Submitted:
04 November 2024
Posted:
04 November 2024
You are already at the latest version
Abstract
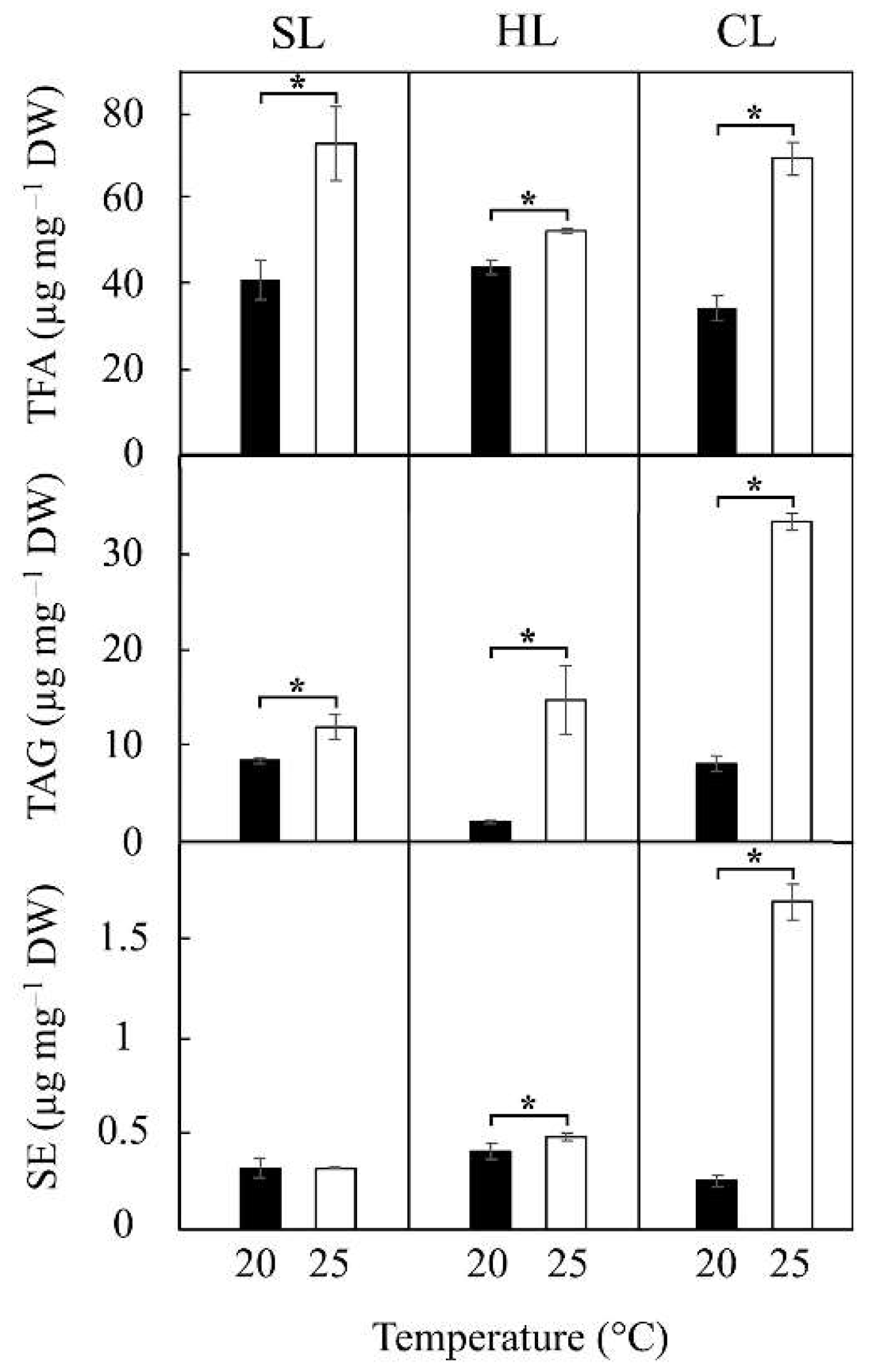
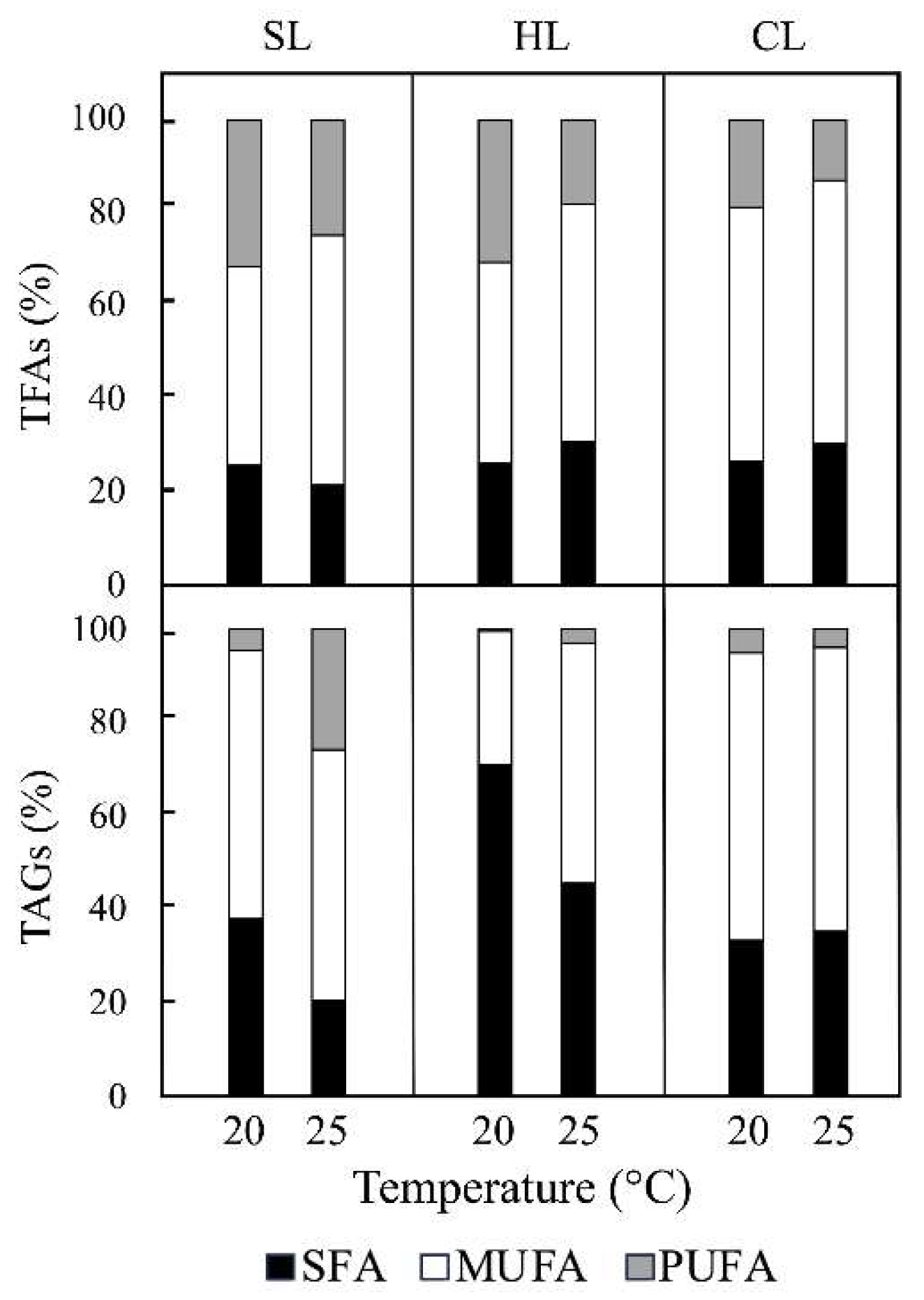
The aim of this study was to investigate the combined effects of temperature and light on the photosynthetic parameters and lipid accumulation in the diatom Phaeodactylum tricornutum, a model organism widely used for studies on diatom physiology, ecology, and biotechnology. Our results highlight the importance of the interaction between temperature and light intensity in influencing growth rates, pigments and active pho-tosystems content, photosynthetic efficiency, lipid production and fatty acid composi-tion in P. tricornutum. Measurements of the maximum electron transport rate (rETRmax) and rETR at maximum PAR (830 µmol m−2 s−1) confirmed that P. tricornutum exhibits significantly higher light sensitivity as growth temperature increases under light/dark cycles at two light intensities (25–60 µmol m−2 s−1). However, this trend was reversed under continuous light (25 µmol m−2 s−1). Moreover, higher rETRmax values (up to dou-ble) were observed at higher irradiance, either in intensity or under continuous light regimes, at the two temperatures tested. On the other hand, increasing light intensity amplified the observed effect of temperature on photosystem I (PSI) activity under light/dark regimes, but not under continuous light conditions. This resulted in a greater deficiency in PSI activity, likely due to limitations in electron supply to this photosys-tem. Furthermore, increasing the culture temperature from 20 °C to 25 °C triggered an increase in the number and size of cytoplasmic lipid droplets under conditions of in-creased light intensity, with an even more pronounced effect under continuous illumi-nation. Notably, the combination of 25 °C and continuous illumination resulted in a more than twofold increase in triacylglyceride (TAG) content, reaching approximately 17 mg L⁻1. This condition also caused a substantial rise (up to ≈90%) in the proportions of palmitoleic and palmitic acids in the TAG fatty acid profile.
