Submitted:
04 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
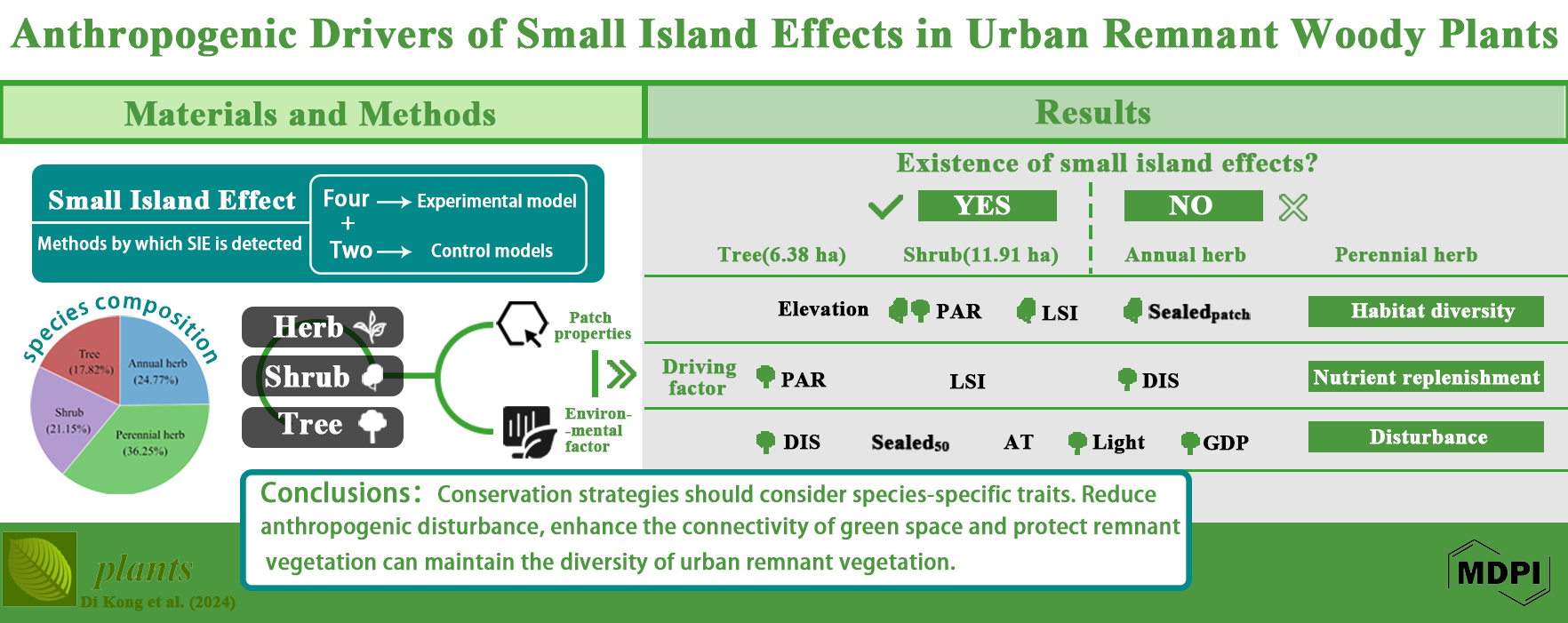
The positive relationship between species richness and area is a fundamental principle in ecology. However, this pattern deviates on small islands, where species richness either changes independently of area or increases at a slower rate—a phenomenon known as the Small-Island Effect (SIE). While the SIE has been well-documented in natural ecosystem, its presence in highly fragmented and disturbed urban ecosystem remains unexplored, posing challenges for urban vegetation conservation. Urban remnant vegetation, isolated by surrounding infrastructures, preserves intact zonal vegetation characteristics, serves as a benchmark for restoring near-natural habitats and offers ideal conditions to test the existence of SIE in human-altered landscapes. In this study, we surveyed 17 remnant vegetation patches in Qingdao City, China. In total of 331 plants attributed to 255 genus in 81 families been recorded. Firstly, by using six species-area relationship regression models tested SIE for remnant vegetation with different life form (i.e. annual herb, perennial herb, shrub and tree), we found SIE detected in only woody plants, with area threshold ranging from 6.38 ha (tree) to 11.91 ha (shrub). According to stepwise and generalized linear regression mode analysis we found SIE detected in only woody plants, with area threshold 6.38 ha for tree and 11.91 ha for shrub. Further analysis revealed that the SIE in shrub was driven by landscape shape index, perimeter-area ratio, and the proportion of sealed surface within patch. For trees, the SIE was influenced by the distance to the source of species, GDP, night light intensity and perimeter-area ratio. This finding justifies that conservation in urban planning, construction and development should focus not only on protecting large areas, but also on maintaining and promoting diverse habitats within these areas. At the same time, reducing anthropogenic disturbance and enhancing the connectivity of green spaces are important for the persistence of metacommunities and can contribute to the local species pool, thus potentially improving the ecological resilience of urban environments.
