Submitted:
04 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
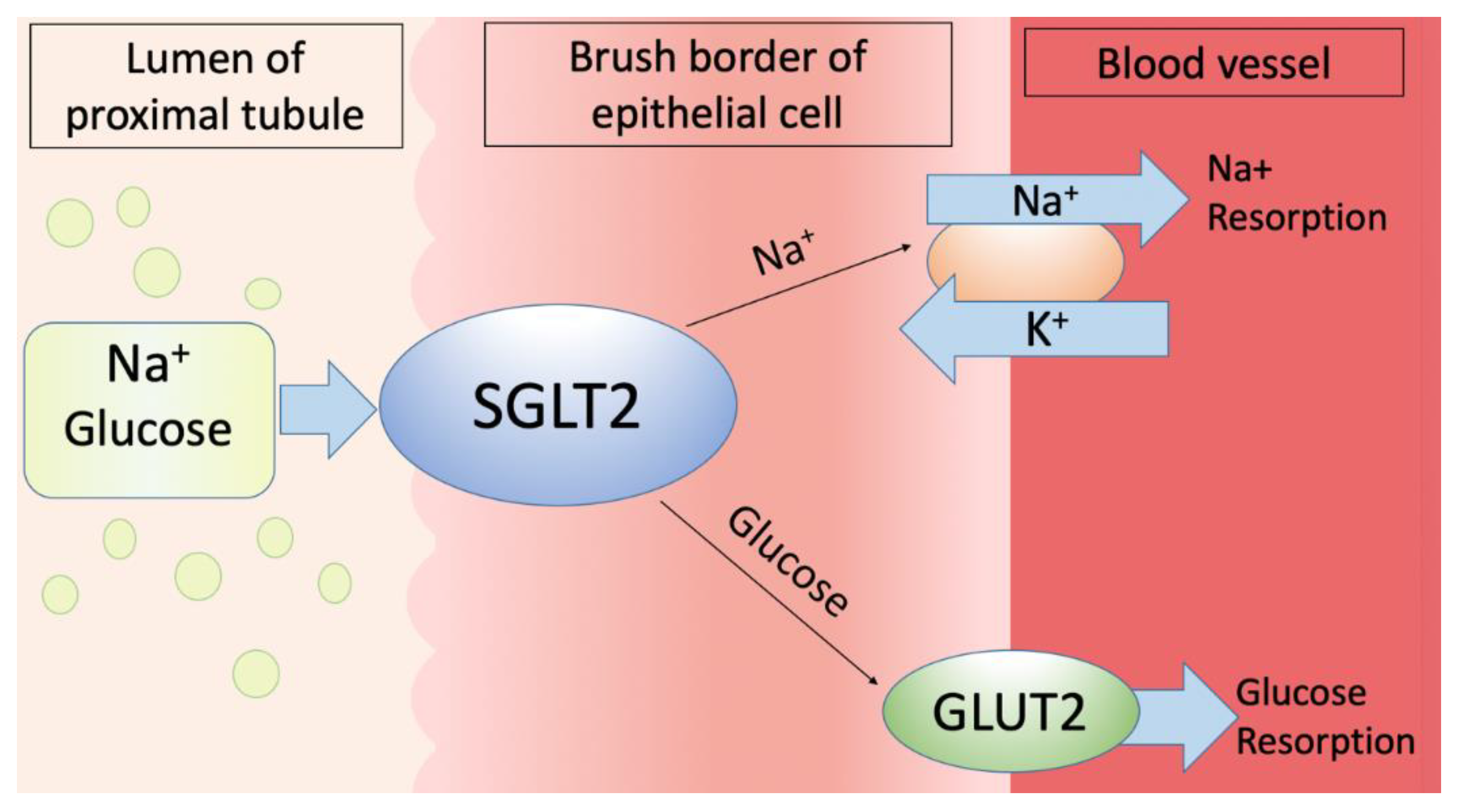
2. Materials and Methods
3. Results
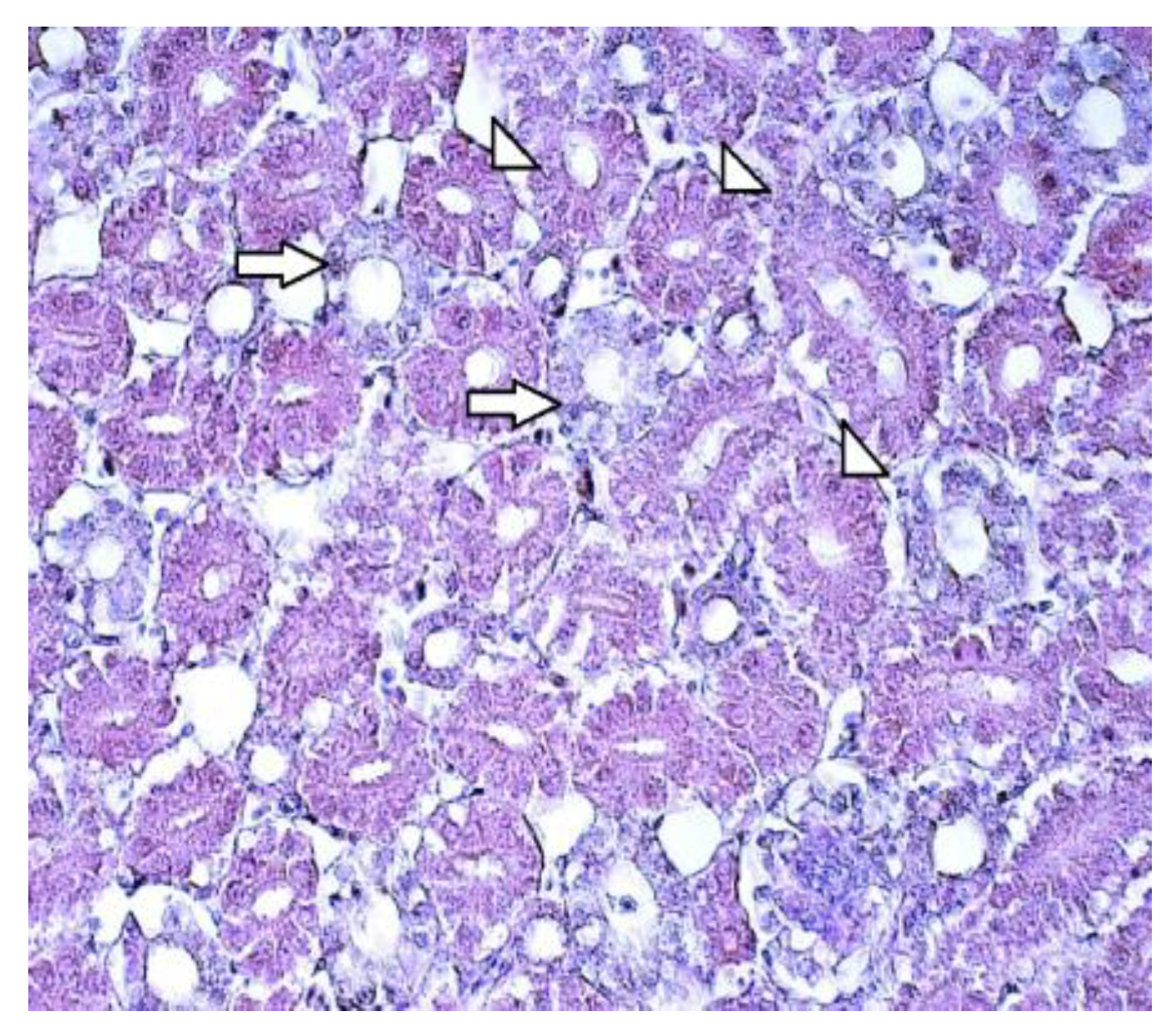
3.1. Routine Histology
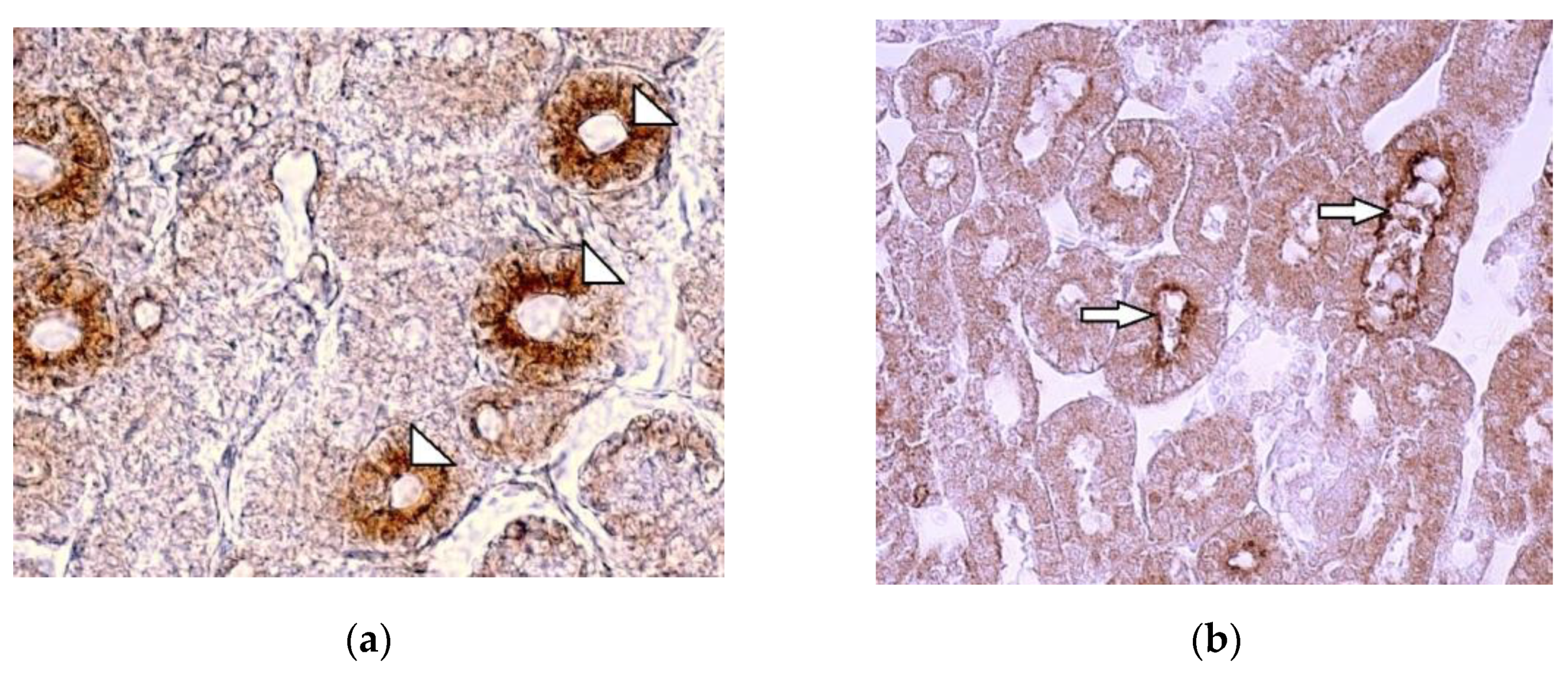
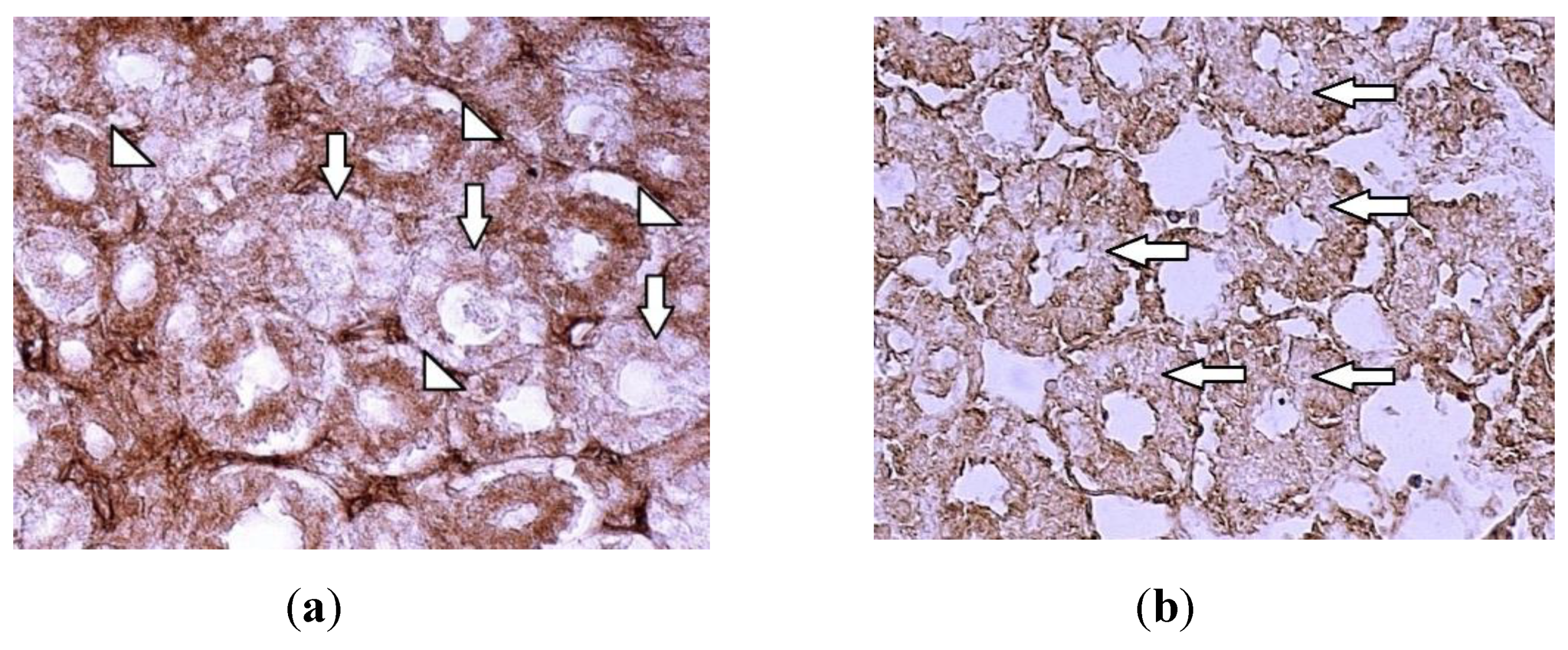
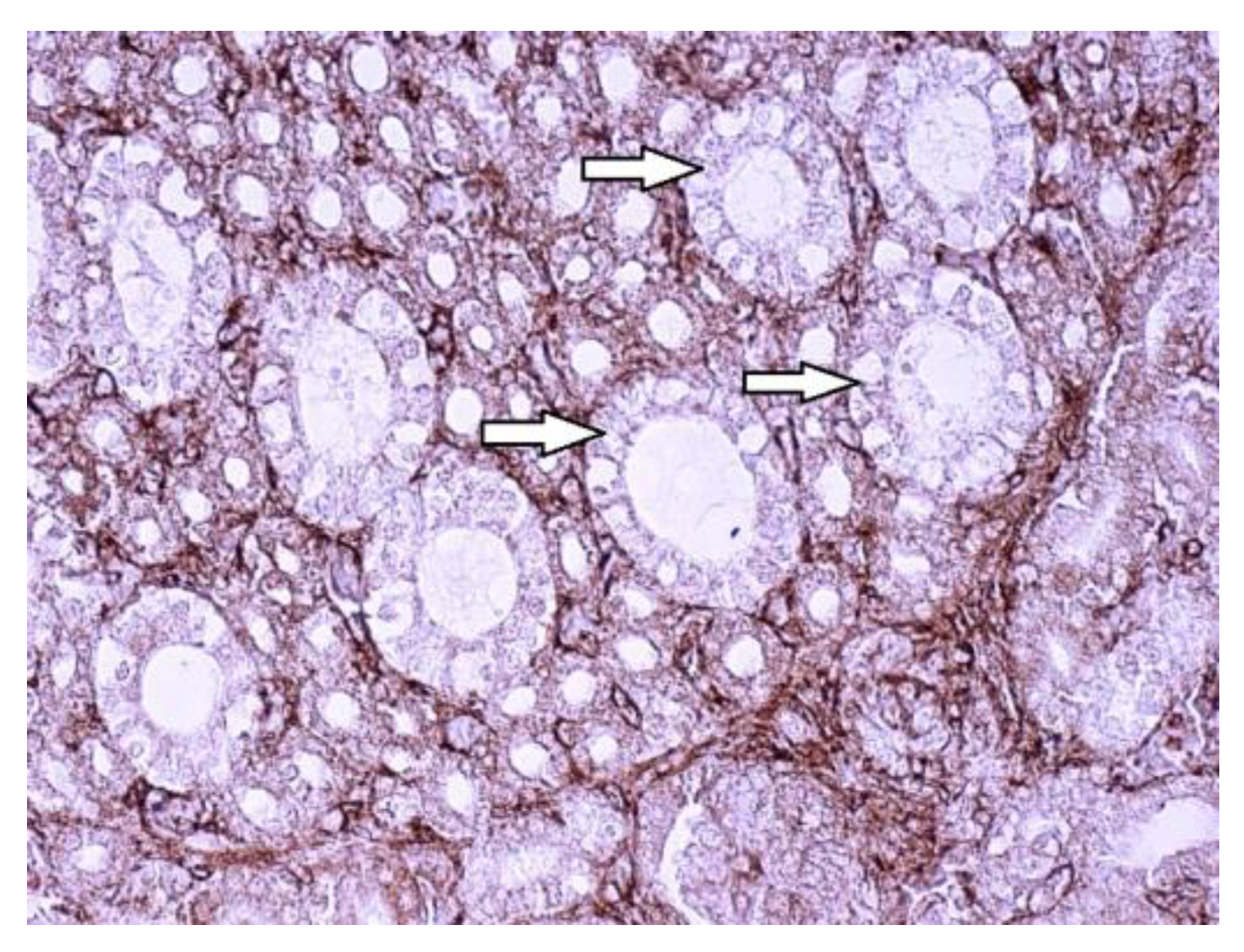
3.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, V.J. In Molecular and Cellular Endocrinology, Bittar, E. ; Bittar, N., Eds.; JAI