Submitted:
02 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
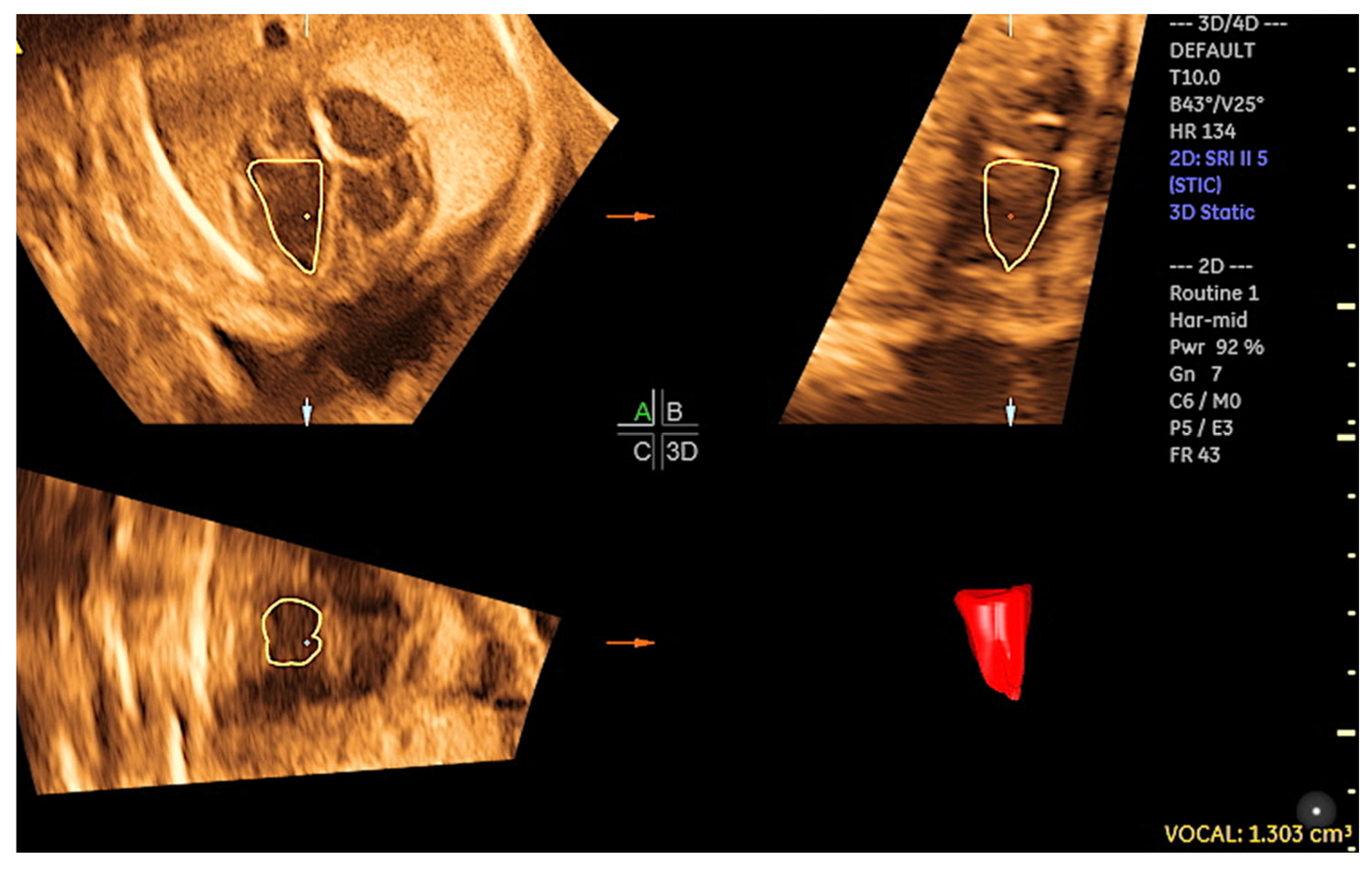
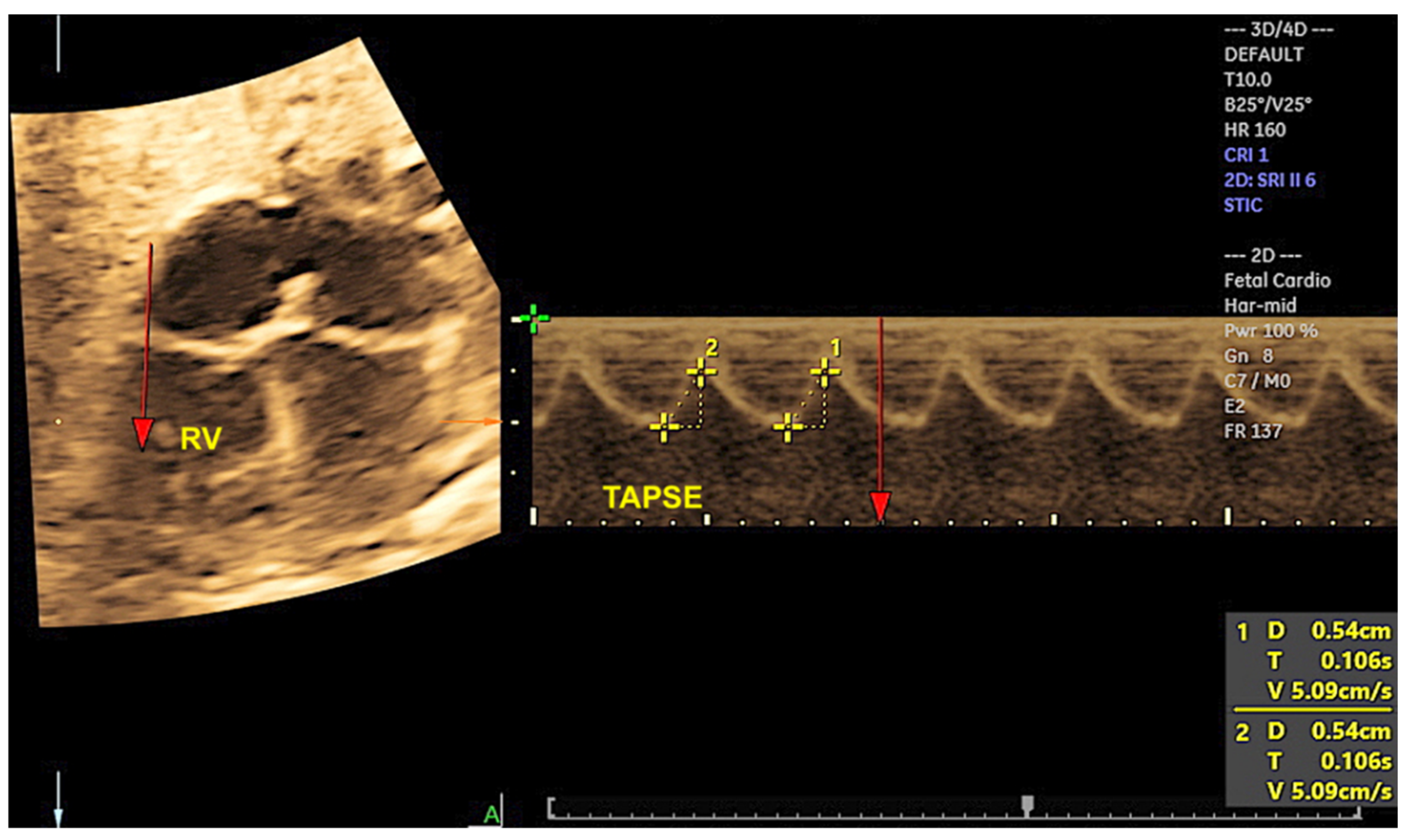
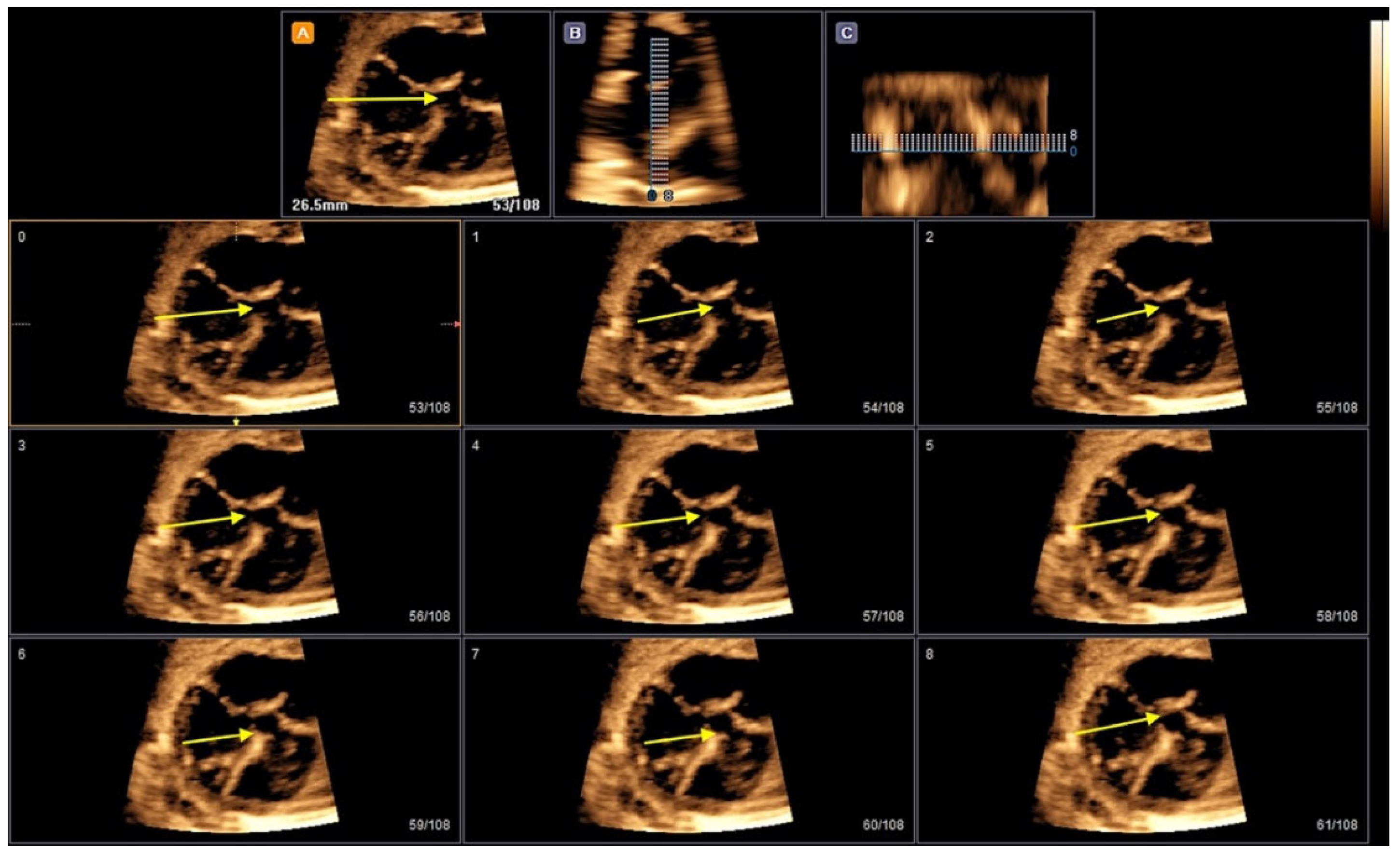
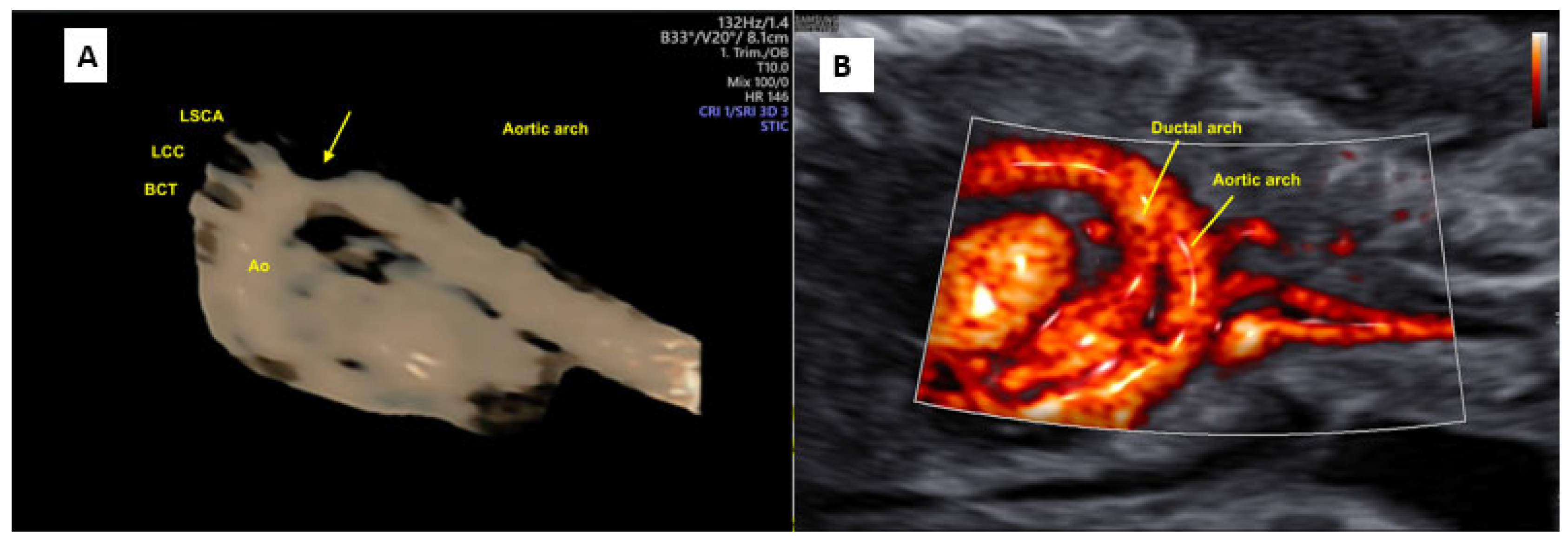
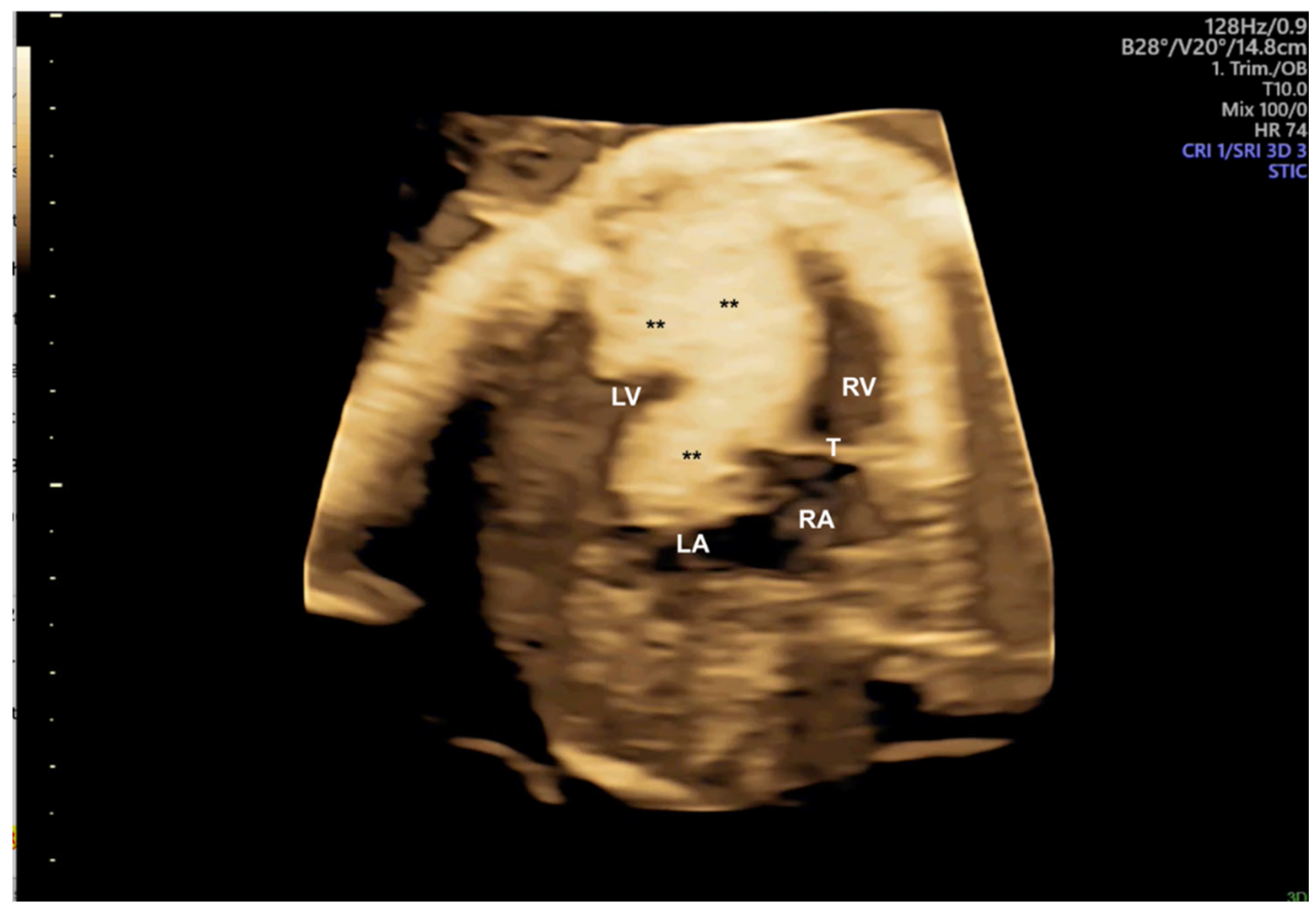
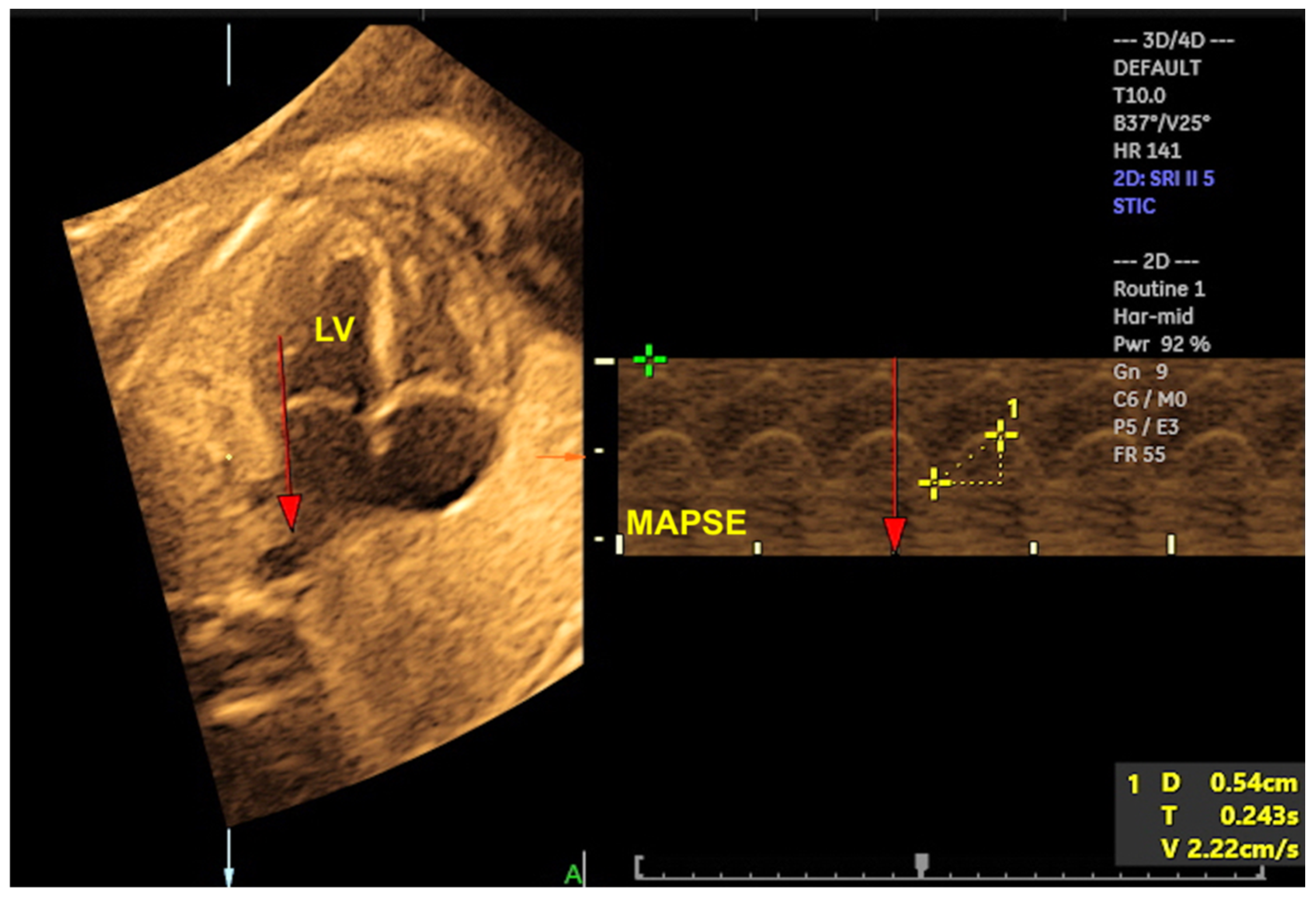
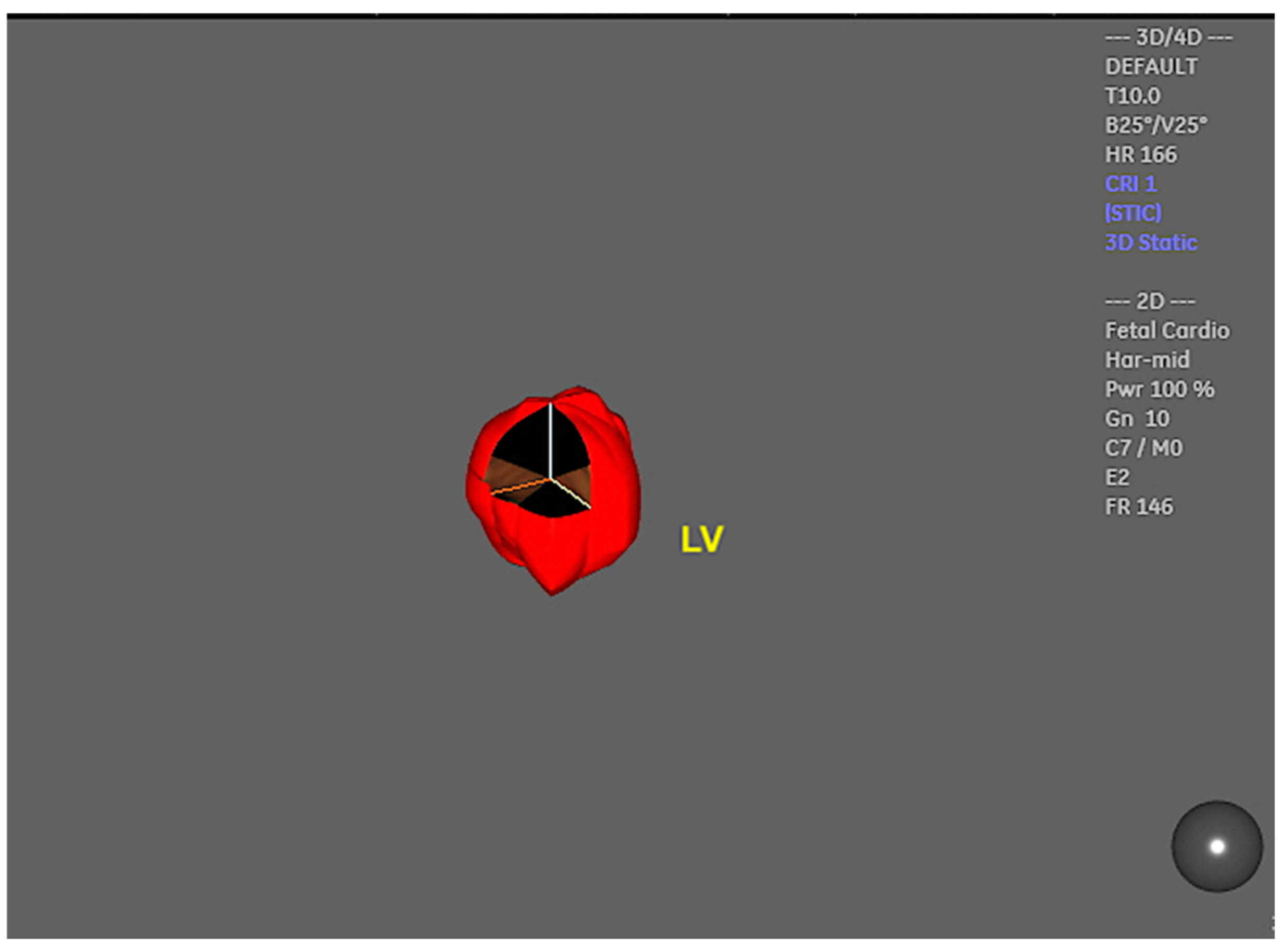
2. Three-Dimensional Ultrasound and Saptiotemporal Image Correlation
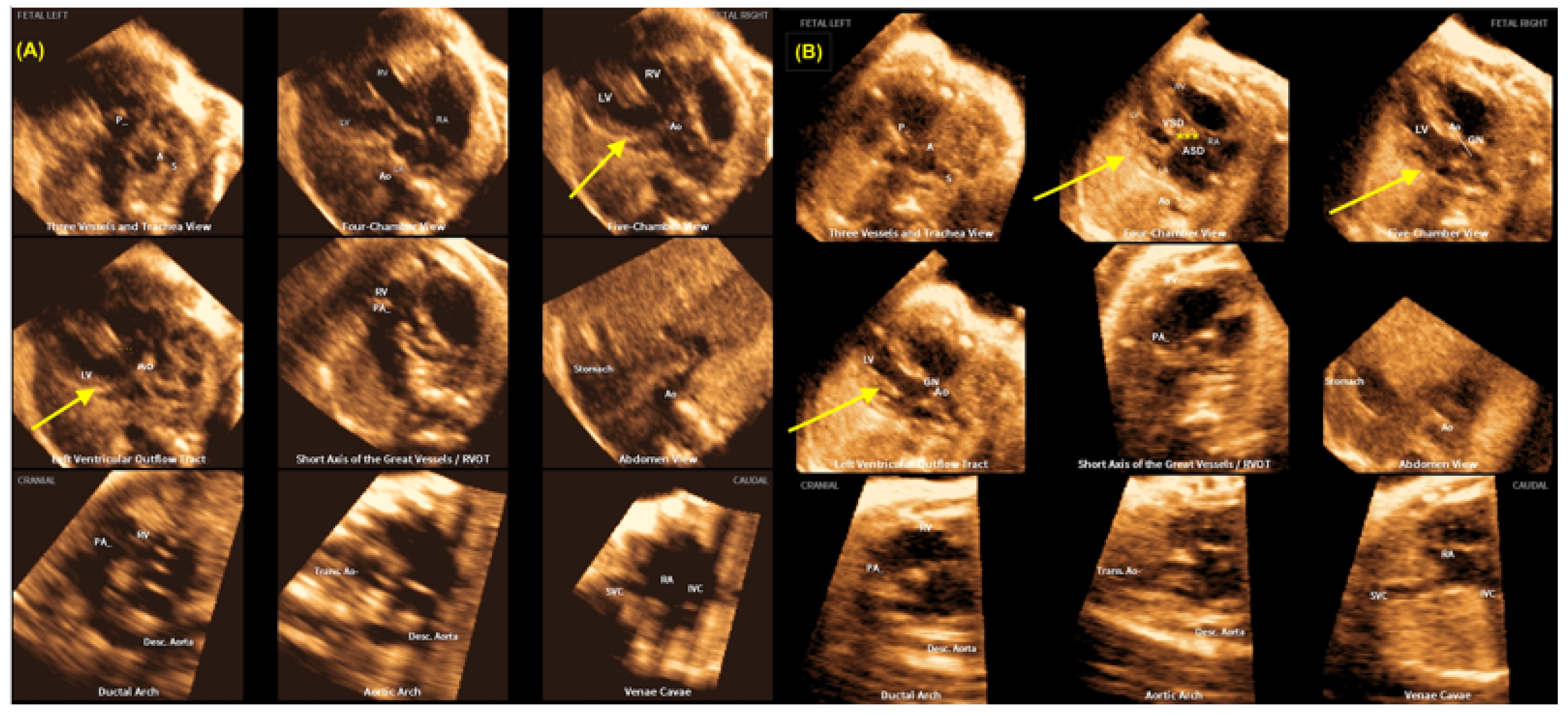
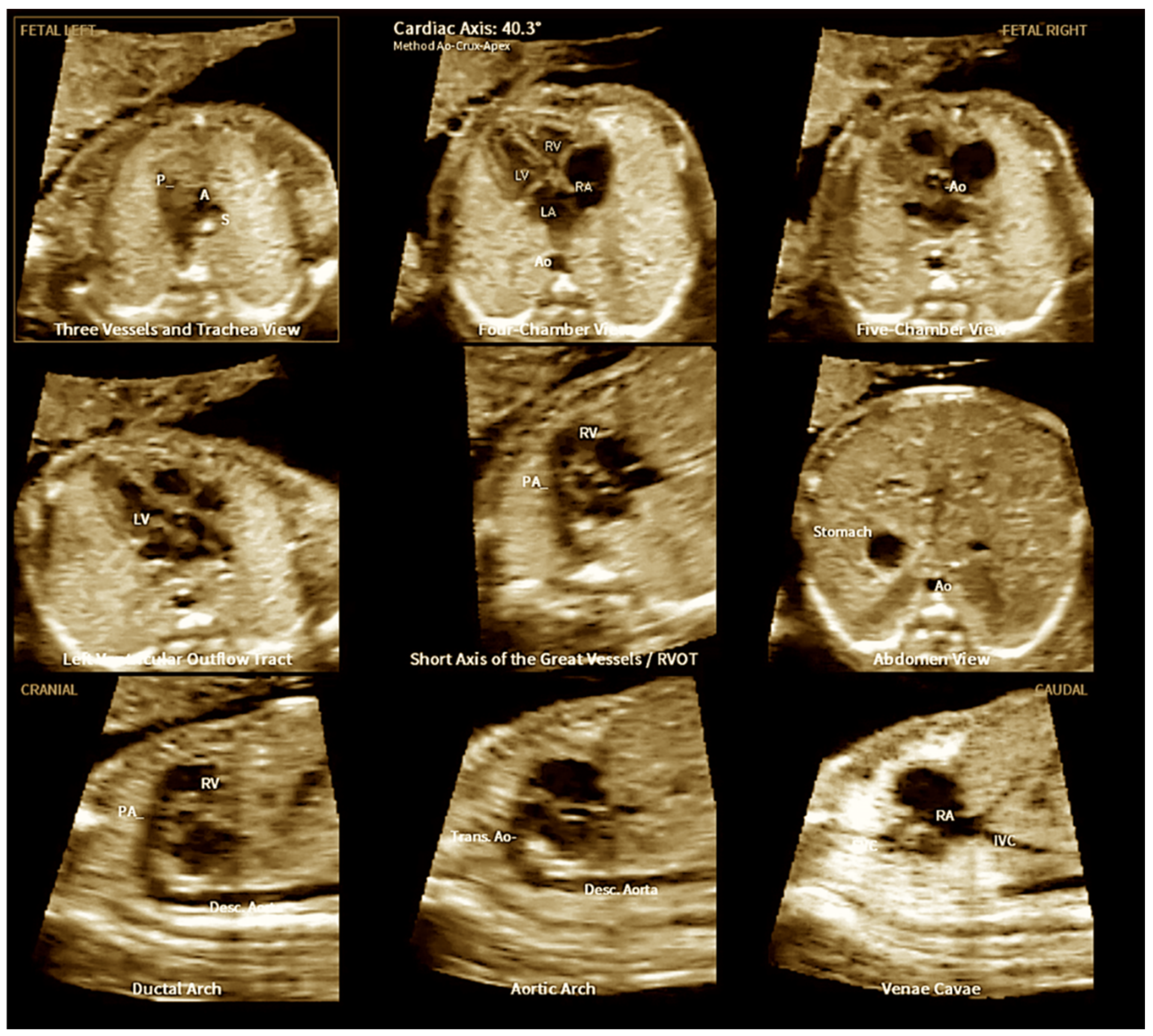
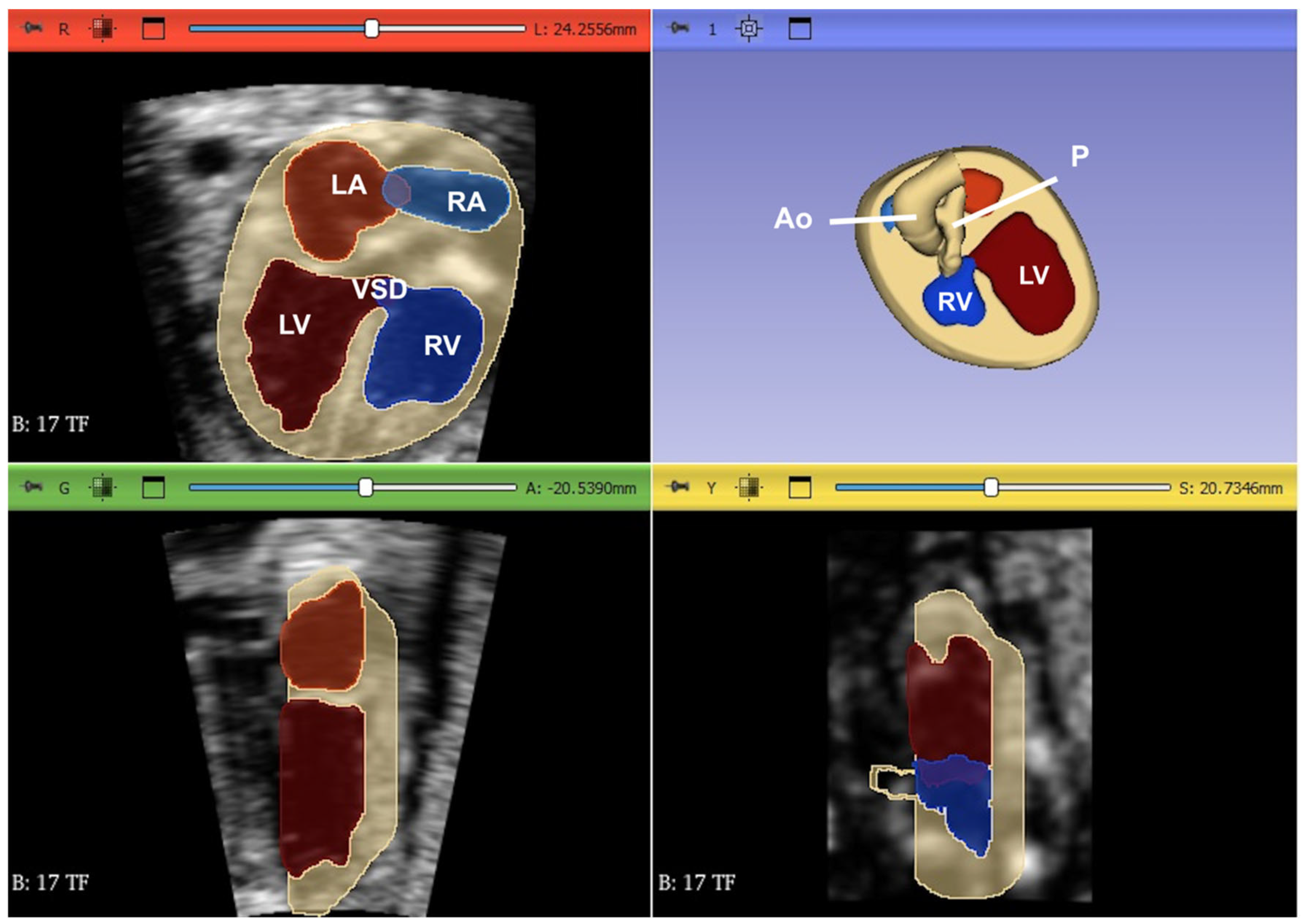
3. Fetal Intelligent Navigation Echocardiography (Fine) and Artificial Intelligence
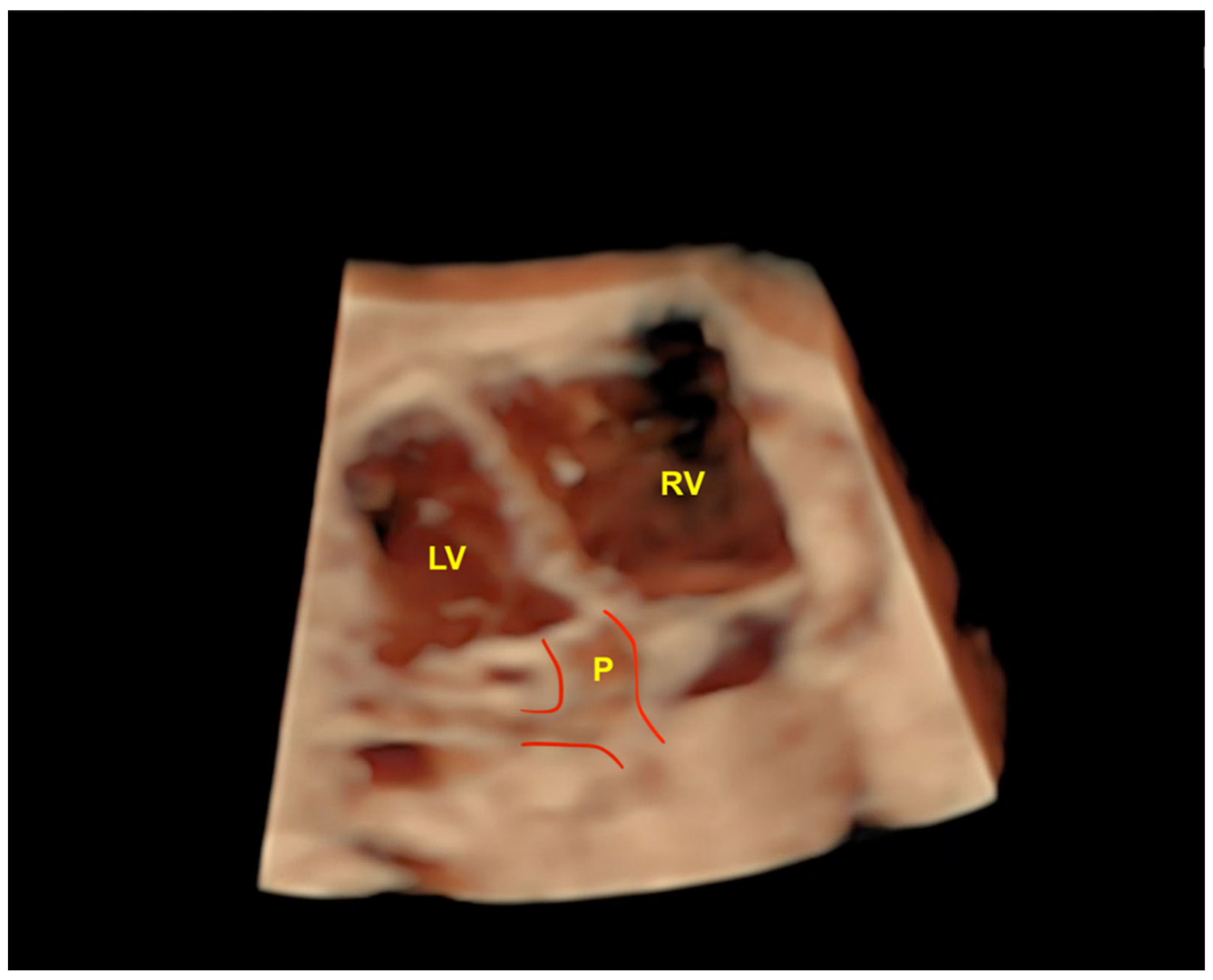
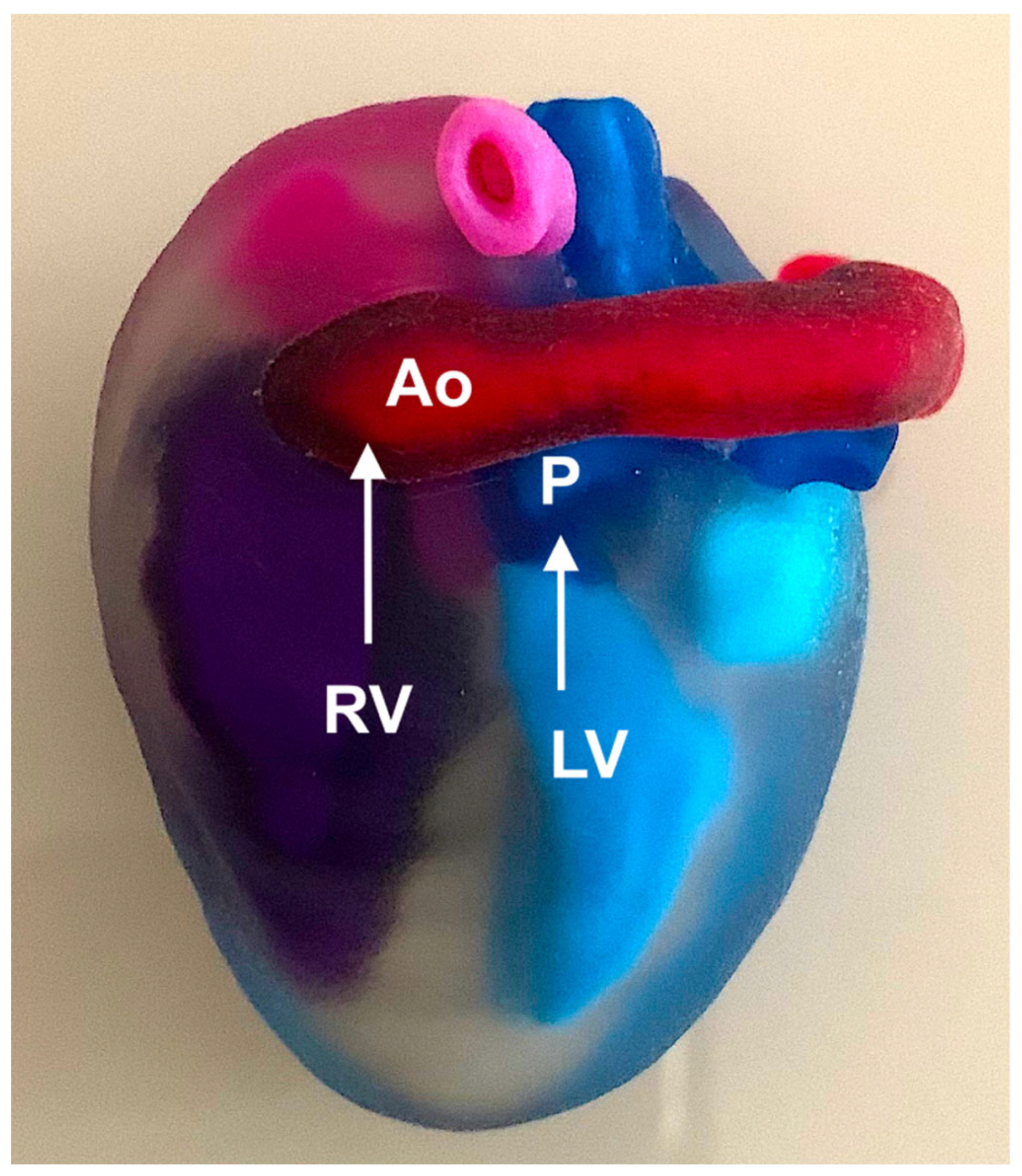
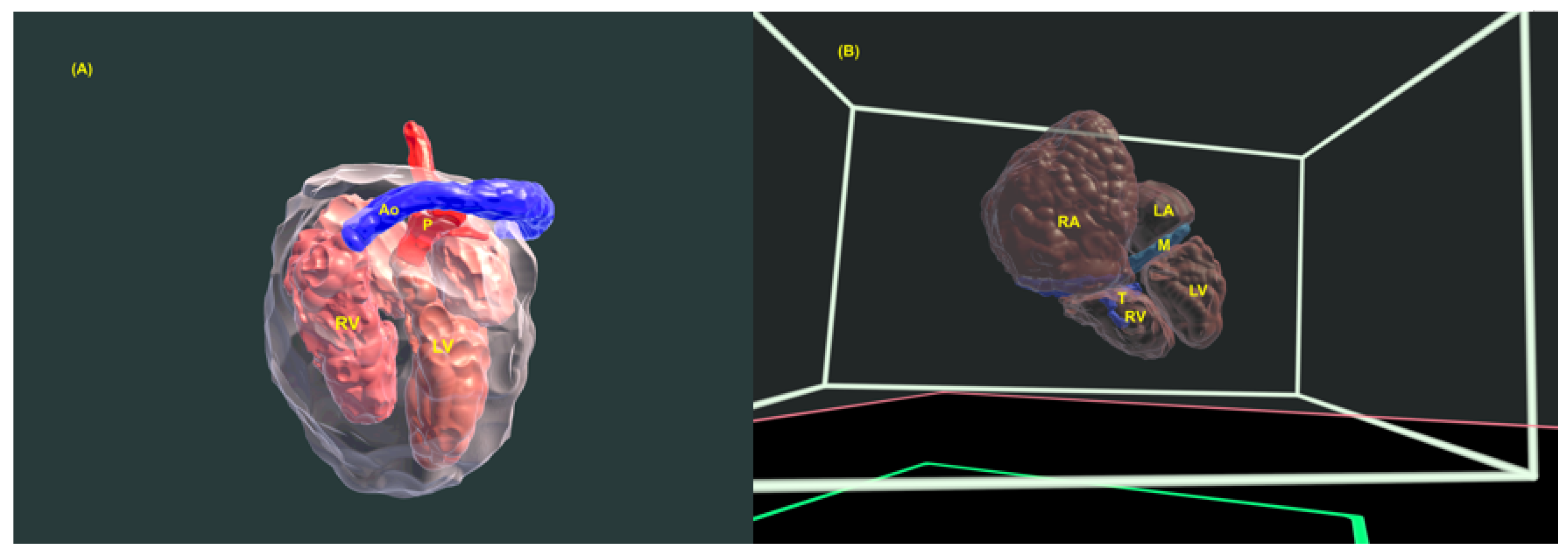
4. Three-Dimensional Physical and Virtual Models of the Fetal Heart
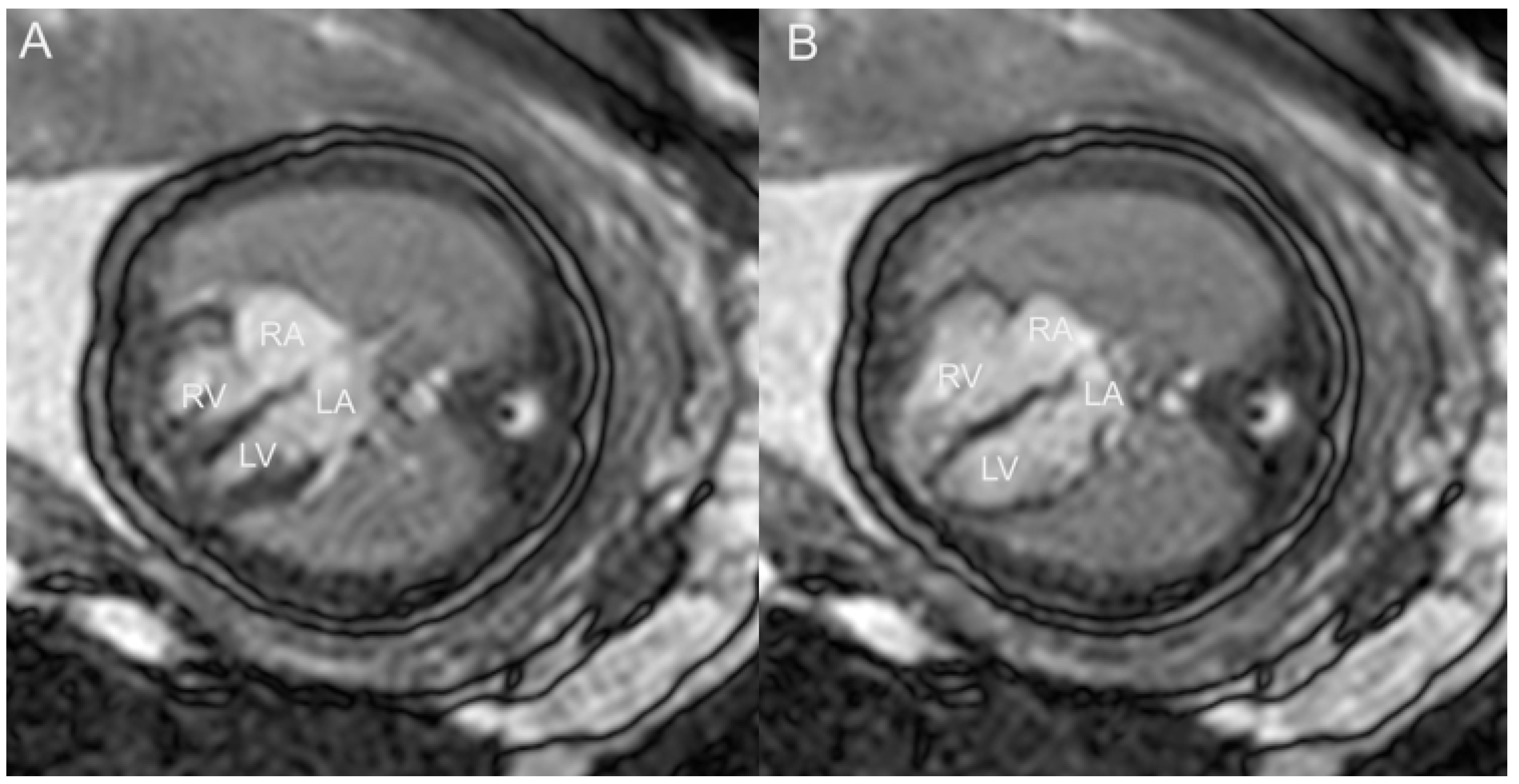
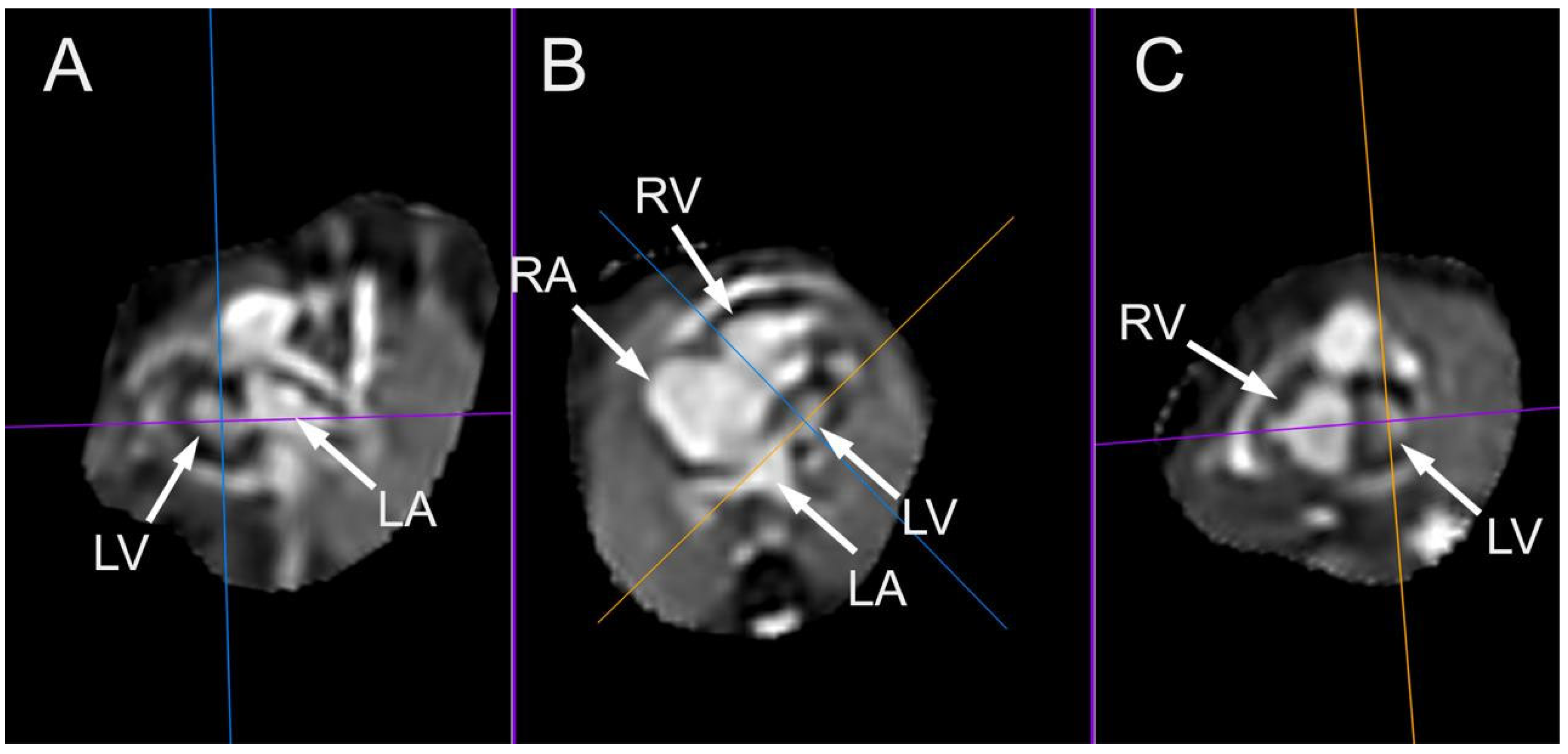