Keywords:
1. Introduction
2. Materials and Methods
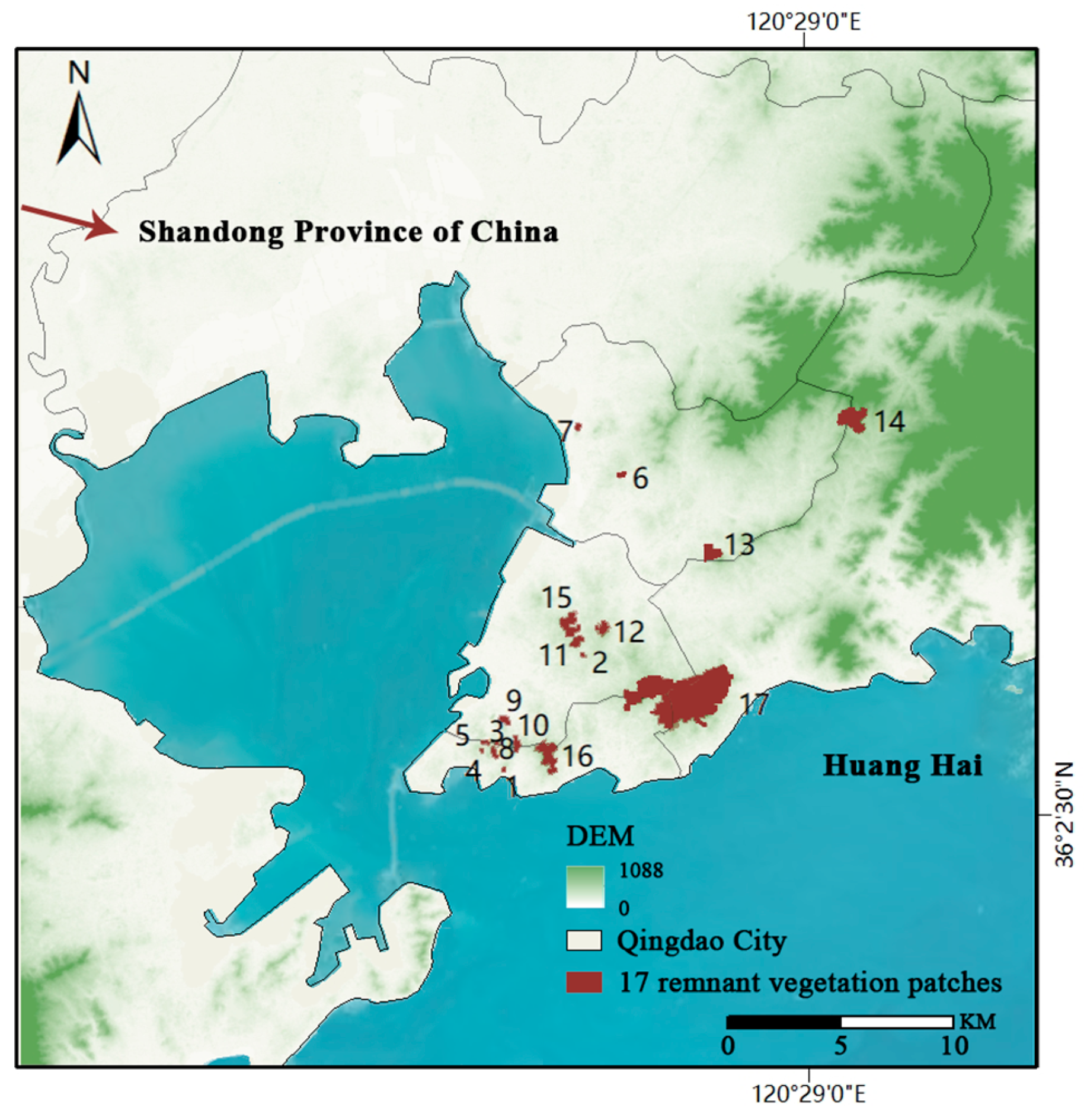
2.1. Study Area
2.2. Field Surveys
2.3. Data Analysis
| model | Equation | Description |
|---|---|---|
| 1 | S=c+(log A≤T)×z1×log A+(log A>T)×((z1×T+z2×(log A-T)) | Regression fragments and slope iteration fragments |
| 2 | S=c+(log A≤T)(z1×log A+(z2-z1)×T)+(log A>T)×z2×log A | Regression fragments and slope iteration fragments |
| 3 | S=c+(log A>T)×z×(log A-T) | Zero-slope regression fragments and slope iteration fragmentss |
| 4 | S=c+(log A≤T)×z1×T+(log A>T)×z1×log A | Regression fragment and direct inheritance fragment |
| 5 | S=c+z×log A | Linear regression |
| 6 | S=c | Zero-slope regression |
3. Results
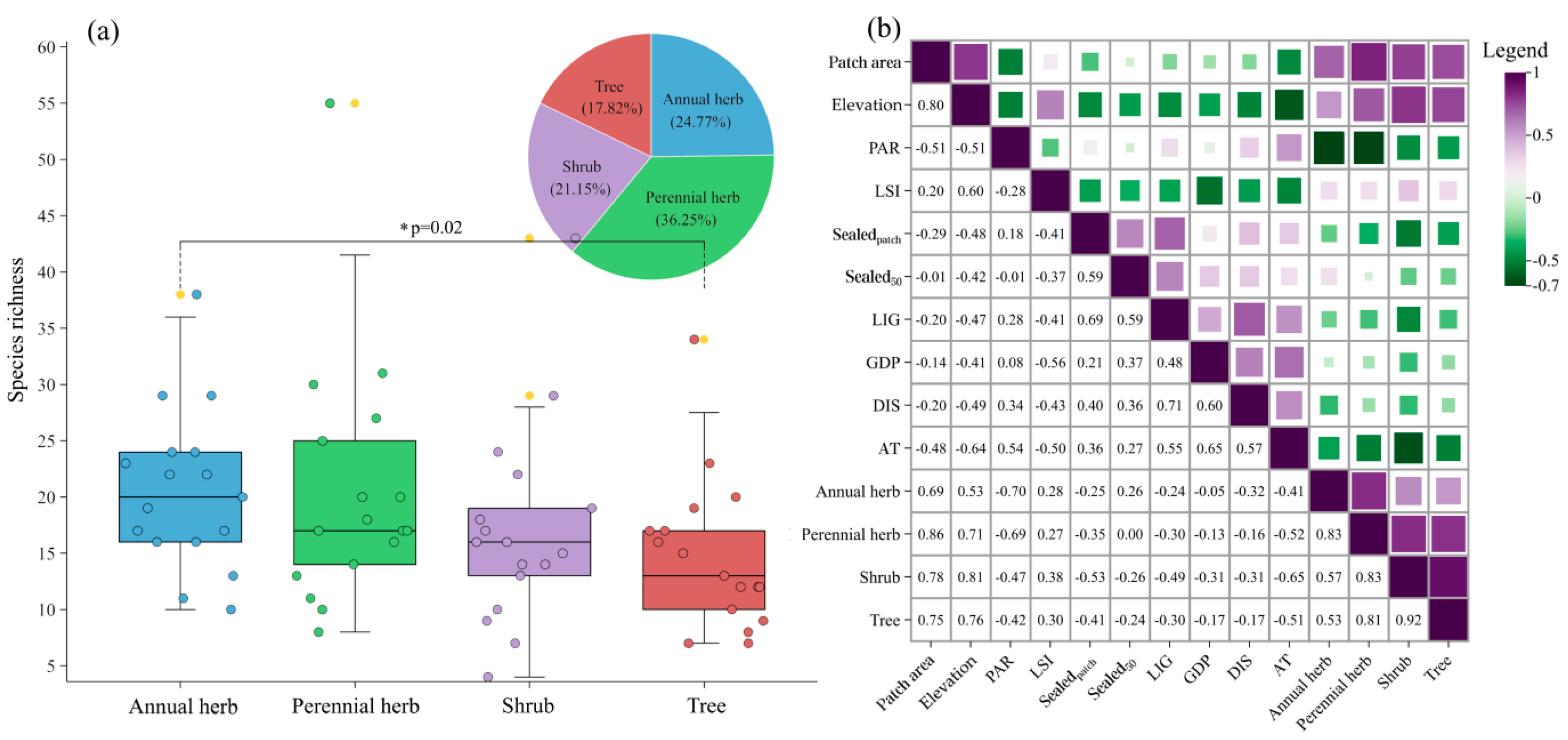
3.1. Species Composition
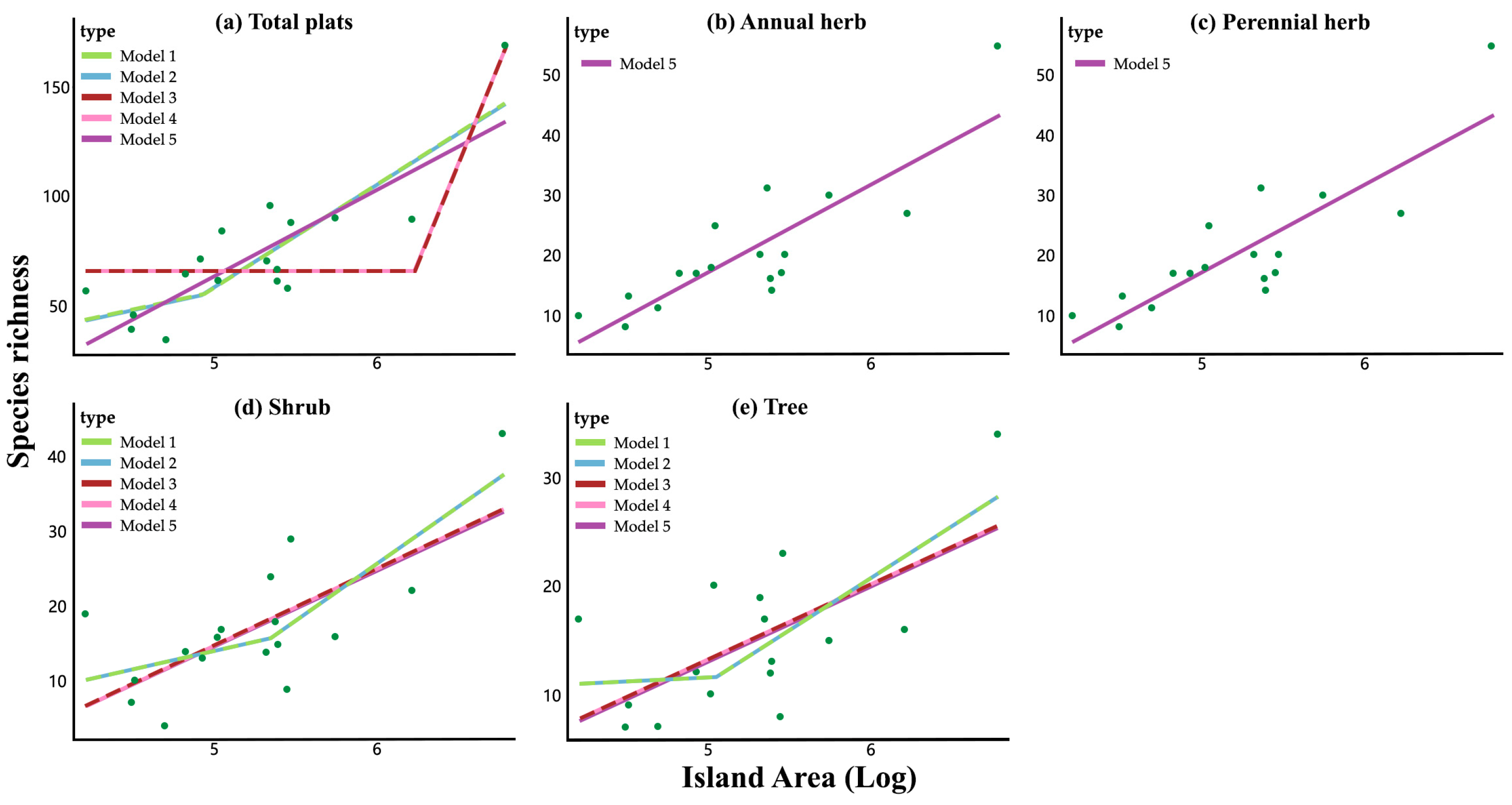
3.2. Detection of the Small Island Effect
| Group | Models | Parameters | Model Comparison | |||||
| C | Z1 | Z2 | T | K | AICc | ΔAICc | ||
| Total plants | 5 | -138.068 | 40.408 | 0 | 0 | 2 | 149.04 | 0 |
| 1 | -25.023 | 16.308 | 31.382 | 4.926 | 3 | 150.654 | 1.613 | |
| 2 | -179.611 | 16.308 | 47.689 | 4.926 | 3 | 150.654 | 1.613 | |
| 3 | 66.875 | 186.37 | 0 | 6.216 | 2 | 150.998 | 1.958 | |
| 4 | -1091.611 | 186.37 | 0 | 6.216 | 2 | 150.998 | 1.958 | |
| 6 | 72.941 | 0 | 0 | 0 | 1 | 166.259 | 17.218 | |
| Annual herb |
5 | -24.248 | 8.586 | NA | NA | 2 | 103.611 | 0 |