Keywords:
1. Introduction
2. Results
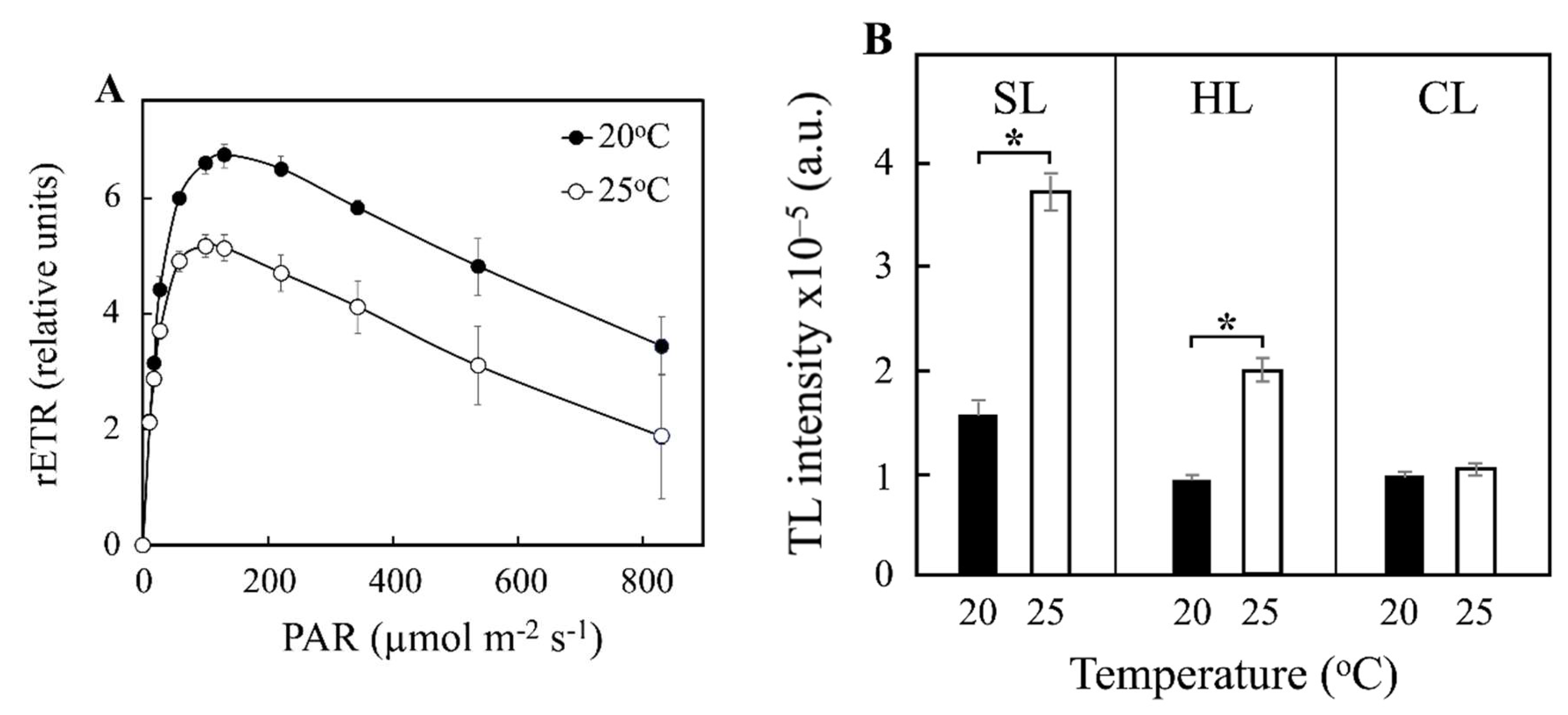
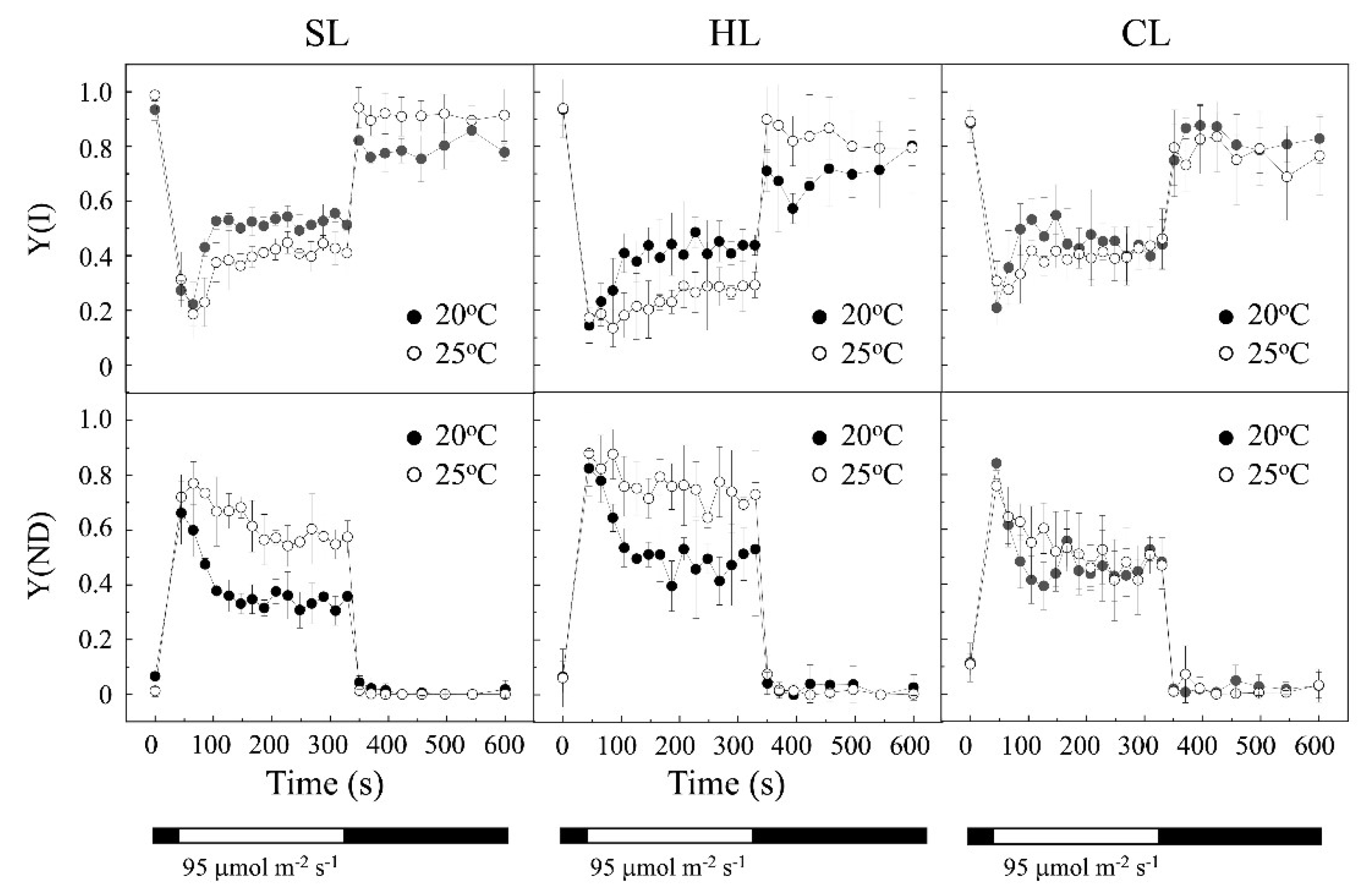
2.1. Influence of Temperature under Two Different Light Intensities in Light/Dark Regime.
2.2. Influence of Temperature under Continuous Light
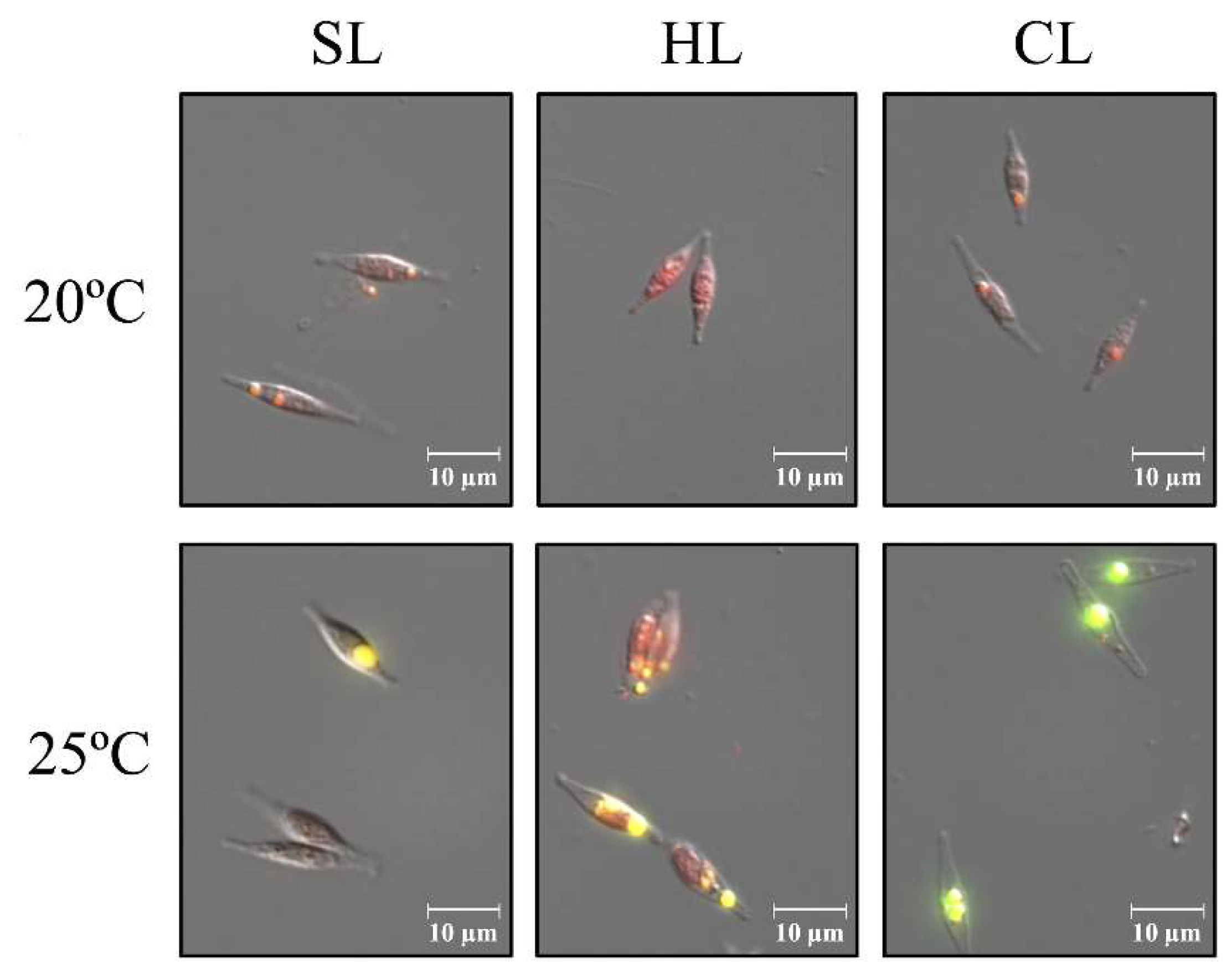
2.3. Combined Effect of Temperature and Light Regimes on Lipid Content and Fatty Acid Composition
3. Discussion
3.1. Combined Effects of Temperature and Light Regimes on Cell Growth and Photosynthetic Parameters
3.2. Combined Effect of Temperature and Light Regimes on Lipid Content and Fatty Acid Composition
4. Materials and Methods
4.1. Microalgal Strain and Culture Conditions
4.2. Analytical Methods
4.3. Nile Red staining and fluorescent microscopy
4.4. Lipid Analysis
4.5. Photosynthetic Measurements
4.6. Statistical Significance Level
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowler, C.; Vardi, A.; Allen, A.E. Oceanographic and biogeochemical insights from diatom genomes. Ann. Rev. Mar. Sci. 2010, 2, 333–365. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C.; Goss, R.; Bailleul, B.; Campbell, D.; Lavaud, J.; Lepetit, B. Photosynthetic light reactions in diatoms. I. The lipids and light-harvesting complexes of the thylakoid membrane. In The Molecular Life of Diatoms, Falciatore, A., Mock, T. Eds.; Springer International