1. Introduction
Stroke is the second leading cause of death and a major contributor to long-term disability worldwide [
1]. Approximately 87% of all strokes are ischemic, primarily caused by arterial occlusions, leading to cerebral ischemia and subsequent neuronal death [
2]. 27% of acute ischemic strokes (AIS) are cardioembolic (CE) in origin [
3], with atrial fibrillation (AF) being a major risk factor, increasing the risk fivefold [
4,
5,
6]. Furthermore, AF patients tend to experience more severe strokes and have poorer outcomes compared to those in sinus rhythm [
7,
8,
9].
In patients with non-valvular atrial fibrillation (NVAF), direct oral anticoagulants (DOACs), such as factor Xa inhibitors and direct thrombin inhibitors, are recommended over vitamin K antagonists (VKAs) for stroke prevention [
10]. Clinical trials have shown that DOACs reduce the risk of stroke by 64% and mortality by 26% compared to placebo [
11,
12], and by 20% and 10%, respectively, compared to VKAs [
13]. Despite these benefits, up to one-third of AF patients on anticoagulation therapy still suffer an AIS [
14,
15,
16].
Secondary prevention strategies, including antithrombotic agents, statins, and antihypertensives, reduce the risk of secondary vascular events by 20% to 30% [
17,
18,
19,
20]. However, patients with AF who experience a stroke while on anticoagulation have an elevated risk of recurrence, with approximately 8.9% suffering an AIS annually [
21]. CE strokes, in particular, carry the highest risk of recurrence, with one in four survivors likely to have another stroke [
7,
22].
Notably, there are currently no specific guidelines from the American Cardiology or Neurology Societies on preventing further ischemic events in patients who suffer a stroke despite being on anticoagulation [
23,
24]. As a result, optimal prevention strategies for these high-risk patients remain uncertain [
21,
25,
26]. This study aims to evaluate the efficacy of OACs in secondary stroke prevention and to explore how the type and quality of anticoagulation impact the risk of recurrence in patients with AF.
2. Materials and Methods
2.1. Study Design and Patient Population
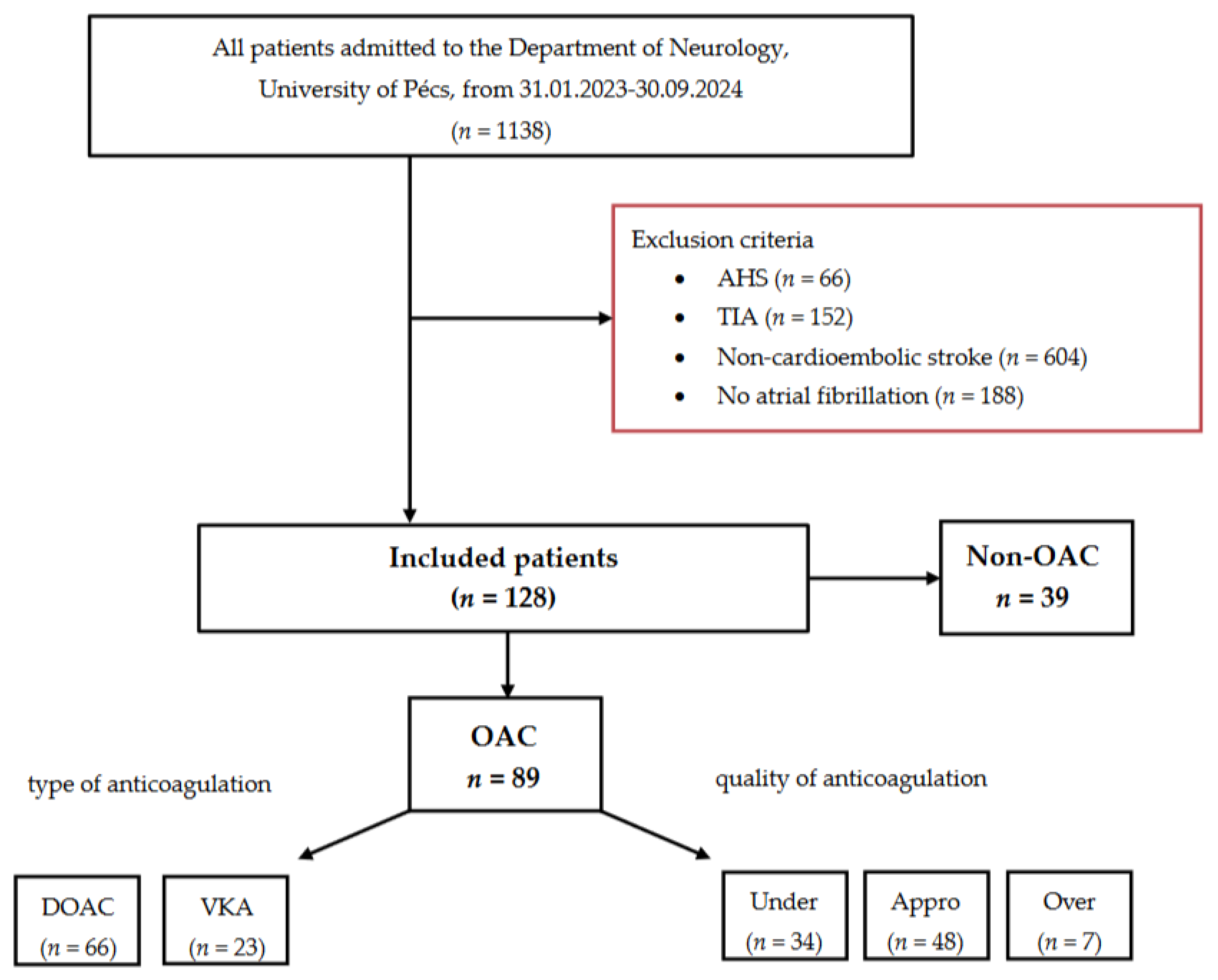
We conducted a retrospective analysis using data from our prospective Transzlációs Idegtudományi Nemzeti Laboratórium (TINL) STROKE-registry. From February 2023 to September 2024, 1138 consecutive adult patients were admitted to the Department of Neurology, University of Pécs. Of these, 1010 patients were excluded for the following reasons: acute hemorrhagic stroke (AHS) (n = 66), transient ischemic attack (TIA) (n = 152), non-cardioembolic stroke (n = 604), or no prior diagnosis of atrial fibrillation (n = 188). Ultimately, 128 patients met the inclusion criteria and were enrolled in the study.
The final cohort was divided into two groups: patients receiving oral anticoagulation (OAC group, n = 89) and those who were anticoagulation-naïve (Non-OAC group, n = 39). The OAC group was further categorized according to the type: DOAC (n = 66) or VKA (n = 23), and quality of anticoagulation: under-anticoagulated (n = 34), appropriately anticoagulated (n = 48), or over-anticoagulated (n = 7).
Inclusion criteria for the study were: (1) AIS of CE origin (referred to as index stroke), diagnosed through a combination of typical clinical presentation (sudden onset of neurological deficits, commonly involving the cerebral cortex), neuroimaging findings (involvement of cortical territory lesions), and evidence of a high-risk cardiac source (2) an electrocardiogram (ECG)-confirmed diagnosis of NVAF established before the index stroke, and (3) the availability of detailed information on anticoagulation therapy before the index stroke. A flowchart summarizing patient exclusion and inclusion criteria is provided in
Figure 1.
2.2. Data Collection and Measurements
This registry includes comprehensive demographic and clinical data such as age, sex, and medical history (e.g., current smoking, alcohol use, hypertension, and diabetes mellitus). Details on anticoagulant treatment (no treatment, VKA or DOAC, including the specific type), along with weight, creatinine, and glomerular filtration rate (GFR) at admission (for patients receiving DOAC therapy) or international normalized ratio (INR) at the index stroke (for patients receiving VKA therapy) were also recorded. Pre-stroke disability was assessed using the premorbidity modified Rankin Scale (pre-mRS), and stroke severity at admission was evaluated using the National Institutes of Health Stroke Scale (NIHSS). Additional data points included the functional and safety outcomes of the index stroke and history of CE stroke (including the interval between strokes and whether AF was known before the previous CE stroke).
DOAC therapy was defined as treatment with one of the following drugs and dosages: apixaban (2.5 mg or 5 mg twice daily), edoxaban (30 mg or 60 mg once daily), rivaroxaban (15 mg or 20 mg once daily) or dabigatran (110 mg or 150 mg twice daily). VKA therapy was defined as treatment with warfarin or acenocoumarol.
Anticoagulation quality was categorized as follows: Under-anticoagulation, defined as inappropriate dose reduction in DOAC patients or an admission-INR below 2.0 for VKA patients. Appropriate anticoagulation: Patients who meet dosing requirements in accordance with current guidelines. Over-anticoagulation, defined as inappropriate dosing without dose reduction in DOAC patients or an admission-INR above 3.0 for VKA patients.
2.3. Follow-up and Outcome Measures
Follow-up was conducted for at least three months post-index stroke to assess the following: Functional outcome at 90 days using the modified Rankin Scale (mRS), all-cause mortality during the follow-up period, and intracerebral hemorrhage (ICH), defined as new neurological symptoms associated with intracerebral hemorrhage confirmed by computer tomography (CT).
The primary endpoint was to assess the relationship between OAC use and the risk of recurrent CE stroke, using prior CE stroke as a proxy measure. Recurrent stroke was defined as new symptomatic neurological deterioration, not due to non-ischemic causes, with imaging evidence confirming new brain infarction.
Additionally, a subgroup analysis was performed on the anticoagulated cohort to examine the impact of anticoagulation type and quality on recurrence risk. For patients on DOACs, dosages were evaluated based on weight, creatinine levels, and GFR at admission. For those on VKAs, anticoagulation quality was assessed using INR measurements at the time of admission.