| 1 | -24.328 | 8.603 | -0.026 | 5.126 | 3 | 106.6 | 2.989 | |
| 2 | -24.195 | 8.603 | 8.577 | 5.126 | 3 | 106.6 | 2.989 | |
| 3 | 19.5 | 33.434 | NA | 6.216 | 2 | 110.172 | 6.561 | |
| 4 | -188.325 | 33.434 | NA | 6.216 | 2 | 110.172 | 6.561 | |
| 6 | 20.588 | NA | NA | NA | 1 | 116.198 | 12.587 | |
| Perennial herb |
5 | -56.323 | 14.717 | NA | NA | 2 | 112.715 | 0 |
| 1 | 23.465 | -3.211 | 18.839 | 4.506 | 3 | 114.95 | 2.235 | |
| 2 | -61.426 | -3.211 | 15.628 | 4.506 | 3 | 114.95 | 2.235 | |
| 3 | 18.375 | 66.19 | NA | 6.216 | 2 | 116.54 | 3.825 | |
| 4 | -393.063 | 66.19 | NA | 6.216 | 2 | 116.54 | 3.825 | |
| 6 | 20.529 | NA | NA | NA | 1 | 131.324 | 18.609 | |
| shrub | 3 | 6.804 | 10.188 | — | 4.216 | 2.000 | 115.033 | 0.000 |
| 4 | -36.147 | 10.188 | — | 4.216 | 2.000 | 115.033 | 0.000 | |
| 5 | -35.959 | 10.153 | — | — | 2.000 | 115.098 | 0.065 | |
| 1 | -9.808 | 4.794 | 10.478 | 5.346 | 3.000 | 115.852 | 0.819 | |
| 2 | -65.823 | 4.794 | 15.272 | 5.346 | 3.000 | 115.852 | 0.819 | |
| 6 | 17.059 | — | — | — | 1.000 | 124.510 | 9.476 | |
| tree | 3 | 7.742 | 6.977 | — | 4.216 | 2.000 | 107.947 | 0.000 |
| 4 | -21.674 | 6.977 | — | 4.216 | 2.000 | 107.947 | 0.000 | |
| 5 | -21.539 | 6.952 | — | — | 2.000 | 107.999 | 0.051 | |
| 1 | 7.882 | 0.754 | 8.868 | 5.046 | 3.000 | 109.413 | 1.465 | |
| 2 | -36.867 | 0.754 | 9.622 | 5.046 | 3.000 | 109.413 | 1.465 | |
| 6 | 14.765 | — | — | — | 1.000 | 114.729 | 6.782 | |
