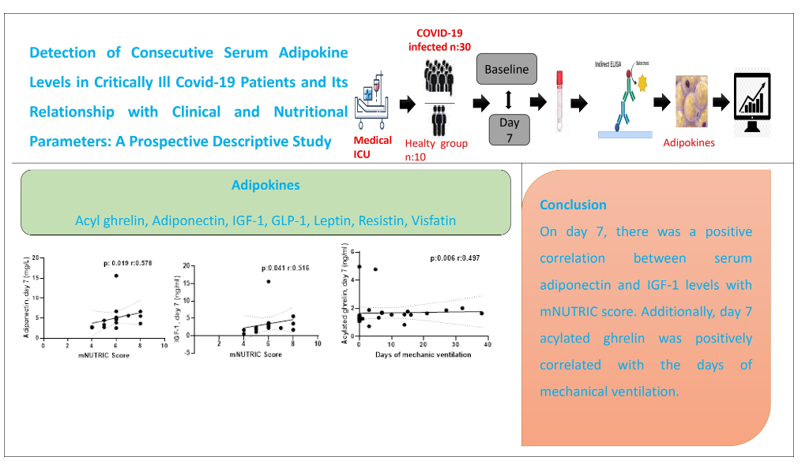
1. Introduction
Critical illness and COVID-19 pose a significant threat to life and necessitate the usage of pharmacological and mechanical interventions to maintain crucial organ function [
1,
2]. Critical illness and COVID-19 cause global mortality and growing economic burden (including sepsis, acute respiratory distress syndrome [ARDS], and multi-organ failure) [
3,
4]. Despite the significant advances in molecular medicine, research into critical illness has yet to produce targeted therapies after decades of effort [
5]. As a result, critical care essentially amounts to supportive care in practice [
6]. It is widely recognized that adipose tissue secretes hormones and cytokines and affects the function of many organs [
7]. These hormones or cytokines regulate metabolic and immune homeostasis by communicating with remote organs through precise receptors [
8]. Adipokines play a role in regulating various inflammatory pathways [
9,
10]. Given their impact on immune system function, adipokines are considered to play key roles in the outcomes of critical illness. Recently, there have been limited reports regarding the correlation between sequential circulation adipokines and critically ill ARDS patients [
11].
Elevated levels of pro-inflammatory adipokines are associated with worse clinical manifestations and a poor prognosis in patients with COVID-19. It has been established that the dysregulation of adipokines plays a role in the heightened inflammation and increased risk of severe outcomes, including acute respiratory distress syndrome (ARDS), observed in patients with COVID-19 [
12].
In this study, our goal is to investigate the sequential changes in blood adipokines and the association between clinical and nutritional outcomes in intensive care unit (ICU) patients with severe respiratory failure caused by SARS-CoV-2.
2. Materials and Methods
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code. Patient and Control Study Group
The prospective study was carried out at the Medical ICU of Kayseri City Hospital from September to November 2022. The study involved 30 patients who are 18 years old or older, along with a control group comprising 10 healthy volunteers. The patients were admitted to the ICU and stayed there for at least seven days. The age and sex of the control group without comorbidities were matched with the patients.
Age, gender, height, weight, body mass index (BMI), The Acute Physiology and Chronic Health Evaluation (APACHE II) score, and modified Nutrition Risk Score (mNUTRIC) data were collected. Patients were monitored for seven days following admission to the ICU. Glasgow Coma Scores (GCS) were recorded after admission to the ICU and Sequential Organ Failure Assessment (SOFA) scores were calculated daily. Mechanical ventilation (MV) status and vasopressor requirement were documented. Daily dietary patterns and calorie intake were also recorded. The length of stay in the ICU and hospital, duration of MV, and the data on mortality in the ICU and hospital, were recorded. The timing, route, dose, and composition of nutrition were adjusted according to the guidelines published by the European Society for Clinical Nutrition and Metabolism (ESPEN) association in 2023 [
13].
2.1. Sample Collection and Laboratory Analyses
The study population comprised patients with laboratory-confirmed SARS-CoV-2 infection and who had been followed up in the ICUs with respiratory failure for more than 24 hours. The diagnosis was confirmed by a PCR assay of nasal and pharyngeal swabs or endotracheal aspirate, in accordance with the World Health Organization (WHO) guidelines [
14]. Samples of blood were obtained from the patients at ICU admission and on the 7th day. Blood samples were collected concurrently with the routine blood samples obtained from the patients at 10:00. The samples were subjected to centrifugation at 5,000 rpm for a period of 10 minutes within a 30-minute timeframe. Laboratory analyses of human AG, adiponectin, GLP-1, IGF-1, leptin, resistin and visfatin proteins were performed with ELISA kits in accordance with the procedures at Erciyes University Biochemistry Department Laboratory.
ELISA measurement of adipokines: ELISA KIT, Bioassay Technology Laboratory, Shanghai, China
Acyl ghrelin: Catalogue code: E3090Hu, Human Acylated ghrelin, Adiponectin: Catalogue code: E1550Hu Human Adiponectin, Ghrelin: Catalogue code: E3091Hu Human Ghrelin, GLP-1: Catalogue code: E0022Hu Human GLP-1, IGF-1: Catalogue code: E0103Hu Human IGF-1, Leptin: Catalogue code: E1559Hu Human Leptin, Resistin: Catalogue code: E0338Hu Human Resistin, Visfatin: Catalogue code: E0025Hu Human Visfatin
2.2. Statistical Analysis
Statistical Package for the Social Sciences (SPSS) 13.0 and Graphpad Prism applications were used for data analysis. Continuous data following a normal distribution were expressed as mean± standard deviation (SD) and non-parametric data as median (25-75 interquartile range (IQR)). The Shapiro-Wilk test was utilized to evaluate normality distribution. Spearman rank correlation analysis was used for statistical analysis of non-normally distributed continuous data and Pearson correlation analysis was used for analysis of normally distributed continuous data. Mann-Whitney U test was used to analyse categorical data with non-normally distributed continuous data and Student t test was used to analyse normally distributed continuous data. Friedman analysis was performed for the analysis of consecutive data. For the significance value of other analyses, P <0.05 was accepted.
