Submitted:
05 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Background: Total gastrectomy with D2 lymph node dissection is standard for resectable esophagogastric junctional and gastric cancer, but high morbidity challenges perioperative care. ERAS guidelines advise against routine drains, yet conflicting evidence leads to inconsistent practice. Methods: The DRAG (DRains After Gastrectomy) Trial is a prospective, non-randomized study conducted from February 2020 to March 2023 at the 1st Propaedeutic Surgery Department, Hippocration General Hospital, Athens. Patients undergoing open D2 total gastrectomy had perianastomotic drains placed based on newly established criteria, offering a more tailored approach. Immediate and short-term postoperative outcomes, including recovery milestones and complications, were assessed. Results: Sixty patients were included, with 40 receiving a drain. The non-drain group had significantly lower pain scores, earlier mobilization, less postoperative nausea and vomiting, and a shorter hospital stay. Among those with complications, significant differences in surgical site infections, delayed mobilization, extra-abdominal issues, length of stay, readmissions, and reoperations suggest that drain use may increase complications and hinder recovery. Conclusion: Our study suggests that routine prophylactic drain use can be avoided in gastrectomy for esophagogastric junctional and gastric cancer when experienced surgeons in high-volume centers follow clearly defined criteria. Implementing these criteria may improve patient outcomes and reduce complications.
Keywords:
1. Introduction
2. Materials and Methods
| ELIGIBILITY CRITERIA |
| INCLUSION CRITERIA |
| Age over 18 years old |
| Histologically proven EGJ (Siewert II or III) or non-EGJ gastric adenocarcinoma (intestinal, diffuse, or mixed Lauren type) |
| Surgical candidates for total gastrectomy plus D2 lymph node dissection |
| ECOG performance status 0 or 1 |
| Signed informed consent from |
| Preoperative evaluation of cTanyNanyM0 according to the American Joint Committee on Cancer Staging Manual, 7th edition |
| EXCLUSION CRITERIA |
| M1 disease |
| Other unplanned organ excision |
| Massive ascites or cachexia |
| Current participation in any other clinical trial |
| Severe cardiovascular, respiratory tract, kidney, liver, or psychiatric disease. |
| Poor compliance to the clinical protocol |
| Pregnancy |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| PREOPERATIVE EVALUATION | |||
| History and physical examination | |||
| Blood exams | FBC, RFTs, LFTs, U&E, blood sugar, albumin and total protein levels | ||
| Blood clotting sufficiency | PT, INR, aPTT | ||
| Thyroid function | T3, T4, TSH, FT3, FT4 | ||
| Hepatitis virulence | anti- HCV antibodies, anti- HBV antibodies, HBs antigen | ||
| Blood type cross-match | |||
| Chest x-ray | |||
| Anesthesiologist check | Incentive spirometry, Mallabati score evaluation, ASA score, necessity for ICU stay after the operation | ||
| Electrocardiogram and cardiology examination | |||
| Previous colonoscopy to exclude secondary/second primary tumor in colon | |||
| aPTT: activated partial thromboplastin time, FBC: Full Blood Count, HBV: hepatitis B virus, HCV: hepatitis C virus, INR: international normalised ratio, LFTs: Liver Function tests, PT: prothrombin time, RFTs: Renal function Tests, T3: Triiodothyronine , T4,: Thyroxine ,TSH: thyroid stimulating hormone, U&E: Urea and Electrolytes. | |||
Appendix B. Timeline of Study Protocol
| STUDY PERIOD | |||||||||
| ENROLLMENT DAYS | DAY OF SURGERY | POSTOPERATIVE DAYS | |||||||
| TIMEPOINT | -7 TO -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | Ω |
| ENROLLMENT | |||||||||
| PATIENT SELECTION | X | ||||||||
| PREOP CHECK | X | ||||||||
| CONSENT SIGN | X | ||||||||
| PROTOCOL EDUCATION | X | ||||||||
| INTERVENTION | |||||||||
| DRAIN PLACEMENT | X | ||||||||
| INTRAOPERATIVE DATA RECORDING | X | ||||||||
| MONITORING | |||||||||
| CLIN. EXAMINATION &VITALS | X | X | X | X | X | X | |||
| LABS | X | X | X | X | X | X | |||
| DRAIN CONTENT MONITORING | X | X | X | X | X | X | X | ||
| ORAL CONTRAST STUDY | X | ||||||||
| PONV | X | X | X | X | X | ||||
| PAIN (VAS SCORE) | X | X | X | X | X | X | |||
| SSI | X | X | X | X | X | ||||
| MOBILIZATION | X | X | X | X | X | ||||
| ORAL FEEDING | X | X | X | X | X | ||||
| GUT MOTILITY | X | X | X | X | X | X | |||
| EXTRAABDOMINAL COMPLICATIONS | X | X | X | X | X | ||||
| LOS | X | X | X | X | X | X | |||
| MORTALITY | X | X | X | X | X | X | |||
| READMISSIONS | X | X | X | X | X | X | |||
| REOPERATIONS | |||||||||
|
LOS: length of stay, SSI: surgical site infection, VAS score: visual analogue score Ω:time between POD#5 and discharge | |||||||||
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; clinicalguidelines@esmo. org, E.G.C.E.a. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D'Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Qin, J.; Shen, K.; Chen, W.; Liu, F.; Fang, Y.; Wang, X.; Wang, H.; Shen, Z.; Sun, Y.; et al. The IGCA staging system is more accurate than AJCC7 system in stratifying survival of patients with gastric cancer in stage III. BMC Cancer 2017, 17, 238. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Young-Fadok, T.; Demartines, N. The History of Enhanced Recovery After Surgery and the ERAS Society. J Laparoendosc Adv Surg Tech A 2017, 27, 860–862. [Google Scholar] [CrossRef]