3.3. Model Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramalho, C.E.; Hobbs, R.J. Time for a Change: Dynamic Urban Ecology. Trends in Ecology & Evolution 2012, 27, 179–188. [Google Scholar] [CrossRef]
- Wadduwage, S.; Millington, A.; Crossman, N.; Sandhu, H. Agricultural Land Fragmentation at Urban Fringes: An Application of Urban-To-Rural Gradient Analysis in Adelaide. Land 2017, 6, 28. [Google Scholar] [CrossRef]
- Fahey, R.; Casali, M. Distribution of Forest Ecosystems over Two Centuries in a Highly Urbanized Landscape. Landscape and Urban Planning 2017, 164. [Google Scholar] [CrossRef]
- Niemelä, J.; Saarela, S.-R.; Söderman, T.; Kopperoinen, L.; Yli-Pelkonen, V.; Väre, S.; Kotze, D.J. Using the Ecosystem Services Approach for Better Planning and Conservation of Urban Green Spaces: A Finland Case Study. Biodiversity and Conservation 2010, 19, 3225–3243. [Google Scholar] [CrossRef]
- Lovell, S.T.; Taylor, J.R. Supplying Urban Ecosystem Services through Multifunctional Green Infrastructure in the United States. Landscape Ecol 2013, 28, 1447–1463. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J. Urbanization Drives Divergence in Functional Diversity and Composition of Woody Plant Communities in Remnant Forest Patches. Global Ecology and Conservation 2023, 48, e02724. [Google Scholar] [CrossRef]
