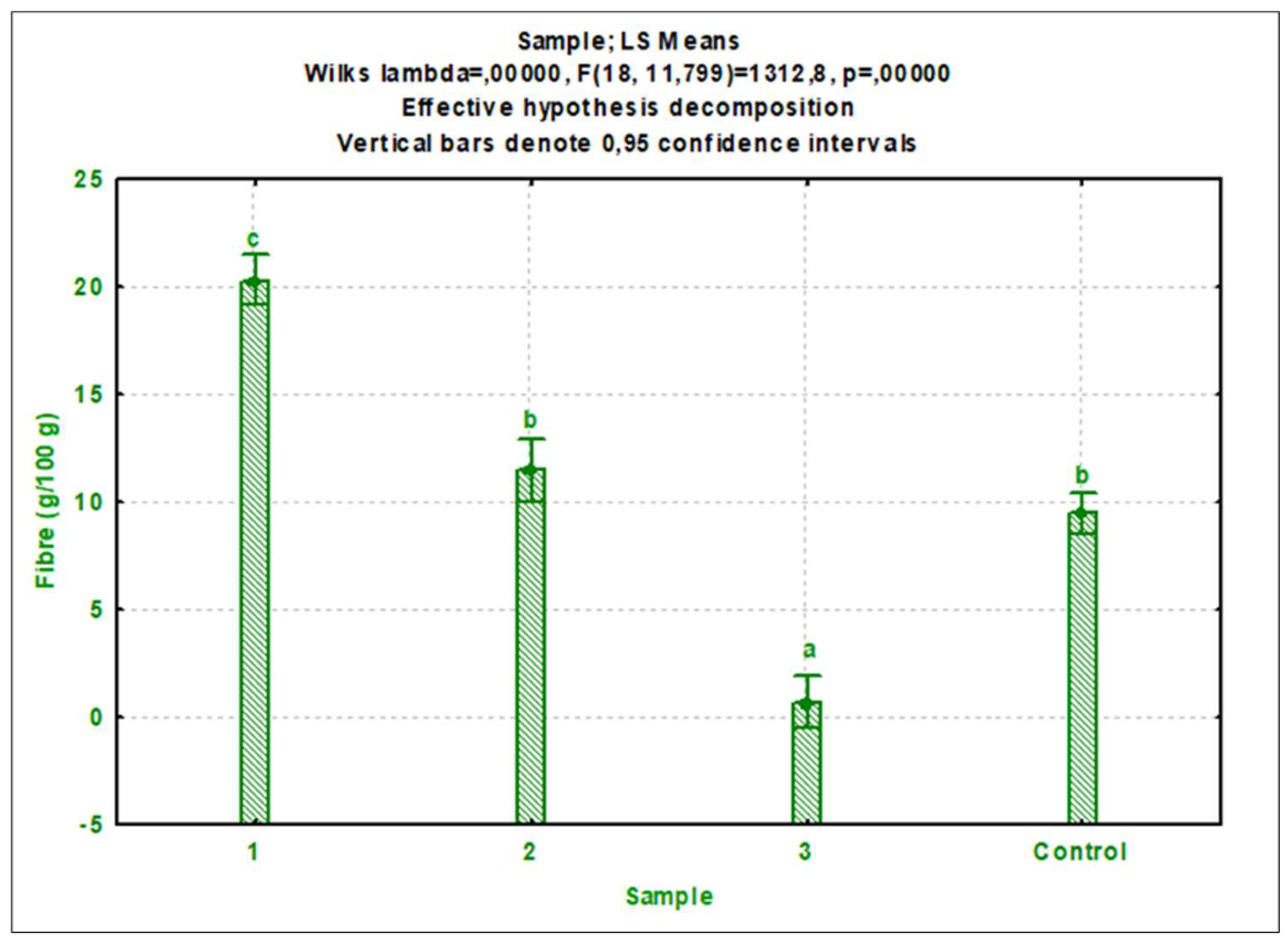
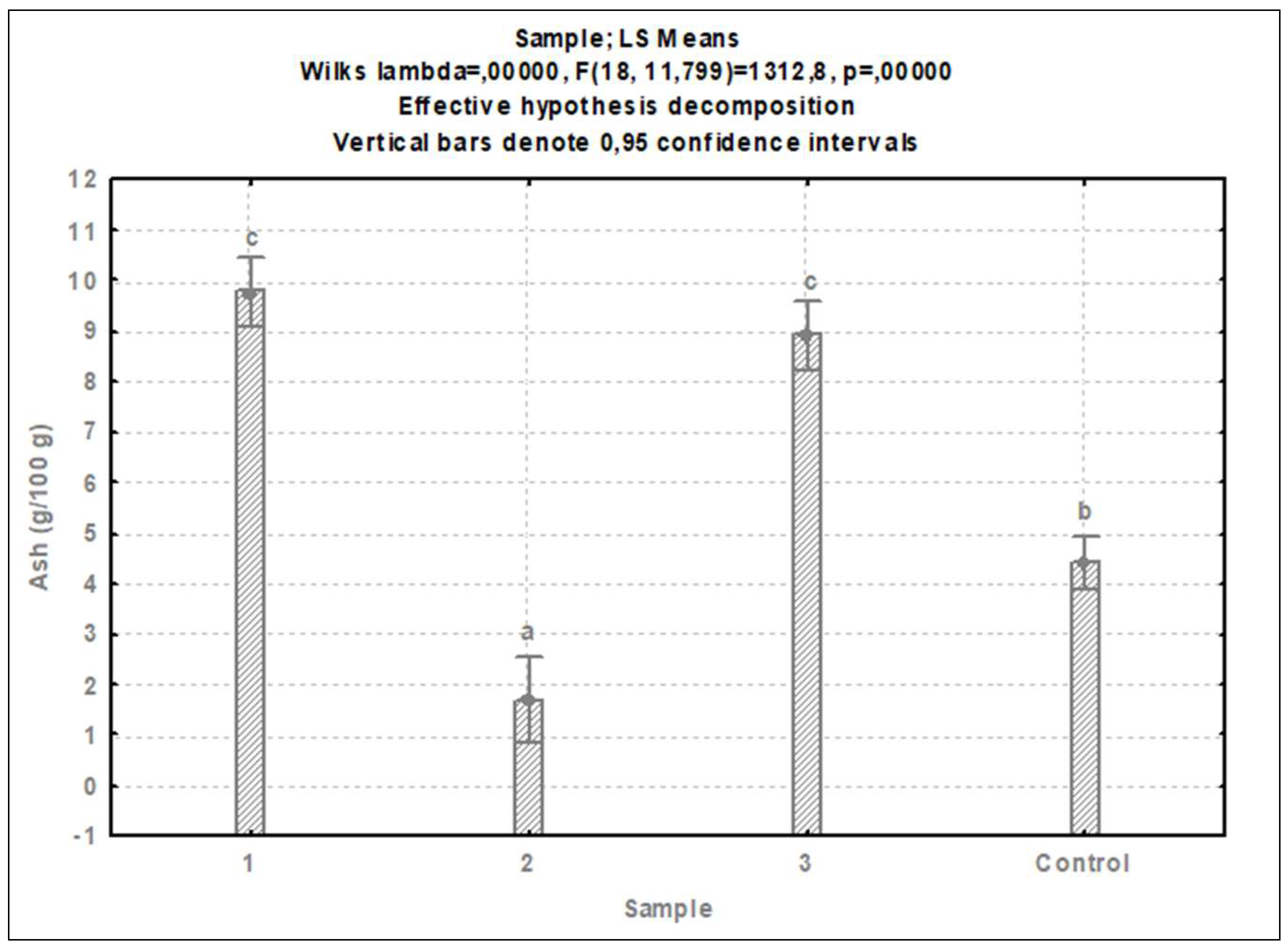
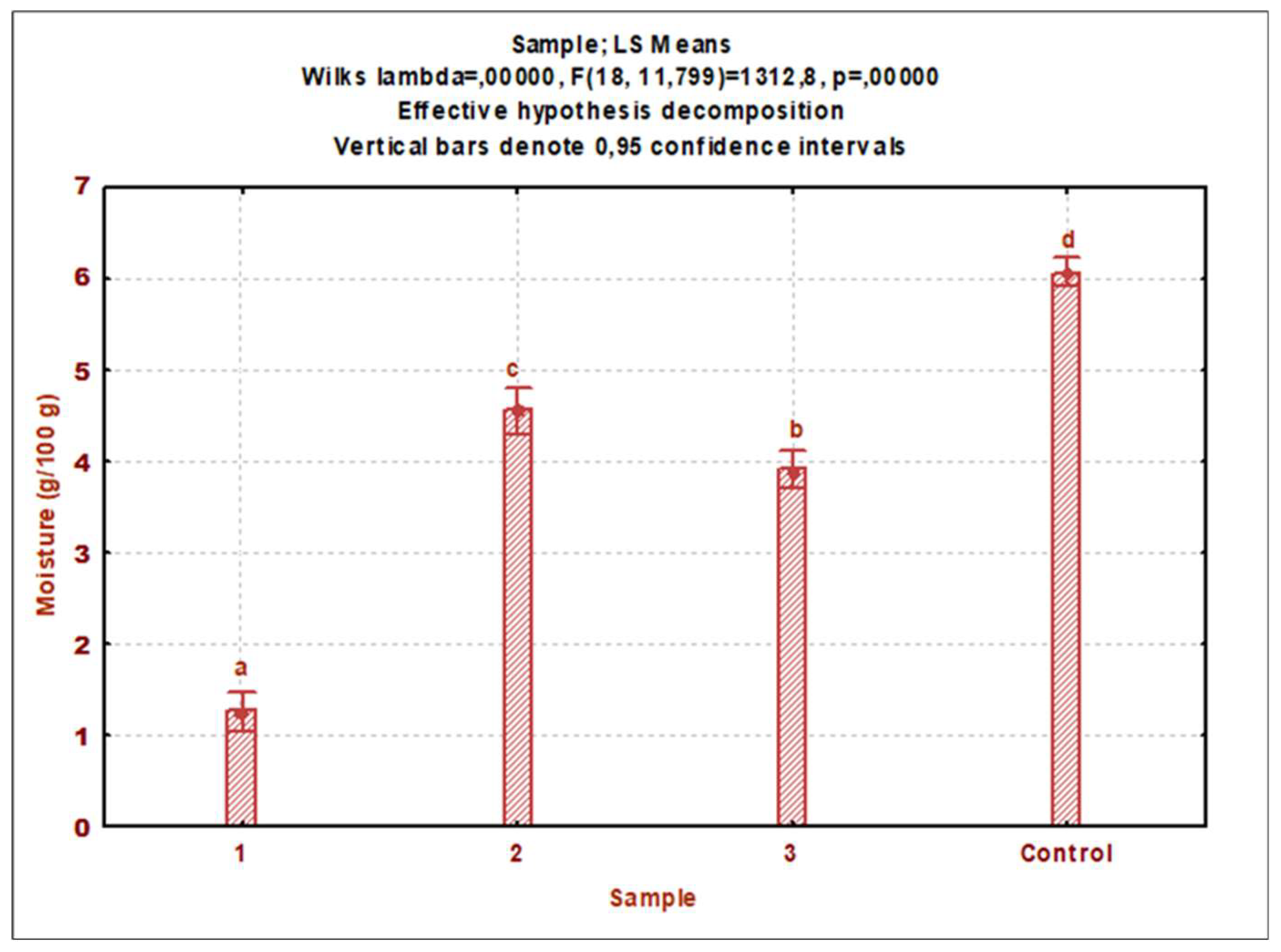
3.1. Nutrient composition
3.1.1. Cricket powder (control)
3.1.2. Cricket protein extracts
3.2. Yields and extraction rate
4. Discussion
4.1. Nutritional composition of cricket powder
4.2. Nutritional composition of cricket protein extracts
4.2.1. Protein
4.2.2. Other nutritional components
4.3. Yields and extraction rate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GNAFC, Global Network Against Food Crises (2022) Global report on food crisis - 2022. Food Security Information Network. Available online: https://docs.wfp.org/api/documents/WFP-0000138913/download/?_ga=2.79685015.2042137803.1683693379-539027021.1683693379 (accessed on 10 August 2024).
- GNAFC, Global Network Against Food Crises (2021) Global report on food crisis - 2021. Food Security Information Network. Available online: https://www.fsinplatform.org/sites/default/files/resources/files/GRFC%202021%20050521%20med.pdf (accessed on 5 August 2024).
- FAO, Food and Agriculture Organization of the United Nations (2009). Global agriculture towards 2050. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 24 July 2024).
- Musungu, A.L.; Muriithi, B.W.; Ghemoh, C.J.; Nakimbugwe, D.; Tanga, C. M. Production, consumption, and market supply of edible crickets: insights from East Africa. Agric. Econ. 2023, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Alvi, T, Sameen, A.; Khan, S.; Blinov, A.V.; Nagdalian, A.A.; Mehdizadeh, M.; Adli, D.N.; Onwezen, M. Consumer Acceptance of Alternative Proteins: A Systematic Review of Current Alternative Protein Sources and Interventions Adapted to Increase Their Acceptability. Sustainability 2022, 14(22), 15370. [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations (2021). Climate change, biodiversity and nutrition nexus. Evidence and emerging policy and programming opportunities. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/dc8635bd-a4c1-427d-ad67-2d886dbb795f/content (assessed on 15th August 2024).