Publishing, Cham. 2022, pp. [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: Research challenges and possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Chen, F.; Leng, Y.; Lu, Q.; Zhou, W. The Application of Microalgae Biomass and Bio-Products as Aquafeed for Aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier Ltd., 2013; pp. [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Bojko, M.; Brzostowska, K.; Kuczyńska, P.; Latowski, D.; Ol-Chawa-Pajor, M.; Krzeszowiec, W.; Waloszek, A.; Strzałka, K. Temperature effect on growth, and selected parameters of Phaeodactylum tricornutum in batch cultures. Acta Biochim. Pol. 2013, 60, 861–4. [Google Scholar] [CrossRef]
- Suzuki, Y.; Takahashi, M. Growth responses of several diatom species isolated from various environments to temperature. J. Phycol. 1995, 31, 880–888. [Google Scholar] [CrossRef]
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003, 295, 145–156. [Google Scholar] [CrossRef]
- Feijão, E.; Gameiro, C.; Franzitta, M.; Duarte, B.; Caçador, I.; Cabrita, M.T.; Matos, A.R. Heat wave impacts on the model diatom Phaeodactylum tricornutum: Searching for photochemical and fatty acid biomarkers of thermal stress. Ecol. Indic. 2018, 95, 1026–1037. [Google Scholar] [CrossRef]