- Alberti, M. Maintaining Ecological Integrity and Sustaining Ecosystem Function in Urban Areas. Current Opinion in Environmental Sustainability 2010, 2, 178–184. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Tripler, C.E. Forest Remnants Along Urban-Rural Gradients: Examining Their Potential for Global Change Research. Ecosystems 2005, 8, 568–582. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Triantis, K.A. Habitat Diversity, Ecological Requirements of Species and the Small Island Effect. Diversity and Distributions 2009, 15, 131–140. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. How Important Are Large vs. Small Forest Remnants for the Conservation of the Woodland Flora in an Urban Context? Global Ecology and Biogeography 2003, 12, 287–298. [Google Scholar] [CrossRef]
- Behera, M.C.; Sahoo, U.K.; Mohanty, T.L.; Prus, P.; Smuleac, L.; Pascalau, R. Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India. Forests 2023, 14, 1931. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities Are Hotspots for Threatened Species. Global Ecology and Biogeography 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Schrader, J.; König, C.; Triantis, K.A.; Trigas, P.; Kreft, H.; Weigelt, P. Species–Area Relationships on Small Islands Differ among Plant Growth Forms. Global Ecology and Biogeography 2020, 29, 814–829. [Google Scholar] [CrossRef]
- Macarthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; REV-Revised.; Princeton University Press, 1967; ISBN 978-0-691-08836-5.