2.3. Ethics Statement
Ethics committee approval (coded 2021/357 and dated 05.05.2021) was obtained for the research. Prior to enrolment, patients or their first-degree relatives were provided with an explanation of the study and asked to sign a written consent form. Individuals whose consciousness improved during their stay in the ICU were re-consented. Patients who declined participation at any stage were excluded from the study.
3. Results
This study included 30 critically ill patients and 10 healthy control individuals. The mean age of the patients was 70±16 years, and 43% were female. The median APACHE-II scores of patients was 20,5 (IQR= 15.75-28) and the mean mNUTRIC score was 5±1. Need to vasopressor and invasive mechanical respiratory support (IMV) of patients was 30%, 40% respectively. The median duration of IVM was 3 days (IQR:2-14). (
Table 1)
Table 2 shows the median calorie intake of the evaluated patients. On the day of admission, 4 patients did not receive any nutrition while 26 patients received some route of nutritional therapy. The patients with the highest median calorie intake were patient 1 (1332, min-max:1296-1818), patient 26 (1440 min-max:580-1584), and patient 30 (1452 min-max:0-1652).
Upon admission, 13% of the patients received enteral tube feeding. By the 7th day of follow-up, this figure had increased to 27%. In terms of parenteral nutrition (PN), 27% of patients received PN upon admission, but this rate decreased to 10% by the 7th day of follow-up. Patients who were conscious and do not need MV were fed a hospital diet, which accounted for 60-63% of all cases. (
Table 3).
Median daily calorie intake per kilogram of weight of the patients during their ICU stay were 6.6-11 kcal/kg/day. On day 5 of the seven-day follow-up in the ICU, the patients reached their maximum calorie intake of 11 kcal/kg/day.
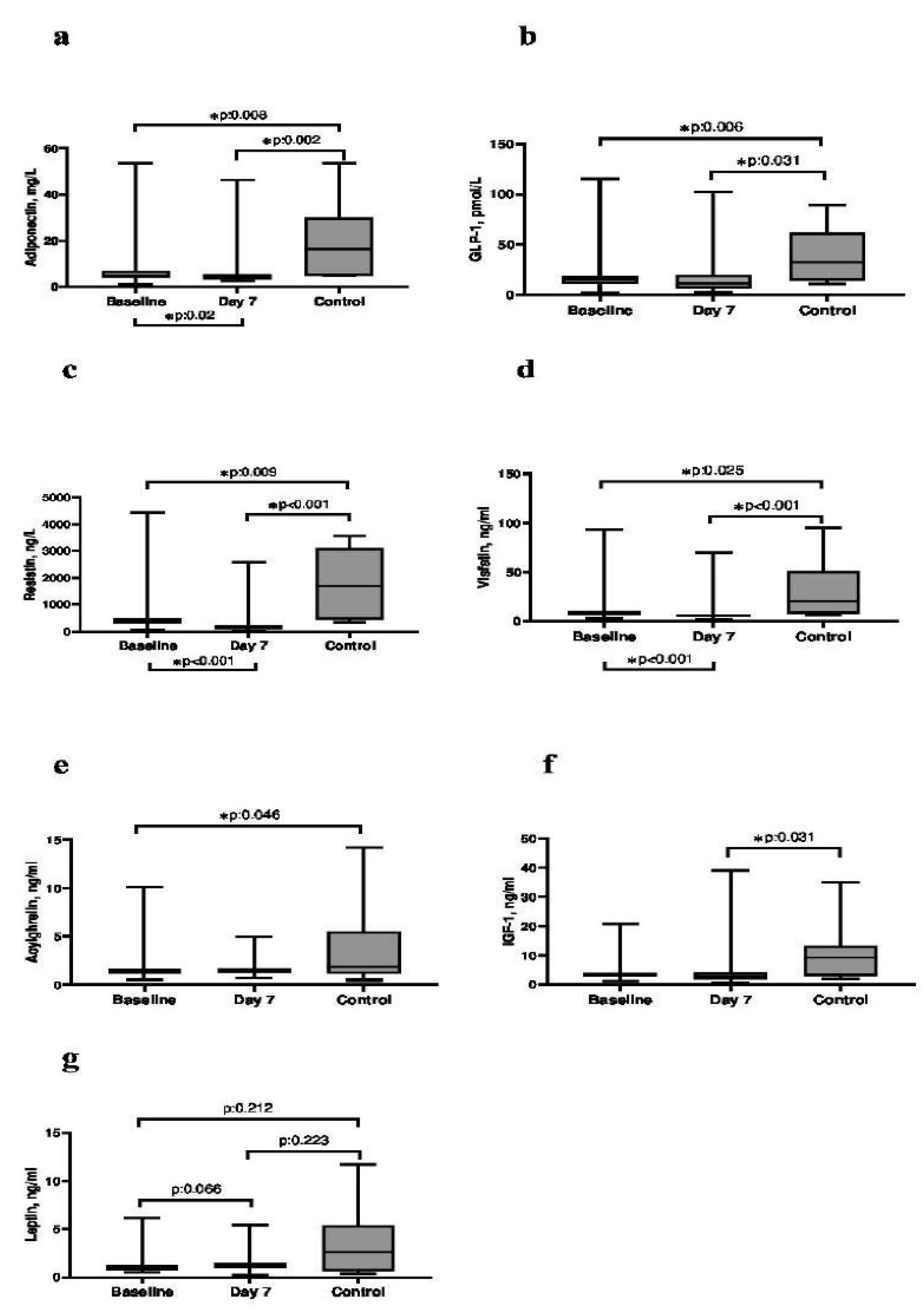
The levels of admission day and 7th day serum adiponectin (p = 0.008 and 0.002), GLP-1(p = 0.006 and 0.031), resistin (p = 0.009 and <0.001) and visfatin (p = 0.025 and <0.001) were significantly lower in the patient compared to the control group. The baseline serum AG level was significantly lower in the patient group compared to the control group (p = 0.046). Day 7 Serum IGF-1 level was significantly lower in the patient group compared to the control group (p = 0.031). Median serum adipokine levels of patients and control subjects and the relationship between control subjects and consecutive days was shown in Figure 2.