- Hong, T.; Huang, N.; Mo, J.; Chen, Y.; Li, T.; Du, H. Transcriptomic and physiological responses of a model diatom (Phaeodactylum tricornutum) to heat shock and heat selection. Ecol. Indic. 2023, 153. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A diatom cell factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour. Technol. Rep. 2022, 101122. [Google Scholar] [CrossRef]
- Kudo, I.; Miyamoto, M.; Noiri, Y.; Maita, Y. Combined effects of temperature and iron on the growth and physiology of the marina diatom Phaeodactylum triconutum (Bacillariophyceae). J. Phyco.l 2000, 36, 1096–1102. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2004, 40, 651–654. [Google Scholar] [CrossRef]
- Cheong, K.Y. , Firlar, E., Ficaro, L., Gorbunov, M.Y., Kaelber, J.T., Falkowski, P.G. Saturation of thylakoid-associated fatty acids facilitates bioenergetic coupling in a marine diatom allowing for thermal acclimation. Glob. Chang. Biol. 3133. [Google Scholar] [CrossRef]
- Wu, H.; Roy, S.; Alami, M.; Green, B.R.; Campbell, D.A. Photosystem II photoinactivation, repair, and protection in marine centric diatoms. Plant Physiol. 2012, 160, 464–476. [Google Scholar] [CrossRef]
- Fisher, N.L.; Halsey, K.H. Mechanisms that increase the growth efficiency of diatoms in low light. Photosynth Res 2016, 129, 183–197. [Google Scholar] [CrossRef]
