1. Introduction
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder impacting millions worldwide, characterized by the gradual deterioration of both motor and non-motor functions [
1,
2,
3]. Among the array of non-motor symptoms, cognitive impairment stands out as a significant concern, as it leads to dementia in nearly half of all patients within a decade of diagnosis [
4].
1.1. Cognitive Decline and Structural Change in PD
Cognitive decline in PD is often accompanied by notable structural brain changes. Specifically, alterations in gray matter predominantly affect the temporal regions, including the hippocampus, as well as the frontal and parietal lobes, while changes in white matter are typically observed in the corpus callosum and cingulate gyrus [
5,
6]. Studies have shown that cognitive dysfunction is increasingly prevalent among individuals with PD, even those who have recently been diagnosed [
6]. Notably, about 30-35% of individuals with early-stage PD experience cognitive decline [
7]. Several studies have highlighted the prevalence of PD mild cognitive impairment (PD-MCI), a precursor to dementia [
8,
9]. At diagnosis, 20%-33% of patients have PD-MCI, with 60%-80% developing Parkinson’s disease dementia (PDD) within 12 years [
10,
11]. Given the profound impact of cognitive decline on PD patients and the ensuing economic challenges, the development of an accurate and cost-effective predictive model for cognitive decline in early PD stages is imperative. However, practical methodologies for early detection of PD-related cognitive decline, integrating baseline demographics, clinical assessments, and blood biomarkers, remain limited. Motivated by the urgent need, this study aims to provide a straightforward, reliable, and easy-to-use model for predicting cognitive decline in early PD using machine learning (ML) techniques and accessible baseline features.
1.2. The Role of Biomarkers
Biomarkers play a pivotal role in PD, offering promise for early diagnosis, disease monitoring, and clinical trial design. A wealth of research has delved into various biomarker types, encompassing clinical, genetic, cerebrospinal fluid (CSF), and imaging biomarkers, which are increasingly pivotal in predicting cognitive decline during early diagnosis and disease prognostication [
12,
13,
14,
15]. Among these, blood biomarkers stand out for their accessibility and cost-effectiveness compared to CSF and imaging biomarkers [
16,
17]. Prior research has explored the relationship between blood biomarkers and cognitive decline in early PD. For instance, increased physical activity has been shown to attenuate the vulnerability associated with the APOE ε4 allele to early cognitive decline in patients with PD [
18]. Another study highlighted the potential of elevated α-synuclein and total tau (t-tau), along with reduced amyloid-beta-40 (Aβ-40) levels, as biomarkers for early detection of cognitive impairment in PD patients [
19]. Additionally, a pilot study suggests that lower serum uric acid levels in the early stages of the disease may be associated with the later development of MCI [
20]. Recent findings by Sekiya, et al. (2022) [
21] further highlight the widespread presence of α-synuclein oligomers in various brain regions of PD patients, especially in the neocortex, and their association with cognitive impairment, suggesting their potential significance in early PD pathology.
1.3. Gaps in Current Research
To the best of our knowledge, no existing risk prediction models have utilized ML methods incorporating baseline clinical, demographic, and blood biomarkers to predict the risk of cognitive decline in early PD. This study aims to fill this gap by developing a risk prediction model using ML algorithms capable of detecting complex patterns and interactions not discernible through traditional analysis methods.
1.4. Predicting Cognitive Decline in Literature
Previous studies have explored various approaches to predicting cognitive decline in PD using different data sources and methodologies. A study used data from the Parkinson’s Progression Markers Initiative (PPMI) to accurately predict cognitive impairment at a 2-year follow-up [
22]. Combining age, non-motor assessments, dopamine transporter (DAT) imaging, and CSF biomarkers effectively predicted Montreal Cognitive Assessment (MoCA) scores at the 2-year follow-up in newly diagnosed PD patients. Another study, also using PPMI data, developed a multimodal ML model to predict cognitive decline in early PD patients by utilizing the change in MoCA scores as the outcome, calculated from the difference between baseline and 4-year follow-up data [
23]. Additionally, a cross-sectional study using data from the Early Parkinson’s Disease Longitudinal Singapore (PALS) study examined cognitive impairment by comparing PD-MCI patients and those with normal cognition (PD-NC). This study highlighted the significant associations between PD-MCI and several factors, including triglycerides (TG), apolipoprotein A1 (ApoA1), and the SNCA rs6826785 genetic marker, suggesting their potential role in early cognitive decline in PD patients [
24].
1.5. Study Objective
This study utilized data from the PALS study to develop a risk prediction model to predict cognitive decline over a five years period in individuals with early PD by using baseline characteristics and various ML algorithms.
2. Materials and Methods
2.1. Study Design and Population
This study utilized data from the PALS prospective cohort study to predict cognitive decline using ML techniques. Data from 214 PD patients, collected over five years between 2014 and 2018, with all participants meeting the National Institute of Neurological Disorders and Stroke (NINDS) clinical criteria for PD. Participants had to have more than 6 years of education and were able to read and write English or Mandarin to enroll in the study. Exclusion criteria included significant medical conditions hindering regular follow-up and orthopedic issues potentially affecting study outcomes. Functional status was measured using the Hoehn and Yahr (HY) rating scale, while motor symptom severity was assessed via the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III. Cognitive function was assessed using the MoCA. Where dopaminergic therapy had begun, the dosage was calculated and reported as cumulative levodopa equivalent daily dose (LEDD) [
25,
26].
Patients were defined as having ‘early PD’ based on the following inclusion criteria: (i) motor symptoms within two years, and (ii) diagnosis of PD within one year according to the NINDS criteria as determined by a specialist in movement disorders. Ethics approval was obtained from the Singapore Health Services Centralized Institutional Review Board (CIRB) for the use of human participants in this study, and all participants provided informed written consent. After excluding patients with missing MoCA scores for the first three years, 193 PD patients were included in the final analysis.
2.2. Outcome Definition
In the context of early PD, the MoCA is used as a key measure to assess cognitive decline. For the purposes of this study, cognitive decline was defined as either a one-unit annual decrease in MoCA score or a one-unit decline observed over two consecutive years during the five-year follow-up period. This threshold of a one-unit decline is clinically relevant, as even a small change can indicate an early sign of cognitive deterioration in early PD.