- Tanious, M.K.; Ljungqvist, O.; Urman, R.D. Enhanced Recovery After Surgery: History, Evolution, Guidelines, and Future Directions. Int Anesthesiol Clin 2017, 55, 1–11. [Google Scholar] [CrossRef]
- Kehlet, H.; Mogensen, T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999, 86, 227–230. [Google Scholar] [CrossRef]
- Mortensen, K.; Nilsson, M.; Slim, K.; Schafer, M.; Mariette, C.; Braga, M.; Carli, F.; Demartines, N.; Griffin, S.M.; Lassen, K.; et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014, 101, 1209–1229. [Google Scholar] [CrossRef]
- Mahendran, R.; Tewari, M.; Dixit, V.K.; Shukla, H.S. Enhanced recovery after surgery protocol enhances early postoperative recovery after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int 2019, 18, 188–193. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations: 2018. World J Surg 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Shimoike, N.; Akagawa, S.; Yagi, D.; Sakaguchi, M.; Tokoro, Y.; Nakao, E.; Tamura, T.; Fujii, Y.; Mochida, Y.; Umemoto, Y.; et al. Laparoscopic gastrectomy with and without prophylactic drains in gastric cancer: a propensity score-matched analysis. World J Surg Oncol 2019, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, N.; Matsubara, T.; Hayashi, H.; Takai, K.; Fujii, Y.; Tajima, Y. Significance of prophylactic intra-abdominal drain placement after laparoscopic distal gastrectomy for gastric cancer. World J Surg Oncol 2015, 13, 181. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.Y.; An, J.Y.; Seo, S.H.; Kim, D.W.; Seo, Y.B.; Nakagawa, M.; Li, S.; Cheong, J.H.; Hyung, W.J.; et al. Do All Patients Require Prophylactic Drainage After Gastrectomy for Gastric Cancer? The Experience of a High-Volume Center. Ann Surg Oncol 2015, 22, 3929–3937. [Google Scholar] [CrossRef]
- Kumar, M.; Yang, S.B.; Jaiswal, V.K.; Shah, J.N.; Shreshtha, M.; Gongal, R. Is prophylactic placement of drains necessary after subtotal gastrectomy? World J Gastroenterol 2007, 13, 3738–3741. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Uslar, R.; Molina, H.; Torres, O.; Cancino, A. Total gastrectomy with or without abdominal drains. A prospective randomized trial. Rev Esp Enferm Dig 2005, 97, 562–569. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Hyung, W.J.; Cheong, J.H.; Chen, J.; Choi, S.H.; Noh, S.H. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg 2004, 8, 727–732. [Google Scholar] [CrossRef]
- Liu, H.P.; Zhang, Y.C.; Zhang, Y.L.; Yin, L.N.; Wang, J. Drain versus no-drain after gastrectomy for patients with advanced gastric cancer: systematic review and meta-analysis. Dig Surg 2011, 28, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Su, K.; Dong, Z. Abdominal drainage versus no drainage post gastrectomy for gastric cancer. Cochrane Database Syst Rev 2011, CD008788. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kang, J.H.; Jung, M.R.; Ryu, S.Y.; Jeong, O. Abdominal Drainage in the Prevention and Management of Major Intra-Abdominal Complications after Total Gastrectomy for Gastric Carcinoma. J Gastric Cancer 2020, 20, 376–384. [Google Scholar] [CrossRef]
- Kanda, M.; Fujiwara, M.; Tanaka, C.; Kobayashi, D.; Iwata, N.; Mizuno, A.; Yamada, S.; Fujii, T.; Nakayama, G.; Sugimoto, H.; et al. Predictive value of drain amylase content for peripancreatic inflammatory fluid collections after laparoscopic (assisted) distal gastrectomy. Surg Endosc 2016, 30, 4353–4362. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kurokawa, Y.; Mikami, J.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; et al. Amylase concentration in drainage fluid as a predictive factor for severe postoperative pancreatic fistula in patients with gastric cancer. Surg Today 2017, 47, 1378–1383. [Google Scholar] [CrossRef]
- Kamiya, S.; Hiki, N.; Kumagai, K.; Honda, M.; Nunobe, S.; Ohashi, M.; Sano, T.; Yamaguchi, T. Two-point measurement of amylase in drainage fluid predicts severe postoperative pancreatic fistula after gastric cancer surgery. Gastric Cancer 2018, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Iwata, N.; Tanaka, C.; Kanda, M.; Yamada, S.; Nakayama, G.; Fujii, T.; Koike, M.; Fujiwara, M.; Kodera, Y. Factors related to occurrence and aggravation of pancreatic fistula after radical gastrectomy for gastric cancer. J Surg Oncol 2015, 112, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Yoshikawa, T.; Yura, M.; Otsuki, S.; Morita, S.; Katai, H.; Nishida, T. Current status of the "enhanced recovery after surgery" program in gastric cancer surgery. Ann Gastroenterol Surg 2019, 3, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Surgeons, A.C.o. American College of Surgeons’ Standards for Gastric Cancer Surgery. Available online: https://decisionpoint.medscape.com/oncology/viewarticle/946018 (accessed on.