- Gao, Z.; Song, K.; Pan, Y.; Malkinson, D.; Zhang, X.; Jia, B.; Xia, T.; Guo, X.; Liang, H.; Huang, S.; et al. Drivers of Spontaneous Plant Richness Patterns in Urban Green Space within a Biodiversity Hotspot. Urban Forestry & Urban Greening 2021, 61, 127098. [Google Scholar] [CrossRef]
- Malkinson, D.; Kopel, D.; Wittenberg, L. From Rural-Urban Gradients to Patch – Matrix Frameworks: Plant Diversity Patterns in Urban Landscapes. Landscape and Urban Planning 2018, 169, 260–268. [Google Scholar] [CrossRef]
- Itescu, Y.; Schwarz, R.; Donihue, C.M.; Slavenko, A.; Roussos, S.A.; Sagonas, K.; Valakos, E.D.; Foufopoulos, J.; Pafilis, P.; Meiri, S. Inconsistent Patterns of Body Size Evolution in Co-Occurring Island Reptiles. Global Ecology and Biogeography 2018, 27, 538–550. [Google Scholar] [CrossRef]
- Lomolino; Weiser Towards a More General Species–Area Relationship: Diversity on All Islands, Great and Small. Journal of Biogeography 2001, 28, 431–445. [CrossRef]
- Gao, de; Wang, Y. A Review of the Small-Island Effect Detection Methods and Method Advancement. Biodiversity Science 2023, 31, 2023299. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Sheng, S.; Zheng, J.; Wu, S.; Cao, Z.; Zhang, K.; Xu, Y. Quantifying the Effects of Landscape and Habitat Characteristics on Structuring Bird Assemblages in Urban Habitat Patches. Sci Rep 2024, 14, 12707. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Scholes, R.J.; Arneth, A.; Barnes, D.K.A.; Burrows, M.T.; Diamond, S.E.; Duarte, C.M.; Kiessling, W.; Leadley, P.; Managi, S.; et al. Overcoming the Coupled Climate and Biodiversity Crises and Their Societal Impacts. Science 2023, 380, eabl4881. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Millien, V. A Global Synthesis of the Small-Island Effect in Habitat Islands. Proceedings of the Royal Society B: Biological Sciences 2018, 285, 20181868. [Google Scholar] [CrossRef] [PubMed]
- Triantis, K.A.; Guilhaumon, F.; Whittaker, R.J. The Island Species–Area Relationship: Biology and Statistics. Journal of Biogeography 2012, 39, 215–231. [Google Scholar] [CrossRef]
- Zhen Cao; Yongying Liu; Shikai Song; Lina Zhang; De Gao. Drivers of the Small-Island Effect in Moss Assemblages on Terrestrial Habitat Islands: A Case Study in Mountaintops of the Middle Taihang Mountains, China. Chinese Journal of Plant Ecology 2023, 47, 65–76. [Google Scholar] [CrossRef]
- Hengeveld, R. MacArthur, R.H. and E.O. Wilson (1967, Reprinted 2001). The Theory of Island Biogeography. Acta Biotheor 2002, 50, 133–136. [Google Scholar] [CrossRef]
- Anderson, W.B.; Wait, D.A. Subsidized Island Biogeography Hypothesis: Another New Twist on an Old Theory. Ecology Letters 2001, 4, 289–291. [Google Scholar] [CrossRef]
- Lomolino, MarK. V. A Call for a New Paradigm of Island Biogeography. Global Ecology and Biogeography 2000, 9, 1–6. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Li, S.; Liang, H.; Lu, H. The Woody Plant Diversity and Landscape Pattern of Fine-Resolution Urban Forest along a Distance Gradient from Points of Interest in Qingdao. Ecological Indicators 2021, 122, 107326. [Google Scholar] [CrossRef]
- Model Selection and Multimodel Inference; Burnham, K. P., Anderson, D.R., Eds.; Springer: New York, NY, 2004; ISBN 978-0-387-95364-9. [Google Scholar]
- Matthews, T.; Triantis, K.; Whittaker, R.J.; Guilhaumon, F. SARS: An R Package for Fitting, Evaluating and Comparing Species–Area Relationship Models. Ecography 2019, 42, 1353–1457. [Google Scholar] [CrossRef]
- Menegotto, A.; Rangel, T.F.; Schrader, J.; Weigelt, P.; Kreft, H. A Global Test of the Subsidized Island Biogeography Hypothesis. Global Ecology and Biogeography 2020, 29, 320–330. [Google Scholar] [CrossRef]
- Hong Liang, Liangjun Da, Kun Song, Chunling Zhou, Zhiwen Gao, Kai Wang. Influence of habitat fragments on the richness of remnant natural forest species[J]. Journal of East China Normal University(Natural Science), 2022, 2022(3): 17-26.