2.4. Statistical Analyses
Data analysis was performed using the Statistical Product and Service Solutions (SPSS) software (version 23) and Python (version 3.13.0). The normality of independent continuous variables was assessed using both descriptive and analytical criteria. Baseline characteristics were summarized through descriptive statistics. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables were presented as counts or percentages and analyzed using χ² test, Fisher's exact test, or one-way analysis of variance (ANOVA) for subgroup comparisons.
Differences between groups in demographic or clinical characteristics were evaluated using Fisher’s exact test for binary data. The rate of stroke recurrence was evaluated using logistic regression, adjusted for potential confounders (e.g., age, sex, hypertension, diabetes, and pre-stroke disability). One-way ANOVA and ridge regression with bootstrapping were used to assess additional factors in patients receiving anticoagulation.
All statistical tests were two-tailed, with a p-value of < 0.05 considered statistically significant. Odds ratios (OR) with 95% confidence intervals (CI) were reported.
3. Results
3.1. Demographic and Clinical Characteristics
A total of 128 AF patients with AIS were retrospectively reviewed, of whom 89 (69.5%) were on anticoagulation therapy (OAC group: 43.8% male, median age 80 years [IQR: 46-95]). The remaining 39 patients (30.5%) were not on anticoagulation therapy (Non-OAC group: 41.0% male, median age 81 years [IQR: 58-93]).
Clinical characteristics included a pre-mRS score of 0 (IQR, 0-4) for the OAC group and 0 (IQR, 0-5) for the Non-OAC group. The median NIHSS score was 6 (IQR, 0-36) in the OAC group and 7 (IQR, 2-21) in the Non-OAC group. Detailed demographic and clinical characteristics are summarized in
Table 1.
3.2. Functional and Safety Outcomes