- Lombardi, P.M.; Mazzola, M.; Giani, A.; Baleri, S.; Maspero, M.; De Martini, P.; Gualtierotti, M.; Ferrari, G. ERAS pathway for gastric cancer surgery: adherence, outcomes and prognostic factors for compliance in a Western centre. Updates Surg 2021, 73, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Salvans, S.; Grande, L.; Dal Cero, M.; Pera, M. State of the art of enhanced recovery after surgery (ERAS) protocols in esophagogastric cancer surgery: the Western experience. Updates Surg 2023, 75, 373–382. [Google Scholar] [CrossRef]
- Gong, W.; Li, J. Combat with esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer: A critical review of the literature. Int J Surg 2017, 47, 18–24. [Google Scholar] [CrossRef]
- Aurello, P.; Magistri, P.; D'Angelo, F.; Valabrega, S.; Sirimarco, D.; Tierno, S.M.; Nava, A.K.; Ramacciato, G. Treatment of esophagojejunal anastomosis leakage: a systematic review from the last two decades. Am Surg 2015, 81, 450–453. [Google Scholar] [CrossRef]
- Trapani, R.; Rausei, S.; Reddavid, R.; Degiuli, M.; Investigators, I.R.G.F.G.C.C. Risk factors for esophago-jejunal anastomosis leakage after total gastrectomy for cancer. A multicenter retrospective study of the Italian research group for gastric cancer. Eur J Surg Oncol 2020, 46, 2243–2247. [Google Scholar] [CrossRef]
- Xing, J.; Liu, M.; Qi, X.; Yu, J.; Fan, Y.; Xu, K.; Gao, P.; Tan, F.; Yao, Z.; Zhang, N.; et al. Risk factors for esophagojejunal anastomotic leakage after curative total gastrectomy combined with D2 lymph node dissection for gastric cancer. J Int Med Res 2021, 49, 3000605211000883. [Google Scholar] [CrossRef]
- Aryaie, A.H.; Singer, J.L.; Fayezizadeh, M.; Lash, J.; Marks, J.M. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc 2017, 31, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kang, D.H.; Kim, H.W.; Park, S.B.; Kim, S.J.; Hwang, S.H.; Lee, S.H. Full covered self-expandable metal stents for the treatment of anastomotic leak using a silk thread. Medicine (Baltimore) 2017, 96, e7439. [Google Scholar] [CrossRef] [PubMed]
- Dallal, R.M.; Bailey, L.; Nahmias, N. Back to basics--clinical diagnosis in bariatric surgery. Routine drains and upper GI series are unnecessary. Surg Endosc 2007, 21, 2268–2271. [Google Scholar] [CrossRef]
- Haverkamp, L.; Weijs, T.J.; van der Sluis, P.C.; van der Tweel, I.; Ruurda, J.P.; van Hillegersberg, R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc 2013, 27, 1509–1520. [Google Scholar] [CrossRef]
- Liu, F.; Huang, C.; Xu, Z.; Su, X.; Zhao, G.; Ye, J.; Du, X.; Huang, H.; Hu, J.; Li, G.; et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol 2020, 6, 1590–1597. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, X.; Zhao, Y.; Qian, F.; Tang, B.; Hao, Y.; Luo, H.; Chen, J.; Yu, P. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc 2018, 32, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Huang, X.; Lu, H.; Zhang, J.; Luo, R.; Xu, H.; Huang, B. Intraoperative blood loss does not independently affect the survival outcome of gastric cancer patients who underwent curative resection. Clin Transl Oncol 2019, 21, 1197–1206. [Google Scholar] [CrossRef]
- Misawa, K.; Kurokawa, Y.; Mizusawa, J.; Takiguchi, S.; Doki, Y.; Makino, S.; Choda, Y.; Takeno, A.; Tokunaga, M.; Sano, T.; et al. Negative impact of intraoperative blood loss on long-term outcome after curative gastrectomy for advanced gastric cancer: exploratory analysis of the JCOG1001 phase III trial. Gastric Cancer 2022, 25, 459–467. [Google Scholar] [CrossRef]
- Chen Cardenas, S.M.; Santhanam, P.; Morris-Wiseman, L.; Salvatori, R.; Hamrahian, A.H. Perioperative Evaluation and Management of Patients on Glucocorticoids. J Endocr Soc 2022, 7, bvac185. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- (ICH), I.C.f.H. Guideline for good clinical practice E6(R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf (accessed on 5th September 2024).