- Gao, Z.; Pan, Y.; Van Bodegom, P.M.; Cieraad, E.; Xing, D.; Yang, Y.; Xia, T.; Luo, X.; Song, K.; Da, L.; et al. Beta Diversity of Urban Spontaneous Plants and Its Drivers in 9 Major Cities of Yunnan Province, China. Landscape and Urban Planning 2023, 234, 104741. [Google Scholar] [CrossRef]
- Chen, X.-S.; Liang, H.; Song, K.; Da, L.-J. [Diversity and classification system of weed community in Harbin City, China]. Ying Yong Sheng Tai Xue Bao 2014, 25, 2221–2228. [Google Scholar] [PubMed]
- Xue, M.; Zhi, O.; Guo, C. Composition of Plant Species and Their Distribution Patterns in Beijing Urban Ecosystem. Acta Ecologica Sinica 2004. [Google Scholar]
- Tian, Z.; Song, K.; Da, L. Distribution patterns and traits of weed communities along an urban–rural gradient under rapid urbanization in Shanghai, China. Weed Biology and Management 2015, 15, 27–41. [Google Scholar] [CrossRef]
- Manzo, E.; Tomasello, S. The First Complete Chloroplast Genome for the Species-Rich Genus Anthemis (Asteraceae). Mitochondrial DNA Part B 2024. [Google Scholar] [CrossRef]
- Saini, I.; Chauhan, J.; Kaushik, P. Medicinal Value of Domiciliary Ornamental Plants of the Asteraceae Family. Journal of Young Pharmacists 2020, 12, 03–10. [Google Scholar] [CrossRef]
- Tamme, R.; Götzenberger, L.; Zobel, M.; Bullock, J.M.; Hooftman, D.A.P.; Kaasik, A.; Pärtel, M. Predicting Species’ Maximum Dispersal Distances from Simple Plant Traits. Ecology 2014, 95, 505–513. [Google Scholar] [CrossRef]
- Zu, K.; Wang, Z. Research Progress on the Elevational Distribution of Mountain Species in Response to Climate Change. Biodiversity Science 2022. [Google Scholar] [CrossRef]
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.N. The Effects of Sealing on Urban Soil Carbon and Nutrients. SOIL 2021, 7, 661–675. [Google Scholar] [CrossRef]
- Edmondson, J.L.; Stott, I.; Davies, Z.G.; Gaston, K.J.; Leake, J.R. Soil Surface Temperatures Reveal Moderation of the Urban Heat Island Effect by Trees and Shrubs. Sci Rep 2016, 6, 33708. [Google Scholar] [CrossRef]
- Schnabel, F.; Liu, X.; Kunz, M.; Barry, K.E.; Bongers, F.J.; Bruelheide, H.; Fichtner, A.; Härdtle, W.; Li, S.; Pfaff, C.-T.; et al. Species Richness Stabilizes Productivity via Asynchrony and Drought-Tolerance Diversity in a Large-Scale Tree Biodiversity Experiment. Science Advances 2021. [Google Scholar] [CrossRef]
- Song, K.; Gao, Z.; Pan, Y.; Zhuge, M.; Wu, T.; Xia, T.; Hu, Y.; Da, L.; Cieraad, E. Urban Plants with Different Seed Dispersal Modes Have Convergent Response but Divergent Sensitivity to Climate Change and Anthropogenic Stressors 2024.