5. Fetal Cardiac Magnetic Resonance Imaging
6. Conclusions
Supplementary Materials
Acknowledgments
References
- Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N; et al. Global birth prevalence of congenital heart defects 1970-2017: Updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019, 48, 455–463.
- Bouma BJ, Mulder BJ. Changing Landscape of Congenital Heart Disease. Circ Res. 2017, 120, 908–922.
- Hoffman JIE. The global burden of congenital heart disease. Cardiovasc J Afr. 2013, 24, 141–145.
- Khasawneh W, Hakim F, Abu Ras O, Hejazi Y, Abu-Aqoulah A. Incidence and patterns of congenital heart disease among Jordanian infants, a cohort study from a university tertiary center. Front Pediatr. 2020, 8, 219.
- Meller CH, Grinenco S, Aiello H, Córdoba A, Sáenz-Tejeira MM, Marantz P; et al. Congenital heart disease, prenatal diagnosis and management. Arch Argent Pediatr. 2020, 118, e149–e161.
- Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013, 131, e1502–e1508.
- Carvalho JS, Axt-Fliedner R, Chaoui R, Copel JA, Cuneo BF, Goff D; et al. ISUOG Practice Guidelines (updated): Fetal cardiac screening. Ultrasound Obstet Gynecol. 2023, 61, 788–803.
- Chaoui, R. The four-chamber view: Four reasons why it seems to fail in screening for cardiac abnormalities and suggestions to improve detection rate. Ultrasound Obstet Gynecol. 2003, 22, 3–10. [Google Scholar] [CrossRef]
- Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation. 2014, 129, 2183–2242.
- Devore GR, Falkensammer P, Sklansky MS, Platt LD. Spatio-temporal image correlation (STIC): New technology for evaluation of the fetal heart. Ultrasound Obstet Gynecol. 2003, 22, 380–387.
- Vinãls F, Poblete P, Giuliano A. Spatio-temporal image correlation (STIC): A new tool for the prenatal screening of congenital heart defects. Ultrasound Obstet Gynecol. 2003, 22, 388–394.