2.3. Input Variables
Input variables included baseline demographics, clinical assessments, and blood biomarkers. Baseline demographics included age, gender, years of education, body mass index (BMI), smoking status, alcohol consumption, coffee consumption, and tea Consumption. Clinical assessments encompassed standing and lying systolic blood pressure (SBP), standing and lying diastolic blood pressure (DBP), HY, total MoCA score, total motor score, diabetes mellitus, hypertension, and hyperlipidemia. Blood biomarkers analyzed were suppression of tumorigenicity 2 (ST2), neurofilament light chain (NfL), t-tau, phosphorylated tau at threonine 181 (p-tau181), apolipoprotein E (APOE), and alpha-synuclein gene promoter (REP1).
2.4. Data Imputation and Transformation
Missing values were imputed using a random forest-based imputation method, which estimates missing values by leveraging relationships observed in existing data [
27]. This approach imputes missing data using mean/mode, and then iteratively fits a random forest (RF) to predict missing values until a stopping criterion or maximum iterations is reached. Continuous input features were transformed into binary variables for easier clinical interpretation. For input variables without well-established cut-off points, including blood biomarkers and total motor score, the Youden Index was used. This method which incorporates both sensitivity and specificity, is a commonly used measure of overall diagnostic performance. It identifies the cut-off point that optimizes the biomarker's differentiating ability when equal weight is assigned to both sensitivity and specificity [
28].
2.5. Feature Selection
Initially, 24 variables were included in the study. The RF-importance was calculated for each feature. The mean RF-importance across all features was used as a threshold, and features with an RF-importance below mean were excluded from the dataset.
2.6. Statistical Analysis and ML Methods
Descriptive statistics, including mean and standard deviation (SD) or median and first- and third-quartile, were reported for numeric variables, depending on normality assumption; while categorical variables were presented as frequency and percentages. Univariate logistic regression analysis was performed to investigate the association of baseline patients’ characteristics with progression outcome; and odds ratios (OR) along with 95% confidence interval (CI) were calculated. Four ML methods including AutoScore, RF, K-Nearest Neighbors (KNN), and Neural Network (NN), along with logistic regression as a baseline statistical approach, were employed. To ensure optimal model performance, hyperparameter tuning was performed for all methods using 10-fold cross-validation. For RF, a grid search was employed to tune the number of trees (ntree), the number of variables selected at each split (mtry), the minimum node size (nodesize), and the maximum number of terminal nodes (maxnodes). The following values were explored: ntree = (100, 200, 500), mtry = (3, 4, 5), nodesize = (5, 10, 15), and maxnodes = (5, 10, 20). The optimal parameters were chosen based on cross-validated performance metrics, specifically the area under the curve (AUC). For NN, one hidden layer was considered, and hyperparameters including the number of hidden units (size = (1, 2, 3, 4, 5)) and weight decay (decay =(0, 0.01, 0.1)) were optimized, with accuracy as the performance metric. Similarly, for KNN, the number of neighbors was optimized within the range of 1 to 10 using grid search, also with accuracy as the performance metric. For AutoScore, as all variables were initially binary, a score table was generated from the model outputs to create interpretable clinical scores. Following hyperparameter tuning, models with the optimal parameters were compared using performance metrics including AUC, sensitivity, and specificity, along with their corresponding 95% CI. Sensitivity and specificity were determined by identifying the optimal threshold on the receiver operating characteristic (ROC) curve, defined as the point closest to the top-left corner, representing a balance between high sensitivity and specificity.
Each ML method was evaluated using two modeling strategies: (i) including all variables, and (ii) selecting the ten most important variables based on feature importance scores derived from the RF-importance approach. AUC as an overall accuracy metric was used to identify the best-performing model. Model calibration was evaluated using a Binned Plot, which is recommended for smaller datasets. In this approach, predicted probabilities are grouped into 10 equal-sized bins, and for each bin, the midpoint of the predicted probability is plotted against the true fraction of positive cases. If the model is well calibrated, the points will fall near the diagonal line [
29]. A risk score table for the model utilizing selected variables was generated using the AutoScore method, an easy-to-use ML algorithm that facilitates risk assessment [
30]. Statistical significance was set at p-value < 0.05. All data analyses were conducted using R software (R Core Team (2024); R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
https://www.R-project.org).
3. Results
3.1. Baseline Characteristics and Descriptive Statistics by Outcome
A total of 193 early PD patients completed baseline assessments and were included in our study, with 58% of participants being male. At baseline, the mean age was 63.6 years (SD=8.94 years), and the mean years of education was 10.7 years (SD=4.37 years). Descriptive statistics of baseline variables are detailed in
Table 1. Cognitive decline as the primary outcome, was observed in 44 (23%) subjects. Significant findings include that patients with 10 or more than 10 years of education had a lower risk of cognitive decline compared to those with fewer years (OR= 0.36, p-value= 0.006). Elevated lying SBP, lying DBP, and standing SBP were associated with a higher risk of cognitive decline (OR =2.13, p-value =0.045; OR=2.60, p-value=0.009; OR=2.13, p-value=0.045, respectively).