- Nymark, M.; Valle, K.C.; Brembu, T.; Hancke, K.; Winge, P.; Andresen, K.; Johnsen, G.; Bones, A.M. An integrated analysis of molecular acclimation to high light in the marine diatom Phaeodactylum tricornutum. PLoS One 2009, 4, e7743. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Algal lipids and effect of the environment on their biochemistry. In Lipids in Aquatic Ecosystems 2009, Springer New York, pp. 24. [CrossRef]
- Tanaka, T.; Yoneda, K.; Maeda, Y. Lipid metabolism in diatoms. In The Molecular Life of Diatoms, Falciatore, A., Mock, T. Eds.; Springer International Publishing, Cham. 2022. [Google Scholar] [CrossRef]
- Leyland, B.; Boussiba, S.; Khozin-Goldberg, I. A review of diatom lipid droplets. Biology (Basel) 2020, 9, 38. [Google Scholar] [CrossRef]
- Fawley, M.W. Effects of light intensity and temperature interactions on growth characteristics of Phaeodactylum tricornutum (bacillariophyceae). J. Phycol. 1984, 20, 67–72. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Price, N.M. Influence of irradiance and temperature on the iron content of the marine diatom Thalassiosira weissflogii (Bacillariophyceae). Mar. Ecol. Prog. Ser. 2000, 206, 107–117. [Google Scholar] [CrossRef]
- Davison, I.R. Enviromental effects on algal photosynthesis: Temperature. J. Phycol. 1991, 27, 2–8. [Google Scholar] [CrossRef]
- Kalituho, L.; Pshybytko, N.; Kabashnikova, L.; Jahns, P. Photosynthetic apparatus and high temperature: role of light. Bulg. J. Plant Physiol.