- Gonçalves LF, Lee W, Chaiworapongsa T, Espinoza J, Schoen ML, Falkensammer P. Four-dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am J Obstet Gynecol. 2003, 189, 1792–1802.
- Yagel S, Benachi A, Bonnet D, Dumez Y, Hochner-Celnikier D, Cohen SM; et al. Rendering in fetal cardiac scanning: The intracardiac septa and the coronal atrioventricular valve planes. Ultrasound Obstet Gynecol. 2006, 28, 266–274.
- Nardozza LM, Rolo LC, Araujo Júnior E, Hatanaka AR, Rocha LA, Simioni C; et al. Reference range for fetal interventricular septum area by means of four-dimensional ultrasonography using spatiotemporal image correlation. Fetal Diagn Ther. 2013, 33, 110–115.
- Vinãls F, Mandujano L, Vargas G, Giuliano A. Prenatal diagnosis of congenital heart disease using four-dimensional spatio-temporal image correlation (STIC) telemedicine via an Internet link: A pilot study. Ultrasound Obstet Gynecol. 2005, 25, 25–31.
- Espinoza J, Gonçalves LF, Lee W, Mazor M, Romero R. A novel method to improve prenatal venous connections using three- and four-dimensional ultrasonography and “inversion mode”. Ultrasound Obstet Gynecol. 2005, 25, 428–434.
- Liu J, Wang Y, Zhao H, Liu W. Spatio-temporal image correlation rendering mode visualizes the specific location and surrounding structure of ventricular septal defect. Clin Anat. 2019, 32, 408–420.
- Malho A, Ximenes RS, Bravo-Valenzuela NJ, Araujo Júnior E. Spatio-Temporal Image Correlation: Three-Dimensional Imaging for Fetal Cardiac Screening and Congenital Heart Disease Assessment. Arq Bras Cardiol. 2024;121(4):e20230580.
- Werner H, Lopes dos Santos JR, Fontes R, Belmonte S, Daltro P, Gasparetto E; et al. Virtual bronchoscopy for evaluating cervical tumors of the fetus. Ultrasound Obstet Gynecol. 2013, 41, 90–94.