3.2. Feature Selection Analysis
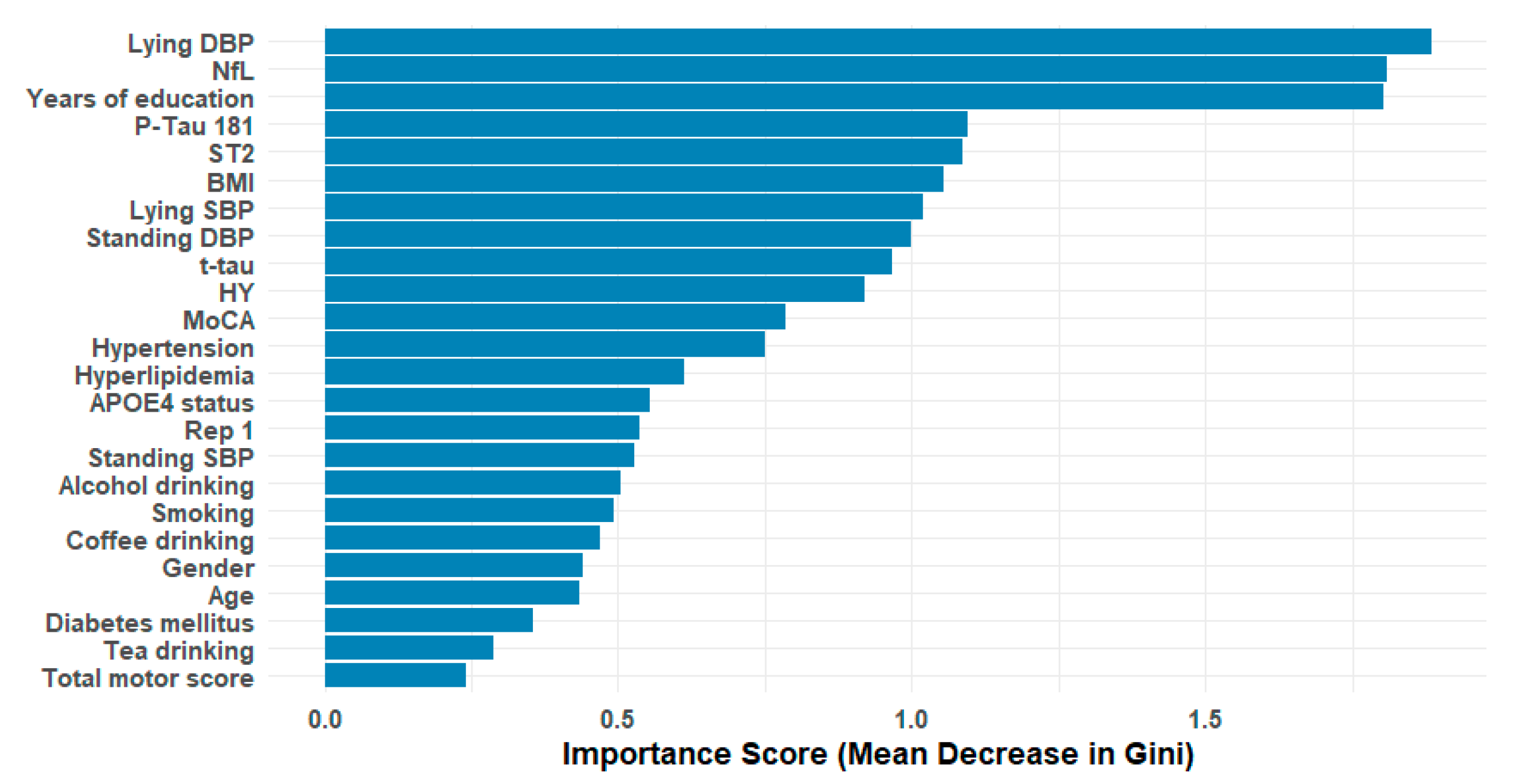
RF-importance scores were calculated for each feature (see
Figure 1). The mean RF-importance across all features was used as a threshold, and features with an RF-importance score below the mean were excluded. This strategy selected 10 out of the initial 24 features: lying DBP, NfL, years of education, p-tau181, ST2, BMI, lying SBP, standing DBP, t-tau, and HY scale.
3.3. Model Performance and ROC Analysis
The results of the various ML methods, evaluated through ROC analysis on the training dataset using 10-fold cross-validation, are summarized in
Table 2. In Model 1, which included all variables, the RF algorithm achieved the highest AUC at 0.999, indicating near-perfect discrimination between patients with and without cognitive decline. The NN also performed exceptionally well, with an AUC of 0.996. AutoScore and logistic regression showed moderate performance, with AUCs of 0.797 and 0.806, respectively. KNN had the lowest AUC at 0.766. In terms of sensitivity, both RF and NN were outstanding, with sensitivities of 1.000 and 0.977, respectively, highlighting their excellent ability to identify patients with cognitive decline. AutoScore had the lowest sensitivity at 0.636. In Model 2
, which included the ten most important variables, RF again had the highest AUC at 0.930, followed by NN and KNN with AUCs of 0.918 and 0.843, respectively. Logistic regression and AutoScore exhibited similar moderate performance, with AUCs of 0.770 and 0.771, respectively. NN achieved the highest sensitivity at 0.841, indicating strong detection capability with fewer variables. AutoScore, RF, and KNN all performed well, each with a sensitivity of 0.818. Overall, RF consistently showed the highest AUC across both models, demonstrating superior performance in distinguishing between patients with and without cognitive decline. NN also performed strongly, particularly in Model 2, where it exhibited the highest sensitivity.
3.4. Calibration of the Predictive Models
Calibration was assessed using a Binned Plot for both models.
Figure 2 presents the calibration results specifically for Model 2, featuring a Binned Plot with a 99% CI based on the internal data. The plot shows that both RF and NN tend to overestimate the predicted probabilities, while KNN underestimates them. In contrast, Logistic Regression and AutoScore demonstrate better calibration performance.
3.5. Score of Risk Factors Based on AutoScore Algorithm
AutoScore, an interpretable ML–based tool for generating automatic clinical score, provides risk factor scores for each feature. This capability translates complex model predictions into a more understandable format for clinical decision-making [
30]. Given its high sensitivity in Model 2, the inclusion of the risk factors generated by AutoScore is particularly valuable. Results indicated that lower education (<10 years), higher NfL (>= 21.5 pg/ml), higher ST2 (>= 14185.8 pg/ml), higher t-tau (>= 2.1 pg/ml), highr p-tau181 (>=27.1 pg/ml), higher standing and lying DBP (>=80 mmHg), higher lying SBP (>=140 mmHg), higher BMI (>=25 kg), and higher HY scale (>=2) are significant risk factors that increase the likelihood of cognitive decline in early PD patients (
Table 3).