Press Inc., Greenwhich, USA, 1997, 10, 387–401. [Google Scholar]
- Mota, M.; Mota, E.; Dinu, I.R. In Treatment of Type 2 Diabetes; Croniger, C., Ed.; IntechOpen: Rijeka, Crotaia, 2015, Chapter 1, pp.13-17.
- König, H.E.; Korbel, R.; Liebich, H.G. Avian Anatomy textbook and colour atlas; 5m Publishing: Sheffield, UK, 2016; Volume 2, pp: 131-133. [Google Scholar]
- Vallon, V.; Thomson, S.C. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M.D.S. Efficacy, Safety and Regulatory Status of SGLT2 Inhibitors: Focus on Canagliflozin. Nutr. Diabetes. 2014, 4, e143. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.; Pollock, C. Glucose Handling by the Kidney. Kidney Int. 2011, 79, S1–S6. [Google Scholar] [CrossRef]
- Takata, K. Glucose Transporters in the Transepithelial Transport of Glucose. J. Electron Microsc. 1996, 45, 275–284. [Google Scholar] [CrossRef]
- Sano, R.; Shinozaki, Y.; Ohta, T. Sodium–Glucose Cotransporters: Functional Properties and Pharmaceutical Potential. J. Diabetes Investig. 2020, 11, 770–782. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose Transporters: Physiological and Pathological Roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Raja, M.; Puntheeranurak, T.; Hinterdorfer, P.; Kinne, R. SLC5 and SLC2 Transporters in Epithelia—Cellular Role and Molecular Mechanisms. In Current Topics in Membranes; Mark O. Bevensee Ed.; Academic Press, 2012; 70, pp.29-76.
- Allmang, C.; Hussar, P.; Järveots, T.; Duritis, I. Sodium-glucose co-transporter SGLT2 in kidneys of chicken in different ages. In ACB 2024, 57, 300: 21st Congress of the International Federation of Associations of Anatomists in conjunction with the 74th Annual Meeting of the Korean Association of Anatomists, Kimdaejung Convention Center, Gwangju, South-Korea, 5-8.09.2024.