- Werner H, Lopes J, Ribeiro G, Jésus NR, Santos GR, Alexandria HAF; et al. Three-dimensional virtual cystoscopy: Noninvasive approach for the assessment of urinary tract in fetuses with lower urinary tract obstruction. Prenat Diagn. 2017, 37, 1350–1352.
- Werner H, Ribeiro G, Lopes J, Sá RAM, Maia F, Castro P; et al. Virtual navigation for the improvement of parents counseling and the planning of fetal endoscopic myelomeningocele repair. Childs Nerv Syst. 2021, 37, 969–972.
- Werner H, Lopes J, Tonni G, Araujo Júnior E. Maternal-fetal attachment in blind women using physical model from three-dimensional ultrasound and magnetic resonance scan data: Six serious cases. J Matern Fetal Neonatal Med. 2016, 29, 2229–2232.
- Werner H, Castro P, Lopes J, Ribeiro G, Araujo Júnior E. Maternal-fetal physical model: Image fusion obtained by white light scanner and magnetic resonance imaging. J Matern Fetal Neonatal Med. 2022, 35, 4427–4430.
- Veronese P, Bertelli F, Cattapan C, Andolfatto M, Gervasi MT, Vida VL. Three-Dimensional Printing of Fetal Heart With d-Transposition of the Great Arteries From Ultrasound Imaging Data. World J Pediatr Congenit Heart Surg. 2021, 12, 291–292.
- Huang J, Shi H, Chen Q, Hu J, Zhang Y, Song H; et al. Three-Dimensional Printed Model Fabrication and Effectiveness Evaluation in Fetuses With Congenital Heart Disease or With a Normal Heart. J Ultrasound Med. 2021, 40, 15–28.
- Werner H, Lopes J, Ribeiro G, Raposo AB, Trajano E, Araujo Júnior E. Three-dimensional virtual traveling navigation and three-dimensional printing models of a normal fetal heart using ultrasonography data. Prenat Diagn. 2019, 39, 175–177.
- Bravo-Valenzuela NJ, Monteiro Pereira Leite MF, Lopes J, Arcoverde V, Ribeiro G, Araujo Júnior E; et al. Three-dimensional navigation inside a normal fetal heart in a virtual reality environment. J Clin Ultrasound. 2024 Aug 21. [CrossRef]
- Giffoni MC, Lopes J, Ribeiro G, Araujo Júnior E, Werner H. Fetal heart segmentation in a virtual reality environment. Int J Cardiovasc Imaging. 2024 Jun 4. [CrossRef]
- Taksøe-Vester CA, Mikolaj K, Petersen OBB, Vejlstrup NG, Christensen AN, Feragen A; et al. Role of artificial-intelligence-assisted automated cardiac biometrics in prenatal screening for coarctation of aorta. Ultrasound Obstet Gynecol. 2024, 64, 36–43.
- Pietrolucci ME, Maqina P, Mappa I, Marra MC, D' Antonio F, Rizzo G. Evaluation of an artificial intelligent algorithm (Heartassist™) to automatically assess the quality of second trimester cardiac views: A prospective study. J Perinat Med. 2023, 51, 920–924.
- Bennasar M, Martinez JM, Gomez O, Bartrons J, Olivella A, Puerto B; et al. Accuracy of four-dimensional spatiotemporal image correlation echocardiography in the prenatal diagnosis of congenital heart defects. Ultrasound Obstet Gynecol. 2010, 36, 458–464.
- Gonçalves LF, Espinoza J, Lee W, Nien K, Hong JS, Santolaya-Forgas J; et al. A new approach to fetal echocardiography: Digital casts of the fetal cardiac chambers and great vessels for detection of congenital heart disease. J Ultrasound Med. 2005, 24, 415–424.
- Barber N, Freud L. Advances in Fetal Cardiac Imaging and Intervention. CJC Pediatr Congenit Heart Dis. 2023, 3, 33–42.
- Vinãls F, Ascenzo R, Naveas R, Huggon I, Giuliano A. Fetal echocardiography at 11 + 0 to 13 + 6 weeks using four-dimensional spatiotemporal image correlation telemedicine via an Internet link: A pilot study. Ultrasound Obstet Gynecol. 2008, 31, 633–638.
- Sharma S, Parness IA, Kamenir SA, Ko H, Haddow S, Steinberg LG; et al. Screening fetal echocardiography by telemedicine: Efficacy and community acceptance. J Am Soc Echocardiogr. 2003(3):202-8.
- Yeo L, Romero R, Jodicke C, Kim SK, Gonzalez JM, Oggè G; et al. Simple targeted arterial rendering (STAR) technique: A novel and simple method to visualize the fetal cardiac outflow tracts. Ultrasound Obstet Gynecol 2011, 37, 549–556.
- Rocha LA, Rolo LC, Barros FS, Nardozza LM, Moron AF, Araujo Júnior E. Assessment of Quality of Fetal Heart Views by 3D/4D Ultrasonography Using Spatio-Temporal Image Correlation in the Second and Third Trimesters of Pregnancy. Echocardiography. 2015, 32, 1015–1021.
- Espinoza J, Lee W, Comstock C, Romero R, Yeo L, Rizzo G; et al. Collaborative study on 4-dimensional echocardiography for the diagnosis of fetal heart defects: The COFEHD study. J Ultrasound Med. 2010, 29, 1573–1580.
- Adriaanse BM, Tromp CH, Simpson JM, Van Mieghem T, Kist WJ, Kuik DJ; et al. Interobserver agreement in detailed prenatal diagnosis of congenital heart disease by telemedicine using four-dimensional ultrasound with spatiotemporal image correlation. Ultrasound Obstet Gynecol. 2012, 39, 203–209.
- Rolo LC, Nardozza LM, Araujo Júnior E, Hatanaka AR, Rocha LA, Simioni C; et al. Reference ranges of atrioventricular valve areas by means of four-dimensional ultrasonography using spatiotemporal image correlation in the rendering mode. Prenat Diagn. 2013, 33, 50–55.
- Rolo LC, Pietrolucci ME, Araujo Júnior E, Barros FS, Nardozza LM, Martina WP; et al. Viewing rate and reproducibility of papillary muscle areas in foetal atrioventricular valves using spatio-temporal image correlation in the rendering mode in congenital heart disease. J Matern Fetal Neonatal Med. 2015, 28, 1375–1380.
- Lei W, Ying Z, Ailu C, Xiaoguang W. Evaluation of normal fetal ductus venosus using B-flow imaging with spatiotemporal image correlation and traditional color Doppler echocardiography. Echocardiography. 2015, 32(2):325–331.
- Qin Y, Zhang Y, Zhou X, Wang Y, Sun, W, Chen L; et al. Four-dimensional echocardiography with spatiotemporal image correlation and inversion mode for detection of congenital heart disease. Ultrasound Med Biol. 2014, 40, 1434–1441.
- Rizzo G, Capponi A, Vendola M, Pietrolucci ME, Arduini D. Role of tomographic ultrasound imaging with spatiotemporal image correlation for identifying fetal ventricular septal defects. J. Ultrasound Med. 2008, 27, 1071–1075.
- Malho AS, Bravo-Valenzuela NJ, Ximenes R, Peixoto AB, Araujo Júnior E. Antenatal diagnosis of congenital heart disease by 3D ultrasonography using spatiotemporal image correlation with HDlive Flow and HDlive Flow silhouette rendering modes. Ultrasonography. 2022, 41, 578–596.
- Tie HX, Ma B, Zhang DC, Li TG. Prenatal diagnosis of fetal inferior vena cava malformation using HDlive flow combined with spatiotemporal image correlation. Echocardiography. 2022, 39, 685–690.
- Li TG, Ma B, Gao YH, Zhang RH, Li PL, Da ZQ. Prenatal diagnosis of total anomalous pulmonary venous connection using 2D and HDlive flow combined with spatiotemporal image correlation. Echocardiography. 2022, 39, 1269–1275.
- Karmegaraj B, Kumar S, Srimurugan B, Sudhakar A, Simpson JM, Vaidyanathan B. 3D/4D spatiotemporal image correlation (STIC) fetal echocardiography provides incremental benefit over 2D fetal echocardiography in predicting postnatal surgical approach in double-outlet right ventricle. Ultrasound Obstet Gynecol. 2021, 57, 423–430.
- Magioli Bravo-Valenzuela NJ, Malho AS, Nieblas CO, Castro PT, Werner H, Araujo Júnior E. Evolution of Fetal Cardiac Imaging over the Last 20 Years. Diagnostics (Basel). 2023, 13, 3509.
- Seale AN, Carvalho JS, Gardiner HM, Mellander M, Roughton M, Simpson J; et al. Total anomalous pulmonary venous connection: Impact of prenatal diagnosis. Ultrasound Obstet Gynecol. 2012, 40, 310–318.
- Bravo-Valenzuela NJM, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of total anomalous pulmonary venous connection: 2D and 3D echocardiographic findings. J Clin Ultrasound. 2021, 49, 240–247.
- Yagel S, Kivilevitch Z, Cohen SM, Valsky DV, Messing B, Shen O, Achiron R. The fetal venous system, part I: Normal embryology, anatomy, hemodynamics, ultrasound evaluation and Doppler investigation. Ultrasound Obstet Gynecol. 2010, 35, 741–750.
- Wolman I, Gull I, Fait G, Amster R, Kupferminc MJ, Lessing JB, et al Persistent right umbilical vein: Incidence and significance. Ultrasound Obstet Gynecol. 2002, 19, 562–564. [CrossRef]
- Weichert J, Hartge D, Germer U, Axt-Fliedner R, Gembruch U. Persistent right umbilical vein: A prenatal condition worth mentioning? Ultrasound Obstet Gynecol. 2011, 37, 543–548.
- Lide B, Lindsley W, Foster MJ, Hale R, Haeri S. Intrahepatic persistent right umbilical vein and associated outcomes: A systematic review of the literature. J Ultrasound Med. 2016, 35, 1–5.
- Strizek B, Zamprakou A, Gottschalk I, Roethlisberger M, Hellmund A, Müller A; et al. Prenatal diagnosis of agenesis of ductus venosus: A retrospective study of anatomic variants, associated anomalies and impact on postnatal outcome. Ultraschall Med. 2019, 40, 333–339.
- Pacheco D, Brandão O, Montenegro N, Matias A. Ductus venosus agenesis and fetal malformations: What can we expect? – a systematic review of the literature. J Perinat Med. 2019, 47, 1–11.
- Turan S, Turan OM, Desai A, Harman CR, Baschat AA. First-trimester fetal cardiac examination using spatiotemporal image correlation, tomographic ultrasound and color Doppler imaging for the diagnosis of complex congenital heart disease in high-risk patients. Ultrasound Obstet Gynecol. 2014, 44, 562–567.
- Rizzo G, Capponi A, Cavicchioni O, Vendola M, Pietrolucci ME, Arduini D. Application of automated sonography on 4-dimensional volumes of fetuses with transposition of the great arteries. J Ultrasound Med. 2008, 27, 771–776.
- Shih JC, Shyu MK, Su YN, Chiang YC, Lin CH, Lee CN. 'Big-eyed frog' sign on spatiotemporal image correlation (STIC) in the antenatal diagnosis of transposition of the great arteries. Ultrasound Obstet Gynecol. 2008, 32, 762–768.
- Araujo Júnior E, Tonni G, Bravo-Valenzuela NJ, Da Silva Costa F, Meagher S. Assessment of Fetal Congenital Heart Diseases by 4-Dimensional Ultrasound Using Spatiotemporal Image Correlation: Pictorial Review. Ultrasound Q. 2018, 34, 11–17.
- Gottschalk I, Walter A, Menzel T, Weber EC, Wendt S, Sreeram N; et al. D-Transposition of the great arteries with restrictive foramen ovale in the fetus: The dilemma of predicting the need for postnatal urgent balloon atrial septostomy. Arch Gynecol Obstet. 2024, 309, 1353–1367.