4. Discussion
4.1. Cognitive Decline in PD and Biomarkers’ Role
Cognitive impairment is one of the most common non-motor symptoms in PD and can be more devastating for both patients and caregivers than motor symptoms [
31]. The pursuit of objective biomarkers in PD is motivated by the potential to aid in early and accurate diagnosis, monitor disease progression effectively, and improve the design and interpretation of clinical trials. While alpha-synuclein remains a promising biomarker candidate, the complex and heterogeneous nature of PD underscores the necessity for a comprehensive biomarker panel [
16,
17]. In light of these considerations, this study aimed to develop accurate predictive models for cognitive decline in early PD patients. Leveraging a combination of blood biomarkers, clinical data, and demographic characteristics, ML techniques were employed to achieve this objective.
4.2. Clinical Relevance of Different Modeling Strategies
This study compared two different modeling strategies to predict cognitive decline in early PD patients, emphasizing the importance of practicality in clinical settings. Model 1, which included all available variables, demonstrated the highest performance metrics, particularly with the RF and NN algorithms. However, the complexity and cost associated with obtaining comprehensive datasets limit its utility in routine clinical practice. In contrast, Model 2, incorporating only the ten most significant variables identified through feature selection, not only maintained strong performance metrics but also enhanced practicality for clinicians. Notably, this model showcased remarkable sensitivity with the NN algorithm, suggesting its potential to effectively detect cognitive decline using a streamlined approach. By focusing on easily obtainable variables, such as years of education and blood pressure, Model 2 could facilitate timely interventions, allowing healthcare providers to identify patients at risk for rapid cognitive decline. Implementing such practical models in clinical workflows could significantly improve early detection and management strategies, ultimately enhancing patient outcomes in early-stage PD.
4.3. Interpretation of Key Predictive Variables
The ten primary variables derived from feature selection, comprising a blend of demographics, clinical parameters, and blood biomarkers, included years of education, BMI, NfL, t-tau, p-tau181, ST2, Standing DBP, Lying DBP, Lying SBP, and HY scale. Notably, several of these features have been identified as significant risk factors in prior research.
4.3.1. Role of BMI
In this study, BMI emerged as a significant demographic variable with potential implications for patient management in early. Although research specifically examining the effect of BMI on early PD is lacking, several studies have explored its association with cognitive decline in PD. For instance, an analysis of data from PPMI identified that higher baseline BMI, along with modifiable comorbidities such as depression and sleep disorders, contributed to an accelerated rate of cognitive decline in PD patients [
32]. Similarly, another study using PPMI data found that PD patients with a metabolically unhealthy normal weight (MUNW) phenotype experienced more rapid cognitive decline, particularly in global cognition and visuospatial perception, over a 48-month period compared to those in other BMI-metabolic status categories [
33]. Conversely, Yoo et al. (2019) reported that PD patients with a higher-than-normal BMI at diagnosis exhibited a slower cognitive decline and a reduced risk of developing dementia over a six-year period compared to those with under/normal weight, suggesting that a higher BMI may have a protective effect against cognitive deterioration in PD [
34]. Additionally, Kim et al. (2012) observed that a decrease in BMI during the initial six months of follow-up in PD patients could serve as an early indicator of future dementia risk, enabling clinicians to predict a faster rate of cognitive decline [
35]. These findings underscore the importance of monitoring BMI in PD patients, as it may inform clinical decisions regarding interventions aimed at preserving cognitive function and improving overall patient outcomes.
4.3.2. Impact of Education on Cognitive Decline
In addition to BMI, years of education also emerged as a significant demographic predictor in this study. This finding is consistent with a recent cross-sectional study using PALS data, which demonstrated that fewer years of education are associated with higher MDS-UPDRS Part III and an elevated risk of MCI in early PD [
24]. Lower educational attainment may therefore be a marker for greater vulnerability to motor and cognitive declines in PD, underscoring the potential role of education in influencing disease progression and patient outcomes.
4.3.3. Blood Pressure as a Predictor of Cognitive Decline
In the present study, standing DBP, lying DBP, and lying SBP emerged as significant clinical predictors of cognitive decline in early PD. This aligns with previous research underscoring the role of hypertension in cognitive decline among early PD patients. Previous research has shown that PD-MCI patients exhibited significantly higher diastolic blood pressure variability (BPV) during follow-up compared to those with non-MCI PD, suggesting BPV as a potential predictive marker of cognitive decline [
36]. Additionally, an analysis using PPMI data indicated that elevated visit-to-visit variability in systolic blood pressure (systolic VIM) was associated with a faster decline in global cognitive function, assessed by MoCA score, in PD-MCI patients [
37]. Further emphasizing the importance of blood pressure management, another study found that, on average, every 10 mmHg increase in pulse pressure was associated with a 0.08 reduction in cognitive Z-scores in early PD [
38]. These findings collectively highlight the critical need for effective blood pressure management in early PD to mitigate the risk of cognitive decline.
4.3.4. Significance of HY Scale in Early PD
Through this study, HY scale emerged as a significant predictor of cognitive decline in early PD, underscoring its clinical relevance beyond motor symptom assessment. This finding aligns with previous research demonstrating a strong association between motor impairment severity, as measured by the HY scale, and cognitive deficits in PD patients. For example, a study by Siciliano et al. (2017) compared cognitive performance in de novo PD patients and found that those at HY stage II scored significantly lower on neuropsychological tests compared to those at HY stage I, indicating that greater motor impairment correlates with increased cognitive dysfunction [
39]. Additionally, the predictive power of the HY scale for disease progression is further supported by studies such as the PASADENA trial, a Phase II randomized, double-blind, placebo-controlled study investigating the efficacy and safety of prasinezumab in early PD [
40], and analyses using PPMI data [
41]. These studies identified the HY stage, along with other biomarkers like dopamine transporter SPECT imaging, as key predictors of clinical progression in early PD. These findings highlight the critical role of the HY scale in the early detection and management of PD, aiding clinicians in predicting and potentially mitigating cognitive decline.