No significant differences were observed between the OAC and Non-OAC groups regarding functional outcomes or mortality at 90 days. Both groups had a median 90-day mRS of 5 (IQR: 0-6, p = 0.622). Mortality at 90 days was similar between groups (33.7% for OAC vs. 30.8% for Non-OAC, p = 0.682). Although the incidence of ICH was higher in the OAC group (6.7% vs. 0.0%), this difference was not statistically significant (p = 0.465).
3.3. Previous History of CE Stroke
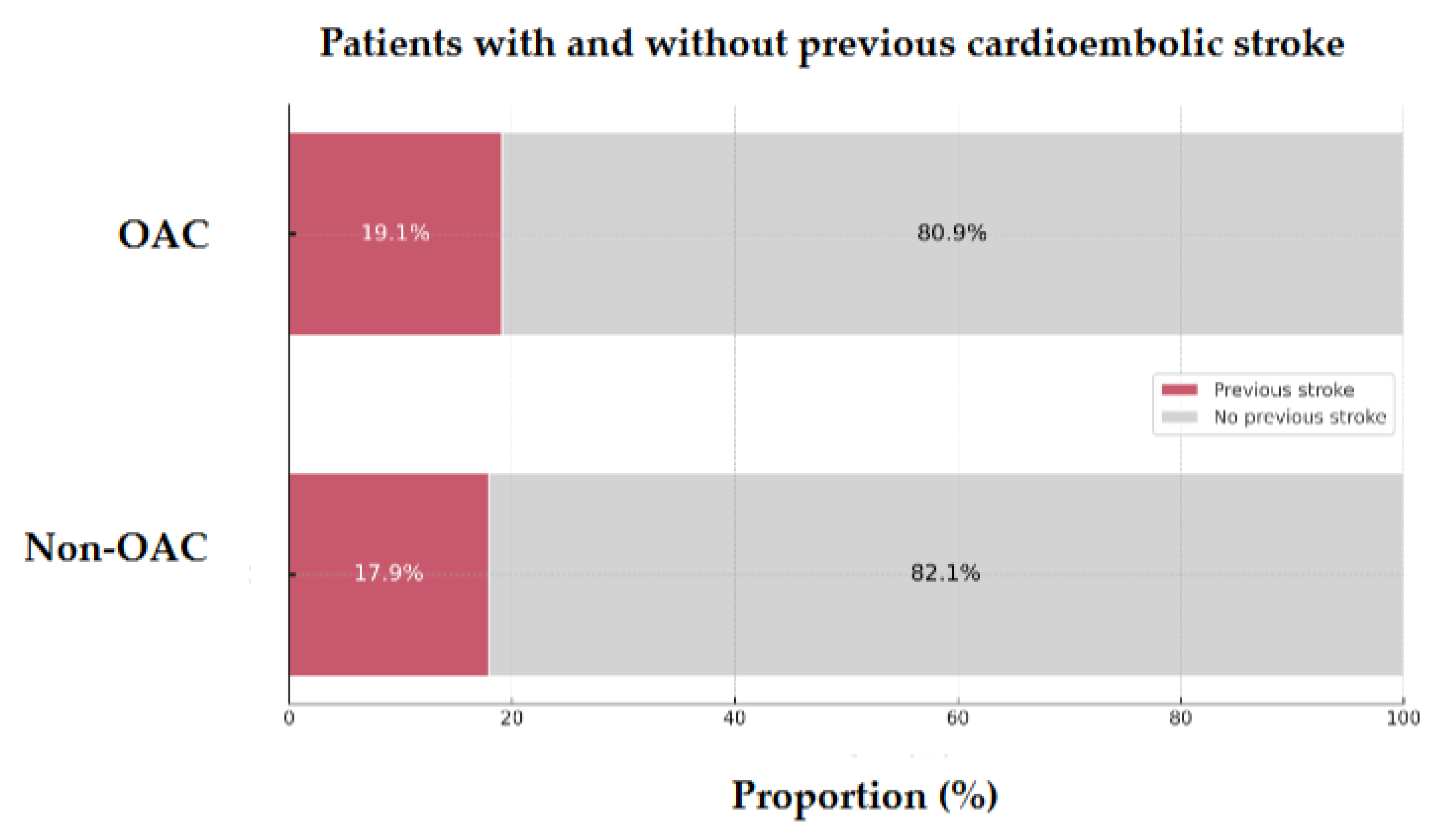
Of the 128 patients, 24 (18.75%) had a history of prior CE stroke. Among these, 19.1% were on anticoagulation therapy, while 17.9% were anticoagulation-naïve before the index stroke (
p = 0.870,
Figure 2). NVAF was present in roughly half of the patients before the previous CE stroke, regardless of prior anticoagulation status (41.2% in those on anticoagulation vs. 42.9% in those without).
Anticoagulated patients who experienced a recurrent stroke had a longer median interval between strokes compared to those who were not anticoagulated (6 years vs. 3 years,
p = 0.672), suggesting a potential protective effect of anticoagulation therapy (
Table 2).
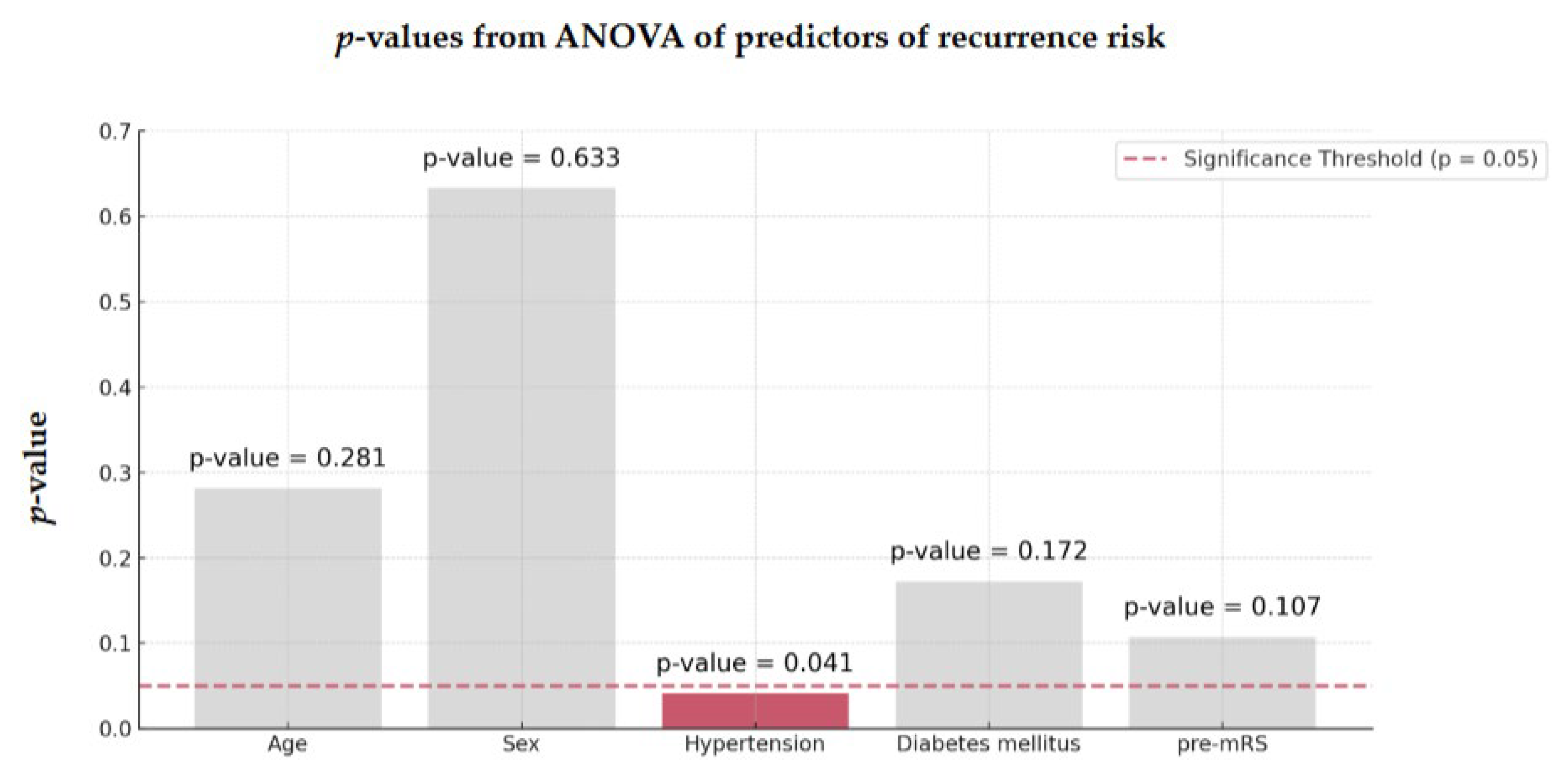
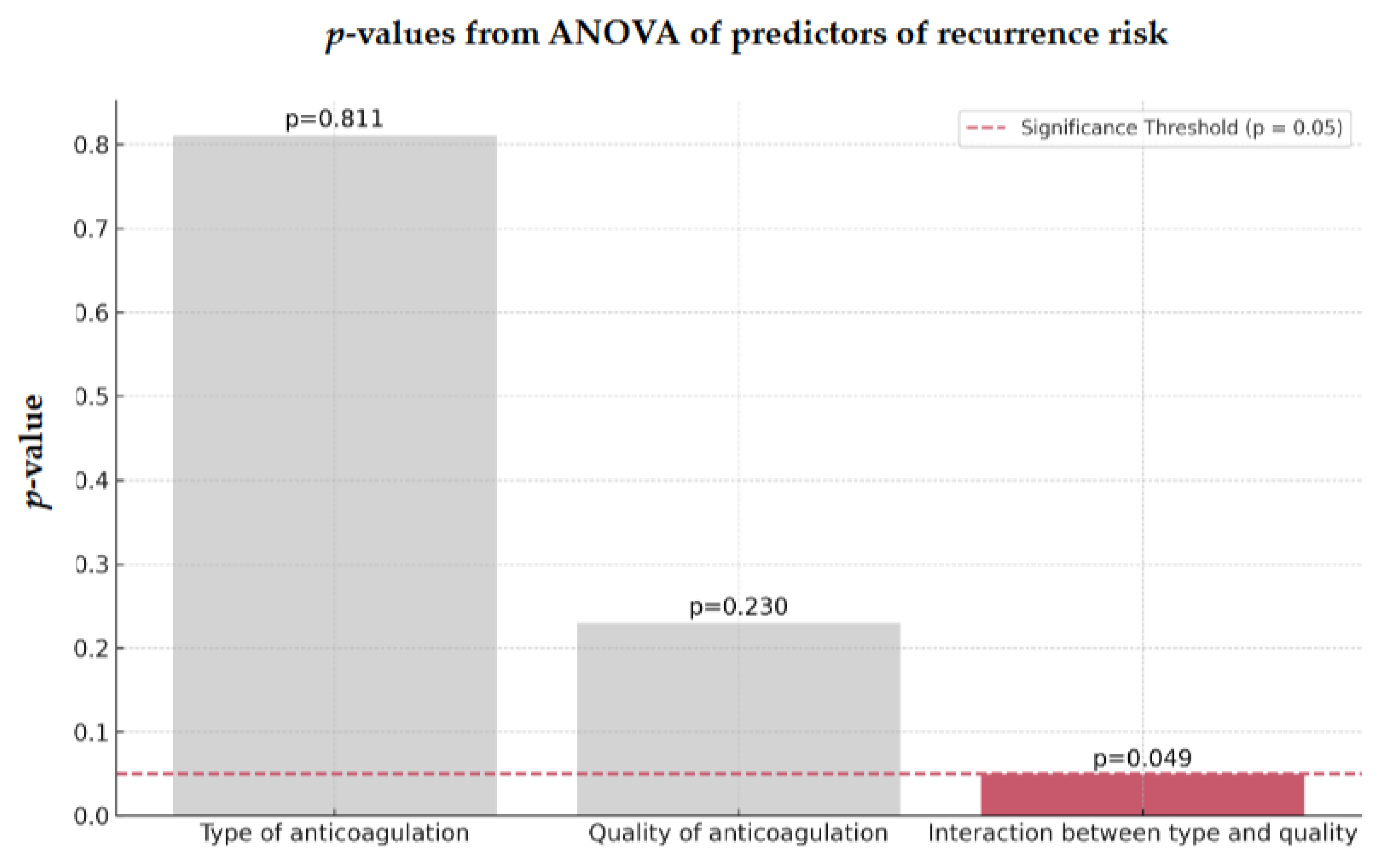
3.4. Factors Influencing Recurrence Risk
ANOVA identified hypertension as the most significant factor associated with recurrent stroke in both groups (
p = 0.041,
Figure 3).
3.5. Subgroup Analysis of Anticoagulated Patients
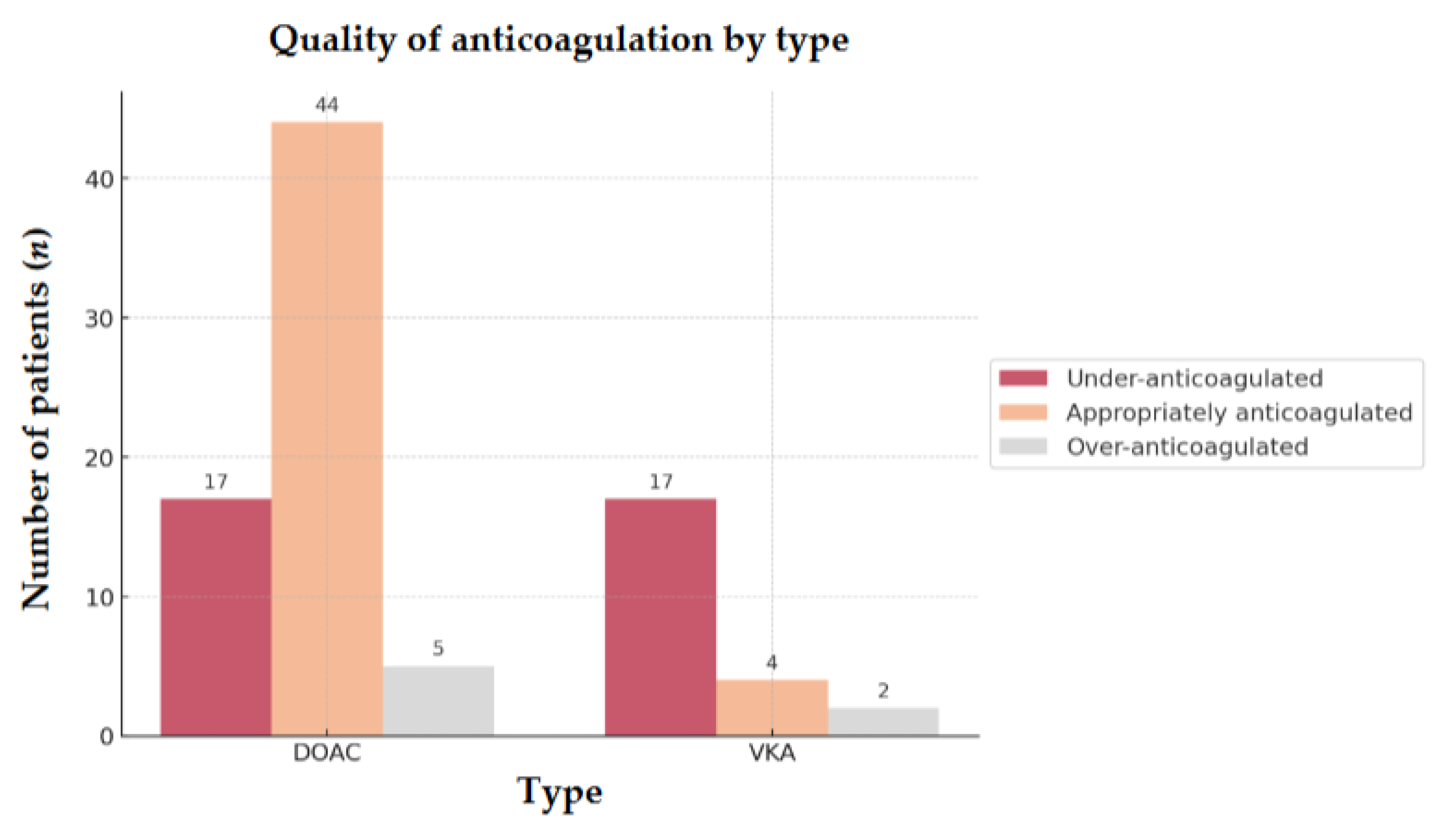
In our subgroup analysis of 89 OAC patients, the majority (74.2%) were on DOACs, with apixaban being the most commonly prescribed (65.2%). The remaining 25.8% were on VKAs, mainly acenocoumarol (56.5%). At admission, 53.9% of all anticoagulated patients were adequately anticoagulated, while 38.2% were under-anticoagulated, and 7.9% were over-anticoagulated (
Table 3).
66.7% of patients taking DOACs were appropriately anticoagulated compared to only 17.4% in the VKA group (OR = 9.5; 95% CI, 0.07-0.37;
p < 0.001). Furthermore, under-anticoagulation was significantly less common in the DOAC group (25.8%) than in the VKA group (73.9%) (OR = 0.12; 95% CI, 0.04-0.36;
p < 0.001) (
Figure 4).
