Submitted:
04 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
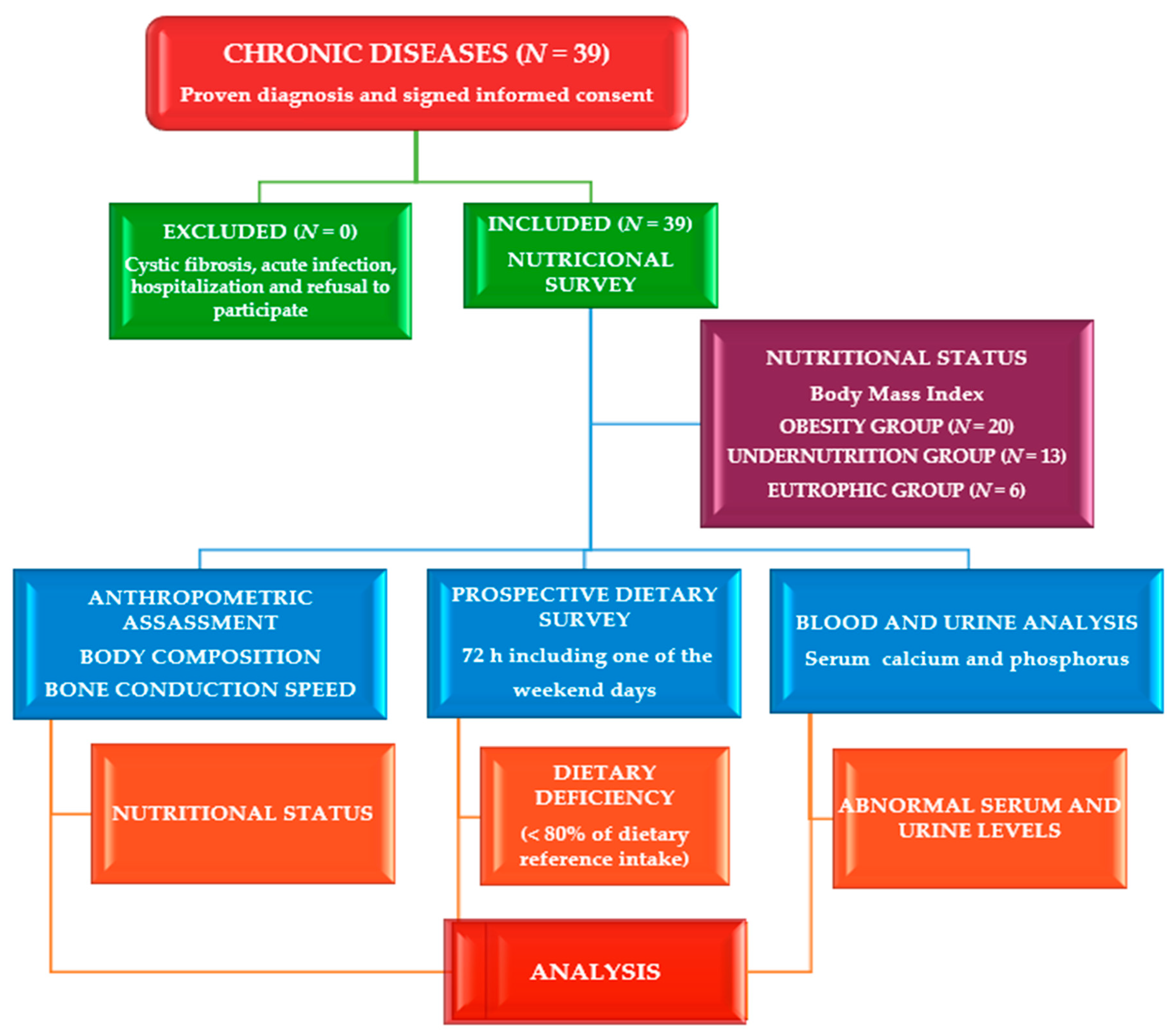
2.1. Study site, Design and Participants
2.2. Ethical Consideration
2.3. Clinical Evaluation
2.4. Assessment of Phenotypic Characteristics
2.5. Dietary Assessment
2.6. Laboratory Exploration
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Status
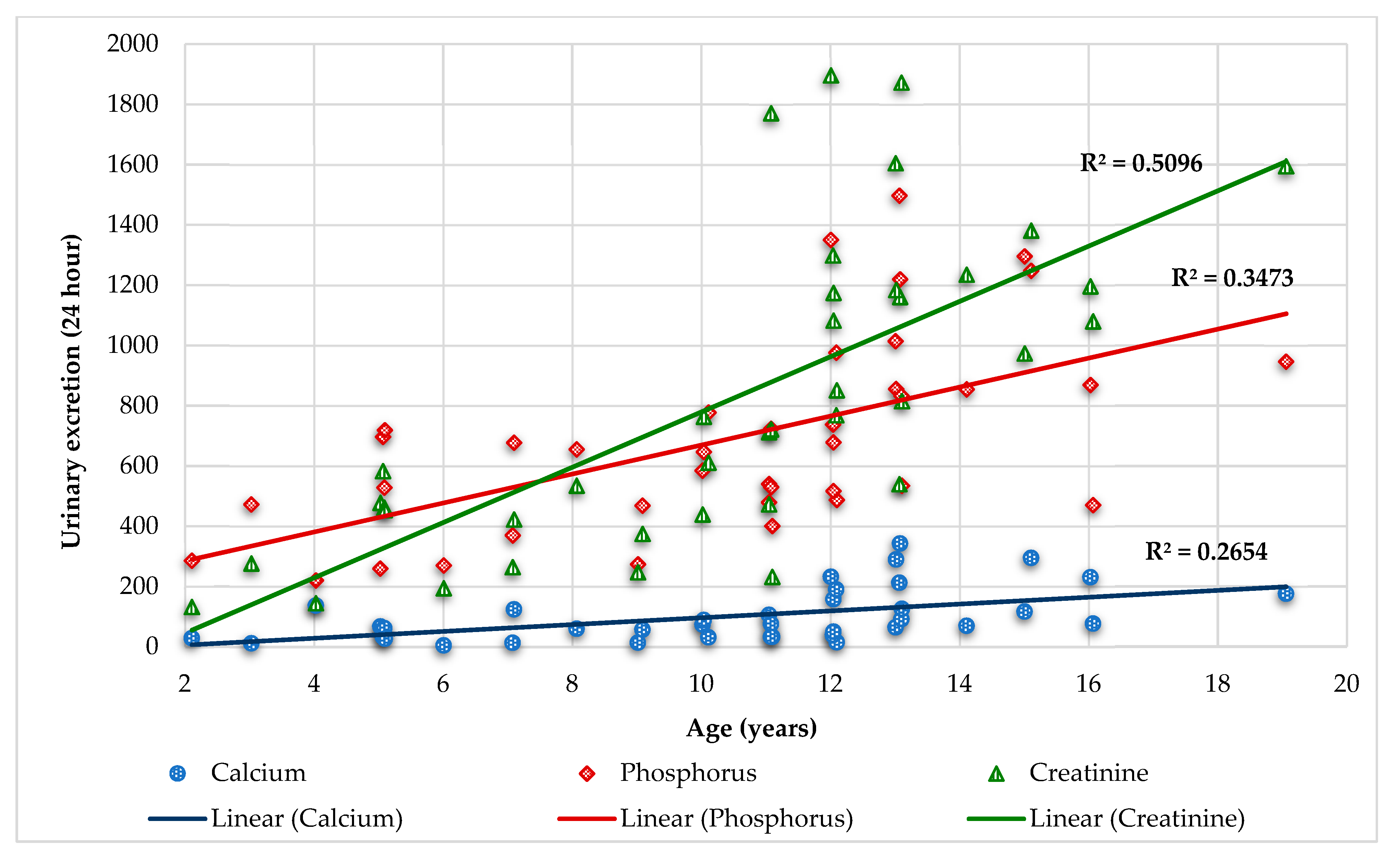
4.2. Calcium and Creatinine
4.3. Phosphorus
4.5. Calcium and Phosphorus relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| FFM | Fat free mass |
| FM | Fat mass |
| BIA | Bioelectrical impedance analysis |
| Ca | Calcium |
| P | Phosphorus or phosphate |
| Cr | Creatinine |
| Vit | Vitamin |
| PA | Physical Activity |
| %DRI | % Dietary Reference Intake |
| ALP | Alkaline phosphatase |
| TRP | Fractional tubular reabsorption of phosphate |
| TmP/GFR | Tubular maximum phosphate reabsorption per glomerular filtration rate |
| EE | Energy expenditure |
| WHO | World Health Organization |
| ANIVA | Anthropometry and Child Nutrition in Valencia |
| ENALIA | The National Survey of Nutrition in the Child and Adolescent Population |
References
- Escobedo-Monge, M.F.; Marcos-Temprano, M.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-Vicente, C.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 1900. [CrossRef]
- Civitelli, R.; Ziambaras, K. Calcium and phosphate homeostasis: Concerted interplay of new regulators. J. Endocrinol. Investig. 2011, 34 (Suppl. S7), 3–7.