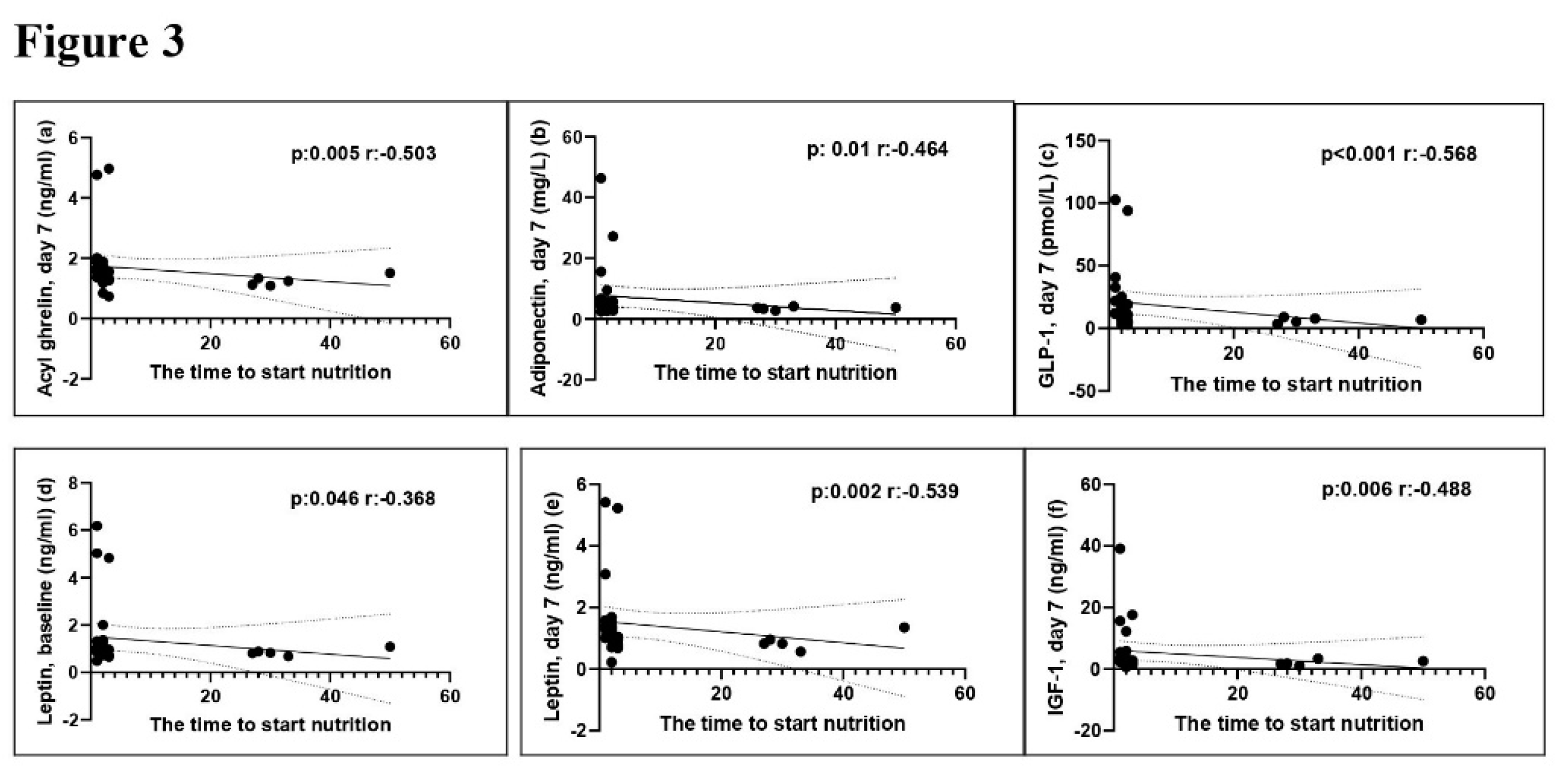
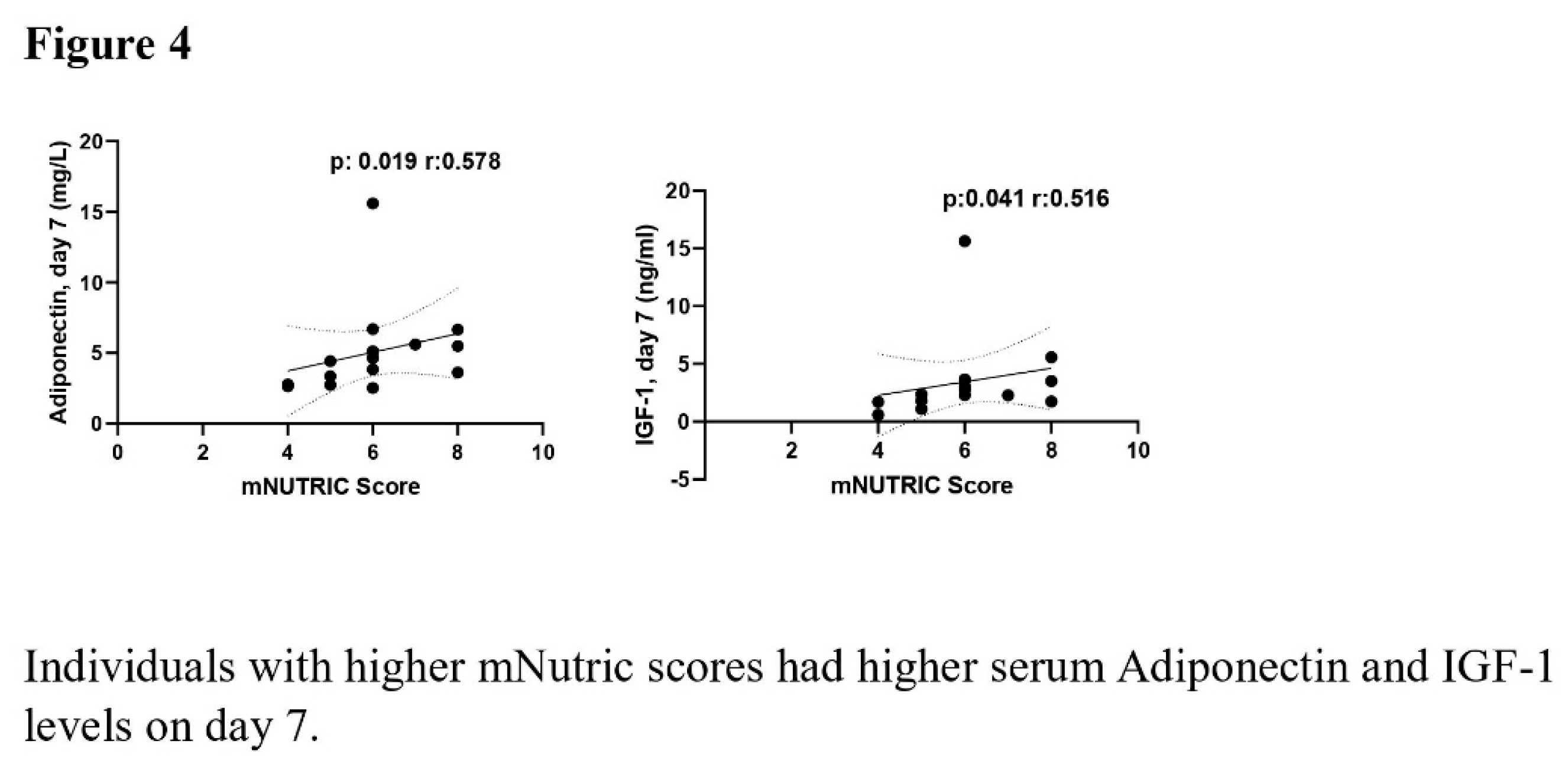
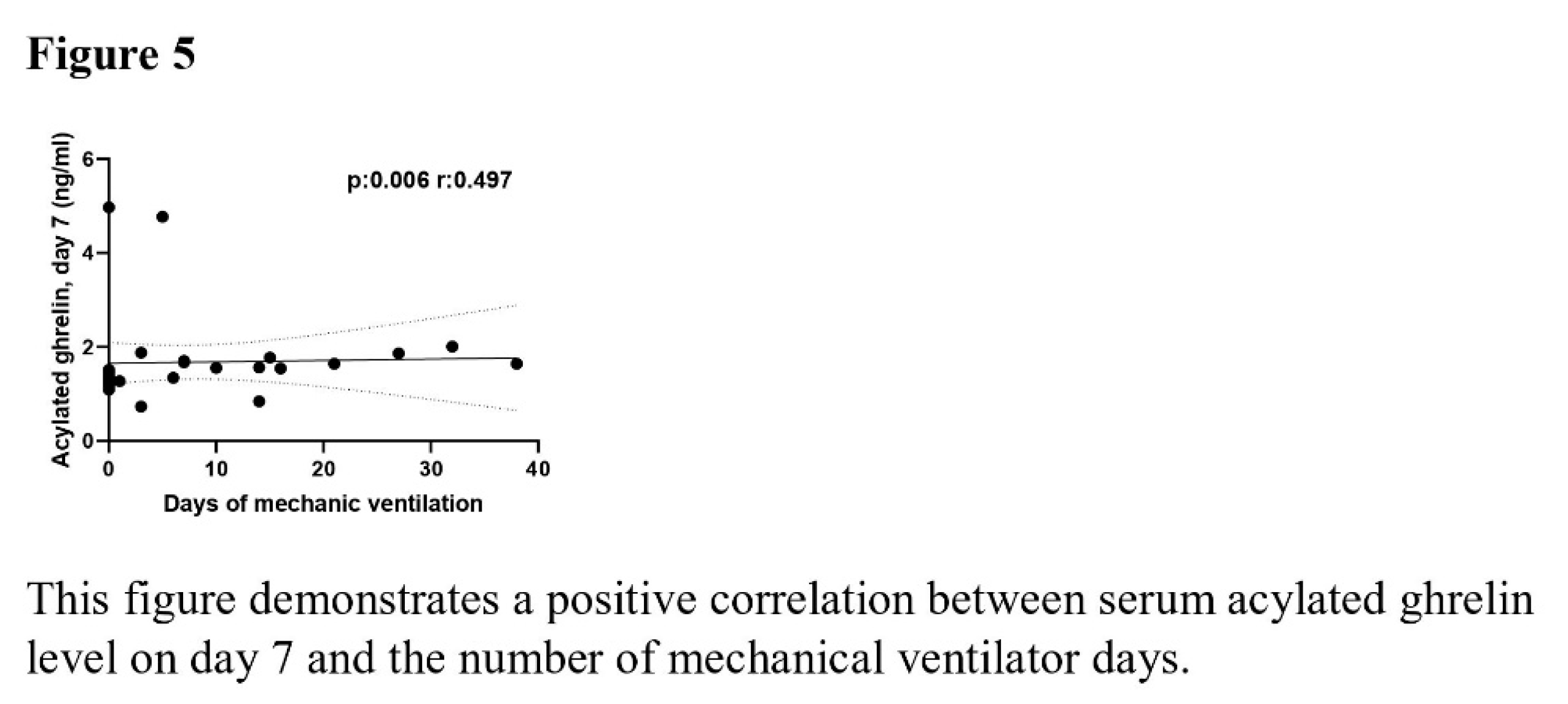
The correlation analysis revealed a negative correlation between time to start nutrition and the levels of baseline (p =0.046, r= -0.368) and day 7 (p =0.002, r= -0.539) serum leptin, day 7 serum AG (p =0.005, r= -0.503), day 7 serum adiponectin (p =0.01, r= -0.464), day 7 serum GLP-1 (p <0.001, r= -0.568), and day 7 serum IGF-1 (p =0.001, r= -0.488). The mNUTRIC score demonstrated a positive association with Day 7 serum adiponectin and IGF-1 levels (p =0.019, r= 0.578 and p=0.041, r= 0.516). The serum AG levels on day 7 demonstrated a positive correlation with the duration of MV (p =0.006, r= 0.497). The relationship between time to start nutrition, mNUTRIC score and duration of MV with serum adipokine levels was shown in Figures 3–5.