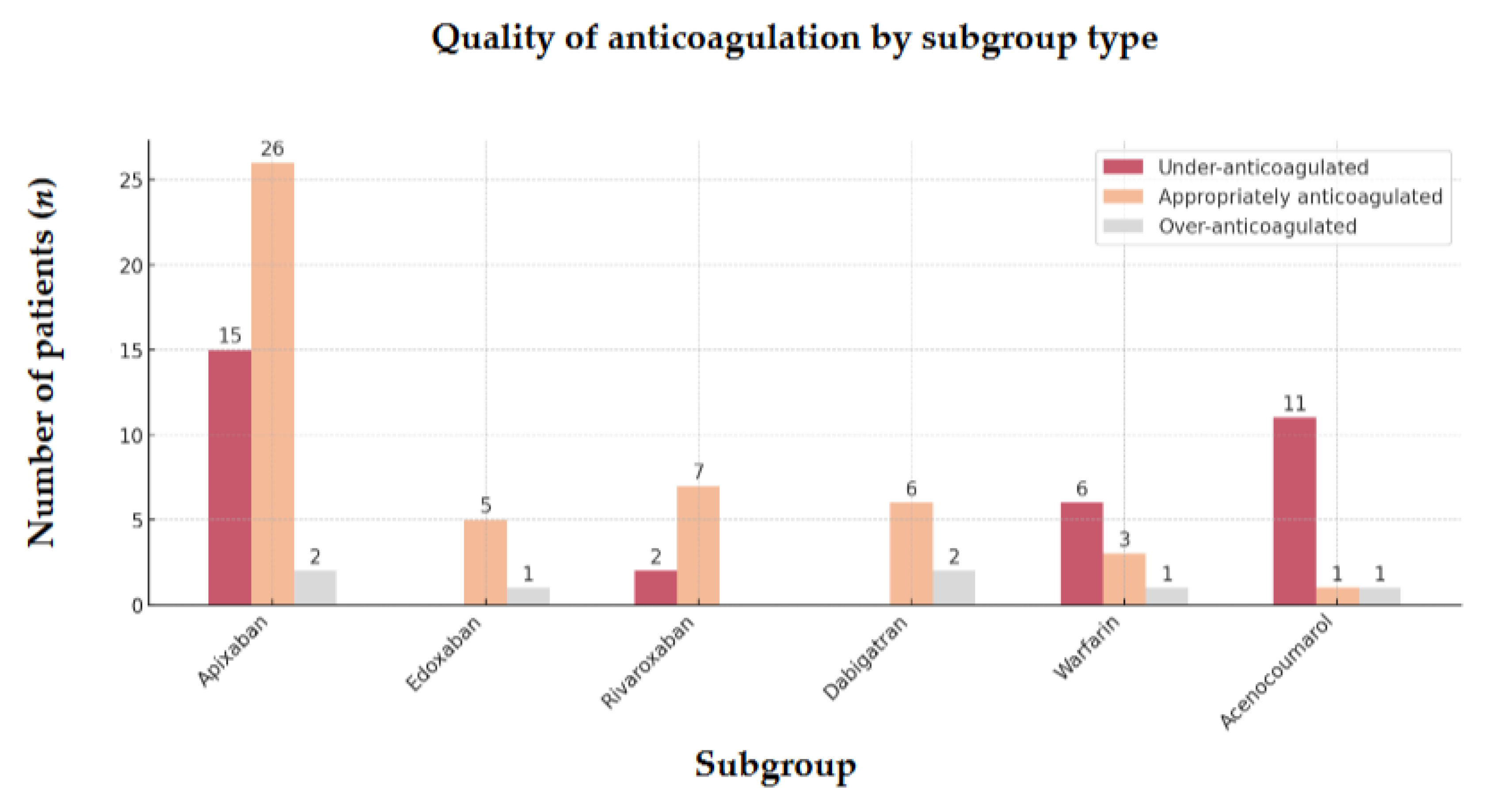
The comparison across different subgroups of VKA and DOAC highlights the same trend (
Figure 5).
3.6. Type of Anticoagulant
Stratification by anticoagulation type revealed no significant differences in baseline characteristics between patients taking DOACs and those taking VKAs (
Table 4).
These findings also hold for comparisons of functional and safety outcomes and the prevalence of previous CE strokes between the groups. Among patients with recurrent strokes, those on VKAs tended to have a shorter interval between strokes (5 years vs. 6 years,
p = 0.741) and were more likely to have been diagnosed with AF prior to their previous CE stroke (13.0% vs. 6.1%,
Table 5).
3.7. Quality of Anticoagulant
Stratification by anticoagulation quality revealed no significant differences in baseline characteristics between under-, appropriately and over-anticoagulated patients (
Table 6).
These findings were consistent when comparing functional and safety outcomes and the prevalence of previous CE strokes across the three groups. Under-anticoagulated patients were more likely to have been diagnosed with AF prior to their previous CE stroke (80.0%). Given that a majority of patients with VKA were under-anticoagulated (73.9%), it is likely that inadequate anticoagulation may have contributed to their prior strokes (
Table 7).
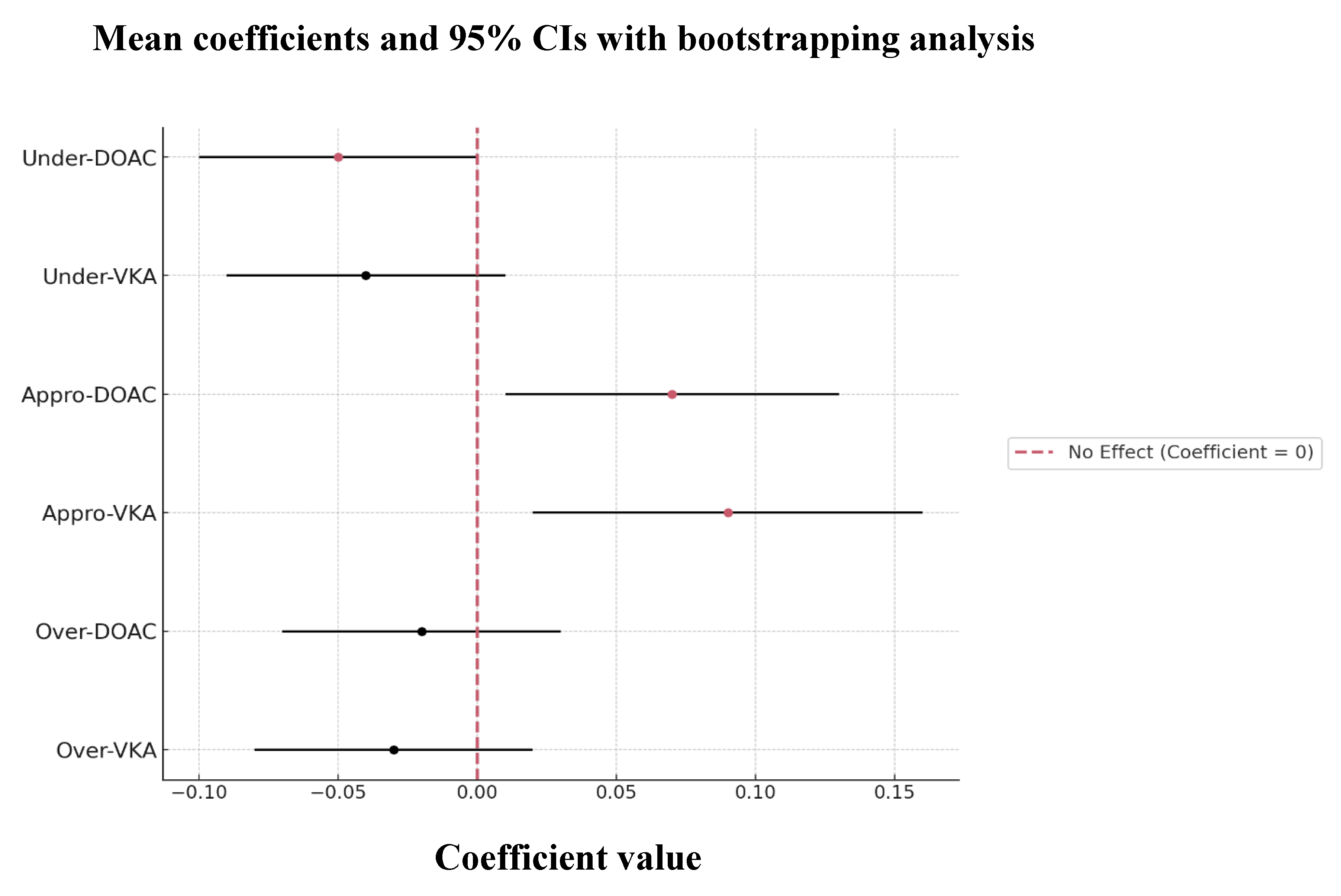
Age (mean = 0.2644; 95% CI, 0.0219-0.4131), hypertension (mean = -0.1404; 95% CI, -0.2562 to -0.0207), diabetes mellitus (mean = 0.1417; 95% CI, 0.0335-0.2599), and pre-mRS (mean = -0.1866; 95% CI, -0.2893 to -0.0795) were significant predictors of recurrence in anticoagulated patients, identified through ridge regression with bootstrapping analysis.
Interestingly, neither the type nor the quality of anticoagulation alone significantly affected the risk of recurrence in the OAC group. However, there was a significant interaction between anticoagulation type and quality (
p = 0.049), indicating that the effectiveness of OACs in reducing a recurrent stroke is strongly influenced by maintaining high-quality treatment (
Figure 6).
Looking closer at this interaction, we observed a trend showing that appropriately anticoagulated patients on either DOAC or VKA therapy may have a reduced risk of recurrence, suggesting that patients receiving optimal anticoagulation are less likely to have another stroke compared to those who are under- or over-anticoagulated (
Figure 7).
4. Discussion
Our study found no significant difference in recurrence rates between OAC-treated and anticoagulation-naïve patients after adjusting for confounding factors. Neither the type nor the quality of anticoagulation alone showed a significant impact on recurrence risk. However, the interaction between type and quality was significant. Achieving optimal anticoagulation, whether with DOACs or VKAs, was associated with a lower risk of recurrence highlighting the importance of maintaining high-quality anticoagulation in AF patients.