- Hanna, R.M.; Ahdoot, R.S.; Kalantar-Zadeh, K.; Ghobry, L.; Kurtz, I. Calcium Transport in the Kidney and Disease Processes. Front Endocrinol (Lausanne). 2022, 12, 762130. [CrossRef]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005, 35(5), 290-304. [CrossRef]
- Kiela, P.R.; Radhakrishnan, V.M.; Ghishan, F.K. Phosphorus: Basic Nutritional Aspects. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 34; pp. 413–427.
- Erem, A.S.; Osuka, S.; Razzaque, M.S. Phosphate Burden and Inflammation. Adv Exp Med Biol. 2022, 1362, 7-13.
- Madeo, B.; Kara, E.; Cioni, K.; Vezzani, S.; Trenti, T.; Santi, D.; Simoni, M.; Rochira, V. Serum Calcium to Phosphorous (Ca/P) Ratio Is a Simple, Inexpensive, and Accurate Tool in the Diagnosis of Primary Hyperparathyroidism. JBMR Plus. 2017, 2, 2, 109-117. [CrossRef]
- Lee, J.J.; Plain, A.; Beggs, M.R.; Dimke, H.; Alexander, R.T. Effects of phospho- and calciotropic hormones on electrolyte transport in the proximal tubule. F1000Res. 2017, 6, 1797. [CrossRef]
- El Mallah, C.; Ghattas, H.; Shatila, D.; Francis, S.; Merhi, K.; Hlais, S.; Toufeili, I.; Obeid, O. Urinary Magnesium, Calcium, and Phosphorus to Creatinine Ratios of Healthy Elementary School Lebanese Children. Biol Trace Elem Res. 2016, 170(2), 264-270. [CrossRef]
- Emmens, J.E.; de Borst, M.H.; Boorsma, E.M.; Damman, K.; Navis, G.; van Veldhuisen, D.J.; Dickstein, K.; Anker, S.D.; Lang, C.C.; Filippatos, G.; Metra, M.; Samani, N.J.; Ponikowski, P.; Ng, L.L.; Voors, A.A.; Ter Maaten, J.M. Assessment of Proximal Tubular Function by Tubular Maximum Phosphate Reabsorption Capacity in Heart Failure. Clin J Am Soc Nephrol. 2022, 17(2), 228-239. [CrossRef]
- Lamberg-Allardt, C.; Kemi, V. Interaction Between Calcium and Phosphorus and the Relationship to Bone Health. In: Gutiérrez, O.; Kalantar-Zadeh, K.; Mehrotra, R. (eds) Clinical Aspects of Natural and Added Phosphorus in Foods. Nutrition and Health. Springer, New York, NY. 2017, 145-157.
- Shaker, J.L.; Deftos, L. Calcium and Phosphate Homeostasis. [Updated 2023 May 17]. In: Feingold, K.R.; Anawalt, B.; Blackman, M.R.; et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279023/.
- Dowhan Hoag, L.; Dharmarajan, T.S. Calcium and Phosphorus. In: Pitchumoni, C.S.; Dharmarajan, T. (eds) Geriatric Gastroenterology. Springer, Cham. 2021, 735-763.
- Escobedo-Monge, M.F.; Bahillo-Curieses, P.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-López, P.; Marugán-Miguelsanz, J.M. Calcium, Phosphate, and Vitamin D in Children and Adolescents with Chronic Diseases: A Cross-Sectional Study. Nutrients 2024, 16, 1349. [CrossRef]
- Escobedo-Monge, M.F.; Torres-Hinojal, M.C.; Barrado, E.; Escobedo-Monge, M.A.; Marugán-Miguelsanz, J.M. Zinc Nutritional Status in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 1121. [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Magnesium Status and Ca/Mg Ratios in a Series of Children and Adolescents with Chronic Diseases. Nutrients 2022, 14, 2941. [CrossRef]
- World Health Organization. Physical activity surveillance. Noncommunicable Disease Surveillance, Monitoring and Reporting. www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance. (Accessed May 11, 2023).
- Frisancho, A.R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [CrossRef]
- Hernández, M.; Sobradillo, B.; Aguirre, A.; Aresti, U.; Bilbao, A.; Fernández-Ramos, C.; Lizárraga, A.; Lorenzo, H.; Madariaga, L.; Rica, I. Curvas y Tablas de Crecimiento (Estudios Longitudinal y Transversal); Fundación Faustino Orbegozo: Bilbao, Spain, 1985.
- Waterlow, J.C. Classification and definition of protein-calorie malnutrition. Br. Med. J. 1972, 3, 566–569. [CrossRef]
- Moraes, A.B.V.; Veiga, G.V.; Azeredo, V.B.; Sichieri, R.; Pereira, R.A. High dietary calcium intake and low adiposity: Findings from a longitudinal study in Brazilian adolescents. Cad. Saude Publica. 2022, 38, e00144521. [CrossRef]
- Martínez, M.J.; Redondo, D.; Conde, F.; Redondo, P.; Franch, M.A. Gráficas Longitudinales de Velocidad de Conducción Media de Ultrasonidos en Falanges. In Estudio Nutricional de Castilla y León; de CyL, J., Ed.; Junta Castilla y León: Valladolid, Spain, 2009.
- Alonso Franch M, Redondo Del Río MP, Suárez Cortina L, Jan, En Nombre del Comité de Nutrición de la Asociación Española de Pediatría. Nutrición infantil y salud ósea [Nutrition and bone health in children]. An Pediatr (Barc). 2010 y 72, 1, 80.e1-11.
- Mataix Verdú, J.; García Diaz, J. NUTRIBER. V. 1.0.; Fundación Universitaria Iberoamericana: Barcelona, Spain, 2005.
- Cuervo, M.; Corbalán, M.; Baladía, E.; Cabrerizo, L.; Formiguera, X.; Iglesias, C.; Lorenzo, H.; Polanco, I.; Quiles, J.; De Avila, M.D.R.; et al. Comparison of dietary reference intakes (DRI) between different countries of the European Union, The United States and the World Health Organization. Nutr. Hosp. 2009, 24, 384–414.
- Costello, R.B.; Rosanoff, A.; Dai, Q., Saldanha, L.G.; Potischman N.A. Perspective: Characterization of Dietary Supplements Containing Calcium and Magnesium and Their Respective Ratio-Is a Rising Ratio a Cause for Concern? Adv. Nutr. 2021 y 10., 12:291–297.