- Agarwal, A.; Levitan, O.; de Carvalho, H.C.; Falkowski, P.G. Light-dependent signal transduction in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. N. Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.C.M.; Wong, J.T.Y. Lipid biosynthesis and its coordination with cell cycle progression. Plant Cell Physiol. 2005, 46, 1973–1986. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Roncel, M.; González-Rodríguez, A.A.; Naranjo, B.; Bernal-Bayard, P.; Lindahl, M.; Hervás, M.; Navarro, J.A.; Ortega, J.M. Iron deficiency induces a partial inhibition of the photosynthetic electron transport and a high sensitivity to light in the diatom Phaeodactylum tricornutum. Front. Plant. Sci. 2016, 7, 208522. [Google Scholar] [CrossRef]
- Castell, C.; Bernal-Bayard, P.; Ortega, J.M.; Roncel, M.; Hervás, M.; Navarro, J.A. The heterologous expression of a plastocyanin in the diatom Phaeodactylum tricornutum improves cell growth under iron-deficient conditions. Physiol. Plant. 2021, 171, 277–290. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Patterson, G.W. Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp. Biochem. Physiol. B 1975, 50, 579–583. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: a review. Renewable and Sustainable Energy Reviews 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Zeng, X.; Jin, P.; Jiang, Y.; Yang, H.; Zhong, J.; Liang, Z.; Guo, Y.; Li, P.; Huang, Q.; Pan, J.; Lu, H.; Wei, Y.; Zou, D.; Xia, J. Light alters the responses of two marine diatoms to increased warming. Mar. Environ. Res. 2020, 154, 158767. [Google Scholar] [CrossRef]
- Thompson, P. The response of growth and biochemical composition to variations in daylength, temperature, and irradiance in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). J. Phycol. 1999, 35, 1215–1223. [Google Scholar] [CrossRef]
- Rehder, L.; Rost, B.; Rokitta, S.D. Abrupt and acclimation responses to changing temperature elicit divergent physiological effects in the diatom Phaeodactylum tricornutum. New Phytologist. 2023, 239, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, C.E.; Baker, K.G.; Nielsen, D.A.; Petrou, K. Temperatures above thermal optimum reduce cell growth and silica production while increasing cell volume and protein content in the diatom Thalassiosira pseudonana. Hydrobiologia 2020, 847, 4233–4248. [Google Scholar] [CrossRef]
- Ragni, M.; Ribera D’ Alcalá, M. Circadian variability in the photobiology of Phaeodactylum tricornutum: Pigment content. J. Plankton Res. 2007, 29, 141–156. [Google Scholar] [CrossRef]
- Zhong. J., Guo, Y., Liang, Z., Huang, Q., Lu, H., Pan, J., Li, P., Jin, P., Xia, J. 2021. Adaptation of a marine diatom to ocean acidification and warming reveals constraints and trade-offs. Sci. Total Environ. 1451. [CrossRef]
- Rutherford, A.W.; Renger, G.; Koike, H.; Inoue, Y. Thermoluminescence as a probe of photosystem II. The redox and protonation states of the secondary acceptor quinone and the O2-evolving enzyme. Biochim. Biophys. Acta (BBA) - Bioenergetics. [CrossRef]
- Friedman, A.L.; Alberte, R.S. Biogenesis and light regulation of the major light harvesting chlorophyll-protein of diatoms. Plant Physiol. 1986, 80, 43–51. [Google Scholar] [CrossRef]
- Salleh, S.; McMinn, A. The effects of temperature on the photosynthetic parameters and recovery of two temperate benthic microalgae, Amphora cf. coffeaeformis and Cocconeis cf. sublittoralis (Bacillariophyceae). J. Phycol. 2011, 47, 1413–1424. [Google Scholar] [CrossRef]
- Gleich, S.J.; Plough, L. V.; Glibert, P.M. Photosynthetic efficiency and nutrient physiology of the diatom Thalassiosira pseudonana at three growth temperatures. Mar. Biol. 2020, 167. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Sharkey, T.D.; Geider, R.J. Activation and deactivation of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in three marine microalgae. Photosynth. Res. 1997, 51, 93–106. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Maeda, Y.; Nojima, D.; Yoshino, T.; Tanaka, T. Structure and properties of oil bodies in diatoms. Philos. Trans. R. Soc Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Kurpan Nogueira, D.P.; Silva, A.F.; Araújo, O.Q.F.; Chaloub, R.M. Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass Bioenergy 2015, 72, 280–287. [Google Scholar] [CrossRef]