- Dzwonko, Z.; Loster, S. Distribution of Vascular Plant Species in Small Woodlands on the Western Carpathian Foothills. Oikos 1989, 56, 77–86. [Google Scholar] [CrossRef]
- Parra-Sanchez, E.; Banks-Leite, C. The Magnitude and Extent of Edge Effects on Vascular Epiphytes across the Brazilian Atlantic Forest. Sci Rep 2020, 10, 18847. [Google Scholar] [CrossRef] [PubMed]
- Sisi, W.U.; Xiaozhen, L.U.; Xinyue, W.; Zhiwei, G.E. Effects of Two Types of Artificial Interferences on Plant Diversity of Urban Landscape Forests. JOURNAL OF NANJING FORESTRY UNIVERSITY 2019, 43, 128. [Google Scholar] [CrossRef]
- Rego, R.M.C.; Moura, M.; Olangua-Corral, M.; Roxo, G.; Resendes, R.; Silva, L. Anthropogenic Disturbance Has Altered the Habitat of Two Azorean Endemic Coastal Plants. BMC Ecol Evo 2024, 24, 111. [Google Scholar] [CrossRef]
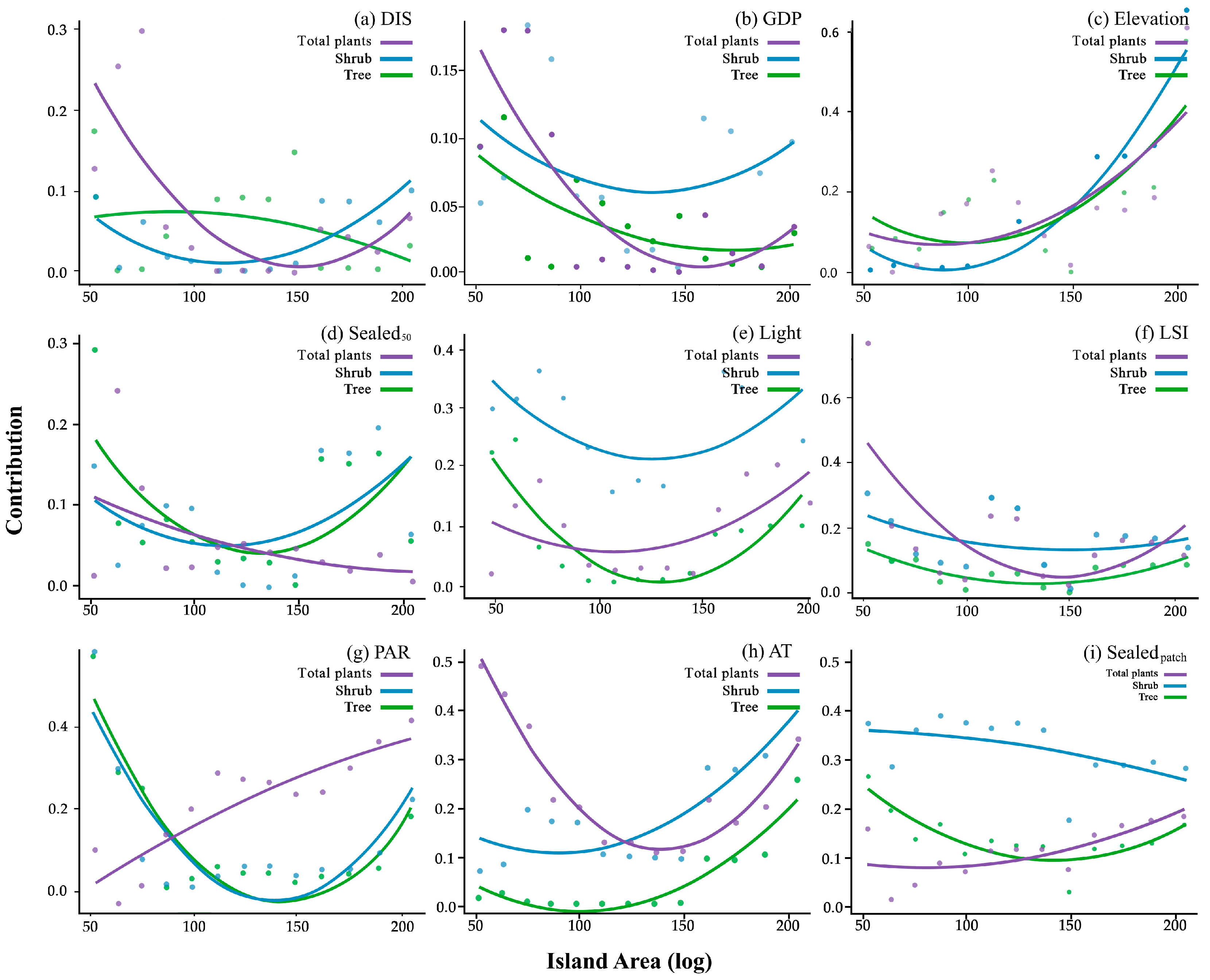
| Explanatory variables | Explanatory variables | Source | proxy hypothesis. |
| Elevation | Elevation | Resource and Environmental Science Data Platform (resolution = 30, https://www.resdc.cn) |
Habitat diversity |
| PAR | Perimeter-area ratio | Nutrient replenishment, Habitat diversity, Disturbance hypothesis |
|
| LSI | Landscape shape index | Nutrient replenishment, Habitat diversity, Disturbance hypothesis |
|
| DIS | Distance to the source of species | Resource and Environmental Science Data Platform (resolution = 30, https://www.resdc.cn) |
Nutrient replenishment. Disturbance hypothesis |
| Sealedpatch | The proportion of sealed surface within patch | National Geomatics Center of China (Resolution = 30, https://www.webmap.cn/) |
Habitat diversity |
| Sealed50 | The proportion of sealed surface around the patch within a radii of 50 m | National Geomatics Center of China (Resolution = 30, https://www.webmap.cn/) |
Disturbance hypothesis |
| AT | Anthropogenic disturbance, class 1 when there is less than one type of interference and the intensity of the interference is small, class 2 when there are one or two types of low-level interference, class 3 when there are three types of medium-level interference, and class 4 when there are four or more types of high-level interference. | ( Hong LIANG, 2022) | Disturbance hypothesis |
| Light | The annual average night light intensity of China in 2020 | Geographic remote sensing ecological network platform (Resolution = 30, www.gisrs.cn) | Disturbance hypothesis |
| GDP | The Gross Domestic Product data of China in 2020 | Geographic remote sensing ecological network platform (Resolution = 30, www.gisrs.cn) | Disturbance hypothesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