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come12345. Adv. Nutr. 2016, 7, 977–993. [CrossRef]
- Li, Q.; Chen, Q.; Zhang, H.; Xu, Z.; Wang, X.; Pang, J.; Ma, J.; Ling, W.; Li, D. Associations of serum magnesium levels and calcium-magnesium ratios with mortality in patients with coronary artery disease. Diabetes Metab. 2020, 46, 384–391. [CrossRef]
- Mathew, A.A.; Panonnummal, R. ‘Mg’-the master cation-as a drug-possibilities and evidences. Biometals 2021, 34, 955–986.
- Rosanoff, A.; Wolf, F.I. A guided tour of presentations at the xiv international magnesium symposium. Magnes. Res. 2016, 29, 55–59.
- Kaslow, J.E. Copper/Zinc Imbalance. Medical Board of California. Available online: http://www.mbc.ca.gov (accessed on 15 August 2021). Nutrients 2022, 14, 2941 26 of 31.
- Eck, P.; Wilson, L. Toxic Metals in Human Health and Disease Eck; Institute of Applied Nutrition and Bioenergetics, Ltd.: Phoenix, AZ, USA, 1989.
- Shroff, R., Wan, M., Nagler, E. V., Bakkaloglu, S., Fischer, D. C., Bishop, N., Cozzolino, M., Bacchetta, J., Edefonti, A., Stefanidis, C. J., Vande Walle, J., Haffner, D., Klaus, G., Schmitt, C. P., & European Society for Paediatric Nephrology Chronic Kidney Disease Mineral and Bone Disorders and Dialysis Working GroupsClinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2-5 and on dialysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc- Eur Ren Assoc. 1 de julio de 2017;32(7):1098-113.
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846.
- Goltzman, D. Approach to Hypercalcemia. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W.; Dhatariya, K.; Dungan, K.; Hershman, J.M.; Hofland, J.; Kalra, S.; et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019.
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic & Laboratory Test Reference, 14th ed.; Elsevier: St. Louis, MO, USA, 2019.
- Stokes, V.J.; Nielsen, M.F.; Hannan, F.M.; Thakker, R.V. Hypercalcemic Disorders in Children. J. Bone Miner. Res. 2017, 32, 2157–2170. [CrossRef]
- Díaz Romero, C.; Henríquez Sánchec, P.; López Blanco, F.; Rodríguez Rodríguez, E.; Serra Majem, L. Concentrations of Na, K, Ca, and P in serum from a representative sample of the Canary Islands population]. Nutr Hosp. 2002, 17, 4, 204-12.
- Madeo, B.; De Vincentis, S.; Repaci, A.; Altieri, P.; Vicennati, V.; Kara, E.; Vescini, F.; Amadori, P.; Balestrieri, A.; Pagotto, U.; Simoni, M.; Rochira, V. The calcium-to-phosphorous (Ca/P) ratio in the diagnosis of primary hyperparathyroidism and hypoparathyroidism: a multicentric study. Endocrine. 2020, 68, 3, 679-687. [CrossRef]
- Klaus, G.; Watson, A.; Edefonti, A.; Fischbach, M.; Rönnholm, K.; Schaefer, F.; Simkova, E.; Stefanidis, C.J.; Strazdins, V.; Vande Walle, J.; Schröder, C.; Zurowska, A.; Ekim, M.; European Pediatric Dialysis Working Group (EPDWG). Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol. 2006, 21(2), 151-159. [CrossRef]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998, 31(4), 607-617. [CrossRef]
- Adeli, K.; Higgins, V.; Trajcevski, K.; White-Al Habeeb, N. The Canadian laboratory initiative on pediatric reference intervals: a CALIPER white paper. Crit Rev Clin Lab Sci 2017, 54, 358–413. [CrossRef]
- García Nieto, V.M.; Luis Yanes, M.I., Tejera Carreño, P., Perez Suarez, G., Moraleda Mesa, T. The idiopathic hypercalciuria reviewed. Metabolic abnormality or disease? La hipercalciuria idiopática revisada. ¿Anomalía metabólica o enfermedad? Nefrologia (Engl Ed). 2019, 39, 6, 592-602.
- Leslie, S.W.; Taneja, A. Treasure Island (FL): Stat Pearls Publishing; Hypercalciuria. Stat Pearls [Internet] 2017 Dec 14 [updated 2018 Dec 6]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448183/ (Accessed February 10, 2023).
- Marwaha, R.K.; Garg, M.K.; Dang, N.; Mithal, A.; Narang, A.; Chadha, A.; Gupta, N.; Kumar, M.R. Reference range of random urinary calcium creatinine ratio in North Indian children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 34–40. [CrossRef]
- Nephrology Calculator and Formulas. https://www.senefro.org/modules.php?name=webstructure&idwebstructure=26. Access on 27 July 2023.
- Seigle, R.L.; Nash, M.A. Chapter 13 – Nephrology. Editor(s): Polin, R.A.; Ditmar, M.F. In: Pediatric Secrets (Fifth Edition), Mosby, 2011, 480-523.
- Landry, D.W.; Gharavi, A.G. Approach to the patient with renal disease. In: Goldman, L.; Cooney, K.A.; eds. Goldman-Cecil Medicine. 27th ed. Philadelphia, PA: Elsevier, 2024, 100.
- Oh M.S.; Briefel, G.; Pincus, M.R. Evaluation of renal function, water, electrolytes, and acid-base balance. In: McPherson, R.A.; Pincus, M.R, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Philadelphia, PA: Elsevier, 2022, chap 15.
- Prasad, N.; Bhadauria, D. Renal phosphate handling: Physiology. Indian J Endocrinol Metab. 2013, 17(4), 620-627. [CrossRef]
- Gil Gómez, R.; Milano Manso, G. Electrolitos urinarios. An. Pediatría Contin. 2014, 12, 133–136. [CrossRef]
- Bellasi, A.; Di Micco, L.; Russo, D.; De Simone, E.; Di Iorio, M.; Vigilante, R.; Di Lullo, L.; Di Iorio, B.R. Fractional Excretion of Phosphate (FeP) Is Associated with End-Stage Renal Disease Patients with CKD 3b and 5. J Clin Med. 2019, 8(7), 1026.
- Alon, U.; Hellerstein, S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994, 8, 250–251. [CrossRef]
- Payne, R.B. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998, 35, 201–206. [CrossRef]
- Brodehl, J. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994, 8, 645. [CrossRef]
- KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009, 53(3 Suppl 2), S11-S104.
- Mansukoski, L.; Qamar, H.; Perumal, N.; Aimone, A.; Bassani, D.G.; Roth, D.E. Growth delay: an alternative measure of population health based on child height distributions. Ann Hum Biol. 2022, 49(2), 100-108. [CrossRef]
- Ethun, Kelly. Chapter 9. Sex and gender differences in body composition, lipid metabolism, and glucose regulation. In Sex differences in physiology. Editor(s): Neigh, G.N.; Mitzelfelt, M.M. Academic Press, 2016. 145-165. [CrossRef]
- Gao, T.; Leonard, M.B.; Zemel, B.; Kalkwarf, H.J., Foster, B.J. Interpretation of Body Mass Index in Children with CKD. Clin J Am Soc Nephrol 2012, 7, 558-564.
- Bonthuis, M.; Jager, K.J.; Abu-Hanna, A.; Verrina, E.; Schaefer, F.; van Stralen, K.J. Application of body mass index according to height-age in short and tall children. PLoS One. 2013, 8(8), e72068. [CrossRef]
- Stöcker, N.; Gaser, D.; Oberhoffer-Fritz, R.; Sitzberger, C. KidsTUMove—A Holistic Program for Children with Chronic Diseases, Increasing Physical Activity and Mental Health. J. Clin. Med. 2024, 13, 3791.
- West, S.L.; Banks, L.; Schneiderman, JE.; Caterini, J.E.; Stephens, S.; White, G.; Dogra, S.; Wells, G.D. Physical activity for children with chronic disease; a narrative review and practical applications. BMC Pediatr. 2019, 19(1), 12. [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; et al.World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. . Br J Sports Med. 2020, 54(24), 1451-1462.