- Boccardo, A.; Hagelaar, G.; Lakemond, C. Evaluation of crises suitability of food systems: a comparison of alternative protein sources. Food Secur. 2023, 15(6), 1647–1665. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations (2023). The state of food security and nutrition in the world. Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/1f66b67b-1e45-45d1-b003-86162fd35dab/content (accessed on 17 August 2024).
- Malila, Y., Owolabi, I.O., Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C. T.; Visessanguan, W.; Karoonuthaisiri; N.; Petchkongkaew, A. Current challenges of alternative proteins as future foods. npj Sci Food 2024, 8, 53. [CrossRef]
- Talwar, R.; Freymond, M.; Beesabathuni, K.; Lingala, S. Current and Future Market Opportunities for Alternative Proteins in Low- and Middle-Income Countries. Curr. Dev. Nutr. 2024, 8(1), 102035. [Google Scholar] [CrossRef]
- Dossey, A. T.; Oppert, B.; Chu, F. C.; Lorenzen, M. D.; Scheffler, B.; Simpson, S.; Koren, S.; Johnston, J. S.; Kataoka, K.; Ide, K. Genome and Genetic Engineering of the House Cricket (Acheta domesticus): A Resource for Sustainable Agriculture. Biomolecules 2023, 13(4), 589. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Romero, G.; Chisaguano-Tonato, A. M.; Herrera-Fontana, M. E.; Chimbo-Gándara, L. F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M. G.; Álvarez-Suárez, J. M. House cricket (Acheta domesticus): A review based on its nutritional composition, quality, and potential uses in the food industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Lange, K. W.; Nakamura, Y. Potential contribution of edible insects to sustainable consumption and production. Front. Sustain. 2023, 4, 1112950. [Google Scholar] [CrossRef]
- Malla, N.; Nørgaard, J. V.; Lærke, H. N.; Heckmann, L. H. L.; Roos, N. Some Insect Species Are Good-Quality Protein Sources for Children and Adults: Digestible Indispensable Amino Acid Score (DIAAS) Determined in Growing Pigs. J. Nutr. 2022, 152(4), 1042–1051. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From Farm to Fork: Crickets as Alternative Source of Protein, Minerals, and Vitamins. Front Nutr. 2021, 10(8), 704002. [Google Scholar] [CrossRef]
- Psarianos, M.; Ojha, S.; Schlüter, O.K. Evaluating an emerging technology-based biorefinery for edible house crickets. Front. Nutr. 2023, 10, 1185612. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Ayu, A.M.; Syiffah, L.; Muthia, H.; Amalina, F. A.; Afifah, D. N.; et al. Physicochemical and sensory properties of cookies with cricket powder as an alternative snack to prevents iron deficiency anemia and chronic energy deficiency. Appl. Food Res. 2024, 4(2), 100485. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Vigani, B.; Sánchez-Espejo, R.; Spano, M.; Totaro Fila, C.; Mannina, L.; Viseras, C.; Rossi, S.; Sandri, G. Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants (Basel) 2023, 12(1), 112. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Shaheen, M.; Tahir, A. B.; Gomes da Silva, A. P.; Manzoor, H. Y.; Zia, S. Unraveling the nutritional, biofunctional, and sustainable food application of edible crickets: A comprehensive review. Trends Food Sci. Technol. 2024, 143, 104254. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- De Souza-Vilela, J.; Andrew, N.; Ruhnke, I. (2019), Insect protein in animal nutrition, Anim. Prod. Sci. 59, 2029–2036. [CrossRef]
- Michel, P.; Begho, T. Paying for sustainable food choices: The role of environmental considerations in consumer valuation of insect-based foods. Food Qual. Pref. 2023, 106, 104816. [Google Scholar] [CrossRef]
- Erhard, A. L.; Águas Silva, M.; Damsbo-Svendsen, M.; Menadeva Karpantschof, B.; Sørensen, H.; Bom Frøst, M. Acceptance of insect foods among Danish children: Effects of information provision, food neophobia, disgust sensitivity, and species on willingness to try. Food Qual. Prefer. 2023, 104, 104713. [Google Scholar] [CrossRef]