Median length of stay in ICU and hospital were 10 (IQR: 7-18), 14 (IQR:10-21) respectively. ICU and hospital mortality were 50% and 53% respectively. (Table-1)
4. Discussion
Our study is the first to consecutively examine the serum levels of 7 adipokines in critically ill Covid-19 patients. We found a negative correlation between these adipokines and time to start nutrition. Additionally, we found that patients with high mNUTRIC scores had high serum adiponectin and IGF-1 levels on day 7. A positive correlation was found between the number of days of MV and day 7 serum AG levels.
During inflammation, levels of adiponectin, which possess anti-inflammatory properties, decrease [
15]. In our study, serum adiponectin levels decreased at basal and day 7 compared to healthy volunteers. A recent study by Flikweert AW et al. evaluated COVID-19 patients admitted to hospital. The study included 30 non-hospitalised patients, 159 patients hospitalised in hospital wards, 101 COVID-19 patients requiring intensive care, 101 healthy control group, and 40 non-COVID-19 intensive care patients. Like our study, the levels of adiponectin were lower in COVID-19 patients who were critically ill compared to the healthy control group. Unlike our study, this study examined adipokines from plasma in a single measurement. The study analysed the levels of plasma leptin, adiponectin, resistin and visfatin. It is worth noting that, unlike our study, critical COVID-19 patients in the other study had lower APACHE-II scores (16 and 21) [
16].
GLP-1, secreted from intestinal L cells in response to food intake, has a direct anti-inflammatory effect on adipose tissue by reducing adipose tissue macrophages [
17]. The study found that basal and 7th day serum GLP-1 levels were lower compared to the control group.
Resistin causes an increase in proinflammatory cytokines and triggers inflammation [
9,
18]. A study conducted in ICU to examine the relationship between single measurement serum resistin levels and inflammation. The study found that serum resistin levels were higher in critically ill patients than in the control group and were positively correlated with IL-6 and TNF-α [
19]. Flikweert AW et al. discovered a positive correlation between IL-6 levels and adiponectin, resistin, and visfatin, and a negative correlation with leptin [
16]. It has been demonstrated that resistin and visfatin levels increase in conditions such as sepsis and ARDS [
18,
20,
21]. In a review of adipokines in critically ill patients, increased visfatin and resistin correlate with higher disease severity in the ICU [
22]. However, our patients exhibited lower baseline and day 7 levels of resistin and visfatin compared to the control group.
Leptin, which is secreted from adipose tissue, controls weight gain in the hypothalamus. It has been shown to increase in Covid-19 patients admitted to the ICU [
16,
23,
24]. However, no relation was found with inflammatory conditions, including endotoxemia, sepsis, and HIV infection [
25]. Our study found no correlation between baseline and day 7 leptin levels in patients and the control group.
In our patients, consecutive serum resistin, visfatin, and leptin levels may have been lower than in the studies mentioned above. This could be due to the use of inflammation-suppressing treatments, such as Tocilizumab and steroids, which inhibit IL-6, a proinflammatory cytokine.
In critically ill patients, increased cytokines resulting from trauma, sepsis or inflammation may lead to low serum levels of AG [
26]. Our study found that baseline AG levels were lower compared to the control group. However, there are conflicting results regarding AG levels in critically ill patients [
27,
28]. This may be due to the fact that the forms of ghrelin analysed in the studies, such as total and AG, differ.
In a study conducted in 2015 in 543 critically ill non-COVID-19 patients, unlike our study, only IGF-1 levels among adipokines were analysed from plasma in the first two days of admission to intensive care or within two days after ARDS developed. The study found that low IGF-1 levels were independently associated with ARDS and mortality in critically ill patients [
29]. In our study, we found that COVID-19 patients had lower day 7 serum IGF-1 levels compared to the control group. However, we did not find any statistical significance in disease severity or mortality. This outcome may be due to the limited size of the patient group.
In this study, a negative correlation was detected between the time of nutritional initiation and all adipokine levels evaluated in the ICU. No studies were found on the relationship between the time of initiation of nutrition and adipokines, particularly in critically ill patients, during the literature review. However, while the risk of malnutrition is high in critically ill patients, delay in initiation time may increase this risk even more [
30].
The NUTRIC score represents the first nutritional risk assessment instrument specifically tailored and validated for intensive care unit patients. If serum IL-6 levels cannot be measured, the mNUTRIC score is mentioned, and the risk of malnutrition is considered high if the score is ≥5 [
31]. In a study conducted in elderly (>65 years) patients hospitalised for heart failure, high adiponectin levels were found in patients with low daily physical activity and malnutrition [
32]. In our study, serum adiponectin and IGF-1 levels on day 7 were high in patients with high mNUTRIC score indicating the risk of malnutrition. Although physical rehabilitation and patient mobilisation are performed in ICU, elevated serum adiponectin and IGF-1 levels on day 7 may have been detected due to inadequate physical activity and difficulties in reaching target calories.