- Thornton, J.S.; Frémont, P.; Khan, K.; Poirier, P.; Fowles, J.; Wells, G.D.; Frankovich, R.J. Physical activity prescription: a critical opportunity to address a modifiable risk factor for the prevention and management of chronic disease: a position statement by the Canadian academy of sport and exercise medicine. Br J Sports Med. 2016, 50(18), 1109–1114. [CrossRef]
- Butte, N.F. 1.3.2 Energy Requirements of Infants, Children, and Adolescents. World Rev Nutr Diet. 2022, 124, 47-54.
- Afroze, F.; Khoshnevisan, F.; Harawa, P.P.; Islam, Z.; Bourdon, C.; Khoswe, S.; Islam, M.; Sarker, S.A.; Islam, F.; Sayeem Bin Shahid, A.S.M.; Joosten, K.; Hulst, J.M.; Eneya, C; Walson, J.L.; Berkley, J.A.; Potani, I.; Voskuijl, W.; Ahmed, T.; Chisti, M.J.; Bandsma, R.H.J. Trajectories of resting energy expenditure and performance of predictive equations in children hospitalized with an acute illness and malnutrition: a longitudinal study. Sci Rep. 2024, 14(1), 3613. [CrossRef]
- Luo, B.; Davidson, Z.E.; O'Brien, K.; Volders, E.; Lu, J.; Dunlea, K.; Lazzari, M.: Billich, N.; Nguo, K. Describing Energy Expenditure in Children with a Chronic Disease: A Systematic Review. Adv Nutr. 2024, 15(4), 100198. [CrossRef]
- García-Ascaso, M.T.; Ares-Segura, S.; Ros-Pérez, P. Is iodine nutrition in the Spanish pediatric population adequate? Historical review and current situation. Endocrinol Diabetes Nutr (Engl Ed). 2018, 65(8), 458-467. [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites 2023, 13, 82. [CrossRef]
- Díaz-López, A.; Paz-Graniel, I.; Alonso-Sanz, R.; Marqués Baldero, C.; Mateos Gil, C.; Arija Val, V. Vitamin D deficiency in primary health care users at risk in Spain. Nutr. Hosp. 2021, 38, 1058–1067. [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; Kostrova, G.; Lebedev, A. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front Endocrinol (Lausanne). 2019, 10, 103. [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [CrossRef]
- Joshi, M.; Uday, S. Vitamin D Deficiency in Chronic Childhood Disorders: Importance of Screening and Prevention. Nutrients 2023, 15, 2805. [CrossRef]
- Bacchetta, J.; Edouard, T.; Laverny, G.; Bernardor, J.; Bertholet-Thomas, A.; Castanet, M.; Garnier, C.; Gennero, I.; Harambat, J.; Lapillonne, A.; Molin, A.; Naud, C.; Salles, J.P.; Laborie, S.; Tounian, P.; Linglart, A. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch Pediatr. 2022, 29(4), 312-325. [CrossRef]
- Edouard, T.; Guillaume-Czitrom, S., Bacchetta, J.; Sermet-Gaudelus, I.; Dugelay, E.; Martinez-Vinson, C.; Salles, J.P.; Linglart, A. Guidelines for the management of children at risk of secondary bone fragility: Expert opinion of a French working group. Arch Pediatr. 2020, 27(7), 393-398. [CrossRef]
- Rana, Z.H.; Bourassa, M.W.; Gomes, F.; Khadilkar, A.; Mandlik, R.; Owino, V.; Pettifor, J.M.; Roth, D.E.; Shlisky, J.; Thankachan, P.; Weaver, C.M. Calcium status assessment at the population level: Candidate approaches and challenges. Ann N Y Acad Sci. 2022, 1517(1), 93-106. [CrossRef]
- Rubio-López, N.; Llopis-González, A.; Picó, Y.; Morales-Suárez-Varela, M. Dietary Calcium Intake and Adherence to the Mediterranean Diet in Spanish Children: The ANIVA Study. Int. J. Environ. Res. Public Health 2017, 14, 637. [CrossRef]
- Tytusa, A.; Wyszyńska, J.; Yatsula, M.; Nyankovskyy, S.; Mazur, A.; Dereń, K. Deficiency of Daily Calcium and Vitamin D in Primary School Children in Lviv, Ukraine. Int J Environ Res Public Health. 2022, 19(9), 5429. [CrossRef]
- Bae, Y.J.; Kratzsch, J. Vitamin D and calcium in the human breast milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 39–45. [CrossRef]
- Çullas İlarslan, N.E.; Şıklar, Z.; Berberoğlu, M. Childhood Sustained Hypercalcemia: A Diagnostic Challenge. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 315–322. [CrossRef]
- Tinawi ,M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus. 2021, 13(1), e12420. [CrossRef]
- Sorkhi, H.; Haji Aahmadi, M. Urinary calcium to creatinine ratio in children. Indian J Pediatr. 2005, 72(12), 1055-1056. [CrossRef]
- Penido, M.G.M.G.; Tavares, M.S. Should pediatric idiopathic hypercalciuria be treated with hypocalciuric agents? World J Nephrol. 2021, 10(4), 47-58.
- Gül, A.; Özer, S.; Yılmaz, R.; Sönmezgöz, E.; Kasap, T.; Takçı, Ş.; Karaaslan, E.; Önder, Y.; Çıtıl, R.; Bütün, İ.; Demir, O. Prevalence of hypercalciuria and urinary calcium excretion in school-aged children in the province of Tokat. Turk Pediatri Ars. 2016, 51(4), 193-197. [CrossRef]
- Penido, M.G M.G.; Tavares, M.S. Beyond kidney stones: Why pediatricians should worry about hypercalciuria. World J Clin Pediatr. 2021, 10(6), 137-150. [CrossRef]
- Cambareri, G.M.; Giel, D.W.; Bayne, A.P.; Corbett, S.; Schurtz, E.; Kovacevic, L.; Sukhu, T.; Yap, M.; Chiang, G. Overweight and Obese Pediatric Stone Formers Have Differences in Metabolic Abnormalities Compared With Normal-weight Stone Formers?. Urology. 2017, 101, 26-30. [CrossRef]
- Bandari, J.; Dangle, P.P.; Lyon, T.D.; Lee, A.; Schneck, F.X.; Cannon, G.M.; Stephany, H.A.; Ost, M.C. 24-Hour Urinary Parameters in Overweight and Obese Children with Urolithiasis. J Urol. 2016, 196(2), 526-530. [CrossRef]
- Al Ghali, R.; El-Mallah, C.; Obeid, O.; El-Saleh, O.; Smail, L.; Haroun, D. Urinary minerals excretion among primary schoolchildren in Dubai-United Arab Emirates. PLoS One. 2021, 16(8), e0255195. [CrossRef]
- Kaneko, K.; Tsuchiya, K.; Kawamura, R.; Ohtomo, Y.; Shimizu, T.; Yamashiro, Y.; Yamada, T.; Yamauchi, K.; Kitagawa, T. Low prevalence of hypercalciuria in Japanese children. Nephron. 2002, 91(3), 439-443. [CrossRef]
- Kaneko, K.; Chiba, M.; Hashizume, M., Kunii, O.; Sasaki, S.; Shimoda, T.; Yamashiro, Y.; Dauletbaev, D.; Caypil, W.; Mazhitova, Z. Extremely high prevalence of hypercalciuria in children living in the Aral Sea region. Acta Paediatrica. 2007, 91(10), 1116-20.