- Eleftheriou, M.-M.; Triantafyllou, Τ.; Bananis, K.; Chrysikos, D.; Theodorou, D. Hybrid intraoperative sutured stenting for anastomotic leak after total gastrectomy.
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Desiderio, J.; Trastulli, S.; D'Andrea, V.; Parisi, A. Enhanced recovery after surgery for gastric cancer (ERAS-GC): optimizing patient outcome. Transl Gastroenterol Hepatol 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Nakaseko, Y.; Haruki, K.; Shiba, H.; Horiuchi, T.; Saito, N.; Sakamoto, T.; Gocho, T.; Yanaga, K. Impact of fresh frozen plasma transfusion on postoperative inflammation and prognosis of colorectal liver metastases. J Surg Res 2018, 226, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Simmons, K.D.; Zaheer, S.; Poe, S.L.; Bartlett, E.K.; Drebin, J.A.; Fraker, D.L.; Kelz, R.R.; Roses, R.E.; Karakousis, G.C. Blood Transfusion in Major Abdominal Surgery for Malignant Tumors: A Trend Analysis Using the National Surgical Quality Improvement Program. JAMA Surg 2016, 151, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A.; Davenport, D.L.; Saha, S.P.; Austin, P.C.; Zwischenberger, J.B. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg 2012, 147, 49–55. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Trautwein, C.; Ludde, T.; Strnad, P.; Gaisa, N.T.; Barabasch, A.; Bruners, P.; Ulmer, T.; et al. Intraoperative Transfusion of Fresh Frozen Plasma Predicts Morbidity Following Partial Liver Resection for Hepatocellular Carcinoma. J Gastrointest Surg 2021, 25, 1212–1223. [Google Scholar] [CrossRef]
- Parisi, A.; Desiderio, J.; Cirocchi, R.; Trastulli, S. Enhanced Recovery after Surgery (ERAS): a Systematic Review of Randomised Controlled Trials (RCTs) in Bariatric Surgery. Obes Surg 2020, 30, 5071–5085. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Donlon, N.E.; Fearon, N.M.; Heneghan, H.M.; Conneely, J.B. Evaluating the Impact of Enhanced Recovery After Surgery Protocols on Surgical Outcomes Following Bariatric Surgery-A Systematic Review and Meta-analysis of Randomised Clinical Trials. Obes Surg 2024, 34, 778–789. [Google Scholar] [CrossRef]
- Feng, F.; Ji, G.; Li, J.P.; Li, X.H.; Shi, H.; Zhao, Z.W.; Wu, G.S.; Liu, X.N.; Zhao, Q.C. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 2013, 19, 3642–3648. [Google Scholar] [CrossRef]
- Albanopoulos, K.; Alevizos, L.; Linardoutsos, D.; Menenakos, E.; Stamou, K.; Vlachos, K.; Zografos, G.; Leandros, E. Routine abdominal drains after laparoscopic sleeve gastrectomy: a retrospective review of 353 patients. Obes Surg 2011, 21, 687–691. [Google Scholar] [CrossRef]
- Ding, J.; Sun, B.; Song, P.; Liu, S.; Chen, H.; Feng, M.; Guan, W. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017, 8, 75699–75711. [Google Scholar] [CrossRef]
- Luzuy-Guarnero, V.; Gronnier, C.; Figuereido, S.; Mantziari, S.; Schäfer, M.; Demartines, N.; Allemann, P. Cost-Benefit Analysis of an Enhanced Recovery Program for Gastrectomy A Retrospective Controlled Analysis. World J Surg 2021, 45, 3249–3257. [Google Scholar] [CrossRef]
- Straatman, J.; Cuesta, M.A.; de Lange-de Klerk, E.S.; van der Peet, D.L. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg 2015, 32, 150–156. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Li, J.; Hong, D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg Oncol 2020, 32, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, T.R.C.o.S.o.E.T.R.C.o.S.o.E.R.C.o.P.a.S.o. Green Theatre Checklist: evidence based resources for greener surgery. Available online: https://ukhealthalliance.org/news-item/green-theatre-checklist-evidence-based-resources-for-greener-surgery/ (accessed on 1/10/2024).
- England, T.R.C.o.S.o.; Sustainability in the operating theatre. Standards & Research. 2022. Available online: https:// www. rcseng. ac. uk/ stand ards- and- resea rch/ stand ardsand- guida nce/ good- pract ice- guides/ susta inabi lity- in- opera ting- theat re/ (accessed on 1/10/2024).