In a study conducted on rats, a ventilator-associated lung injury (VILI) model was developed. The administration of ghrelin to rats was shown to decrease VILI. The decrease in VILI was explained by the decrease in TLR4 and NF-κB expression of ghrelin and the decrease in proinflammatory cytokines [
33]. In a 2010 study of critically ill patients, serum ghrelin levels were higher in spontaneously breathing patients compared to those in need of MV [
27]. However, in our study, we found a positive correlation between day 7 AG levels and the number of days of MV. The study did not mention the form of ghrelin used in the previous studies. According to Karaca Z. et al. there may be a difference in AG and total ghrelin levels [
28].
Our study had several strengths. Firstly, we analysed 7 consecutive serum adipokine levels in ICU patients with COVID-19. Secondly, evidence has shown that heparin affects plasma adipokine levels [
34,
35,
36], therefore, serum adipokine levels were analysed instead of plasma. Additionally, this was the only study to investigate the potential correlation between serum adipokine levels and the duration of MV and time to start nutrition in critically ill COVID-19 patients.
Our study had limitations as well as strengths. Firstly, the number of patients and control group was small. We did not perform power analysis. Secondly, we did not analyse COVID-19 phenotypes in which inflammation and the cytokines secreted accordingly differ. Thirdly, while we examined cytokines and serum adipokine levels affected by inflammation, we did not record treatments affecting these cytokines such as IL-6 receptor inhibitors, steroids or immunosuppressive agents in these critically ill COVID-19 patients.
5. Conclusions
This study investigated the relationship between sequential serum adipokine levels and clinical outcomes and nutritional parameters in adult critical COVID-19 patients. Statistical differences were found between baseline and day 7 serum adiponectin, GLP-1, resistin, and visfatin levels compared to the control group. Additionally, there were statistical differences between baseline serum achyl ghrelin and the control group, as well as between day 7 IGF-1 and the control group.
There was a negative correlation between feeding time and serum adipokine levels. Serum Adiponectin and IGF-1 levels were positively correlated with mNUTRIC score. Additionally, a positive correlation was observed between the number of mechanical ventilator days and serum AG. This is a pilot study. Further studies with a larger number of patients are required to investigate changes in blood adipokine levels and their impact on critically ill patients.
Author Contributions
Conceptualization, Şahin Temel and Kürşat Gündoğan.; methodology, Şahin Temel and Kürşat Gündoğan.; software, Şahin Temel, Serap Şahin Ergül and Kürşat Gündoğan.; validation, Şahin Temel, Serap Şahin Ergül and Kürşat Gündoğan.; formal analysis, Şahin Temel and Serap Şahin Ergül.; investigation, Şahin Temel, Serap Şahin Ergül, Ali Yeşiltepe, Recep Civan Yüksel and Ahmet Safa Kaynar.; resources, Şahin Temel, Serap Şahin Ergül and Kürşat Gündoğan.; data curation, Şahin Temel, Serap Şahin Ergül, Ali Yeşiltepe, Recep Civan Yüksel and Ahmet Safa Kaynar.; writing—original draft preparation, Şahin Temel, Serap Şahin Ergül and Kürşat Gündoğan.; writing—review and editing, Şahin Temel, Serap Şahin Ergül and Murat Sungur, Kürşat Gündoğan.; visualization, Şahin Temel and Serap Şahin Ergül.; supervision, Şahin Temel and Serap Şahin Ergül.; project administration, Şahin Temel and Serap Şahin Ergül.; funding acquisition, Şahin Temel, Serap Şahin Ergül, Murat Sungur and Kürşat Gündoğan. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of ERCIYES UNIVERSITY MEDICAL FACULTY ETHICS INSTUTION (coded 2021/357 and dated 05.05.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pallanch O, Ortalda A, Pelosi P, Latronico N, Sartini C, Lombardi G, et al. Effects on health-related quality of life of interventions affecting survival in critically ill patients: a systematic review. Crit Care. 2022, 26, 126. [Google Scholar] [CrossRef] [PubMed]
- Gundogan K, Akbudak IH, Hanci P, Halacli B, Temel S, Gullu Z, et al. Clinical Outcomes and Independent Risk Factors for 90-Day Mortality in Critically Ill Patients with Respiratory Failure Infected with SARS-CoV-2: A Multicenter Study in Turkish Intensive Care Units. Balkan Med J. 2021, 38, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Gehlot M, Mohanty S, Venkateshan M, Gomathi B, Shetty A, Das PK, et al. Socioeconomic Burden of Critically Ill Patients: A Descriptive Study. Cureus. 2023, 15, e35598. [Google Scholar]
- Nakhaee, M.; Khandehroo, M.; Esmaeili, R. Cost of illness studies in COVID-19: a scoping review. Cost Eff Resour Alloc. 2024, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman JJ, Harmon LA, Smithburger PL, Chaykosky D, Heffner AC, Hravnak M, et al. Choosing Wisely For Critical Care: The Next Five. Crit Care Med. 2021, 49, 472–81. [Google Scholar] [CrossRef]
- Adhikari, N.K.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet. 2010, 376, 1339–46. [Google Scholar] [CrossRef]
- Cypess, A.M. Reassessing Human Adipose Tissue. N Engl J Med. 2022, 386, 768–79. [Google Scholar] [CrossRef]
- Hajri T, Gharib M, Kaul S, Karpeh MS, Jr. Association between adipokines and critical illness outcomes. J Trauma Acute Care Surg. 2017, 83, 507–19. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005, 174, 5789–95. [Google Scholar] [CrossRef]
- Desruisseaux MS, Nagajyothi, Trujillo ME, Tanowitz HB, Scherer PE. Adipocyte, adipose tissue, and infectious disease. Infect Immun. 2007, 75, 1066–78. [Google Scholar] [CrossRef]
- Bluher, M.; Mantzoros, C.S. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015, 64, 131–45. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Birkhahn, L.; Leucuta, D.C.; Al Srouji, N.; Popa, S.L.; Dumitrascu, D.L. Are adipokines related to COVID-19 and its severity? A systematic review and meta-analysis. Med Pharm Rep. 2024, 97, 120–31. [Google Scholar] [CrossRef] [PubMed]
- Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin Nutr. 2023, 42, 1671–89. [Google Scholar] [CrossRef] [PubMed]
- World Health, O. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. Geneva: World Health Organization; 2020 2020. Contract No.: WHO/nCoV/Clinical/2020.3.