- Abdalbary, M.; Chishti, E.; Shakhashiro, M.; Mohamed, R.; Parikh, T.; Nassar, M.K.; Sayed-Ahmed, N.; Faugere, M.C.; Sawaya, B.P.; El-Husseini, A. Impact of urinary calcium excretion on kidney, bone, and cardiovascular systems in patients with bone biopsy proven osteoporosis: a longitudinal long-term follow-up study. Osteoporos Int. 2023, 34(4), 763-774. [CrossRef]
- Stam, S.P.; Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; de Meijer, V.E.; van Beek, A.P.; Gansevoort, R.T.: Bakker, S.J.L. Muscle mass determined from urinary creatinine excretion rate, and muscle performance in renal transplant recipients. J Cachexia Sarcopenia Muscle. 2019; (3), 621-629. [CrossRef]
- Stam, S.P.; Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; de Meijer, V.E.; van Beek, A.P.; Gansevoort, R.T.: Bakker, S.J.L. Muscle mass determined from urinary creatinine excretion rate, and muscle performance in renal transplant recipients. J Cachexia Sarcopenia Muscle. 2019, 10(3), 621-629. [CrossRef]
- Ubetagoyena Arrieta, M.; Areses Trapote, R.; Mendia Ubetagoyena, J.; Perez Revuelta, M.; García Albizua, I. Basal renal function in paediatric patients: correlation of methods that depend on a 24h urine collection with simpler methods that do not require a timed urine. An Pediatr. 2020, 92(2), 65-70. [CrossRef]
- Remer, T.; Neubert, A.; Maser-Gluth, C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002, 75(3), 561-569. [CrossRef]
- Ullal, J.; Kutney, K.; Williams, K. M.; Weber, D. R. Treatment of cystic fibrosis related bone disease. J Clin Transl Endocrinol. 2021, 27, 100291. [CrossRef]
- Gordon, R.J.; Misra, M.; Mitchell, D.M. Osteoporosis and Bone Fragility in Children. [Updated 2023 Jul 20]. In: Feingold, K.R.; Anawalt, B.; Blackman, M.R; et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; from, 2000.
- Williams, K.M.; Darukhanavala, A.; Hicks, R.; Kelly, A. An update on methods for as-sessing bone quality and health in Cystic fibrosis. J Clin Transl Endocrinol. 2021, 27, 100281.
- Ouyang, Y.; Quan, Y.; Guo, C.; Xie, S.; Liu, C.; Huang, X.; Huang, X.; Chen, Y.; Xiao, X.; Ma, N.; Xie, R. Saturation Effect of Body Mass Index on Bone Mineral Density in Adolescents of Different Ages: A Population-Based Study. Front Endocrinol (Lausanne). 2022, 13, 922903. [CrossRef]
- Acar, D.B.; Kavuncuoğlu, S.; Çetinkaya, M.; Petmezci, E.; Dursun, M.; Korkmaz, O.; Altuncu, E. K. Assessment of the place of tubular reabsorption of phosphorus in the diagnosis of osteopenia of prematurity. Turk Pediatri Ars. 2015, 50(1), 45-50. [CrossRef]
- Koljonen, L.; Enlund-Cerullo, M.; Hauta-Alus, H.; Holmlund-Suila, E.; Valkama, S.; Rosendahl, J.; Andersson, S.; Pekkinen, M.; Mäkitie, O. Phosphate Concentrations and Modifying Factors in Healthy Children From 12 to 24 Months of Age. J Clin Endocrinol Metab. 2021, 106(10), 2865-2875. [CrossRef]
- Arnold, A.; Dennison, E.; Kovacs, C.S.; Mannstadt, M.; Rizzoli, R.; Brandi, M.L.; Clarke, B.; Thakker, R.V. Hormonal regulation of biomineralization. Nat Rev Endocrinol. 2021; 17(5):261-275. [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46.
- Bacchetta, J.; Bernardor, J.; Garnier, C.; Naud, C.; Ranchin, B. Hyperphosphatemia and Chronic Kidney Disease: A Major Daily Concern Both in Adults and in Children. Calcif Tissue Int. 2021, 108(1), 116-127. [CrossRef]
- Sharma, S.; Hashmi, M.F.; Kaur, J.; Castro, D. Hypophosphatemia. [Updated 2024 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493172/.
- Uday, S.; Sakka, S.; Davies, J.H.; Randell, T.; Arya, V.; Brain, C.; Tighe, M.; Allgrove, J.; Arundel, P.; Pryce, R.; Högler, W.; Shaw, N.J. Elemental formula associated hypophosphataemic rickets. Clin Nutr. 2019, 38(5), 2246-2250. [CrossRef]
- Al Harbi, S.A.; Al-Dorzi, H.M.; Al Meshari, A.M.; Tamim, H.; Abdukahil, S.A.I.; Sadat, M.; Arabi, Y. Association between phosphate disturbances and mortality among critically ill patients with sepsis or septic shock. BMC Pharmacol Toxicol. 2021, 28, 22(1), 30. [CrossRef]
- Shah, S.K.; Irshad, M.; Gupta, N.; Kabra, S.K.; Lodha, R. Hypophosphatemia in Critically Ill Children: Risk Factors, Outcome and Mechanism. Indian J Pediatr. 2016, 83(12-13), 1379-1385. [CrossRef]
- Berger, M.M.; Appelberg, O.; Reintam-Blaser, A.; Ichai, C.; Joannes-Boyau, O.; Casaer, M.; Schaller, S.J.; Gunst, J.; Starkopf, J.; ESICM-MEN section. Prevalence of hy-pophosphatemia in the ICU - Results of an international one-day point prevalence survey. Clin Nutr. 2021, 40(5), 3615-3621.
- Sands D.; Mielus M.; Umławska W.; Lipowicz A.; Oralewska B.; Walkowiak J. Evaluation of factors related to bone disease in Polish children and adolescents with cystic fibrosis. Adv Med Sci. 2015, 60(2), 315–320. [CrossRef]
- Taranta-Janusz, K.; Łabieniec, Ł.; Porowski, T.; Szymański, K.; Porowska, H.; Wasilewska, A. Determining normal values of urinary phosphorus excretion in 3913 healthy children aged 2-18 to aid early diagnosis and treatment for urolithiasis. Acta Paediatr. 2017, 106(7), 1170-1175. [CrossRef]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflugers Arch. 2019, 471(1), 83-98. [CrossRef]
- Gil Gómez, R.; Milano Manso, G. Electrolitos urinarios. Anales de Pediatría Continuada. 2014, 12, 3, 133-136.
- Harrison, C.M, Johnson, K., McKechnie, E. Osteopenia of prematurity: a national survey and review of practice. Acta Pediatr. 2008, 97, 407–13. [CrossRef]
- Verploegen, M.F.A.; Vargas-Poussou, R.; Walsh, S.B.; Alpay, H.; Amouzegar, A.; Ariceta, G.; et.al. Parathyroid hormone and phosphate homeostasis in patients with Bartter and Gitelman syndrome: an international cross-sectional study. Nephrol Dial Transplant. 2022, 37(12), 2474-2486. [CrossRef]
- Mosca, M.; Bernardor, J.; Lemoine, S.; Bertholet-Thomas, A.; Bacchetta, J. Rare diseases of phosphate and calcium metabolism: Crossing glances between nephrology and endocrinology. Ann Endocrinol (Paris). 2021, 82(1), 30-35. [CrossRef]
- Loughrill, E.; Wray, D.; Christides, T.; Zand, N. Calcium to phosphorus ratio, essential elements and vitamin D content of infant foods in the UK: Possible implications for bone health. Matern. Child. Nutr. 2017, 13, e12368. [CrossRef]
- Sun, M.; Wu, X.; Yu, Y.; Wang, L.; Xie, D.; Zhang, Z.; Chen, L.; Lu, A.; Zhang, G.; Li, F. Disorders of Calcium and Phosphorus Metabolism and the Proteomics/Metabolomics-Based Research. Front Cell Dev Biol. 2020, 8, 576110.). [CrossRef]
- Cuadrado-Soto, E.; López-Sobaler, A.M.; Jiménez-Ortega, A.I.; Aparicio, A.; Bermejo, L.M., Hernández-Ruiz, Á.; et. al. Usual Dietary Intake, Nutritional Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D of Spanish Children Aged One to <10 Years. Findings from the EsNuPI Study. Nutrients. 2020, 12(6), 1787.