- Pontes ALS, Chagas CC, Bravo-Valenzuela NJ, Peixoto AB, Mappa I, Rizzo G; et al. Fetal heart foramen ovale area by three-dimensional ultrasound using stic in the rendering mode: Reference range and applicability in congenital heart diseases. Int J Cardiovasc Imaging. 2023, 39, 531–539.
- Freud LR, Wilkins-Haug LE, Beroukhim RS, LaFranchi T, Phoon CK, Glickstein JS; et al. Effect of In Utero Non-Steroidal Anti-Inflammatory Drug Therapy for Severe Ebstein Anomaly or Tricuspid Valve Dysplasia (NSAID Therapy for Fetal Ebstein anomaly). Am J Cardiol. 2021, 141, 106–112.
- Hirano Y, Masuyama H, Hayata K, Eto E, Nobumoto E, Hiramatsu Y. Prenatal Diagnosis of Interrupted Aortic Arch: Usefulness of Three-Vessel and Four-Chamber Views. Acta Med Okayama. 2016, 70, 485–491.
- Hata T, Koyanagi A, Yamanishi T, Bouno S, Takayoshi R, Miyagi Y; et al. Success rate of five fetal cardiac views using HDlive Flow with spatiotemporal image correlation at 18-21 and 28-31 weeks of gestation. J Perinat Med. 2020 Mar 3:/j/jpme.ahead-of-print/jpm-2019-0434/jpm-2019-0434.xml. [CrossRef]
- Pappalardo E, Gulino FA, Ettore C, Cannone F, Ettore G. Body Stalk Anomaly Complicated by Ectopia Cordis: First-Trimester Diagnosis of Two Cases Using 2- and 3-Dimensional Sonography. J Clin Med. 2023, 12, 1896.
- Zhang S, Wang N, Qu P, Shu X, Mi Y, Gao X. Prenatal echocardiography diagnosis of a novel combination of bilateral ductus arteriosus and cardiovascular anomalies: A case report and literature review. Front Cardiovasc Med. 2024, 11, 1389759.
- Pluym ID, Sklansky M, Wu JY, Afshar Y, Holliman K, Devore GR; et al. Fetal cardiac rhabdomyomas treated with maternal sirolimus. Prenat Diagn. 2020, 40, 358–364.
- Maász A, Bodó T, Till Á, Molnár G, Masszi G, Labossa G; et al. Three-Year Follow-Up after Intrauterine mTOR Inhibitor Administration for Fetus with TSC-Associated Rhabdomyoma. Int J Mol Sci. 2023, 24, 12886.
- Qaderi S, Javinani A, Blumenfeld YJ, Krispin E, Papanna R, Chervenak FA; et al. Mammalian target of rapamycin inhibitors: A new-possible approach for in-utero medication therapy. Prenat Diagn. 2024, 44, 88–98.
- Kamil D, Geipel A, Schmitz C, Breuer J, Herberg U, Knöpfle G; et al. Fetal pericardial teratoma causing cardiac insufficiency: Prenatal diagnosis and therapy. Ultrasound Obstet Gynecol. 2006, 28, 972–973.
- Messing B, Cohen SM, Valsky DV, Rosenak D, Hochner-Celnikier D, Savchev S; et al. Fetal cardiac ventricle volumetry in the second half of gestation assessed by 4D ultrasound using STIC combined with inversion mode. Ultrasound Obstet Gynecol. 2007, 30, 142–151.
- Simioni C, Nardozza LM, Araujo Júnior E, Rolo LC, Zamith M, Caetano AC; et al. Heart stroke volume, cardiac output, and ejection fraction in 265 normal fetus in the second half of gestation assessed by 4D ultrasound using spatio-temporal image correlation. J Matern Fetal Neonatal Med. 2011, 24, 1159–1167.
- Tedesco GD, de Souza Bezerra M, Barros FS, Martins WP, Nardozza LM, Carrilho MC; et al. Reference Ranges of Fetal Cardiac Biometric Parameters Using Three-Dimensional Ultrasound with Spatiotemporal Image Correlation M Mode and Their Applicability in Congenital Heart Diseases. Pediatr Cardiol. 2017, 38, 271–279.
- Bravo-Valenzuela NJ, Peixoto AB, Mattar R, Melo Júnior JF, da Silva Pares DB, Araujo Júnior E. Fetal Cardiac Function and Ventricular Volumes Determined by Three-Dimensional Ultrasound Using STIC and VOCAL Methods in Fetuses from Pre-gestational Diabetic Women. Pediatr Cardiol. 2020, 41, 1125–1134.
- Tanis JC, Mohammed N, Bennasar M, Martinez JM, Bijnens B, Crispi F; et al. Online versus offline spatiotemporal image correlation (STIC) M-mode for the evaluation of cardiac longitudinal annular displacement in fetal growth restriction. J Matern Fetal Neonatal Med. 2018 Jul;31(14):1845-1850.
- Yeo L, Romero R. Fetal Intelligent Navigation Echocardiography (FINE): A Novel Method for Rapid, Simple, and Automatic Examination of the Fetal Heart. Ultrasound Obstet Gynecol. 2013, 42, 268–284.
- Carrillo MC, Rolo LC, Tonni G, Araujo Júnior E. Evaluation of the quality of standard fetal heart views using the FAST, STAR and FINE four-dimensional ultrasound techniques in congenital heart disease screening. Echocardiography. 2020, 37, 114–123.
- Yeo L, Romero R. Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart. Ultrasound Obstet Gynecol. 2017, 50, 476–491.
- Yeo L, Romero R. Intelligent navigation to improve obstetrical sonography. Ultrasound Obstet Gynecol. 2016, 47, 403–409.
- Rizzo G, Pietrolucci ME, Capponi A, Mappa I. Exploring the role of artificial intelligence in the study of the foetal heart. Int J Cardiovasc Imaging. 2022, 38, 1017–1019.
- Wu H, Wu B, Lai F, Liu P, Lyu G, He S; et al. Application of Artificial Intelligence in Recognising the Anatomical Structure of Fetal Heart Standard Section. Comput Math Methods Med. 2023, 2023, 5650378.
- Pietrolucci ME, Maqina P, Mappa I, Marra MC, D'Antonio F, Rizzo G. Evaluation of an artificial intelligent algorithm (Heart Assist™) to automatically assess the quality of second trimester cardiac views: A prospective study. J Perinat Med. 2023, 51, 920–924.
- Rodenbarger A, Thorsson T, Stiver C, Jantzen D, Chevenon M, Yu S; et al. Third trimester predictors of interventional timing and accuracy of fetal anticipatory guidance in tetralogy of Fallot: A multi-center study. Prenat Diagn. 2020, 40, 870–877.
- Ungureanu A, Marcu AS, Patru CL, Ruican D, Nagy R, Stoean R; et al. Learning deep architectures for the interpretation of first-trimester fetal echocardiography (LIFE) - a study protocol for developing an automated intelligent decision support system for early fetal echocardiography. BMC Pregnancy Childbirth. 2023, 23, 20.
- Nieblas CO, Bravo-Valenzuela NJ, Araujo Júnior E, Werner H. Fetal transposition of the great arteries: 3D virtual and physical models from ultrasound datasets. Int J Cardiovasc Imaging. 2024, 40, 1157–1158.
- Pires GDV, Nieblas CO, Bravo-Valenzuela NJ, Araujo Júnior E, Castro PT, Werner H. Ebstein anomaly: 3D virtual and physical models from obstetrical ultrasound data. Echocardiography. 2024, 41, e15806.
- Acar P, Hadeed K, Dulac Y. Advances in 3D echocardiography: From foetus to printing. Arch Cardiovasc Dis. 2016, 109, 84–86.
- Chen SA, Ong CS, Hibino N, Baschat AA, Garcia JR, Miller JL. 3D printing of fetal heart using 3D ultrasound imaging data. Ultrasound Obstet Gynecol. 2018, 52, 808–809.
- Aly DM, Shah S. Three-Dimensional Echocardiography Derived Printing: A Review of Workflow, Current, and Future Applications. Curr Cardiol Rep. 2023, 25, 597–605.
- Werner H, Ribeiro G, Arcoverde V, Lopes J, Velho L. The use of metaverse in fetal medicine and gynecology. Eur J Radiol. 2022, 150, 110241.
- Lloyd DFA, Pushparajah K, Simpson JM, van Amerom JFP, van Poppel MPM, Schulz A; et al. Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: A prospective, single-centre cohort study. Lancet. 2019, 393, 1619–1627.
- Lloyd DFA, van Poppel MPM, Pushparajah K, Vigneswaran TV, Zidere V, Steinweg J; et al. Analysis of 3-Dimensional Arch Anatomy, Vascular Flow, and Postnatal Outcome in Cases of Suspected Coarctation of the Aorta Using Fetal Cardiac Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2021, 14, e012411.
- Zhang X, Zhu M, Dong SZ. Utility of fetal cardiovascular magnetic resonance imaging in assessing the fetuses with complete vascular ring. Front Pediatr. 2023, 11, 1159130.
- Ulm B, Muin D, Scharrer A, Prayer D, Dovjak G, Kasprian G. Prenatal ultrasound and magnetic resonance evaluation and fetal outcome in high-risk fetal tumors: A retrospective single-center cohort study over 20 years. Acta Obstet Gynecol Scand. 2020, 99, 1534–1545.
- Prayer, D. Fetal MRI. Top Magn Reson Imaging. 2011, 22, 89. [Google Scholar] [CrossRef]
- Manganaro L, Savelli S, Di Maurizio M, Francioso A, Fierro F, Tomei A; et al. Fetal MRI of the cardiovascular system: Role of steady-state free precession sequences for the evaluation of normal and pathological appearances. Radiol Med. 2009, 114, 852–870.
- Salehi D, Fricke K, Bhat M, Arheden H, Liuba P, Hedström E. Utility of fetal cardiovascular magnetic resonance for prenatal diagnosis of complex congenital heart defects. JAMA Netw Open. 2021, 4, e213538.
- Liu K, Zhu M, Zhang YQ, Chen LJ, Dong SZ. Utility of fetal cardiac magnetic resonance imaging in assessing the cardiac axis in fetuses with congenital heart disease. Pediatr Radiol. 2023, 53, 910–919.
- Goncalves LF, Lindblade CL, Cornejo P, Patel MC, McLaughlin ES, Bardo DME. Contribution of fetal magnetic resonance imaging in fetuses with congenital heart disease. Pediatr Radiol. 2022, 52, 513–526.
- Rubert NC, Jategaonkar G, Plasencia JD, Lindblade CL, Bardo DME, Goncalves LF. Four-dimensional fetal cardiac imaging in a cohort of fetuses with suspected congenital heart disease. Pediatr Radiol. 2023, 53, 198–209.