4.3.5. Blood Biomarkers: NfL, p-tau181, t-tau and ST2
Regarding blood biomarkers, four biomarkers NfL, p-tau181, t-tau, and ST2 were identified as significant predictors of cognitive decline in early PD. Our findings for NfL align closely with previous research, reinforcing its role as a valuable prognostic biomarker. For instance, one study demonstrated that elevated serum NfL levels are positively associated with an increased risk of early PD-related symptoms, suggesting that serum NfL could serve as a promising biomarker for early PD [
42]. Additionally, another study discovered, through a study using PALS data, that higher plasma NfL levels were linked to a frontal pattern of neurodegeneration, which also correlated with cognitive performance in early PD [
43]. This supports the potential future role of plasma NfL as an accessible biomarker for neurodegeneration and cognitive dysfunction in PD. Ng et al. (2020) further highlighted that higher plasma NfL levels were associated with worse cognition and motor function in the postural instability gait disorder (PIGD) subtype of PD, predicting motor and cognitive decline over two years [
44]. Similarly, Aamodt et al. (2021) reported that PD participants with high plasma NfL levels were significantly more likely to develop incident cognitive impairment (HR= 5.34, p-value = 0.005). Although their ROC analysis demonstrated only modest performance for plasma NfL alone in predicting the conversion from normal cognition to MCI or dementia, they noted that incorporating plasma NfL into a multi-marker panel could enhance predictive accuracy [
45]. In line with these findings, Batzu et al. (2022) reported that higher plasma NfL levels in PD patients were associated with lower Mini-Mental State Examination (MMSE) scores at baseline, even after adjusting for age, gender, and education [
46].
To our knowledge, there have been no extensive studies specifically exploring the roles of blood biomarkers including t-tau, p-tau181 and ST2 in predicting cognitive decline in early PD. In this context Batzu et al. (2022) conducted a cross-sectional study that found significantly higher plasma p-tau181 concentrations in PD subjects compared to healthy controls at baseline [
46]. However, their follow-up over two years did not reveal a significant association between plasma p-tau181 levels and either baseline or longitudinal cognitive performance. Another study highlighted the potential of elevated α-synuclein and t-tau, along with reduced Aβ-40 levels, as biomarkers for early detection of cognitive impairment in PD patients [
19].
Most research in this area has focused on CSF biomarkers. For instance, Almgren et al. (2023) used PPMI data to develop a ML model for predicting cognitive decline in de novo PD, incorporating CSF biomarkers, clinical test scores, basic demographics, and baseline cognition [
23]. Their findings showed that higher levels of CSF beta-amyloid were significantly associated with less cognitive decline, while higher baseline MoCA scores, elevated CSF t-tau, anxiety, and autonomic dysfunction were linked to greater cognitive decline. Similarly, Tao et al. (2022) investigated the associations between non-motor symptoms and CSF biomarkers in early PD using PPMI data [
47]. They found that PD patients with cognitive impairment had significantly lower levels of CSF α-synuclein, Aβ
1-42, and t-tau compared to PD patients without cognitive impairment. Additionally, Terrelonge et al. (2016) explored the role of CSF biomarkers in predicting cognitive impairment in early PD, revealing that lower baseline levels of CSF Aβ
1-42 were significantly associated with a higher risk of cognitive impairment over a two-year period, while no significant associations were found for t-tau or p-tau181 [
48]. These findings emphasize the importance of CSF biomarkers, as early indicators of cognitive decline risk in PD, underscoring their potential clinical utility for early diagnosis and targeted intervention in PD-related cognitive impairment.
In terms of ST2, a study measuring plasma soluble decoy receptor form of ST2 (sST2) levels in controls and patients with, Alzheimer’s disease (AD), frontotemporal dementia (FTD), and PD found that sST2 levels were elevated across all disease groups compared to controls, with the highest levels observed in FTD, followed by AD and PD [
49]. However, to our knowledge, no studies have specifically investigated plasma ST2 levels in the context of early-stage PD. This highlights the novelty of our study in exploring the association of ST2 with cognitive decline in early PD.
In other words, this study is among the first to explore the association of plasma biomarkers, including t-tau, p-tau181, and ST2, with cognitive decline in early PD. This novel approach provides new insights into how these plasma biomarkers might predict cognitive deterioration in early-stage PD.
4.4. Comparison of ML Algorithms
The performance of different ML methods in predicting cognitive decline in early PD was evaluated, with RF and NN consistently showing superior results compared to AutoScore and KNN. Model 1, which included all available variables, demonstrated the highest performance, while Model 2, focusing on the top ten variables, provided a more practical approach with notable performance in predicting cognitive decline in early PD.
Previous studies have leveraged ML algorithms to enhance the prediction of cognitive decline and other outcomes in PD. Zhang et al. (2023) used grouped predictors based on their cost and accessibility, finding that incorporating genetic factors into models built with demographic variables, hospital admission examinations, and clinical assessments to prediction of PD risk. Penalized logistic regression and XGBoost emerged as the most accurate algorithms, with penalized logistic regression achieving an AUC of 0.94 [
3]. Deng et al. (2023) conducted a cross-sectional study on PALS data, identifying eight key variables associated with MCI in early PD using ShapleyVIC-assisted and backward selection methods [
24]. Their final model included fewer years of education, a shorter history of hypertension, higher MDS-UPDRS motor scores, elevated levels of TG and ApoA1, and noncarrier status of the SNCA rs6826785 genetic marker. These findings align with the present study, which also identified fewer years of education and a history of hypertension as significant predictors of cognitive decline. The combined insights from these studies underscore the importance of a multifaceted approach in using ML to predict cognitive outcomes as a longitudinal outcome in early PD, integrating demographic, clinical, biochemical, and genetic factors for more accurate and practical predictive models.
4.5. Limitations and Future Avenues
This study employed 10-fold cross-validation to assess model performance; however, several limitations should be noted. The high AUC observed could be influenced by the small sample size, which may lead to overly optimistic performance estimates, even with 10-fold cross-validation. Although 1/10 of the data is set aside for validation in each iteration, small datasets can result in higher variance in performance metrics, and the results may not generalize well to larger, independent datasets. This raises concerns about producing biased performance estimates. Therefore, a more conservative approach, such as nested cross-validation, may provide more reliable performance estimates. Additionally, the calibration results for Model 2 showed that while RF and NN tend to overestimate predicted probabilities, KNN underestimates them. In contrast, Logistic Regression and AutoScore exhibit better calibration performance. However, these results should be interpreted with caution due to the small sample size and the use of the same dataset for both training and evaluation. These limitations highlight the necessity for further validation with independent, external datasets to ensure the robustness and generalizability of the findings.