The absence of a significant impact of anticoagulant type or quality alone can be explained by multicollinearity (when analyzed independently, both factors may lose meaningful interactions that are critical for understanding their actual effects) or power and sample size (evaluating anticoagulation type and quality separately might have resulted in insufficient statistical power to detect significant effects). In contrast, the combined interaction between both was powerful enough to demonstrate a significant influence on recurrence risk.
4.1. Mechanisms of Stroke Despite OACs
Despite the use of OACs, a residual risk of stroke persists, which could be due to several factors, including: competing stroke mechanisms unrelated to AF (such as large-artery disease and small-vessel disease), insufficient anticoagulation (which may result from non-compliance, low anticoagulant activity at admission, inappropriate low-dose DOAC usage according to product labeling, or temporary withdrawal of therapy), and other CE mechanisms distinct from AF [
27,
28,
29].
4.1.1. Non-AF-related Mechanisms
Approximately 30% of recurrenct strokes are not related to AF [
24]. Within this subgroup, the most common competing mechanism is large artery atherosclerosis (60.6%), followed by small vessel disease (26.3%) [
25]. For many of these stroke mechanisms, anticoagulation offers no advantage over aspirin. For example, the Warfarin-Aspirin Recurrent Stroke Study (WARSS) demonstrated no benefit of warfarin compared to aspirin in patients with non-CE stroke [
30]. Similarly, the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial found that warfarin was not superior to aspirin for secondary stroke prevention in patients with intracranial atherosclerosis [
31].
4.1.2. Insufficient Anticoagulation Therapy
Despite having significant embolic risk factors, only half of patients with NVAF currently use Food and Drug Administration (FDA)-approved methods for stroke prevention [
32]. Likewise, adherence to anticoagulation therapy remains a challenge, with many patients discontinuing treatment or receiving inadequate doses [
33]. In a retrospective cohort analysis of 16,075 AF patients who initiated either warfarin or a DOAC, it was found that more than one in eight patients discontinued therapy within the first year.[
34] Additionally, a 2016 study reported declining persistence rates over a 12 month-period, with warfarin showing a persistence rate of 63.6%, while DOACs had a slightly higher persistence rate of 79.2% among newly diagnosed AF patients [
35].
Recent evidence underscores the significant risks associated with discontinuing OACs following an ischemic stroke. A Danish nationwide cohort study involving 8,119 patients 50 years and older (54.1% male, mean age 78.4 years) showed that approximately 4.3% experienced a recurrent stroke within one year after discharge from their initial stroke. Importantly, those who discontinued anticoagulation were found to be more than twice as likely to experience another stroke during a mean follow-up period of 2.9 years compared to those who continued therapy [
36]. These findings highlight the critical need for strategies to improve adherence and reduce discontinuation rates.
Among patients enrolled in the warfarin arms of the 3 most widely used DOAC trials (ROCKET-AF, RE-LY, and ARISTOTLE), the average time within the therapeutic range was notably low, ranging from 55% to 62% [
37,
38,
39]. Underdosing of DOACs also presents a significant concern. Many patients are prescribed reduced doses, often with the aim of mitigating bleeding risks under the principle of "do no harm." Similarly, in our study, under-anticoagulation was a prevalent issue, affecting a significant portion of both VKA and DOAC patients. The FDA has previously issued warnings against the off label underdosing of anticoagulants, highlighting the associated risks of compromised safety and efficacy [
40,
41].
4.1.3. CE Stroke Despite Adequate Anticoagulation Therapy
CE Stroke in AF patients despite optimal anticoagulation therapy represents a significant challenge in clinical practice, mainly because the underlying mechanisms are not yet fully understood. Evidence suggests that advanced atrial disease, including atrial enlargement and increased AF burden, along with high CHA
2DS
2-VASc scores, may contribute to ischemic strokes in these patients. This phenomenon indicates that CE risk may persist even under adequate anticoagulation, pointing to a gap in current therapeutic strategies for stroke prevention in high-risk AF patients [
42].
4.2. Embolic Stroke of Undetermined Source (ESUS)
Embolic Stroke of Undetermined Source (ESUS) refers to a subset of ischemic strokes where an embolic origin is suspected, but no definitive source can be identified after ruling out other possible stroke etiologies. Two major randomized clinical trials “Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of Dabigatran Etexilate Versus Acetylsalicylic Acid in Patients With Embolic Stroke of Undetermined Source” (RE-SPECT ESUS) and “New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial Versus Aspirin to Prevent Embolism in Embolic Stroke of Undetermined Source” (NAVIGATE ESUS) aimed to determine the best treatment approach for ESUS. Both trials concluded that neither dabigatran nor rivaroxaban offered a significant advantage over aspirin in preventing recurrent strokes in ESUS patients [
43,
44]. Likewise, ATTICUS and ARCADIA, demonstrated no benefit of apixaban over aspirin in preventing recurrent AIS among patients with ESUS [
45,
46]. These results challenged the expectation that empiric anticoagulation could significantly lower the risk of stroke recurrence compared to antiplatelet therapy.
Two additional trials examined the effectiveness of anticoagulation in patients with atrial high-rate episodes (AHRE), providing insights into the limitations of anticoagulation for secondary stroke prevention: “Non-vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes – Atrial Fibrillation Network 6“ (NOAH-AFNET 6) and “Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Subclinical Atrial Fibrillation“ (ARTESiA). The NOAH-AFNET 6 trial was terminated prematurely due to increased major bleeding in the edoxaban group and futility assessment to achieve efficacy endpoints [
47]. The second trial showed a significant reduction in stroke or systemic embolism in apixaban-assigned patients, but this benefit was counterbalanced by a significantly increased risk of major bleeding. The AIS rate was 0.64% per year in the apixaban group compared to 1.02% per year in the aspirin group, which, while lower, still represented a modest benefit relative to the risk of bleeding [
48].
4.3. Risk Factors
In patients with AF several risk factors are associated with an increased risk of ischemic stroke, including older age, hypertension, diabetes, heart failure, previous stroke or TIA, vascular disease, renal dysfunction, low body mass index (BMI), and female sex [
49]. Likewise, in our study, older age, hypertension, and diabetes mellitus emerged as independent factors associated with recurrent stroke in anticoagulated patients.
The high residual stroke risk in these patients must be quantified for targeted treatment. The currently used CHA2DS2-VASc score may not be adequate to assess residual stroke risk, as it weighs the history of previous strokes with a score of only 2. Furthermore, the CHA2DS2-VASc score does not account for the added risk associated with recurrent strokes in patients already receiving anticoagulation therapy.
4.3.1. Hypertension
Hypertension is a well-established, independent risk factor for stroke recurrence. A meta-analysis involving 13,944 stroke survivors found that hypertension increased the odds of recurrent stroke by 67% (95% CI, 45%–92%) over a follow-up period ranging from one to five years [
50]. Additionally, data from the “Prevention Regimen for Effectively avoiding Second Strokes“ (PROFESS) trial, demonstrated that systolic blood pressure (SBP) variability was linked to an increased risk of recurrent stroke, each 10-point increment in SBP variability was associated with a 15% higher hazard (95% CI, 2%–32%) of recurrence [
51]. In our study, hypertension was identified as the main risk factor for stroke recurrence in both anticoagulated and anticoagulation-naïve patients.
4.3.2. Diabetes mellitus
Diabetes mellitus is also a significant predictor of stroke recurrence. A meta-analysis of 27 studies involving 274,631 patients with prior ischemic stroke found that diabetes was associated with a 50% increase in the risk of stroke recurrence (pooled HR, 1.50; 95% CI, 1.36–1.65) [
52].
4.4. Alternative Strategies and Future Directions
4.4.1. Switching Anticoagulants
Observational studies show no benefit in reducing stroke recurrence by switching anticoagulation therapies or adding antiplatelet agents. Among 2,337 patients with NVAF, those who switched from one DOAC to another or converted to warfarin had higher rates of recurrent ischemic stroke (12.8% and 12.6%, respectively) compared to those who continued their initial DOAC (8.7%). Moreover, adding antiplatelet agents offered no additional benefit and was associated with an increased risk of bleeding complications [
25,
26].
4.4.2. Percutaneous Left Atrial Appendage Closure (LAAC)
As of the time of this article, the only FDA-approved alternatives for stroke prophylaxis that eliminate the need for lifelong anticoagulation in patients with NVAF are LAAC devices, specifically the WATCHMAN FLX and Amplatzer Amulet [
53]. Two large-scale multinational randomized control trials (RCTs), the CHAMPION-AF trial for WATCHMAN FLX and the CATALYST trial for Amplatzer Amulet, are currently enrolling patients with NVAF and compare their efficacy against DOACs.
Moreover, several ongoing RCTs are investigating the potential benefits of combining endovascular LAAC with DOAC therapy, including the “Evaluation of Percutaneous Left Atrial Appendage Occlusion in Stroke Prevention on Top of Direct Oral Anticoagulant Therapy“ (ELPASE) trial.
A real-world comparative study showed that LAAC reduced the risk of stroke and long-term bleeding by approximately 35% compared to long-term oral anticoagulant use in patients with NVAF [
54]. Furthermore, the “Left Atrial Appendage Occlusion Study III“ (LAAOS III) RCT demonstrated that closing the left atrial appendage (LAA) during cardiac surgery for anticoagulated patients with AF led to a 33% reduction in the risk of AIS after the procedure [
55]. In a separate study involving 115 patients who underwent endovascular LAAC after experiencing an AIS despite being on OAC, the observed annual rate of ischemic events was 2.6% after the procedure [
56].
4.4.3. Factor XIa Inhibitors
Following promising safety outcomes, phase 3 trials have been launched to evaluate asundexian (“Oral Factor XIa Inhibitor Asundexian in Patients with Atrial Fibrillation” [OCEANIC-AF]) and milvexian (“LIBREXIA-Atrial Fibrillation: Evaluation of Milvexian in Stroke Prevention for Atrial Fibrillation Patients” [LIBREXIA-AF]) for stroke prevention in patients with atrial fibrillation. Unfortunately, the OCEANIC-AF trial was prematurely stopped after an interim analysis revealed no significant efficacy.