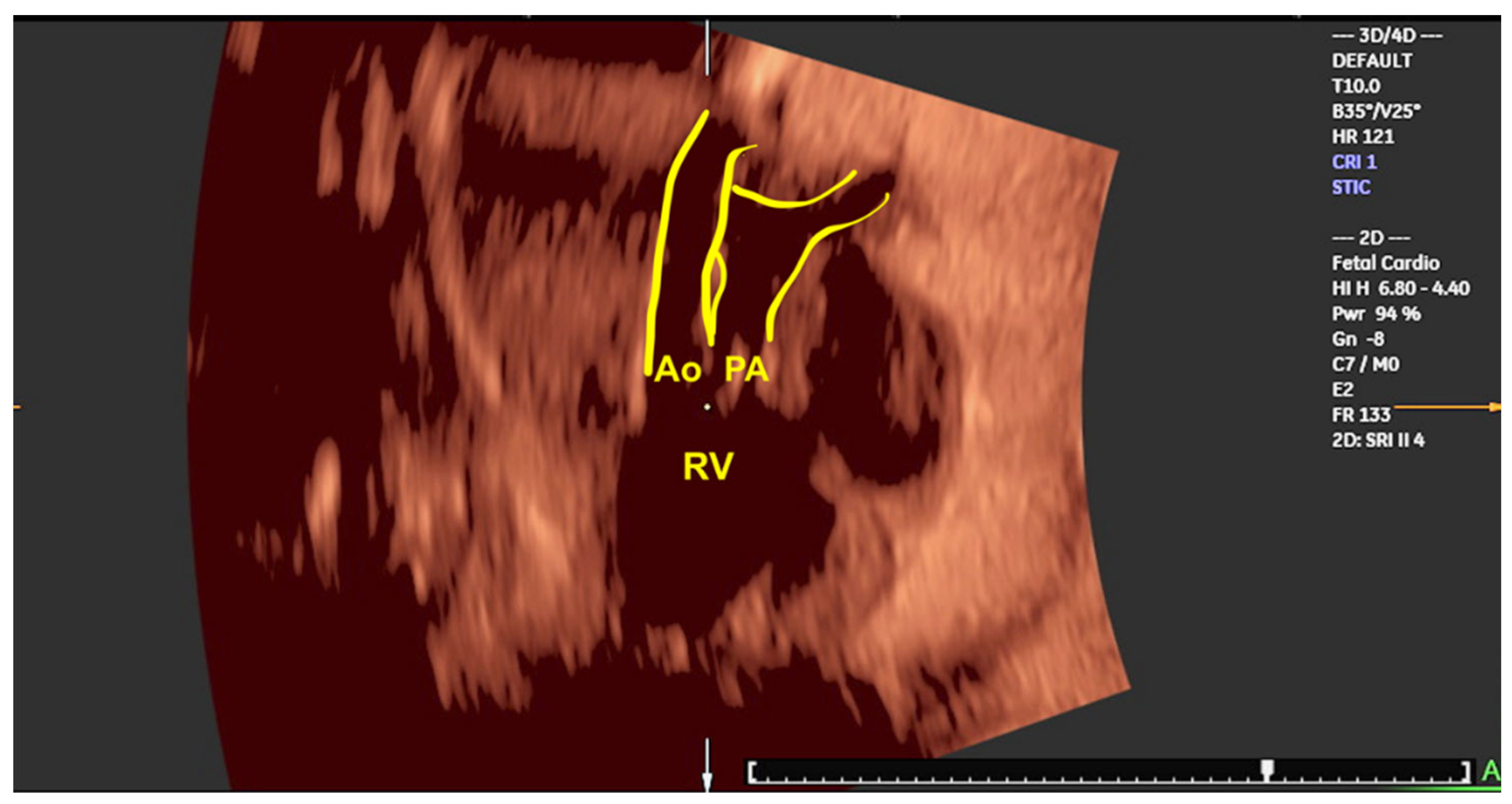
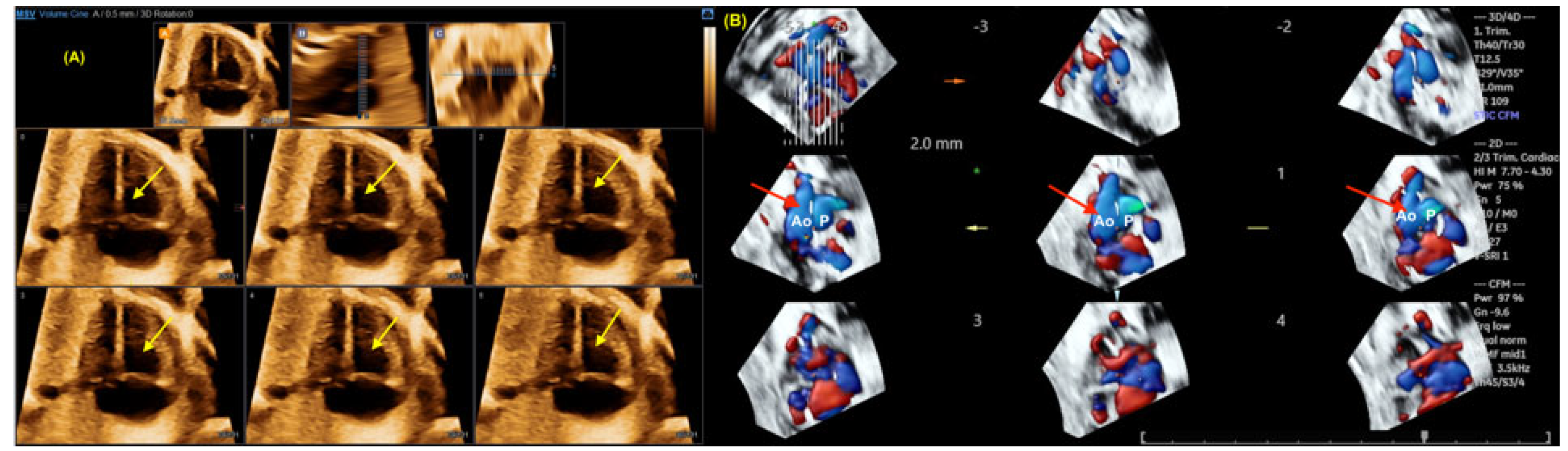
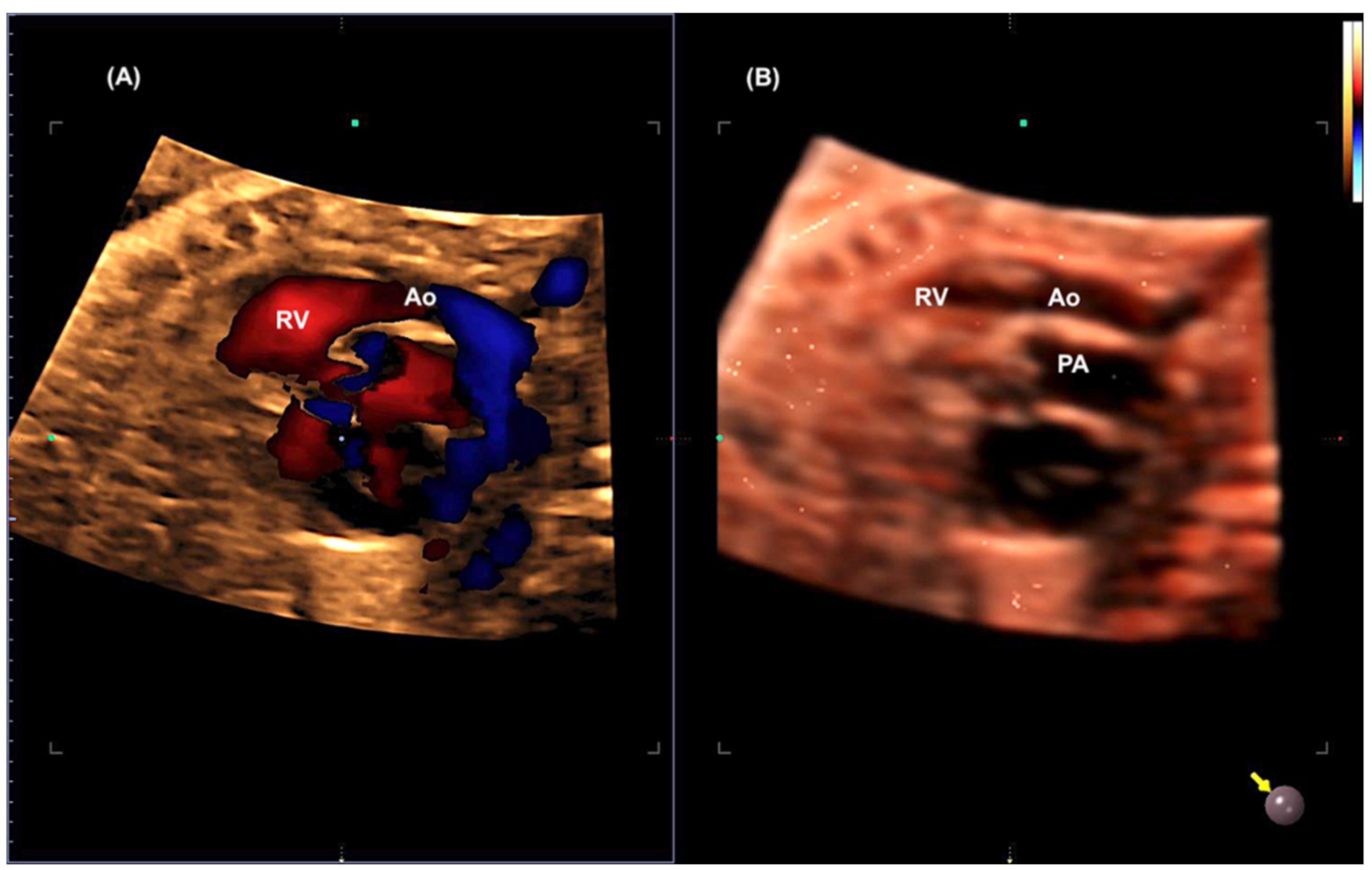

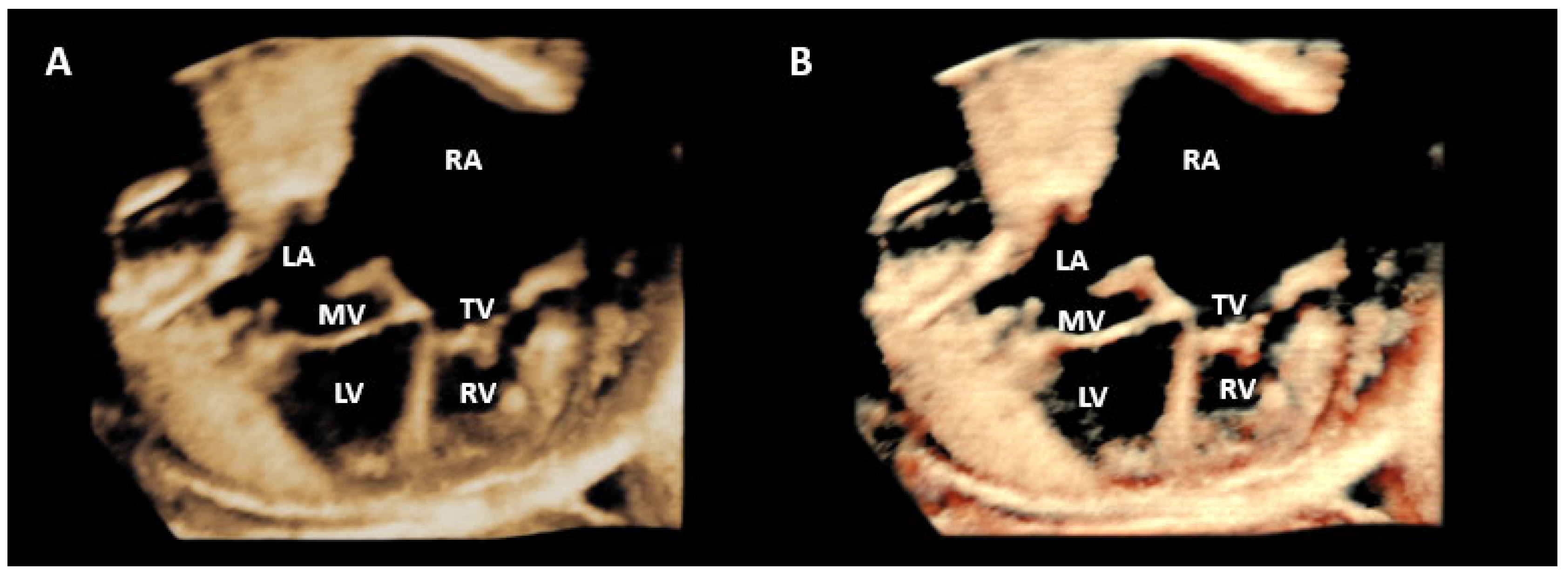
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