- Bestepe, N.; Cuhaci, F.N.; Polat, B.; Ogmen, B.E.; Ozdemir, D.; Ersoy, R.; Cakir, B. Serum Calcium/Phosphorus Ratio in Biochemical Screening of Primary Hyperparathyroidism. Rev. Assoc. Med. Bras. (1992) 2022, 68, 1668–1674. [CrossRef]
- De Vincentis, S.; Del Sindaco, G.; Pagnano, A.; Brigante, G.; Moretti, A.; Zirilli, L.; Rochira, V.; Simoni, M.; Mantovani, G.; Madeo, B. Application of calcium-to-phosphorus (Ca/P) ratio in the diagnosis of pseudohypoparathyroidism: Another piece in the puzzle of diagnosis of Ca-P metabolism disorders. Front. Endocrinol. 2023, 14, 1268704. [CrossRef]
- Lin, S.H.; Chu, P.; Cheng, C.J.; Chu, S.J.; Hung, Y.J.; Lin, Y.F. Early diagnosis of thyrotoxic periodic paralysis: spot urine calcium to phosphate ratio. Crit Care Med. 2006, 34(12), 2984-2989. [CrossRef]
- Grez-Capdeville, M.; Crenshaw, T.D. Evaluation of calcium to phosphorus ratio in spot urine samples as a practical method to monitor phosphorus intake adequacy in sows. J. Anim. Sci. 2021, 99, skab335. [CrossRef]
| Characteristics | Total (n = 39) |
Obesity (n = 20) |
Undernutrition (n = 13) |
Eutrophic (n = 6) |
p-Value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Dietary | |||||
| Calcium (%DRI) | 94 ± 28 | 93 ± 28 | 93 ± 27 | 100 ± 36 | 0.865 |
| Vitamin D (%DRI) | 75 ± 145 | 93 ± 194 | 61 ± 59 | 47 ± 74 | 0.734 |
| Serum | |||||
| Vitamin D (ng/mL) | 23.6 ± 15.9 | 18.2 ± 8.2 | 28.0 ± 21.6 | 32.3 ± 17.2 | 0.075 |
| Calcium (mg/dL) | 9.8 ± 0.3 | 9.9 ± 0.4 | 9.8 ± 0.2 | 9.9 ± 0.4 | 0.779 |
| Creatinine (mg/dL) | 0.53 ± 0.21 | 0.51 ± 0.16 | 0.47 ± 0.14 | 0.72 ± 0.39 | 0.059 |
| Phosphorus (mg/dL) | 4.7 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.5 | 4.8 ± 0.4 | 0.894 |
| Calcium/phosphorus ratio | 2.12 ± 0.27 | 2.12 ± 0.28 | 2.13 ± 0.29 | 2.07 ± 0.16 | 0.919 |
| Calcium - phosphorus product | 46.8 ± 6.5 | 47.1 ± 7.6 | 45.9 ± 5.3 | 48.1 ± 5.2 | 0.818 |
| Alkaline phosphatase (U/L) | 353 ± 235 | 329 ± 252 | 435 ± 243 | 254 ± 91 | 0.244 |
| Urine | |||||
| Urine calcium (mg/kg/d) | 2.5 ± 2.3 | 1.7 ± 1.1 | 3.5 ± 3.0 | 2.9 ± 3.2 | 0.078 |
| 24-urine calcium (mg/L) | 101.2 ± 86.5 | 108.7 ± 82.6 | 79.1 ± 51.1 | 123.6 ± 150.5 | 0.509 |
| Creatinine (mg/kg/d) | 18.6 ± 7.3 | 15.6 ± 5.4 | 23.5 ± 8.1 | 18.2 ± 6.2 | 0.007* |
| 24-urine creatinine (mg/d) | 819.8 ± 504.5 | 999.5 ± 545.9 | 624.4 ± 383.9 | 644.5 ± 427.3 | 0.070 |
| Calcium/creatinine rate | 0.15 ± 0.16 | 0.14 ± 0.09 | 0.18 ± 0.25 | 0.14 ± 0.11 | 0.723 |
| Urine calcium excretion | 0.08 ± 0.07 | 0.06 ± 0.06 | 0.08 ± 0.09 | 0.09 ± 0.07 | 0.597 |
| Urine phosphorus (mg/kg/d) | 17.6 ± 7.4 | 13.8 ± 4.9 | 21.6 ± 7.6 | 21.7 ± 7.8 | 0.002* |
| 24-urine phosphorus (mg/d) | 690.8 ± 320.2 | 794.1 ± 305.5 | 530.8 ± 297.6 | 693.3 ± 320.7 | 0.066 |
| Fractional excretion of phosphorus | 11.4 ± 7.3 | 10.5 ± 7.0 | 9.6 ± 2.7 | 18.1 ± 11.6 | 0.043* |
| Calcium/phosphorus ratio | 0.14 ± 0.11 | 0.13 ± 0.07 | 0.17 ± 0.16 | 0.14 ± 0.14 | 0.571 |
| Calcium x phosphorus product | 50.2 ± 57.5 | 26.1 ± 21.8 | 78.9 ± 64.5 | 68.5 ± 92.0 | 0.021* |
| Tubular Reabsorption of Phosphate | 88.6 ± 7.3 | 89.5 ± 7.0 | 90.4 ± 2.7 | 81.9 ± 11.6 | 0.043* |
| TmP/GFR (mg/dL) | 4.6 ± 0.9 | 4.8 ± 1.1 | 4.6 ± 0.8 | 4.0 ± 0.7 | 0.261 |
| Gender | Female (n = 15) | Male (n = 24) | p-value |
|---|---|---|---|
| Body Fat Percentage | 28.9 ± 12.2 | 20.4 ± 11.2 | 0.036 |
| Bone Conduction Speed (Absolute value) | 1961 ± 79 | 1894 ± 50 | 0.006 |
| Serum phosphorus (mg/dL) | 4.6 ± 0.6 | 4.9 ± 0.5 | 0.029 |
| Serum calcium/phosphorus ratio | 2.2 ± 0.3 | 2.0 ± 0.2 | 0.049 |
| Serum calcium-phosphorus product | 45.0 ± 6.5 | 49.5 ± 5.6 | 0.035 |
| Alkaline phosphatase (mg/dL) | 286 ± 167 | 461 ± 291 | 0.022 |
| Urine phosphorus (mg/kg/d) | 15.2 ± 5.6 | 21.5 ± 8.4 | 0.008 |
| Age group | Children (n = 17) | Adolescents (n = 22) | |
| Calcium intake (%DRI) | 115 ± 26 | 78 ± 19 | <0.001 |
| Serum phosphorus (mg/dL) | 5.0 ± 0.4 | 4.5 ± 0.6 | 0.003 |
| Serum calcium/phosphorus ratio | 1.9 ± 0.2 | 2.2 ± 0.3 | 0.003 |
| Serum calcium-phosphorus product | 49.7 ± 4.7 | 44.5 ± 6.8 | 0.011 |
| 24-hour urine calcium | 50.2 ± 38.5 | 140.5 ± 93.2 | <0.001 |
| Urine creatinine (mg/kg/d) | 15.5 ± 5.9 | 21.0 ± 7.5 | 0.018 |
| 24-hour urine creatinine | 388.2 ± 177.9 | 1153.4 ± 411.1 | <0.001 |
| 24-hour urine phosphorus | 487.9 ± 187.3 | 847.6 ± 315.9 | <0.001 |
| Calcium intake (%DRI) | Deficient (n = 18) | Normal (n = 21) | |
| Bone conduction speed AV | 1966 ± 68 | 1906 ± 72 | 0.013 |