- Westwood, E.; Walshaw, J.; Boag, K.; Chua, W.; Dimashki, S.; Khalid, H.; Lathan, R.; Wellington, J.; Lockwood, S.; Yiasemidou, M. Time for change: compliance with RCS green theatre checklist-facilitators and barriers on the journey to net zero. Front Surg 2023, 10, 1260301. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.; Rizan, C. The Green Surgery report: a guide to reducing the environmental impact of surgical care, but will it be implemented? Ann R Coll Surg Engl 2024, 106, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, F.; Cobianchi, L.; Piccolo, D.; Balch, J.; Biancuzzi, H.; Biffl, W.L.; Campostrini, S.; Cicuttin, E.; Coccolini, F.; Damaskos, D.; et al. Are we ready for "green surgery" to promote environmental sustainability in the operating room? Results from the WSES STAR investigation. World J Emerg Surg 2024, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Lee, Y.C.; Kim, J.H.; Chung, H.; Park, J.C.; Shin, S.K.; Lee, S.K.; Kim, H.I.; Hyung, W.J.; Noh, S.H. Postoperative Helicobacter pylori Infection as a Prognostic Factor for Gastric Cancer Patients after Curative Resection. Gut Liver 2017, 11, 635–641. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Li, S.; Bai, F.; Xie, H.; Shan, H.; Liu, Z.; Ma, T.; Tang, X.; Tang, H.; et al. The effect of Helicobacter pylori eradication on prognosis of postoperative early gastric cancer: a multicenter study. World J Surg Oncol 2021, 19, 285. [Google Scholar] [CrossRef]
- Kang, S.Y.; Han, J.H.; Ahn, M.S.; Lee, H.W.; Jeong, S.H.; Park, J.S.; Cho, Y.K.; Han, S.U.; Kim, Y.B.; Kim, J.H.; et al. Helicobacter pylori infection as an independent prognostic factor for locally advanced gastric cancer patients treated with adjuvant chemotherapy after curative resection. Int J Cancer 2012, 130, 948–958. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Wang, Z.; Xu, J.; Cui, J.; Cai, S.; Zhan, W.; He, Y. Gastric cancer patients with Helicobacter pylori infection have a poor prognosis. J Surg Oncol 2013, 108, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, R.; Chen, G.; Nie, M.; Zhang, F.; Chen, X.; Lin, J.; Chen, Z.; Lin, F.; Wei, C.; et al. Anti-Helicobacter pylori Treatment in Patients With Gastric Cancer After Radical Gastrectomy. JAMA Netw Open 2024, 7, e243812. [Google Scholar] [CrossRef] [PubMed]
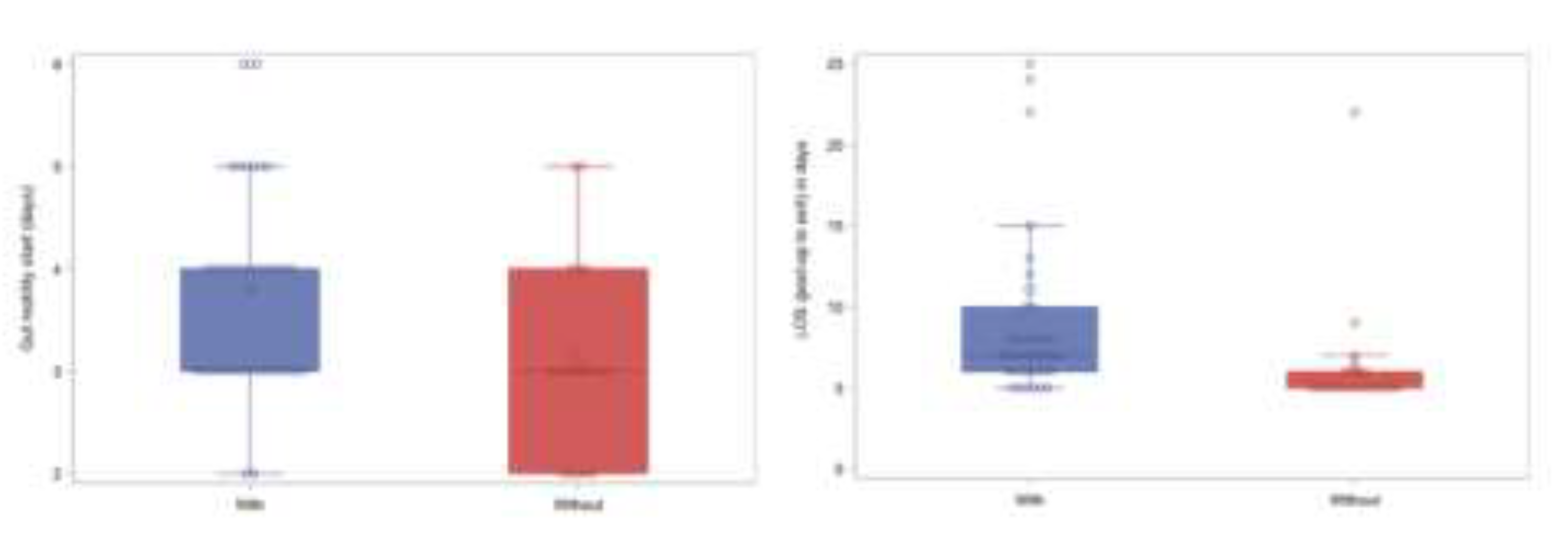
| Group | Characteristic | Value |
| Demographics and patient characteristics | Age | 71±9.6, median (Q1-Q3): 73 (65-77.5), min: 48, max: 87 |