- Salvator H, Grassin-Delyle S, Naline E, Brollo M, Fournier C, Couderc LJ, et al. Contrasting Effects of Adipokines on the Cytokine Production by Primary Human Bronchial Epithelial Cells: Inhibitory Effects of Adiponectin. Front Pharmacol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Flikweert AW, Kobold ACM, van der Sar-van der Brugge S, Heeringa P, Rodenhuis-Zybert IA, Bijzet J, et al. Circulating adipokine levels and COVID-19 severity in hospitalized patients. Int J Obes (Lond). 2023, 47, 126–37. [Google Scholar] [CrossRef]
- Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012, 55, 2456–68. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Christodoulatos, G.S.; Dalamaga, M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr Obes Rep. 2019, 8, 434–57. [Google Scholar] [CrossRef]
- Koch, A.; Gressner, O.A.; Sanson, E.; Tacke, F.; Trautwein, C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care. 2009, 13, R95. [Google Scholar] [CrossRef]
- Lee YC, Lin CY, Chen YH, Chiu WC, Wang YY, Hsu C, et al. Essential Role of Visfatin in Lipopolysaccharide and Colon Ascendens Stent Peritonitis-Induced Acute Lung Injury. Int J Mol Sci. 2019, 20. [Google Scholar]
- Sethi, J.K.; Vidal-Puig, A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005, 11, 344–7. [Google Scholar] [CrossRef]
- Alipoor, E.; Mohammad Hosseinzadeh, F.; Hosseinzadeh-Attar, M.J. Adipokines in critical illness: A review of the evidence and knowledge gaps. Biomed Pharmacother. 2018, 108, 1739–50. [Google Scholar] [CrossRef] [PubMed]
- van der Voort PHJ, Moser J, Zandstra DF, Muller Kobold AC, Knoester M, Calkhoven CF, et al. Leptin levels in SARS-CoV-2 infection related respiratory failure: A cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon. 2020, 6, e04696. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Lipcsey, M.; Hultstrom, M.; Frithiof, R.; Eriksson, M. Plasma Leptin Is Increased in Intensive Care Patients with COVID-19-An Investigation Performed in the PronMed-Cohort. Biomedicines. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Preas, H.L.; Chrousos, G.P.; Suffredini, A.F. Circulating leptin levels during acute experimental endotoxemia and antiinflammatory therapy in humans. J Infect Dis. 1998, 178, 887–90. [Google Scholar] [CrossRef] [PubMed]
- Santacruz CA, Quintairos A, Righy C, Crippa IA, Couto L, Jr. , Imbault V, et al. Is There a Role for Enterohormones in the Gastroparesis of Critically Ill Patients? Crit Care Med. 2017, 45, 1696–701. [Google Scholar] [CrossRef]
- Koch, A.; Sanson, E.; Helm, A.; Voigt, S.; Trautwein, C.; Tacke, F. Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit Care. 2010, 14, R94. [Google Scholar] [CrossRef]
- Karaca Z, Yuksel RC, Gunes Sahin G, Sungur N, Temel S, Baskol G, et al. Measurement of serial serum total and acylated ghrelin levels in critically ill patients: A prospective and observational pilot study. Nutr Clin Pract. 2022, 37, 192–8. [Google Scholar] [CrossRef]
- Ahasic AM, Tejera P, Wei Y, Su L, Mantzoros CS, Bajwa EK, et al. Predictors of Circulating Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor-Binding Protein-3 in Critical Illness. Crit Care Med. 2015, 43, 2651–9. [Google Scholar] [CrossRef]
- Razzera, E.L.; Milanez, D.S.J.; Silva, F.M. Derivation of the Screening of Nutritional Risk in Intensive Care risk prediction score: A secondary analysis of a prospective cohort study. JPEN J Parenter Enteral Nutr. 2024, 48, 82–92. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011, 15, R268. [Google Scholar] [CrossRef]
- Horibe H, Ando K, Maekawa Y, Narisawa M, Yamase Y, Funabiki J, et al. The association of serum adiponectin level with activities of daily living in hospitalized elderly patients with heart failure. J Cardiol. 2024, 83, 130–7. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.; Xia, W.F.; Zhou, C.L.; Lv, L.Q. Protective effects of ghrelin in ventilator-induced lung injury in rats. Int Immunopharmacol. 2017, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Scholman RC, Giovannone B, Hiddingh S, Meerding JM, Malvar Fernandez B, van Dijk MEA, et al. Effect of anticoagulants on 162 circulating immune related proteins in healthy subjects. Cytokine. 2018, 106, 114–24. [Google Scholar] [CrossRef] [PubMed]
- Henno LT, Storjord E, Christiansen D, Bergseth G, Ludviksen JK, Fure H, et al. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines - Consequences for defining reference values in healthy humans. Cytokine. 2017, 97, 86–95. [Google Scholar] [CrossRef]
- Allione A, Di Gaetano C, Dani N, Barberio D, Sieri S, Krogh V, et al. Anticoagulants used in plasma collection affect adipokine multiplexed measurements. Cytokine. 2016, 80, 43–7. [Google Scholar] [CrossRef]
Table 1.