- Liceaga, A. M. Approaches for Utilizing Insect Protein for Human Consumption: Effect of Enzymatic Hydrolysis on Protein Quality and Functionality. Ann. Entomol. Soc. Am. 2019, 112(6), 529–532. [Google Scholar] [CrossRef]
- Ververis, E.; Boué, G.; Poulsen, M.; Pires, S. M.; Niforou, A.; Thomsen, S. T.; et al. A systematic review of the nutrient composition, microbiological and toxicological profile of Acheta domesticus (house cricket). J. Food Compos. Anal. 2022, 114, 104859. [Google Scholar] [CrossRef]
- Brena-Melendez, A.; Garcia-Amezquita, L. E.; Liceaga, A.; Pascacio-Villafán, C.; Tejada-Ortigoza, V. Novel food ingredients: Evaluation of commercial processing conditions on nutritional and technological properties of edible cricket (Acheta domesticus) and its derived parts, Innovative Food Sci. Emerg. Technol. 2024, 92, 103589. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K. I.; Maciuk, A.; et al. Safety of frozen and dried formulations from whole house crickets (Acheta domesticus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19(8), e06779. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B. K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; Ma, H. Recent Insight on Edible Insect Protein: Extraction, Functional Properties, Allergenicity, Bioactivity, and Applications. Foods 2022, 11(19), 2931. [Google Scholar] [CrossRef]
- Davalos-Vazquez, A.; Mojica, L.; Sánchez-Velázquez, O. A.; Castillo-Herrera, G.; Urías-Silvas, J. EW.; Doyen, A.; Moreno-Vilet, L. Techno-functional properties and structural characteristics of cricket protein concentrates affected by pre-treatments and ultrafiltration/diafiltration processes. Food Chem. 2024, 461, 140908. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Haubruge, É.; Francis, F. Insects, the next european foodie craze? In Edible insects in sustainable food systems, 1st ed.; Halloran, A, Flore, R, Vantomme, P, Roos, N., Eds.; Publisher: Springer International Publishing New York, USA, 2018; Volume, pp. 353–361. [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J. L.; Johansson, D. P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes - Extraction and functional properties. PLoS ONE 2016, 11(2), e0147791. [Google Scholar] [CrossRef] [PubMed]
- Amarender, R. V.; Bhargava, K.; Dossey, A. T.; Gamagedara, S. Lipid and protein extraction from edible insects – Crickets (Gryllidae) LWT 2020, 125, 109222. [CrossRef]
- Hall, F. G.; Jones, O. G.; O’Haire, M. E.; Liceaga, A. M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Quinteros, M. F.; Martínez, J.; Barrionuevo, A.; Rojas, M.; Carrillo, W. Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology, 2022, 11(5), 776. [CrossRef]
- Pustaka, D.; Badan, P.K.; Obat, P.M.; Makanan, D.; Mansur, L.K.; Sinandang, U.D. et al. Guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals, 16 th ed.; Publisher: AOAC, Association of Official Analytical Chemists Rockville, Maryland, USA, 2005.
- Bassett, F. S.; Dunn, M. L.; Pike, O. A.; Jefferies, L. K. Physical, nutritional, and sensory properties of spray-dried and oven-roasted cricket (Acheta domesticus) powders. J. Insects Food Feed 2021, 7(6), 987–1000. [Google Scholar] [CrossRef]
- Ndiritu, A. K.; Kinyuru, J. N.; Kenji, G. M.; Gichuhi, P. N. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2017, 11(4), 2013–2021. [Google Scholar] [CrossRef]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; Hou, X. Edible Insects: A New Sustainable Nutritional Resource Worth Promoting. Foods 2023, 12(22), 4073. [Google Scholar] [CrossRef]
- Kröncke N, Benning R. Influence of Dietary Protein Content on the Nutritional Composition of Mealworm Larvae (Tenebrio molitor L.). Insects 2023, 14(3), 261. [CrossRef]
- El Hajj, R.; Mhemdi, H.; Besombes, C.; Allaf, K.; Lefrançois, V.; Vorobiev, E. Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field. Processes 2022, 10(5), 970. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9(6), 782. [Google Scholar] [CrossRef]