| Gender (male) | 32 (53.33%) | |
| Charlson score | 2.45±0.85, median (Q1-Q3): 2 (2-2.5), min:2, max: 5 | |
| ECOG | 8 patients (13.3%) had score 1, 52 patients had score 0 | |
| Histology | Diffuse | 22 (36.67%) |
| Intestinal | 28 (46.67%) | |
| Other | 7 (11.67%) | |
| Location | EGJ | 12 (20%) |
| Fundus | 5 (8.33%) | |
| Body | 26 (43.33%) | |
| Prepyloric antrum | 16 (26.67%) | |
| Pylorus | 1 (1.67%) | |
| Preoperative chemotherapy | Positive | 15 (25.42%) |
| Laboratory results abnormality | FBC | 6 (10%) |
| Biochemical | 4 (6.67%) | |
| Clotting | none | |
| Cancer antigens positivity * | CA 19.9 | 8 (13.33%) |
| CA 15.3 | 6 (10%) | |
| CA 125 | 3 (5%) | |
| CEA | 7 (12%) | |
| Intraoperative characteristics | Duration of operation (min) | 197.5±37.2, median (Q1-Q3): 200 (180-227.5), min 120, max 180 |
| Blood transfusion | 8 (13.33%) | |
| FFP transfusion | 5 (8.33%) | |
| Allergic reaction to drugs | 0 (%) | |
| Intraoperative incidents | 7 (11.67%) | |
| Criteria for drain placement | Blood loss >250mls | 20 (33.33%) |
| Vessel injury | 3 (5%) | |
| Anastomosis concerns | 11 (18.33%) | |
| Adjacent structures injury | None | |
| Stump integrity concerns | 3 (5%) | |
| Chronic respiratory comorbidity | 3 (5%) | |
| Post-operative characteristics | Derangements of vital signs | 14 (23.33%) |
| Drain quantity abnormality | 4 (9.76%) | |
| Drain quality abnormality | 5 (11.9%) | |
| FBC abnormality | 16 (26.67%) | |
| FBC abnormality day (POD) | 1.1±2.3, median (Q1-Q3): 1 (0-1), min 0, max 11 Day 0: 71.7%, day 1: 10%, after day 1: 18.3% |
|
| Biochemical abnormality | 15 (25%) | |
| Biochemical abnormality day (POD) | 0.7±1.8, median (Q1-Q3): 0 (0-1), min 0, max 11 Day 0: 73.3%, day 1: 10%, after day 1: 16.7% |
|
| Clotting abnormality (POD) | 1 (1.67%) | |
| Swallow test negative | 58 (96.67%) | |
| VAS score (high) | 34 (56.67%) | |
| SSI | 12 (20%) | |
| Mobilization delay | 22 (36.67%) | |
| Oral feeding delay | 17 (28.33%) | |
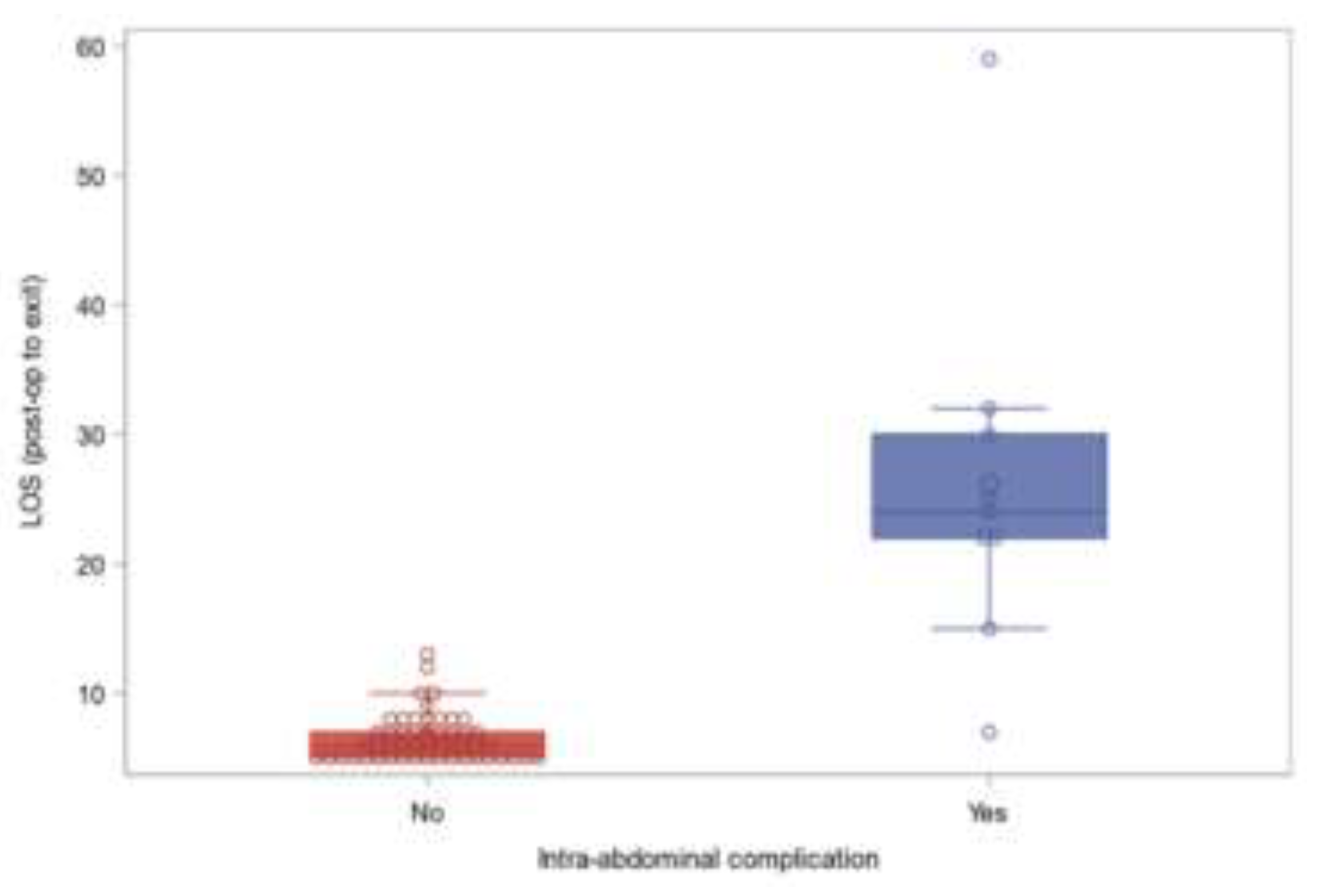
| Intra-abdominal complication | 9 (15%) | |
| Extra-abdominal complication | 5 (8.33%) | |
| ICU treatment | 2 (3.33%) | |
| PONV | 29 (48.33%) | |
| Bowel movement start (POD) | 3.58±1.04, median (Q1-Q3): 3 (3-4), min: 2, max 6 | |
| LOS (post-op to exit) | 9.5±9.01, median (Q1-Q3): 6.5 (5-8), min: 5, max: 59 | |
| Outcomes | Mortality | 3 (5%) |
| Re-admission | 5 (8.33%) | |
| Re-operation | 6 (10%) |