| Kilocalories (%DRI) | 85 ± 19 | 104 ± 26 | 0.013 |
| Serum phosphorus (mg/dL) | 4.5 ± 0.5 | 4.9 ± 0.5 | 0.016 |
| Serum calcium/phosphorus ratio | 2.2 ± 0.3 | 2.0 ± 0.2 | 0.016 |
| Serum Ca-P product (mg2/dL2) | 44.3 ± 5.8 | 48.9 ± 6.4 | 0.029 |
| Urine creatinine (mg/kg/d) | 21.1 ± 7.0 | 16.5± 7.3 | 0.049 |
| 24-hour urine creatinine | 1091.9 ± 509.3 | 586.6 ± 373.2 | 0.001 |
| Serum phosphorus | Deficiency (n = 14) | Normal (n = 25) | |
| Bone conduction speed AV | 1974 ± 61 | 1909 ± 73 | 0.011 |
| Serum calcium/phosphorus ratio | 2.4 ± 0.2 | 1.9 ± 0.1 | <0.001 |
| Serum calcium-phosphorus product | 40.4 ± 4.1 | 50.6 ± 4.4 | <0.001 |
| 24-hour urine creatinine | 1078.6 ± 558.9 | 674.9 ± 415.7 | 0.014 |
| TmP/GFR | 3.9 ± 0.6 | 5.0 ± 0.9 | <0.001 |
| Fisher’s Exact Test | Odds Ratio | 95% Confidence Interval |
Cochran’s | Mantel–Haenszel | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Deficient calcium intake | ||||||
| Deficit kilocalories intake | 0.016 | 7.600 | 1.350 | 42.799 | 0.013 | 0.036 |
| Adolescents | <0.001 | 20.000 | 3.4807 | 114.9202 | <0.001 | <0.001 |
| High calcium/creatinine rate | ||||||
| High urine calcium (mg/kg/d) | 0.001 | 28.000 | 3.810 | 205.791 | <0.001 | <0.001 |
| High urine calcium (24-hour) | 0.018 | 11.200 | 1.600 | 78.400 | 0.006 | 0.028 |
| Hypercalciuria (mg/kg/d) | ||||||
| High urine calcium excretion | 0.006 | 26.000 | 2.287 | 295.637 | 0.001 | 0.008 |
| Hyperphosphaturia (mg/kg/d) | ||||||
| Hypercalciuria (mg/kg/d) | 0.026 | 9.692 | 1.060 | 88.653 | 0.022 | 0.060 |
| Low serum phosphorus | ||||||
| Adolescents | 0.029 | 1.786 | 1.062 | 3004 | 0.037 | 0.084 |
| Normal urine phosphorus (24-hour) | ||||||
| Very active/active | 0.035 | 1.429 | 0.986 | 2.069 | 0.023 | 0.074 |
| Age (years) |
Sex | BMI | Dietary | Serum | Urinary | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Vit-D | Ca | P | Vit-D | Ca/P ratio |
Ca-PP | Cr | ALP | Calcium | Creatinine | Ca/Cr rate |
UCaE | Phosphorus | FEP | TmP/ GFR |
Ca/P ratio |
Ca-PP | ||||||
| mg/kg/d | 24hU | mg/kg/d | 24hU | mg/kg/d | 24hU | ||||||||||||||||||
| 2 | F | 1.24 | 107 | 5# | 10.6* | 5.2 | 13# | 2.04 | 55.12* | 0.4 | 256 | 1.40 | 28 | 6.66# | 131 | 0.21* | 0.08 | 14.47 | 285 | 16.71 | 4.33 | 0.10 | 20.26 |
| 4 | M | -2.00# | 103 | 15# | 9.7 | 5.2 | 85 | 1.87 | 50.44 | 0.4 | 437* | 11.04* | 136 | 11.71# | 144 | 0.94* | 0.38* | 17.85* | 220 | 4.69 | 11.73 | 0.62 | 197 |
| 5 | M | -1.68 | 130 | 14# | 9.9 | 5.4 | 33 | 1.83 | 53.46 | 0.4 | 382* | 4.57* | 66 | 32.91* | 477 | 0.14 | 0.06 | 17.86* | 259 | 4.02 | 6.70* | 0.26 | 81.62 |
| 7 | M | -1.72 | 151 | 206 | 9.6 | 5.4 | 59 | 1.78 | 51.84 | 0.4 | 636* | 5.90* | 123 | 20.17 | 421 | 0.29* | 0.12 | 32.39* | 677 | 11.9 | 4.84 | 0.18 | 191.1 |
| 11 | F | -2.31# | 72# | 25 | 9.9 | 4.5 | 15# | 2.20 | 44.55 | 0.4 | 156 | 4.33* | 105 | 19.41 | 472 | 0.22* | 0.09 | 19.71* | 479 | 9.0 | 4.51 | 0.22 | 85.34 |
| 12 | F | -1.79 | 53# | 78# | 9.9 | 4.2# | 21 | 2.36 | 41.58 | 0.5 | 467* | 5.12* | 157 | 35.26* | 1082 | 0.15 | 0.07 | 22.08* | 678 | 7.45 | 4.49 | 0.23 | 113.0 |
| 12 | M | 1.95 | 112 | 32# | 10.8* | 5.8 | 20 | 1.86 | 62.40* | 0.2 | 223* | 3.90 | 189 | 15.80 | 768 | 0.24* | 0.05 | 20.08* | 976* | 4.38 | 7.08* | 0.19 | 78.31 |
| 12 | M | 3.53* | 76# | 33# | 9.7 | 4.5 | 10# | 2.16 | 43.65 | 0.6 | 349* | 2.57 | 232* | 21.04 | 1896 | 0.12 | 0.07 | 14.98 | 1350* | 9.49 | 4.43 | 0.17 | 38.50 |
| 13 | F | 1.52 | 67# | 20# | 9.8 | 4.7 | 58 | 2.09 | 46.06 | 0.7 | 167 | 5.20* | 289* | 21.29* | 1184 | 0.24* | 0.17* | 15.38 | 855 | 10.76 | 4.40 | 0.34 | 79.98 |
| 13 | F | -0.08 | 73# | 13# | 9.9 | 5.2 | 15# | 1.90 | 51.48 | 0.6 | 216 | 8.36* | 342* | 28.37* | 1160 | 0.29* | 0.18* | 29.80* | 1219* | 12.1 | 4.62 | 0.28 | 249.1 |
| 13 | F | 3.65* | 115 | 705 | 9.6 | 4.6 | 25 | 2.09 | 44.16 | 0.6 | 312* | 2.50 | 212* | 6.36# | 539 | 0.39* | 0.24* | 17.65* | 1497* | 36.20* | 2.93 | 0.14 | 44.13 |
| 15 | F | 3.39* | 55# | 103 | 10.1 | 4.5 | 12# | 2.24 | 45.45 | 0.8 | 254* | 4.08* | 294* | 19.15 | 1381 | 0.21* | 0.17* | 17.30* | 1247* | 16.06 | 3.78 | 0.24 | 70.58 |
| 16 | F | 3.44* | 64# | 18# | 10.0 | 3.6# | 23 | 2.78* | 36.00 | 0.7 | 87 | 3.01 | 230* | 15.67 | 1196 | 0.19 | 0.13 | 11.38 | 868 | 14.12 | 3.09 | 0.26 | 34.25 |
| 3 | M | -1.46 | 113 | 46# | 10.0 | 4.3# | 16# | 2.33 | 43.00 | 0.3 | 537* | 2.89 | 32.4 | 2.89# | 21 | 0.14 | 0.04 | 35.71* | 400 | 12.08 | 3.82 | 0.08 | 103.2 |