- Spilling, K.; Ylöstalo, P.; Simis, S.; Seppälä, J. Interaction effects of light, temperature and nutrient limitations (N, P and Si) on growth, stoichiometry and photosynthetic parameters of the cold-water diatom Chaetoceros wighamii. PLoS One 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Scodelaro Bilbao, P.G.; Garelli, A.; Díaz, M.; Salvador, G.A.; Leonardi, P.I. Crosstalk between sterol and neutral lipid metabolism in the alga Haematococcus pluvialis exposed to light stress. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158767. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. “Designer” Biodiesel: optimizing fatty ester composition to improve fuel properties. Energ. Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Manzoor, M.; Hussain, A.; Ahmad, Q. ul A.; Chaudhary, A.; Schenk, P.M.; Deepanraj, B.; Loke Show, P. Biodiesel quality assessment of microalgae cultivated mixotrophically on sugarcane bagasse. Sustain. Energy Technol. Assess. 2022, 53, 102359. [Google Scholar] [CrossRef]
- Dodson, V.J.; Mouget, J.L.; Dahmen, J.L.; Leblond, J.D. The long and short of it: temperature-dependent modifications of fatty acid chain length and unsaturation in the galactolipid profiles of the diatoms Haslea ostrearia and Phaeodactylum tricornutum. Hydrobiologia 2014, 727, 95–107. [Google Scholar] [CrossRef]
- McLachlan, J. Some considerations of the growth of marine algae in artificial media. Can. J. Microbiol. 2011, 10, 769–782. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Ruban, A. V. Detachment of the fucoxanthin chlorophyll a/c binding protein (FCP) antenna is not involved in the acclimative regulation of photoprotection in the pennate diatom Phaeodactylum tricornutum. Biochim. Biophys. Acta (BBA) - Bioenergetics 2017, 1858, 218–230. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pfl. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 1972, 310, 2nd edition, Ottawa, Canada, Fisheries Research Board of Canada, (Bulletin Fisheries Research Board of Canada, Nr. 167). [CrossRef]
- Ren, X.; Wei, C.; Yan, Q.; Shan, X.; Wu, M.; Zhao, X.; Song, Y. Optimization of a novel lipid extraction process from microalgae. Sci. Rep. 2021, 11, 20221. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Guschina, I.A.; Martínez-Rivas, J.M.; Mancha, M.; Harwood, J.L. The utilization and desaturation of oleate and linoleate during glycerolipid biosynthesis in Olive (Olea Europaea L.) callus cultures. J. Exp. Bot. 2008, 59, 2425–2435. [Google Scholar] [CrossRef]
- Garcés, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem 1993, 211, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Martínez, A.M.; Jiménez-López, J.; Hernández, M.L.; Pérez-Ruiz, J.M.; Cejudo, F.J. Plastid 2-Cys peroxiredoxins are essential for embryogenesis in Arabidopsis. Redox Biol. 2023, 62, 102645. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, J.M.; Miranda, T. Graphical and numerical analysis of thermoluminescence and fluorescence F0 emission in photosynthetic material. Photosynth. Res. 1992, 33, 15–27. [Google Scholar] [CrossRef]
- Ducruet, J.M.; Serrano, A.; Roncel, M.; Ortega, J.M. Peculiar properties of chlorophyll thermoluminescence emission of autotrophically or mixotrophically grown Chlamydomonas reinhardtii. J. Photochem. Photobiol. B 2011, 104, 301–307. [Google Scholar] [CrossRef]
| SL (25 µmol m-2 s-1) | HL (60 µmol m-2 s-1) | CL (25 µmol m-2 s-1) | ||||
|---|---|---|---|---|---|---|
| Parametera) | 20ºC | 25ºC | 20ºC | 25ºC | 20ºC | 25ºC |
| Specific growth rate, µ(day-1) | 0.294±0.021(100%)b) | 0.218±0.001(74%) | 0.311±0.021(100%) | 0.290±0.001(93%) | 0.317±0.018(100%) | 0.266±0.001 (84%) |
| Cells per mL (x10–7) | 1.680±0.180(100%) | 1.250±0.003(74%) | 1.880±0.201(100%) | 1.550±0.004(82%) | 1.120±0.120 (100%) | 1.210±0.003(108%) |
| Net photosynthetic rate (µmol O2 h-1 per 106 cells) | 35.536±0.176(100%) | 15.451±0.091(43%) | 22.309±1.499(100%) | 17.690±0.969(79%) | 36.696±1.668(100%) | 16.926±0.445(46%) |
| Total Chl per cell(pg cell-1) | 0.851±0.057(100%) | 0.863±0.074(102%) | 0.606±0.025(100%) | 0.816±0.029(135%) | 1.033±0.053(100%) | 0.818±0.035(79%) |
| Chl a/Chl c | 4.163(100%) | 6.238(150%) | 6.032(100%) | 8.263(137%) | 3.331(100%) | 2.582(78%) |
| Total carotenoids per cell (pg cell-1) | 0.260±0.004(100%) | 0.287±0.001(110%) | 0.245±0.001(100%) | 0.512±0.001(209%) | 0.361±0.009(100%) | 0.140±0.004(39%) |
| Fv/Fm | 0.61 ± 0.02 | 0.66 ± 0.01 | 0.59 ± 0.01 | 0.64 ± 0.02 | 0.59 ± 0.07 | 0.58 ± 0.04 |
| rETRmax | 6.75 ± 0.18 | 5.17± 0.13 | 11.82 ± 0.22 | 7.67 ± 0.55 | 7.66 ± 0.19 | 10.54 ± 0.39 |
| rETR (at PARmax) | 3.44 ± 0.51 | 1.88 ± 1.13 | 11.35 ± 0.63 | 6.79 ± 0.69 | 6.89 ± 0.41 | 10.55 ± 0.18 |
| Pm | 0.238 ± 0.022 | 0.426 ± 0.005 | 0.176 ± 0.025 | 0.254 ± 0.015 | 0.123 ± 0.016 | 0.236 ± 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