- You, G.; Lee, W.-S.; Barros, E.J.G.; Kanai, Y.; Huo, T.-L.; Khawaja, S.; Wells, R.G.; Nigam, S.K.; Hediger, M.A. Molecular Characteristics of NA+-Coupled Glucose Transporters in Adult and Embryonic Rat Kidney. J. Biol. Chem. 1995, 270, 29365–29371. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence. Diabetes Metab. Syndr. Obes. 2020, 13, 161–174. [Google Scholar] [CrossRef]
- Horiba, N.; Masuda, S.; Takeuchi, A.; Takeuchi, D.; Okuda, M.; Inui, K.-I. Cloning and Characterization of a Novel NA+-Dependent Glucose Transporter (NAGLT1) in Rat Kidney. J. Biol. Chem. 2003, 278, 14669–14676. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P.; Dūrītis, I.; Popovska-Percinic, F.; Järveots, T. Short communication : Immunohistochemical study of sodium-dependent glucose co-transporters in ostriches kidneys. Agraarteadus J. Agric. Sci. 2020, 31, 147–150. [Google Scholar] [CrossRef]
- Hussar, P.; Allmang, C.; Popovska-Percinic, F.; Järveots, T.; Dūrītis, I. Comparative Study of Sodium-Dependent Glucose Co-Transporters in Kidneys of Ostrich Chickens. Sci. Hori. 2022, 25, 30–35. [Google Scholar] [CrossRef]
- Hussar, P.; Kaerner, M.; Duritis, I.; Plivca, A.; Pendovski, L.; Jaerveots, T.; Popovska-Percinic, F. Temporospatial Study of Hexose Transporters and Mucin in the Epithelial Cells of Chicken (Gallus Gallus Domesticus) Small Intestine. Pol. J. Vet. Sci. 2017, 20, 627–633. [Google Scholar] [CrossRef]
- Varzakas, T.; Agriopoulou, S.; Stamatelopoulou, E.; Mycotoxin. Encyclopedia. 2024. Available online: https://encyclopedia.pub/entry/22939 (accessed on 26 September 2024).
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of Temperature on Infection, Growth, and Mycotoxin Production by Fusarium Langsethiae and F. Sporotrichioides in Durum Wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium Mycotoxins T-2 Toxin and HT-2 Toxin in Wheat. J. Agric. Food. Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef]
- Nayakwadi, S.; Ramu, R.; Sharma, A.K.; Gupta, V.K.; Rajukumar, K.; Kumar, V.; Shirahatti, P.S.; L, R.; Basalingappa, K.M. Toxicopathological Studies on the Effects of T-2 Mycotoxin and Their Interaction in Juvenile Goats. PLoS One. 2020, 15, e0229463. [Google Scholar] [CrossRef]
- Edwards, S.G.; Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C. Molecular Studies to Identify the Fusarium Species Responsible for HT-2 and T-2 Mycotoxins in UK Oats. Int. J. Food Microbiol. 2012, 156, 168–175. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Maragos, C.M.; Visconti, A. Improvement of Detection Sensitivity of T-2 and HT-2 Toxins Using Different Fluorescent Labeling Reagents by High-Performance Liquid Chromatography. Talanta 2008, 74, 1476–1483. [Google Scholar] [CrossRef]
- Kiš, M.; Vulić, A.; Kudumija, N.; Šarkanj, B.; Tkalec, V.J.; Aladić, K.; Škrivanko, M.; Furmeg, S.; Pleadin, J. A Two-Year Occurrence of Fusarium T-2 and HT-2 Toxin in Croatian Cereals Relative of the Regional Weather. Toxins. 2021, 13, 39. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules. 2021, 26, 6868. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Wu, W.; Mitchell, N.J.; Bursian, S.; Pestka, J.J.; Wu, F. Modeling the Emetic Potencies of Food-Borne Trichothecenes by Benchmark Dose Methodology. Food Chem. Toxicol. 2016, 94, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.S.; Mirocha, C.J.; Kurtz, H.J.; Weaver, G.; Bates, F.; Shimoda, W. Effects of T-2 Toxin on Reproductive Performance and Health of Laying Hens, Poult. Sci. 1977, 56, 628–637. [Google Scholar] [CrossRef]
- Henghold, W.B. 2nd. Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin, 22. [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A Review on Mycotoxins in Food and Feed: Malaysia Case Study. Compr Rev Food Sci Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Cope, R.B. In Veterinary Toxicology; 3rd ed.; Gupta, R.C., Ed., Academic Press: Cambridge, MA, USA, 2018, 3, 1043–1053.