| Group of characteristics | Characteristic | Without drain= 20 | With drain= 40 | p- value |
| Demographics and patient characteristics | Age(years) | 73 (66.5-77.5) | 73.5 (63-78) | 0.8138 |
| Gender (male) | 9/45% | 23/57.5% | 0.4180 | |
| Charlson score | 2 (2-2) | 2 (2-3) | 0.1567 | |
| ECOG | 0 (0-0) | 0 (0-0) | 0.5943 | |
| Histology | Diffuse | 7/35% | 15/37.5% | 1.0000 |
| Intestinal | 11/55% | 17/42.5% | 0.4180 | |
| Mixed | 1/5% | 6/15% | 0.4065 | |
| Location | EGJ | 6/30% | 6/15% | 0.1893 |
| Fundus | 3/15% | 2/5% | 0.3216 | |
| Body | 7/35% | 19/47.5% | 0.4162 | |
| Pre-pyloric antrum | 4/20% | 12/30% | 0.5408 | |
| Pylorus | 1/5% | 0/0% | 0.3333 | |
| Preoperative chemotherapy | Positive | 5/25% | 10/25.64% | 1.0000 |
| Laboratory results abnormality | FBC | 2/10% | 4/10% | 1.0000 |
| Biochemical | 2/10% | 2/5% | 0.5948 | |
| Clotting | None | None | NA | |
| Cancer antigens positivity* | CA 19.9 | 2/10% | 6/15% | 0.7068 |
| CA 15.3 | 1/5% | 5/12.5% | 0.6532 | |
| CA 125 | 0/0% | 3/7.5% | 0.5441 | |
| CEA | 3/ 15% | 4/10% | 0.6760 |
| Characteristic | Without drainage device n=20 | With drainage device n=40 | p | OR and 95% CI* |
| Duration of operation (minutes) | 195 (160-205) | 200 (180-230) | 0.2095 | NA |
| Blood transfusion | 4/20% | 4/10% | 0.4218 | 2.25 (0.5-10.14) |
| FFP transfusion | 2/10% | 3/7.5% | 1.0000 | 1.37 (0.21-8.94) |
| Allergic reaction to drugs | None | None | NA | NA |
| Intraoperative incidents | 2/10% | 5/12.5% | 1.0000 | 0.78 (0.14-4.41) |
| Group of characteristics | Characteristic | Without drain (n=20) | With drain (n=40) | p | OR and 95% CI* |
| Post operative characteristics | Derangements of vital signs | 4/20% | 10/25% | 0.7556 | 0.75 (0.2-2.78) |
| Drain quantity abnormality | 0/0% | 4/10% | 1.0000 | NA | |
| Drain quality abnormality | 0/0% | 5/12.5% | 1.0000 | NA | |
| FBC abnormality | 3/15% | 13/32.5% | 0.2182 | 0.37 (0.09-1.48) | |
| FBC abnormality on day 0 | 17/85% | 26/65% | 0.1051 | 3.1 (0.76 - 12.23) | |
| Biochemical abnormality | 4/20% | 11/27.5% | 0.7529 | 0.66 (0.18-2.41) | |
| Biochemical abnormality on day 0 | 15/75% | 29/72.5% | 0.8365 | 1.1 (0.33 - 3.89) | |
| Clotting abnormality | 0/0% | 1/2.5% | 1.0000 | NA | |
| Swallow test positive | 0/100% | 2/5% | 0.5480 | NA | |
| VAS score (high) | 3/15% | 31/77.5% | <0.0001 | 19.52 (4.65-81.91) | |
| SSI | 4/20% | 8/20% | 1.0000 | 1 (0.26-3.83) | |
| Mobilization delay | 2/10% | 20/50% | 0.0038 | 0.11 (0.02-0.54) | |