| 3 | M | -0.08 | 74# | 3# | 10.2 | 5.2 | 47 | 1.96 | 53.04 | 0.4 | 176 | 0.78 | 11.0 | 19.52 | 275 | 0.04 | 0.02 | 33.48* | 472 | 13.19 | 4.43 | 0.02 | 26.11 |
| 5 | M | 4.14* | 95 | 595 | 9.5 | 5.5 | 19# | 1.73 | 52.25 | 0.4 | 190 | 0.90 | 32.6 | 16.09 | 582 | 0.06 | 0.02 | 19.23* | 696 | 8.69 | 5.59* | 0.05 | 17.31 |
| 5 | M | 2.27* | 105 | 35# | 9.8 | 5.4 | 11# | 1.81 | 52.92 | 0.2 | 203 | 2.62 | 61.0 | 19.08 | 461 | 0.13 | 0.03 | 22.66* | 528 | 4.24 | 6.63 | 0.12 | 59.37 |
| 5 | F | 1.75 | 127 | 19# | 10.0 | 5.1 | 12# | 1.96 | 51.00 | 0.4 | 204 | 0.81 | 26.2 | 14.02 | 453 | 0.06 | 0.02 | 22.23* | 718 | 12.44 | 4.47 | 0.04 | 18.01 |
| 6 | F | -3.31# | 75* | 11# | 9.5 | 4.9 | 28 | 1.94 | 46.55 | 0.3 | 530* | 0.27 | 3.8 | 13.83 | 194 | 0.02 | 0.01 | 19.21* | 269 | 8.50 | 5.02 | 0.01 | 5.19 |
| 9 | M | -0.33 | 145 | 195 | 10.4 | 4.9 | 33 | 2.12 | 50.96 | 0.9 | 308 | 1.96 | 56.45 | 12.98# | 374 | 0.15 | 0.14 | 16.25 | 468 | 22.99* | 3.77 | 0.12 | 31.85 |
| 12 | F | -3.31# | 72# | 23# | 9.8 | 3.4# | 8# | 2.88* | 33.32 | 0.7 | 442* | 0.60 | 14.4 | 35.44* | 851 | 0.02 | 0.01 | 20.29* | 487 | 11.79 | 3.06 | 0.03 | 12.17 |
| 13 | M | 1.60 | 80 | 9# | 10.3 | 4.1# | 43 | 2.51 | 42.23 | 0.6 | 1056* | 0.89 | 63.64 | 22.44 | 1604 | 0.04 | 0.02 | 14.18 | 1014* | 9.25 | 4.07 | 0.06 | 12.62 |
| 14 | F | -1.82# | 92 | 59# | 9.9 | 4.4# | 29 | 2.25 | 43.56 | 0.7 | 201 | 1.40 | 69.0 | 25.03* | 1234 | 0.06 | 0.04 | 17.32* | 854 | 11.01 | 4.08 | 0.08 | 24.25 |
| 11 | F | -2.28# | 70# | 150 | 10.2 | 4.8 | 22 | 2.13 | 48.96 | 0.6 | 656* | 3.93 | 116 | 24.13* | 712 | 0.16 | 0.09 | 18.30* | 540 | 9.48 | 4.73 | 0.21 | 70.92 |
| 15 | M | -2.00# | 107 | 32# | 10.0 | 5.1 | 24 | 1.96 | 51.00 | 0.5 | 956* | 3.10 | 116 | 26.02* | 972 | 0.12 | 0.06 | 34.63* | 1295* | 13.05 | 4.37 | 0.09 | 107.3 |
| 10 | M | -0.62 | 97 | 12# | 9.3 | 4.5 | 17# | 2.07 | 41.85 | 1.4 | 408* | 0.74 | 30.7 | 14.71# | 610 | 0.05 | 0.07 | 18.72* | 777 | 39.59* | 2.72 | 0.04 | 13.85 |
| 19 | F | 3.21* | 119 | 17# | 9.4 | 3.7# | 10# | 2.54 | 34.78 | 0.7 | 165* | 1.58 | 175 | 14.36 | 1594 | 0.11 | 0.08 | 8.52 | 945* | 11.22 | 3.40 | 0.19 | 13.46 |
| Serum P | Serum Ca/P ratio | Serum Ca-PP |
Urine Ca mg/kg/d |
Urine Ca 24-hours |
Ca/Cr rate | Urine Ca excretion |
Urine P mg/kg/d |
Urine P 24-hour |
Urine P excretion | Urine Ca/P ratio |
Urine Ca-PP |
TRP | TmP/GFR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear | regression | analysis | |||||||||||
| IgG3 R2 = 0.867 p = <0.001 |
WA R2 = 0.425 p = <0.001 |
||||||||||||
| TmP/GFR R2 = 0.474 p = <0.001 |
TmP/GFR R2 = 0.510 p = <0.001 |
Urine Ca excretion R2 = 0.847 p = <0.001 |
Ca/Cr rate R2 = 0.847 p = <0.001 |
Hit perimeter R2 = 0.466 p = <0.001 |
TRP R2 = 0.924 p = <0.001 |
Urine Ca (mg/kg/d) R2 = 0.867 p = <0.001 |
Urine P excretion R2 = 0.924 p = <0.001 |
||||||
| Serum Ca/P ratio R2 = 0.900 p = <0.001 |
Serum P R2 = 0.900 p = <0.001 |
Serum Ca-PP R2 = 0.723 p = <0.001 |
Urine Ca-PP R2 = 0.867 p = <0.001 |
Urine P 24-hour R2 = 0.459 p = <0.001 |
Urine Ca R2 = 0.700 p = <0.001 |
FM (kg) A R2 = 0.545 p = <0.001 |
GFR R2 = 0.454 p = <0.001 |
GFR R2 = 0.431 p = <0.001 |
|||||
| Serum Ca-PP R2 = 0.938 p = <0.001 |
Serum Ca-PP R2 = 0.723 p = <0.001 |
Serum P R2 = 0.938 p = <0.001 |
Urine Ca/P ratio R2 = 0.657 p = <0.001 |
Urine Ca/P ratio R2 = 0.714 p = <0.001 |
Urine Ca excretion R2 = 0.847 p = <0.001 |
FM index R2 = 0.432 p = <0.001 |
Excreted nitrogen R2 = 0.969 p = <0.001 |
Serum P R2 = 0.537 p = <0.001 |
|||||
| Multilinear | regression | analysis | Ca intake | ||||||||||
| TmP/GFR UCr 24-h R2 = 0.670 p = <0.001 |
Serum P, Serum Ca-PP R2 = 0.977 p = <0.001 |
Serum P, Serum Ca R2 = 0.999 p = <0.001 |
Urine Ca, Urine Ca/P ratio R2 = 0.988 p = <0.001 |
U. Ca/P ratio, U. P 24-hour R2 = 0.918 p = <0.001 |
Urine Ca*, Urine Ca excretion R2 = 0.878 p = <0.001 |
Ca/Cr rate Urine Ca 24-hour R2 = 0.902 p = <0.001 |
Triceps skinfold and Zs R2 = 0.544 p = <0.001 |
FFM (kg) A and FFM BIA R2 = 0.420 p = 0.003 |
BUN, Cr R2 = 0.754 p = <0.001 |
Urine Ca 24-hour, Ca/Cr rate R2 = 0.776 p = <0.001 |
Urine Ca*, Urine Ca/P ratio R2 = 0.976 p = <0.001 |
BUN, Cr R2 = 0.718 p = <0.001 |
Mg, Vit-E and A R2 = 0.538 p = <0.001 |
| Serum Ca/P ratio S. Ca-PP R2 = 0.995 p = <0.001 |
Urine Ca-PP, Urine Ca R2 = 0.650 p = <0.001 |
Urine Cr, urine Ca 24-hour R2 = 0.637 p = <0.001 |
HDL-C, Serum Vit-C R2 = 0.456 p = <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