5. Conclusions
This study demonstrates the potential of ML methods in accurately predicting cognitive decline in individuals with early-stage PD. By integrating baseline demographic, clinical, and blood biomarker data, these models offer valuable insights for early identification of patients at high risk of cognitive deterioration, providing opportunities for timely interventions and improved patient outcomes. While a comprehensive model incorporating all available variables achieved the highest predictive performance, the practicality of utilizing certain biomarkers in clinical settings may be limited due to their cost and accessibility. A more streamlined model focusing on key biomarkers, however, maintained strong predictive capabilities, offering a more practical and feasible approach for real-world clinical implementation.
Author Contributions
Conceptualization, R.M., A.N., E.T., L.T., S.S.; Methodology, R.M., E.S., W.G., S.S.; Software, R.M., S.S.; Validation, R.M., S.S.; Formal analysis, R.M., S.S.; Investigation, R.M., S.S.; Resources, S.N., J.T., A.N., X.D., X.C., D.H., S.N., Z.X., K.T., W.A., E.T., L.T., S.S.; Data curation, S.N., J.T., A.N., X.D., X.C., S.S.; Writing—original draft preparation, R.M., S.S.; Writing—review and editing, R.M., S.E., J.T., A.N., X.D., X.C., D.H., S.N., Z.X., K.T., W.A., E.T., L.T., E.S., W.G., S.S.; Visualization, R.M., S.S.; Supervision, A.N., E.T., L.T., W.G., S.S.; Project administration, R.M., S.N., J.T.; Funding acquisition, E.T., L.T., S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Singapore Ministry of Health’s National Medical Research Council (MOH-OFLCG18May-0002, MOH-CSAINV21-0005, CNIG22jul-0004).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of SingHealth (IRB reference number: CIRB 2019-2433) and National University of Singapore (IRB reference number: NUS-IRB-2022-899).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The study data will be made available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| PD |
Parkinson's disease |
| CD |
Cognitive decline |
| MoCA |
Montreal Cognitive Assessment |
| AUC |
Area under the curve |
| MCI |
Mild cognitive impairment |
| PDD |
Parkinson’s disease dementia |
| ML |
Machine learning |
| RF |
Random Forest |
| CSF |
Cerebrospinal fluid |
| PPMI |
Parkinson’s Progression Markers Initiative |
| ApoA1 |
Apolipoprotein A1 |
| TG |
Triglycerides |
| HY |
Hoehn and Yahr |
| MDS-UPDRS |
Movement Disorder Society Unified Parkinson’s Disease Rating Scale |
| BMI |
Body mass index |
| SBP |
Systolic blood pressure |
| DBP |
Diastolic blood pressure |
| ST2 |
Suppression of tumorigenicity 2 |
| NfL |
Neurofilament light chain |
| t-tau |
Total tau |
| p-tau 181 |
Phosphorylated tau at threonine 181 |
| APOE |
Apolipoprotein E |
| REP1 |
Alpha-synuclein gene promoter |
| OR |
Odds ratios |
| SD |
Standard deviation |
| CI |
Confidence interval |
| KNN |
K-Nearest Neighbors |
| NN |
Neural Network |
| ROC |
Receiver operating characteristic |
| AD |
Alzheimer’s disease |
| FTD |
Frontotemporal dementia |
References
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive
decline in Parkinson disease. Nat. Rev. Neurol 2017, 13, 217–231. https://www.ncbi.nlm.nih.gov/pubmed/28257128.
- Ciucci, M.R.; Grant, L.M.; Rajamanickam, E.S. P.; Hilby, B.L.; Blue, K.V.; Jones, C.A.; Kelm-Nelson, C.A. In Early identification and treatment of communication and swallowing deficits in Parkinson disease, Semin. Speech Lang., Thieme Medical Publishers: 2013; pp 185-202.
- Zhang, J.; Zhou, W.; Yu, H.; Wang, T.; Wang, X.; Liu, L.; Wen, Y. Prediction of Parkinson’s Disease Using Machine Learning Methods. Biomolecules 2023, 13, 1761. https://www.ncbi.nlm.nih.gov/pubmed/38136632.
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J. Neurol.
Neurosurg. Psychiatry 2013, 84, 1258–1264. https://www.ncbi.nlm.nih.gov/pubmed/23781007.
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. , Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. , Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinson’s disease 2020, 2020, 2076942.
- Poletti, M.; Emre, M.; Bonuccelli, U. , Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 579–586. https://www.ncbi.nlm.nih.gov/pubmed/21489852.
- Kandiah, N.; Mak, E.; Ng, A.; Huang, S.; Au, W.L.; Sitoh, Y.Y.; Tan, L.C. S. , Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat. Disord. 2013, 19, 680–683. https://www.ncbi.nlm.nih.gov/pubmed/23623194.
- Pigott, K.; Rick, J.; Xie, S.X.; Hurtig, H.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; Chahine, L.M.; Dahodwala, N.; Akhtar, R.S. , Longitudinal study of normal cognition in Parkinson disease. Neurology 2015,
85, 1276–1282. https://www.ncbi.nlm.nih.gov/pubmed/26362285.
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. , The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Burn, D.J. , Stability of mild cognitive impairment in newly diagnosed Parkinson's disease. J. Neurol.
Neurosurg. Psychiatry 2017, 88, 648–652. https://www.ncbi.nlm.nih.gov/pubmed/28250029.
- Deng, X.; Saffari, S.E.; Ng, S.Y. E.; Chia, N.; Tan, J.Y.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L. , Blood lipid biomarkers in early Parkinson’s disease and Parkinson’s disease with mild cognitive impairment. J. Parkinsons Dis. 2022, 12, 1937–1943. https://www.ncbi.nlm.nih.gov/pubmed/35723114.