Demographic and clinical information of the patients.
Table 1.
Demographic and clinical information of the patients.
| |
Patient, n=30 |
Healthy control, n=10 |
| Age, mean±SD, year |
70±16 |
68±11 |
| Gender, n (%) Female |
13 (43) |
4 (40) |
| BMI, median (IQR), kg/m2 |
25 (22-31) |
26 (23-32) |
| mNUTRIC score, mean±SD |
5±1 |
|
| APACHE II, median (IQR) |
20.5 (15.75-28) |
|
| SOFA score, median (IQR) |
6 (5-8) |
|
| Infection, n (%) |
18 (60) |
|
| Vasopressor, n (%) |
9 (30) |
|
| Mechanical Ventilation (MV), n (%) |
12 (40) |
|
| Duration of MV, median (IQR), day |
3 (2-14) |
|
| Initiation time of feeding, median (IQR), hour |
2 (1,75-3) |
|
| Length of stay in ICU, median (IQR), day |
10 (7-18) |
|
| Length of Hospital stay, median (IQR), day |
14 (10-21) |
|
| ICU Mortality, n (%) |
15 (50) |
|
| Hospital Mortality, n (%) |
16 (53) |
|
Table 2.
The Daily Calorie Intake of Patients.
Table 2.
The Daily Calorie Intake of Patients.
| Patients No: |
Daily Calorie Intake, kcal/day |
|
|
|
|
|
|
|
| |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Median calorie intake, (min-max) |
| 1 |
1332 |
1442 |
1818 |
1296 |
1296 |
1728 |
1296 |
1332 (1296-1818) |
| 2 |
|
180 |
300 |
300 |
300 |
400 |
400 |
300 (0-400) |
| 3 |
480 |
1008 |
960 |
864 |
1152 |
336 |
864 |
864 (336-1152) |
| 4 |
1680 |
576 |
768 |
300 |
600 |
800 |
900 |
768 (300-1680) |
| 5 |
150 |
200 |
100 |
1800 |
1260 |
756 |
800 |
756 (100-1800) |
| 6 |
400 |
400 |
400 |
500 |
600 |
400 |
400 |
400 (400-600) |
| 7 |
600 |
800 |
1000 |
1000 |
600 |
600 |
650 |
650 (600-1000) |
| 8 |
180 |
432 |
540 |
504 |
600 |
450 |
600 |
504 (180-600) |
| 9 |
1008 |
1152 |
934 |
1156 |
1152 |
444 |
600 |
1008 (444-1152) |
| 10 |
500 |
600 |
500 |
800 |
1000 |
800 |
900 |
800 (500-1000) |
| 11 |
1152 |
1152 |
1152 |
1152 |
1152 |
1152 |
1152 |
1152 (1152-1152) |
| 12 |
468 |
612 |
1080 |
972 |
1620 |
1134 |
1100 |
1080 (468-1620) |
| 13 |
672 |
1380 |
1552 |
1552 |
400 |
400 |
400 |
672 (400-1552) |
| 14 |
400 |
600 |
500 |
600 |
500 |
500 |
500 |
500 (400-600) |
| 15 |
300 |
400 |
500 |
400 |
450 |
500 |
500 |
450 (300-500) |
| 16 |
816 |
1416 |
684 |
864 |
840 |
900 |
800 |
864 (684-1416) |
| 17 |
720 |
1728 |
1296 |
1152 |
1152 |
1152 |
1152 |
1152 (720-1728) |
| 18 |
192 |
576 |
1152 |
1152 |
984 |
912 |
900 |
912 (192-1152) |
| 19 |
|
100 |
576 |
576 |
800 |
576 |
576 |
576 (0-800) |
| 20 |
864 |
864 |
864 |
864 |
864 |
864 |
468 |
864 (468-864) |
| 21 |
384 |
1152 |
1152 |
1152 |
1668 |
600 |
1000 |
1152 (384-1668) |
| 22 |
|
|
300 |
600 |
600 |
650 |
700 |
600 (0-700) |
| 23 |
540 |
600 |
700 |
900 |
700 |
800 |
800 |
700 (540-900) |
| 24 |
|
300 |
250 |
200 |
200 |
300 |
400 |
250 (0-400) |
| 25 |
900 |
1000 |
600 |
700 |
800 |
800 |
800 |
800 (600-1000) |
| 26 |
580 |
1452 |
1302 |
1360 |
1440 |
1440 |
1584 |
1440 (580-1584) |
| 27 |
640 |
876 |
876 |
548 |
700 |
1000 |
1000 |
876 (548-1000) |
| 28 |
816 |
1152 |
1152 |
1404 |
924 |
870 |
1050 |
1050 (816-1404) |
| 29 |
400 |
500 |
400 |
500 |
600 |
300 |
400 |
400 (300-600) |
| 30 |
|
1168 |
528 |
1452 |
1652 |
1552 |
1500 |
1452 (0-1652) |
Table 3.
Daily nutrition type of patients.
Table 3.
Daily nutrition type of patients.
| Patients |
Enteral Tube Feeding, n (%) |
Parenteral Nutrition,
n (%) |
Oral supplement/diet n (%) |
| Baseline |
4 (13) |
8 (27) |
18 (60) |
| Day 2 |
5 (17) |
7 (23) |
18 (60) |
| Day 3 |
5 (17) |
6 (20) |
19 (63) |
| Day 4 |
6 (20) |
5 (17) |
19 (63) |
| Day 5 |
7 (24) |
4 (13) |
19 (63) |
| Day 6 |
7 (24) |
4 (13) |
19 (63) |
| Day 7 |
8 (27) |
3 (10) |
19 (63) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).