In parallel, a phase 2b study (AZALEA-TIMI 71) comparing abelacimab to rivaroxaban showed a 67% reduction in the primary endpoint of major or clinically relevant non-major bleeding. Abelacimab is currently being studied in patients with atrial fibrillation who are not eligible for anticoagulation, however, individuals with a history of stroke are excluded from this trial.
4.4.4. Early Rhythm Control
Recent RCT data showed that early rhythm control can decrease the risk of AIS among patients with NVAF, including patients with a history of stroke [
57,
58].
4.4.5. Pill-in-the-Pocket
The 'pill-in-the-pocket' approach to anticoagulation is currently being evaluated in the REACT-COM and TACTIC-AF trials. This strategy aims to determine whether it is safe for patients to use DOACs only during episodes of AF and discontinue them during extended periods of sinus rhythm [
59,
60].
4.4.6. Colchicine
Colchicine has recently emerged as a promising candidate for stroke prevention, especially in patients who have experienced a recent stroke. Unlike many other therapies, colchicine is less likely to elevate the risk of intracranial or extracranial bleeding, making it a potentially safer alternative. The CONVINCE trial (“Colchicine for Prevention of Vascular Inflammation in Non-Cardio Embolic Stroke”) is currently evaluating the efficacy of low-dose colchicine (0.5 mg/day) for preventing major vascular events following mild ischemic stroke or high-risk TIA [
61].
4.5. Limitations
This study has several limitations that should be considered when interpreting the results. Firstly, the relatively small sample size from a single center and the non-randomized study design introduce potential selection bias, which may limit the generalizability of our findings. Although we used multivariable analyses to adjust for confounding variables, it was not possible to fully eliminate biases inherent to an observational design.
Additionally, the influence of unmeasured confounders cannot be ruled out. Important parameters associated with the risk of ischemic stroke in NVAF patients, such as the type of atrial fibrillation (sustained or paroxysmal), AF duration, untreated obstructive sleep apnea, and levels of natriuretic peptides or troponins, were not captured, which may have impacted the observed results [
62,
63,
64].
Moreover, the study did not evaluate other pharmacological treatments beyond anticoagulation, and no data on plasma DOAC levels were available for our patients. This limits our assessment of the full efficacy of treatment, particularly concerning optimal anticoagulant dosing.
It should also be acknowledged that the effectiveness and safety of anticoagulation therapy may differ for patients who have experienced a more recent stroke and are at a heightened risk of recurrence [
65].
Despite these limitations, this study provides valuable insights as it reflects real-world clinical experiences. Such a real-world perspective is crucial for understanding the practical challenges and potential benefits of anticoagulation therapy, offering important guidance for developing strategies aimed at reducing recurrent strokes in AF patients receiving OAC therapy.
5. Conclusions
Adequate anticoagulation, not just its use, is critical for minimizing residual risk of stroke, especially in high-risk patients with AF who have suffered a previous CE stroke. Clinicians should focus on optimizing anticoagulation therapy, as inadequate treatment quality is associated with an increased recurrence risk. Future strategies should concentrate on individualized patient management, regular monitoring, and optimal dosing to effectively prevent further strokes.
Beyond anticoagulation, managing modifiable risk factors, such as hypertension and diabetes, is crucial. Emerging therapies such as LAAC have shown promise as alternative prevention strategies, offering outcomes comparable to DOACs. However, persistent recurrence rates suggest the need for combined approaches, such as integrating LAAC with anticoagulation, to further improve patient outcomes. Ultimately, this comprehensive approach may best serve high-risk AF patients and significantly reduce their risk of recurrent stroke.
Author Contributions
Conceptualization, J.S. and B.C.; methodology, J.S. and L.S.; validation, Z.N.K. and E.B.; formal analysis, J.S.; data curation, J.S.; writing—original draft preparation, J.S. and B.C.; writing—review and editing, Z.N.K. and E.B.; visualization, J.S.; supervision, L.S.; project administration, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by Scientific and Research Ethics Committee of the Medical Research Council of the University of Pécs (RRF-2.3.1-21-2022-00011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| OACs |
oral anticoagulants |
| AF |
atrial fibrillation |
| TINL |
Transzlációs Idegtudományi Nemzeti Laboratórium |
| CE |
cardioembolic |
| AIS |
acute ischemic stroke |
| NVAF |
non-valvular atrial fibrillation |
| DOACs |
direct oral anticoagulants |
| VKAs |
vitamin K antagonists |
| TIA |
transient ischemic attack |
| AHS |
acute hemorrhagic stroke |
| ECG |
electrocardiogram |
| GFR |
glomerular filtration rate |
| INR |
international normalized ratio |
| pre-mRS |
premorbidity modified Rankin Scale |
| NIHSS |
National Institute of Health Stroke Scale |
| mRS |
modified Rankin Scale |
| ICH |
intracranial hemorrhage |
| CT |
computer tomography |
| SPSS |
Statistical Product and Service Solutions |
| SD |
standard deviation |
| IQR |
interquartile range |
| ANOVA |
analysis of variance |
| OR |
odds ratio |
| CI |
confidence interval |
| WARSS |
Warfarin-Aspirin Recurrent Stroke Study |
| WASID |
Warfarin-Aspirin Symptomatic Intracranial Disease |
| FDA |
Food and Drug Administration |
| ESUS |
Embolic Stroke of Undetermined Source |
| RE-SPECT ESUS |
Randomized Evaluation in Secondary Stroke Prevention Comparing the Thrombin Inhibitor Dabigatran Etexilate with Acetylsalicylic Acid (Aspirin) in Embolic Stroke of Undetermined Source |
| NAVIGATE ESUS |
New Approach Rivaroxaban Inhibition in Embolic Stroke of Undetermined Source |
| AHRE |
Atrial high-rate episodes |
| NOAH-AFNET 6 |
Non–Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High-Rate Episodes |
| ARTESiA |
Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Subclinical Atrial Fibrillation |
| BMI |
body mass index |
| PROFESS |
Prevention Regimen for Effectively avoiding Second Strokes |
| SBP |
systolic blood pressure |
| LAAC |
left atrial appendage closure |
| RCT |
randomized control trial |
| ELPASE |
Evaluation of Percutaneous Left Atrial Appendage Occlusion in Stroke Prevention on Top of Direct Oral Anticoagulant Therapy |
| LAA |
left atrial appendage |
| OCEANIC-AF |
Oral Anticoagulant to Prevent Ischemic Stroke in Patients With Atrial Fibrillation (Asundexian) Trial |
| LIBREXIA-AF |
LIBREXIA-Atrial Fibrillation: Evaluation of Milvexian in Stroke Prevention for Atrial Fibrillation Patients |
| LAAOS III |
Left Atrial Appendage Occlusion Study III |
| CONVINCE |
Colchicine for Prevention of Vascular Inflammation in Non-Cardio Embolic Stroke |
References
- Pu L, Wang L, Zhang R, et al. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke. 2023;54:1330–9. [CrossRef]
- Martin SS, Aday AW, Almarzooq ZI, et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2024;149. [CrossRef]
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [CrossRef]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. [CrossRef]
- Lin H-J, Wolf PA, Kelly-Hayes M, et al. Stroke Severity in Atrial Fibrillation. Stroke. 1996;27:1760–4. [CrossRef]
- Kimura K. Atrial fibrillation as a predictive factor for severe stroke and early death in 15 831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:679–83. [CrossRef]
- Marini C, De Santis F, Sacco S, et al. Contribution of Atrial Fibrillation to Incidence and Outcome of Ischemic Stroke. Stroke. 2005;36:1115–9. [CrossRef]
- Hylek EM, Go AS, Chang Y, et al. Effect of Intensity of Oral Anticoagulation on Stroke Severity and Mortality in Atrial Fibrillation. New England Journal of Medicine. 2003;349:1019–26. [CrossRef]
- Petty GW, Brown RD, Whisnant JP, et al. Ischemic Stroke Subtypes. Stroke. 2000;31:1062–8. [CrossRef]
- Carnicelli AP, Hong H, Connolly SJ, et al. Direct Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials With Interaction Testing by Age and Sex. Circulation. 2022;145:242–55. [CrossRef]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. [CrossRef]
- Lip GYH, Freedman B, De Caterina R, et al. Stroke prevention in atrial fibrillation: Past, present and future. Thromb Haemost. 2017;117:1230–9. [CrossRef]
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. The Lancet. 2014;383:955–62. [CrossRef]
- Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol Neurosurg Psychiatry. 2021;92:1062–7. [CrossRef]
- Xian Y, O’Brien EC, Liang L, et al. Association of Preceding Antithrombotic Treatment With Acute Ischemic Stroke Severity and In-Hospital Outcomes Among Patients With Atrial Fibrillation. JAMA. 2017;317:1057. [CrossRef]
- Paciaroni M, Caso V, Agnelli G, et al. Recurrent Ischemic Stroke and Bleeding in Patients With Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment With Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke. 2022;53:2620–7. [CrossRef]
- Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [CrossRef]
- Lakhan SE, Sapko MT. Blood pressure lowering treatment for preventing stroke recurrence: a systematic review and meta-analysis. Int Arch Med. 2009;2:30. [CrossRef]
- Gubitz G, Counsell C, Sandercock P, et al. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd 1999.