- Hoogland, J.; De Bie, R.M. A.; Williams-Gray, C.H.; Muslimović, D.; Schmand, B.; Post, B. , Catechol-O-methyltransferase val158met and cognitive function in Parkinson's disease. Mov. Disord. 2010, 25, 2550–
2554. https://www.ncbi.nlm.nih.gov/pubmed/20878993.
- Kim, R.; Kim, H.J.; Shin, J.H.; Lee, C.Y.; Jeon, S.H.; Jeon, B. , Serum inflammatory markers and progression of nonmotor symptoms in early Parkinson's disease. Mov. Disord. Mov. Disord. 2022, 37, 1535–1541.
https://www.ncbi.nlm.nih.gov/pubmed/35596676.
- Michael, J. Fox Foundation. FDA Issues Letter of Support Encouraging Use of Synuclein-Based Biomarker (Asyn-SAA) in Clinical Trials (archived on 30 September 2024 at https://web.archive.org/web/20240930072011/https://www.michaeljfox.org/publication/fda-issues-letter-support-encouraging-use-synuclein-based-biomarker-asyn-saa-clinical). 30 September.
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. , CSF and blood biomarkers for Parkinson's disease. The Lancet Neurology 2019, 18, 573–586. https://www.ncbi.nlm.nih.gov/pubmed/30981640.
- Youssef, P.; Hughes, L.; Kim, W.S.; Halliday, G.M.; Lewis, S.J.; Cooper, A.; Dzamko, N. , Evaluation of plasma levels of NFL, GFAP, UCHL1 and tau as Parkinson's disease biomarkers using multiplexed single molecule counting. Sci. Rep. 2023, 13, 5217. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Park, S.; Yoo, D.; Jun, J.-S.; Jeon, B. , Association of physical activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology 2021, 96, e2429–e2437.
https://www.ncbi.nlm.nih.gov/pubmed/33790041.
- Chen, N.-C.; Chen, H.-L.; Li, S.-H.; Chang, Y.-H.; Chen, M.-H.; Tsai, N.-W.; Yu, C.-C.; Yang, S.-Y.; Lu, C.-H.; Lin, W.-C. , Plasma levels of α-synuclein, Aβ-40 and T-tau as biomarkers to predict cognitive impairment in Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.T.; Savastano, R.; Moccia, M.; Picillo, M.; Siano, P.; Erro, R.; Vallelunga, A.; Amboni, M.; Vitale, C.; Santangelo, G. , Lower serum uric acid is associated with mild cognitive impairment in early Parkinson’s disease: a 4-year follow-up study. J. Neural Transm. 2016, 123, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, H.; Tsuji, A.; Hashimoto, Y.; Takata, M.; Koga, S.; Nishida, K.; Futamura, N.; Kawamoto, M.; Kohara, N.; Dickson, D.W. , Discrepancy between distribution of alpha-synuclein oligomers and Lewy-related pathology in Parkinson’s disease. Acta neuropathol. commun. 2022, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. , Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. The Lancet Neurology 2017, 16, 66–75. https://www.ncbi.nlm.nih.gov/pubmed/27866858.
- Almgren, H.; Camacho, M.; Hanganu, A.; Kibreab, M.; Camicioli, R.; Ismail, Z.; Forkert, N.D.; Monchi, O. , Machine learning-based prediction of longitudinal cognitive decline in early Parkinson’s disease using multimodal features. Sci. Rep. 2023, 13, 13193. https://www.ncbi.nlm.nih.gov/pubmed/37580407.
- Deng, X.; Ning, Y.; Saffari, S.E.; Xiao, B.; Niu, C.; Ng, S.Y. E.; Chia, N.; Choi, X.; Heng, D.L.; Tan, Y.J. , Identifying clinical features and blood biomarkers associated with mild cognitive impairment in Parkinson disease using machine learning. Eur. J. Neurol. 2023, 30, 1658–1666.
https://www.ncbi.nlm.nih.gov/pubmed/36912424.
- Ng, S.Y.-E.; Chia, N.S.-Y.; Abbas, M.M.; Saffari, E.S.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L.; Tan, E.-K. , Physical activity improves anxiety and apathy in early Parkinson's disease: a longitudinal follow-up study. Front. Neurol. 2021, 11, 625897. [Google Scholar] [CrossRef] [PubMed]
- Yong, A.C. W.; Tan, Y.J.; Zhao, Y.; Lu, Z.; Ng, E.Y. L.; Ng, S.Y. E.; Chia, N.S. Y.; Choi, X.; Heng, D.; Neo, S. , SNCA Rep1 microsatellite length influences non-motor symptoms in early Parkinson’s disease. Aging (Albany N. Y.) 2020, 12, 20880.
- Stekhoven, D.J. , Using the missForest package. R package 2011, 1–11. [Google Scholar]
- Youden, W.J. , Index for rating diagnostic tests. Cancer 1950, 3, 32–35.
https://www.ncbi.nlm.nih.gov/pubmed/15405679.
- Niculescu-Mizil, A.; Caruana, R. In Predicting good probabilities with supervised learning, 2005; pp 625-632.
- Saffari, S.E.; Ning, Y.; Xie, F.; Chakraborty, B.; Volovici, V.; Vaughan, R.; Ong, M.E. H.; Liu, N. , AutoScore-Ordinal: an interpretable machine learning framework for generating scoring models for ordinal outcomes. BMC Med. Res. Methodol. 2022, 22, 286. https://www.ncbi.nlm.nih.gov/pubmed/36333672.
- Roheger, M.; Kalbe, E.; Liepelt-Scarfone, I. , Progression of cognitive decline in Parkinson’s disease. J. Parkinsons Dis. 2018, 8, 183–193. https://www.ncbi.nlm.nih.gov/pubmed/29914040.
- Forbes, E.; Tropea, T.F.; Mantri, S.; Xie, S.X.; Morley, J.F. , Modifiable comorbidities associated with cognitive decline in Parkinson's disease. "Mov. Disord. Clin. Pract. 2021, 8, 254–263.
https://www.ncbi.nlm.nih.gov/pubmed/33553496.