- Ayensu, J.; Annan, R. A.; Edusei, A.; Lutterodt, H. Beyond nutrients, health effects of entomophagy: a systematic review. NFS 2019, 49(1), 2–17. [Google Scholar] [CrossRef]
- Trinh, B. T.; Supawong, S. Enzymatic Hydrolysis of Cricket (Gryllodes sigillatus) Protein: Influence of Alcalase and Neutrase Enzyme on Functional Properties of Recovered Protein. Biol. Sci. 2021, 10(3), 342–353. [Google Scholar] [CrossRef]
- Salt, D.J.; Leslie, R.B.; Lillford, P.J.; Dunnill, P. Factors influencing protein structure during acid precipitation: A study of soya proteins. European J. Appl. Microbiol. Biotechnol. 1982, 14, 144–148. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Bhaswant, M.; Kaur, S.; Tripathi, M.; Lanterbecq, D.; Chawla, P.; Sharma, M. Extraction, Modification, Biofunctionality, and Food Applications of Chickpea (Cicer arietinum) Protein: An Up-to-Date Review. Foods 2024, 13, 1398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, D.; Liu, W.; Kan, J.; Zhang, Y. Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins. Foods 2024, 13, 2496. [Google Scholar] [CrossRef] [PubMed]
- Makishi, G. L. A.; Lacerda, R. S.; Mamani, H. N. C.; Costa, P. A.; Bittante, A. M. Q. B.; Gomide, C. A.; Sobral, P. J. A. Effect of alkaline agent and pH on the composition of freeze-dried proteins extracted from castor bean (Ricinus communis, l.) cake. Chem. Eng. Trans. 2014, 37, 697–702. [Google Scholar] [CrossRef]
- Harris, G.K., Marshall, M.R. Ash Analysis. In Food Analysis, 5th ed.; Nielsen, S.S. Eds;. Publisher: Springer Link New York, USA, 2017, pp 287–297.
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines (Basel) 2020, 7(8), 45. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S. A.; Altemimi, A. B.; Hashmi, A. A.; Shahzadi, S.; Mujahid, W.; Ali, A.; Bhat, Z. F.; Naz, S.; Nawaz, A.; Abdi, G.; Aadil, R. M. Edible crickets, as a possible way to curb protein-energy malnutrition: Nutritional status, food applications, and safety concerns. Food Chem: X 2024, 23, 101533. [Google Scholar] [CrossRef]
- Kapoor, R.; Karabulut, G.; Mundada, V.; Feng, H. Unraveling the potential of non-thermal ultrasonic contact drying for enhanced functional and structural attributes of pea protein isolates: A comparative study with spray and freeze-drying methods. Food Chem 2024, 439, 138137. [Google Scholar] [CrossRef]
- Pellerin, G.; Doyen, A. Effect of thermal and defatting treatments on the composition, protein profile and structure of house cricket (Acheta domesticus) protein extracts. Food Chem. 2024, 448, 139149. [Google Scholar] [CrossRef]
- Oshin, T. A.; Abhulimen, K. E.; Abadi, J. C.; Adekanye, T. A. Comparative simulation studies on the countercurrent multi-stage solid–liquid extraction of soybean oil by ethanol and hexane. Chem. Pap. 2024, 78, 5657–5669. [Google Scholar] [CrossRef]
- Sosa-Flores, M. L.; García-Hernández, D. G.; Amaya-Guerra, C. A.; Bautista-Villarreal, M.; González-Luna, A. R. Obtención de aislados e hidrolizados proteicos de grillo (Acheta domesticus) y evaluación de su actividad antioxidante. Investigación y Desarrollo en Ciencia y Tecnología de Alimentos 2023, 8(1), 608–618. [CrossRef]

| Methodџ of extraction | Group | Proximate analysis (samples number) | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein | Lipids | Carbohydrates | Fiber | Moisture | Ash | |||
| 1 | Alkaline | Experimental | 3 | 3 | 3 | 3 | 3 | 3 |
| Control* | 5 | 5 | 5 | 5 | 5 | 5 | ||
| 2 | Acidic | Experimental | 3 | 3 | 3 | 3 | 3 | 3 |
| 3 | Enzymatic | Experimental | 3 | 3 | 3 | 3 | 3 | 3 |
| Nutritional components of cricket powder (%w/w), dry basis | |
|---|---|
| Protein | 57±0.23 |
| Fat | 19±0.1 |
| Carbohydrates | 6±0.95 |
| Fiber | 9±0.57 |
| Moisture | 6±0.03 |
| Ash | 4±0.09 |
| Method | Extraction Yield (%) | Protein (g/100 g) | Protein extraction rate (%) |
|---|---|---|---|
| 1 NaOH | 75.07 | 66.33 | 74.32 |
| 2 Ascorbic acid | 69.10 | 55.25 | 56.99 |
| 3 Alcalase | 77.81 | 74.03 | 85.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