- Ní Chróinín D, Asplund K, Åsberg S, et al. Statin Therapy and Outcome After Ischemic Stroke. Stroke. 2013;44:448–56. [CrossRef]
- Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann Neurol. 2020;87:677–87. [CrossRef]
- Kaplan RC, Tirschwell DL, Longstreth WT, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. 2005;65:835–42. [CrossRef]
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149. [CrossRef]
- Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52. [CrossRef]
- Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2022;93:588–98. [CrossRef]
- Ip YMB, Lau KK, Ko H, et al. Association of Alternative Anticoagulation Strategies and Outcomes in Patients With Ischemic Stroke While Taking a Direct Oral Anticoagulant. Neurology. 2023;101. [CrossRef]
- Rota E, Testa L, Di Brigida G, et al. The management of patients with acute ischemic stroke while on direct oral anticoagulants (DOACs): data from an Italian cohort and a proposed algorithm. J Thromb Thrombolysis. 2020;50:732–8. [CrossRef]
- Stretz C, Wu TY, Wilson D, et al. Ischaemic stroke in anticoagulated patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2021;92:1164–72. [CrossRef]
- Galea R, Seiffge D, Räber L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. J Clin Med. 2023;12:5784. [CrossRef]
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis. New England Journal of Medicine. 2005;352:1305–16. [CrossRef]
- Hankey GJ. Warfarin-Aspirin Recurrent Stroke Study (WARSS) Trial. Stroke. 2002;33:1723–6. [CrossRef]
- Hsu JC, Freeman J V. Underuse of Vitamin K Antagonist and Direct Oral Anticoagulants for Stroke Prevention in Patients With Atrial Fibrillation: A Contemporary Review. Clin Pharmacol Ther. 2018;104:301–10. [CrossRef]
- Perreault S, de Denus S, White-Guay B, et al. Oral Anticoagulant Prescription Trends, Profile Use, and Determinants of Adherence in Patients with Atrial Fibrillation. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2020;40:40–54. [CrossRef]
- Kefale AT, Bezabhe WM, Peterson GM. Oral Anticoagulant Discontinuation and Its Predictors in Patients with Atrial Fibrillation. J Clin Med. 2022;11:6022. [CrossRef]
- Martinez C, Katholing A, Wallenhorst C, et al. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. Thromb Haemost. 2016;115:31–9. [CrossRef]
- Hindsholm MF, García Rodríguez LA, Brandes A, et al. Recurrent Ischemic Stroke in Patients With Atrial Fibrillation While Receiving Oral Anticoagulants. JAMA Neurol. 2024;81:805. [CrossRef]
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2011;365:981–92. [CrossRef]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2009;361:1139–51. [CrossRef]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine. 2011;365:883–91. [CrossRef]
- Steinberg BA, Shrader P, Thomas L, et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes. J Am Coll Cardiol. 2016;68:2597–604. [CrossRef]
- Beasley BN, Unger EF, Temple R. Anticoagulant Options — Why the FDA Approved a Higher but Not a Lower Dose of Dabigatran. New England Journal of Medicine. 2011;364:1788–90. [CrossRef]
- Paciaroni M, Agnelli G, Caso V, et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non–Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke. 2019;50:2168–74. [CrossRef]
- Diener H-C, Chutinet A, Easton JD, et al. Dabigatran or Aspirin After Embolic Stroke of Undetermined Source in Patients With Patent Foramen Ovale. Stroke. 2021;52:1065–8. [CrossRef]
- Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. New England Journal of Medicine. 2018;378:2191–201. [CrossRef]
- Geisler T, Keller T, Martus P, et al. Apixaban versus Aspirin for Embolic Stroke of Undetermined Source. NEJM Evidence. 2023;3. [CrossRef]
- Kamel H, Longstreth WT, Tirschwell DL, et al. Apixaban to Prevent Recurrence After Cryptogenic Stroke in Patients With Atrial Cardiopathy. JAMA. 2024;331:573. [CrossRef]
- Kirchhof P, Toennis T, Goette A, et al. Anticoagulation with Edoxaban in Patients with Atrial High-Rate Episodes. New England Journal of Medicine. 2023;389:1167–79. [CrossRef]
- Healey JS, Lopes RD, Granger CB, et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. New England Journal of Medicine. 2024;390:107–17. [CrossRef]
- Okumura K, Tomita H, Nakai M, et al. Risk Factors Associated With Ischemic Stroke in Japanese Patients With Nonvalvular Atrial Fibrillation. JAMA Netw Open. 2020;3:e202881. [CrossRef]
- Zheng S, Yao B. Impact of risk factors for recurrence after the first ischemic stroke in adults: A systematic review and meta-analysis. Journal of Clinical Neuroscience. 2019;60:24–30. [CrossRef]
- de Havenon A, Fino NF, Johnson B, et al. Blood Pressure Variability and Cardiovascular Outcomes in Patients With Prior Stroke. Stroke. 2019;50:3170–6. [CrossRef]
- Zhang L, Li X, Wolfe CDA, et al. Diabetes As an Independent Risk Factor for Stroke Recurrence in Ischemic Stroke Patients: An Updated Meta-Analysis. Neuroepidemiology. 2021;55:427–35. [CrossRef]
- Collado FMS, Lama von Buchwald CM, Anderson CK, et al. Left Atrial Appendage Occlusion for Stroke Prevention in Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2021;10. [CrossRef]
- Zeitler EP, Kearing S, Coylewright M, et al. Comparative Effectiveness of Left Atrial Appendage Occlusion Versus Oral Anticoagulation by Sex. Circulation. 2023;147:586–96. [CrossRef]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. New England Journal of Medicine. 2021;384:2081–91. [CrossRef]
- Cruz-González I, González-Ferreiro R, Freixa X, et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Revista Española de Cardiología (English Edition). 2020;73:28–34. [CrossRef]
- Jensen M, Suling A, Metzner A, et al. Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST-AFNET 4 trial. Lancet Neurol. 2023;22:45–54. [CrossRef]
- Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. New England Journal of Medicine. 2020;383:1305–16. [CrossRef]
- PASSMAN R, LEONG-SIT P, ANDREI A, et al. Targeted Anticoagulation for Atrial Fibrillation Guided by Continuous Rhythm Assessment With an Insertable Cardiac Monitor: The Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACT.COM) Pilot Study. J Cardiovasc Electrophysiol. 2016;27:264–70. [CrossRef]
- Waks JW, Passman RS, Matos J, et al. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: Results from the Tailored Anticoagulation for Non-Continuous Atrial Fibrillation (TACTIC-AF) pilot study. Heart Rhythm. 2018;15:1601–7. [CrossRef]
- Katsanos AH, Palaiodimou L, Price C, et al. An Updated Meta-Analysis of RCTs of Colchicine for Stroke Prevention in Patients with Coronary Artery Disease. J Clin Med. 2021;10:3110. [CrossRef]
- Koga M, Yoshimura S, Hasegawa Y, et al. Higher Risk of Ischemic Events in Secondary Prevention for Patients With Persistent Than Those With Paroxysmal Atrial Fibrillation. Stroke. 2016;47:2582–8. [CrossRef]
- Chen LY, Chung MK, Allen LA, et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018;137. [CrossRef]
- Calenda BW, Fuster V, Halperin JL, et al. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13:549–59. [CrossRef]
- Seiffge DJ, Cancelloni V, Räber L, et al. Secondary stroke prevention in people with atrial fibrillation: treatments and trials. Lancet Neurol. 2024;23:404–17. [CrossRef]
Figure 1.
Flowchart of patients included in the study. Abbreviations: AHS = acute hemorrhagic stroke, TIA = transient ischemic attack, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated.
Figure 1.
Flowchart of patients included in the study. Abbreviations: AHS = acute hemorrhagic stroke, TIA = transient ischemic attack, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated.
Figure 2.
Proportion of previous cardioembolic strokes in 128 AIS-AF patients Abbreviations: AIS = acute ischemic stroke, AF = atrial fibrillation, OAC = oral anticoagulant.
Figure 2.
Proportion of previous cardioembolic strokes in 128 AIS-AF patients Abbreviations: AIS = acute ischemic stroke, AF = atrial fibrillation, OAC = oral anticoagulant.
Figure 3.
ANOVA of predictors of recurrence risk of 128 AIS-AF patients Abbreviations: ANOVA = analysis of variance, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 3.
ANOVA of predictors of recurrence risk of 128 AIS-AF patients Abbreviations: ANOVA = analysis of variance, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 4.
Quality of anticoagulation by type of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist.
Figure 4.
Quality of anticoagulation by type of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist.
Figure 5.
Quality of anticoagulation by subgroup type of 89 OAC-AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 5.
Quality of anticoagulation by subgroup type of 89 OAC-AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 6.
ANOVA predictors of recurrence risk of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 6.
ANOVA predictors of recurrence risk of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation.
Figure 7.
Ridge regression with bootstrapping analysis of predictors of recurrence risk of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated.
Figure 7.
Ridge regression with bootstrapping analysis of predictors of recurrence risk of 89 OAC AIS-AF patients Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated.
Table 1.
Demographic and clinical characteristics of 128 OAC and Non-OAC AIS-AF patients.
Table 1.
Demographic and clinical characteristics of 128 OAC and Non-OAC AIS-AF patients.
| |
OAC (n = 89) |
Non-OAC (n = 39) |
p-value/OR [95% CI] |
| Demographic characteristics |
|
|
|
| Age, years, median (IQR) |
80 (46-95) |
81 (58-93) |
p = 0.904 |
| Sex, male, n (%) |
39 (43.8%) |
16 (41.0%) |
OR = 1.12 [0.52-2.40], p = 0.780 |
| Medical history, n (%) |
|
|
|
Current smoking
Alcohol
Hypertension
Diabetes mellitus |
6 (6.7%)
39 (43.8%)
83 (93.3%)
37 (41.6%) |
3 (7.7%)
14 (35.9%)
37 (94.9%)
19 (48.7%) |
OR = 0.87 [0.21-3.66], p = 0.850
OR = 1.39 [0.64-3.03], p = 0.399
OR = 0.81 [0.18-3.62], p = 0.677
OR = 0.86 [0.39-1.90], p = 0.717 |
| pre-mRS score, median (IQR) |
0 (0-4) |
0 (0-5) |
p = 0.720 |
| pre-mRS score, n (%) |
|
|
|
0
1
2
>2 |
51 (57.3%)
12 (13.5%)
11 (12.4%)
15 (16.9%) |
22 (56.4%)
8 (20.5%)
0 (0.0%)
9 (23.1%) |
OR = 0.86 [0.43-1.73], p = 0.677
OR = 0.65 [0.24-1.74], p = 0.394
p = 0.008
OR = 0.68 [0.27-1.71], p = 0.323 |
| NIHSS score at admission, median (IQR) |
6 (0-36) |
7 (2-21) |
p = 0.873 |
| Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, OR = (common) odds ratio, IQR = interquartile range, pre-mRS = premorbidity modified Rankin Scale, NIHSS = National Institute of Health Stroke Scale |
Table 2.