- Zhang, L.; Gu, L.-Y.; Dai, S.-b.; Zheng, R.; Jin, C.-Y.; Fang, Y.; Yang, W.-Y.; Tian, J.; Yin, X.-Z.; Zhao, G.-H. , Associations of body mass index-metabolic phenotypes with cognitive decline in Parkinson’s disease. Eur. Neurol. 2022, 85, 24–30. https://www.ncbi.nlm.nih.gov/pubmed/34689144.
- Yoo, H.S.; Chung, S.J.; Lee, P.H.; Sohn, Y.H.; Kang, S.Y. , The influence of body mass index at diagnosis on cognitive decline in Parkinson's disease. J Clin Neurol 2019, 15, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, E.S.; Lee, J.H.; Moon, J.S.; Oh, J.E.; Shin, J.W.; Lee, K.J.; Baek, I.C.; Jeong, S.-H.; Song, H.-J. , Relationship between changes of body mass index (BMI) and cognitive decline in Parkinson's disease (PD). Arch. Gerontol. Geriatr. 2012, 55, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-Y.; Pyo, S.J.; Lee, H.M.; Seo, W.-K.; Koh, S.-B. , Cognition and visit-to-visit variability of blood pressure and heart rate in de novo patients with Parkinson’s disease. J. Mov. Disord. 2016, 9, 144.
https://www.ncbi.nlm.nih.gov/pubmed/27667186.
- Xiao, Y.; Yang, T.; Zhang, L.; Wei, Q.; Ou, R.; Hou, Y.; Liu, K.; Lin, J.; Jiang, Q.; Shang, H. , Association between the blood pressure variability and cognitive decline in Parkinson's disease. Brain Behav. 2023, 13, e3319. [Google Scholar] [CrossRef] [PubMed]
- Doiron, M.; Langlois, M.; Dupré, N.; Simard, M. , The influence of vascular risk factors on cognitive function in early Parkinson's disease. Int. J. Geriatr. Psychiatry 2018, 33, 288–297.
https://www.ncbi.nlm.nih.gov/pubmed/28509343.
- Siciliano, M.; De Micco, R.; Trojano, L.; De Stefano, M.; Baiano, C.; Passaniti, C.; De Mase, A.; Russo, A.; Tedeschi, G.; Tessitore, A. , Cognitive impairment is associated with Hoehn and Yahr stages in early, de novo Parkinson disease patients. Parkinsonism Relat. Disord. 2017, 41, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Boess, F.G.; Taylor, K.I.; Ricci, B.; Mollenhauer, B.; Poewe, W.; Boulay, A.; Anzures-Cabrera, J.; Vogt, A.; Marchesi, M. , A phase II study to evaluate the safety and efficacy of prasinezumab in early Parkinson's disease (PASADENA): rationale, design, and baseline data. Front. Neurol. 2021, 12, 705407.
https://www.ncbi.nlm.nih.gov/pubmed/34659081.
- Jackson, H.; Anzures-Cabrera, J.; Taylor, K.I.; Pagano, G.; Investigators, P.; Prasinezumab Study, G. , Hoehn and Yahr stage and striatal Dat-SPECT uptake are predictors of Parkinson’s disease motor progression. Front. Neurosci. 2021, 15, 765765. https://www.ncbi.nlm.nih.gov/pubmed/34966256.
- Wang, X.; Yang, X.; He, W.; Song, X.; Zhang, G.; Niu, P.; Chen, T. , The association of serum neurofilament light chains with early symptoms related to Parkinson's disease: A cross-sectional study. J. Affect. Disord. 2023, 343, 144–152. https://www.ncbi.nlm.nih.gov/pubmed/37805158.
- Welton, T.; Tan, Y.J.; Saffari, S.E.; Ng, S.Y.; Chia, N.S.; Yong, A.C.; Choi, X.; Heng, D.L.; Shih, Y.-C.; Hartono, S. , Plasma neurofilament light concentration is associated with diffusion-tensor MRI-based measures of neurodegeneration in early Parkinson’s disease. J. Parkinsons Dis. 2022, 12, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.S. L.; Tan, Y.J.; Yong, A.C. W.; Saffari, S.E.; Lu, Z.; Ng, E.Y.; Ng, S.Y. E.; Chia, N.S. Y.; Choi, X.; Heng, D. , Utility of plasma Neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol. Neurodegener. 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, W.W.; Waligorska, T.; Shen, J.; Tropea, T.F.; Siderowf, A.; Weintraub, D.; Grossman, M.; Irwin, D.; Wolk, D.A.; Xie, S.X. , Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord. 2021, 36, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Batzu, L.; Rota, S.; Hye, A.; Heslegrave, A.; Trivedi, D.; Gibson, L.L.; Farrell, C.; Zinzalias, P.; Rizos, A.; Zetterberg, H. , Plasma p-tau181, neurofilament light chain and association with cognition in Parkinson’s disease. npj Parkinson's Disease 2022, 8, 154. https://www.ncbi.nlm.nih.gov/pubmed/36371469.
- Tao, M.; Dou, K.; Xie, Y.; Hou, B.; Xie, A. , The associations of cerebrospinal fluid biomarkers with cognition, and rapid eye movement sleep behavior disorder in early Parkinson’s disease. Front. Neurosci. 2022, 16,
1049118. https://www.ncbi.nlm.nih.gov/pubmed/36507360.
- Terrelonge, M.; Marder, K.S.; Weintraub, D.; Alcalay, R.N. , CSF β-amyloid 1-42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J. Mol. Neurosci. 2016, 58, 88–92.
https://www.ncbi.nlm.nih.gov/pubmed/26330275.
- Tan, Y.J.; Saffari, S.E.; Zhao, Y.; Ng, E.Y.; Yong, A.C.; Ng, S.Y.; Chia, N.S.; Choi, X.; Heng, D.; Neo, S. , Longitudinal Study of SNCA Rep1 Polymorphism on Executive Function in Early Parkinson’s Disease. J. Parkinsons Dis. 2022, 12, 865–870. https://www.ncbi.nlm.nih.gov/pubmed/35068417.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).