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer ofFusariummycotoxins and ‘Masked’ Deoxynivalenol (Deoxynivalenol-3-Glucoside) from Field Barley through Malt to Beer. Ood Addit. Contam. - Chem. Anal. Control Expo. Risk Assess. 2008, 25, 732–744. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains – an Update. Toxins. 2019, 11, 634. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and Biological Transformations for Detoxification of Trichothecene Mycotoxins in Human and Animal Food Chains: A Review. Trends Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Carson, F.L. Histotechnology: A Self-Instructional Text; 2nd ed. ASCP Press: Chicago, IL, USA, 1997. [Google Scholar]
- Deepa, K.P.; Sreeranjini, A.R.; Soumya, C.B.; Mayadany, S.; Sunilkumar, N.S.; Sumena, K.B. Comparative histological studies on the renal medulla in broiler chicken and broiler duck. Int J Vet Sci Anim Husbandry 2021, 6, 11–14. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Medical physiology. 3rd ed.; Elsevier, Philadelphia, USA, 2016; p. 727.
- Triplitt, C.L. Understanding the kidneys' role in blood glucose regulation. Am J Manag Care. 2012, 18, S11–S16. [Google Scholar] [PubMed]
- Allmang, C.; Hussar, P. ; Duriti,s I. ; Järveots, T. Immunolocalization of sodium-dependent glucose co-transporter 1 and sodium-dependent glucose co-transporter 2 in chicken’s (Gallus gallus domesticus) kidneys, Pol. J. Vet. Sci. 2024, 27, 323–328. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Yu, J.; Ehrlich, K.C. Toxins of filamentous fungi. Chem Immunol. 2002, 81, 167–206. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc., 2011, 2, 129–144. [Google Scholar] [CrossRef]
- Türker, L.; Gümüş, S. A Theoretical Study on Vomitoxin and Its Tautomers. J. Hazard. Mater. 2009, 163, 285–294. [Google Scholar] [CrossRef]
- Devreese, M.; De Backer, P.; Croubels, S. Verschillende Methoden Om Mycotoxineproductie En de Impact Op de Diergezondheid Tegen Te Gaan. Vlaams Diergeneeskundig Tijdschrift. 2013, 82. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: toxicological effects and decontamination strategies. Oncotarget. 2017, 8, 33933–33952. [Google Scholar] [CrossRef]
- Stoev, S.D.; Diakov, L.; Koynarski, V.; Angelov, A. In Special pathology and diagnostics of mycoses, mycotoxicoses, parasitoses, intoxications and avitaminoses; Publishing House CD Contrast: Stara Zagora; Bulgaria, 2010, pp. 1–239.
- Wright, E.M. Renal Na+-Glucose Cotransporters. Am. J. Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Sudakin, D.L. Trichothecenes in the Environment: Relevance to Human Health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Ueno, Y. Toxicological Features of T-2 Toxin and Related Trichothecenes. Fundam. Appl. Toxicol. 1984, 4, S124–S132. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.-Y.; Kuča, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative Stress-Mediated Cytotoxicity and Metabolism of T-2 Toxin and Deoxynivalenol in Animals and Humans: An Update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
| Antibody | Chicken nr. | Control group | T-2 toxin group |
|---|---|---|---|
| SGLT1* | 1 2 3 4 5 6 7 |
++ +++ ++ ++ ++ ++ ++ |
++ + + + ++ + + |
| SGLT2* | 1 | +++ | + |
| 2 | +++ | ++ | |
| 3 | ++ | ++ | |
| 4 | +++ | + | |
| 5 | ++ | + | |
| 6 | +++ | + | |
| 7 | +++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