| Oral feeding delay | 2/10% | 15/37.5% | 0.0340 | 0.19 (0.04-0.91) | |
| Intra-abdominal complication | 2/10% | 7/17.5% | 0.704 | 0.52 (0.1-2.79) | |
| Extra-abdominal complication | 2/10% | 3/7.5% | 1.0000 | 1.37 (0.21-8.94) | |
| ICU treatment | 1/5% | 1/2.5% | 1.0000 | 2.06 (0.12 -34.62) | |
| PONV | 5/25% | 24/60% | 0.0142 | 0.22 (0.07-0.73) | |
| Bowel movement start(POD) | 3 (2-4) | 4 (3-4) | 0.0141 | NA | |
| LOS (post-op to exit) | 5 (5-6) | 7 (6-10) | 0.0001 | NA | |
| Short- Term outcomes | Mortality | 2/10% | 1/2.5% | 0.2554 | 4.33 (0.37-50.95) |
| Re-admission | none | 5/12.5% | 0.1588 | NA | |
| Re-operation | 2/10% | 4/10% | 1.0000 | 1 (0.17-5.98) |
| Without drain (n=2) | With drain (n=7) | P-value | |
| Anastomotic leak detection | |||
| No | 2 (100%) | 3 (42.9%) | 0.444 |
| Yes | 0 (0%) | 4 (57.1%) | |
| POD | |||
| Mean (SD) | 5.00 (2.83) | 6.14 (2.73) | 0.445 |
| Median [Min, Max] | 5.00 [3.00, 7.00] | 7.00 [1.00, 9.00] | |
| Drain relation | |||
| (-) | 1 (50.0%) | 3 (42.9%) | 1 |
| (+) | 1 (50.0%) | 4 (57.1%) | |
| Diagnosis by drain content | |||
| No | 1 (50.0%) | 3 (42.9%) | 1 |
| Yes | 1 (50.0%) | 4 (57.1%) | |
| Diagnosis by vitals | |||
| No | 1 (50.0%) | 2 (28.6%) | 1 |
| Yes | 1 (50.0%) | 5 (71.4%) | |
| Diagnosis by fbc | |||
| No | 2 (100%) | 4 (57.1%) | 0.5 |
| Yes | 0 (0%) | 3 (42.9%) | |
| Prolonged hospitalization | |||
| No | 1 (50.0%) | 1 (14.3%) | 0.417 |
| Yes | 1 (50.0%) | 6 (85.7%) | |
| Reoperation | |||
| No | 1 (50.0%) | 4 (57.1%) | 1 |
| Yes | 1 (50.0%) | 3 (42.9%) | |
| Death | |||
| No | 1 (50.0%) | 6 (85.7%) | 0.417 |
| Yes | 1 (50.0%) | 1 (14.3%) |
| Diagnosis by other means(n=3) | Diagnosis by drain content(n=4) | P-value | |
| Anastomotic leak | |||
| No | 1 (33.3%) | 2 (50.0%) | 1 † |
| Yes | 2 (66.7%) | 2 (50.0%) | |
| POD | |||
| Mean (SD) | 6.67 (2.52) | 5.75 (3.20) | 0.714 |
| Median [Min, Max] | 7.00 [4.00, 9.00] | 7.00 [1.00, 8.00] | |
| Diagnosis by vitals | |||
| No | 1 (33.3%) | 1 (25.0%) | 1 † |
| Yes | 2 (66.7%) | 3 (75.0%) | |
| Diagnosis by labs | |||
| No | 2 (66.7%) | 2 (50.0%) | 1 † |
| Yes | 1 (33.3%) | 2 (50.0%) | |
| Prolonged hospitalization | |||
| Yes | 3 (100%) | 3 (75.0%) | 1 † |
| No | 0 (0%) | 1 (25.0%) | |
| Reoperation | |||
| No | 1 (33.3%) | 3 (75.0%) | 0.486 † |
| Yes | 2 (66.7%) | 1 (25.0%) | |
| Death | |||
| No | 3 (100%) | 3 (75.0%) | 1 † |
| Yes | 0 (0%) | 1 (25.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