Outcomes of 128 OAC and Non-OAC AIS-AF patients.
Table 2.
Outcomes of 128 OAC and Non-OAC AIS-AF patients.
| |
OAC (n = 89) |
Non-OAC (n = 39) |
p-value/OR [95% CI] |
| Functional and safety outcome |
|
|
|
90-day mRS, median (IQR)
Mortality at 90 days, n (%)
ICH, n (%) |
5 (0-6) n = 82
30 (33.7%)
6 (6.7%) |
5 (0-6) n = 36
12 (30.8%)
0 (0.0%) |
p = 0.622
OR = 1.14 [0.51-2.57], p = 0.682
p = 0.465 |
| Previous CE stroke, n (%) |
17 (19.1%) |
7 (17.9%) |
OR = 1.08 [0.41-2.86], p = 0.870 |
| Time between strokes, years, median (IQR) |
6 (1-27) |
3 (1-21) |
p = 0.682 |
| AF known before previous CE stroke, n (%) |
7 (41.2%) |
3 (42.9%) |
OR = 0.93 [0.16-5.54], p = 0.856 |
| Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, OR = (common) odds ratio, IQR = interquartile range, mRS = modified Rankin Scale, ICH = intracranial hemorrhage, CE = cardioembolic |
Table 3.
Quality and type of anticoagulation of 89 OAC AIS-AF patients.
Table 3.
Quality and type of anticoagulation of 89 OAC AIS-AF patients.
| Quality of anticoagulation, n (%) |
|
Under-anticoagulated
Appropriately anticoagulated
Over-anticoagulated |
34 (38.2%)
48 (53.9%)
7 (7.9%) |
| Type of anticoagulation, n (%) |
|
DOAC
Apixaban
Edoxaban
Rivaroxaban
Dabigatran |
66 (74.2%)
43 (65.2%)
6 (9.1%)
9 (13.6%)
8 (12.1%) |
VKA
Warfarin
Acenocoumarol |
23 (25.8%)
10 (43.5%)
13 (56.5%) |
| Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, OR = (common) odds ratio, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist |
Table 4.
Demographic and clinical characteristics of 89 OAC AIS-AF patients stratified by type of anticoagulation.
Table 4.
Demographic and clinical characteristics of 89 OAC AIS-AF patients stratified by type of anticoagulation.
| |
DOAC (n = 66) |
VKA (n = 23) |
p-value/OR [95% CI] |
| Demographic characteristics |
|
|
|
| Age, years, median (IQR) |
81 (46-95) |
78 (50-91) |
p = 0.184 |
| Sex, male, n (%) |
29 (43.9%) |
10 (43.5%) |
OR = 1.02 [0.42-2.51], p = 0.964 |
| Medical history, n (%) |
|
|
|
Current smoking
Alcohol
Hypertension
Diabetes mellitus |
4 (6.1%)
30 (45.5%)
61 (92.4%)
27 (40.9%) |
2 (8.7%)
9 (39.1%)
22 (95.7%)
10 (43.5%) |
OR = 0.68 [0.12-3.86], p = 0.657
OR = 1.29 [0.51-3.28], p = 0.588
OR = 0.61 [0.07-5.36], p = 0.653
OR = 0.90 [0.36-2.22], p = 0.820 |
| Monitoring parameters, median (IQR) |
|
|
|
Weight, kg
Creatinine, µmol/l
GFR, ml/min/1.73 m²
INR at admission |
77.4 (43-130)
91 (48-200)
55 (19->90)
1.2 (0.9-2.4) |
-
-
-
1.6 (1.0-6.9) |
- -
- -
- -
p = 0.345 |
| pre-mRS score, median (IQR) |
0 (0-4) |
0 (0-4) |
p = 0.629 |
| pre-mRS score, n (%) |
|
|
|
0
1
2
>2 |
37 (56.1%)
11 (16.7%)
9 (13.6%)
9 (13.6%) |
14 (60.9%)
1 (4.4%)
2 (8.7%)
6 (26.1%) |
OR = 0.84 [0.34-2.09], p = 0.693
OR = 4.04 [0.49-33.4], p = 0.187
OR = 1.66 [0.31-8.94], p = 0.558
OR = 0.45 [0.13-1.39], p = 0.166 |
| NIHSS score at admission, median (IQR) |
6 (0-36) |
7 (2-21) |
p = 0.964 |
| Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, OR = (common) odds ratio, IQR = interquartile range, GFR = glomerular filtration rate, INR = international normalized ratio, pre-mRS = premorbidity modified Rankin Scale, NIHSS = National Institute of Health Stroke Scale |
Table 5.
Outcomes of 89 OAC AIS-AF patients stratified by type of anticoagulation.
Table 5.
Outcomes of 89 OAC AIS-AF patients stratified by type of anticoagulation.
| |
DOAC (n = 66) |
VKA (n = 23) |
p-value/OR [95% CI] |
| Functional and safety outcome |
|
|
|
90-day mRS, median (IQR)
Mortality at 90 days, n (%)
ICH, n (%) |
5 (0-6) n = 60
23 (34.9%)
3 (4.6%) |
3 (0-6) n = 22
7 (30.4%)
3 (13.0%) |
p = 0.379
OR = 1.23 [0.47-3.24], p = 0.683
OR = 0.32 [0.06-1.66], p = 0.176 |
| Previous CE stroke, n (%) |
13 (19.7%) |
4 (17.4%) |
OR = 1.17 [0.34-4.05], p = 0.800 |
| Time between strokes, years, median (IQR) |
6 (1-27) |
5 (3-19) |
p = 0.741 |
| AF known before previous CE stroke, n (%) |
4 (6.1%) |
3 (13.0%) |
OR = 0.43 [0.10-1.91], p = 0.267 |
Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, OR = (common) odds ratio, IQR = interquartile range, mRS = modified Rankin Scale, ICH = intracranial hemorrhage, CE = cardioembolic
|
Table 6.
Demographic and clinical characteristics of 89 OAC AIS-AF patients stratified by quality of anticoagulation.
Table 6.
Demographic and clinical characteristics of 89 OAC AIS-AF patients stratified by quality of anticoagulation.
| |
Under (n = 34) |
Appro (n = 48) |
Over (n = 7) |
p-value |
| Demographic characteristics |
|
|
|
|
Age, years, median (IQR)
Sex, male, n (%) |
82.5 (50-95)
14 (41.2%) |
79.5 (46-95)
23 (47.9%) |
80 (63-90)
2 (28.6%) |
p = 0.506
p = 0.590 |
| Medical history, n (%) |
|
|
|
|
Current smoking
Alcohol
Hypertension
Diabetes mellitus |
1 (2.9%)
16 (47.1%)
32 (94.1%)
13 (38.2%) |
5 (10.4%)
21 (43.8%)
44 (91.7%)
19 (39.6%) |
0 (0.0%)
2 (28.6%)
7 (100%)
5 (71.4%) |
p = 0.322
p = 0.676
p = 0.699
p = 0.253 |
| pre-mRS score, median (IQR) |
0 (0-4) |
0 (0-4) |
0 (0-1) |
p = 0.156 |
| pre-mRS score, n (%) |
|
|
|
|
0
1
2
>2 |
18 (52.9%)
4 (11.8%)
3 (8.8%)
9 (26.5%) |
27 (56.3%)
7 (14.6%)
8 (16.7%)
6 (12.5%) |
6 (85.7%)
1 (14.3%)
0 (0.0%)
0 (0.0%) |
p = 0.280
p = 0.935
p = 0.341
p = 0.118 |
| NIHSS score at admission, median (IQR) |
8 (0-36) |
6 (0-24) |
3 (1-11) |
p = 0.239 |
| Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated, IQR = interquartile range, pre-mRS = premorbidity modified Rankin Scale, NIHSS = National Institute of Health Stroke Scale |
Table 7.
Outcomes of 89 OAC AIS-AF patients stratified by quality of anticoagulation.
Table 7.
Outcomes of 89 OAC AIS-AF patients stratified by quality of anticoagulation.
| |
Under (n = 34) |
Appro (n = 48) |
Over (n = 7) |
p-value |
| Functional and safety outcome |
|
|
|
|
90-day mRS, median (IQR)
Mortality at 90 days, n (%)
ICH, n (%) |
5 (0-6) n = 33
12 (35.3%)
3 (8.8%) |
5 (0-6) n = 43
17 (35.4%)
3 (6.3%) |
1.5 (0-6) n = 6
1 (14.3%)
0 (0.0%) |
p = 0.349
p = 0.536
p = 0.692 |
| Previous CE stroke, n (%) |
5 (14.7%) |
9 (18.8%) |
3 (42.9%) |
p = 0.231 |
Time between strokes, years, median (IQR)
AF known before previous CE stroke, n (%) |
6 (3-26)
4 (80.0%) |
5 (1-27)
3 (33.3%) |
6 (2-6)
0 (0.0%) |
p = 0.592
p = 0.067 |
Abbreviations: OAC = oral anticoagulant, AIS = acute ischemic stroke, AF = atrial fibrillation, Under = under-anticoagulated, Appro = appropriately anticoagulated, Over = over-anticoagulated, IQR = interquartile range, mRS = modified Rankin Scale, ICH = intracranial hemorrhage, CE = cardioembolic
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).