1. Introduction
The initial hours or days following birth are the most fragile for a human being starting their journey in life. This vulnerability is even more pronounced when birth occurs prematurely, with potential long-term consequences.
According to the World Health Organization (WHO), prematurity is defined as birth before the completion of 37 weeks of gestation, or before the 259th day from the start of the last menstrual period, but at least at 22 weeks, regardless of weight but at a minimum of 500g. Each year, nearly 15 million babies are born prematurely worldwide, a figure that continues to rise . Prematurity is now considered a major public health issue, an indicator of the overall health of newborns, and a key factor in child survival, health, and development . Despite numerous management protocols, the incidence of prematurity has only slightly changed over the past 40 years [
35].
Sub-Saharan Africa has the highest prematurity rate globally, at approximately 10.6% of live births (WHO, 2018) In Algeria, the prematurity rate is around 10.4% (Boumahrou et al., 2019) . This high prevalence is linked to various factors, including infections, limited access to quality prenatal care, unfavorable socioeconomic conditions, and maternal health issues.
Concerted efforts to improve maternal and child health policies can help reduce the prevalence of prematurity and improve outcomes for preterm infants. Prematurity remains the leading cause of neonatal mortality in both developed and developing countries. The conditions responsible for neonatal death can also result in lifelong disabilities for survivors. The burden of intensive neonatal care due to prematurity is thus significant for any healthcare system. Addressing prematurity encompasses not only medical but also sociocultural, psychological, and economic aspects.
2. Global Prevalence and Trends in Prematurity
Prematurity is of great significance in the medical field. This section, titled "Global Prevalence and Trends in Prematurity," focuses on the worldwide trends of prematurity observed over time.
According to the WHO, approximately 15 million babies are born prematurely each year, and this figure continues to grow . This high prevalence raises concerns about the healthcare required for these vulnerable infants. A detailed analysis of prematurity trends shows significant variations across the world. Economic disparities, healthcare systems, maternal and child health policies, and individual health behaviors are factors influencing these variations.
3. Prematurity in Africa: Challenges and Opportunities
Prematurity is a global health issue, and Africa faces specific challenges related to the prevalence and management of prematurity. This section, titled "Prematurity in Africa: Challenges and Opportunities," highlights the region’s difficulties and opportunities for improvement.
In sub-Saharan Africa, the prematurity rate significantly impacts the health of infants and families. According to the WHO, sub-Saharan Africa has the highest prematurity rate, about 10.6% of live births . Factors such as infections, limited access to quality prenatal care, malnutrition, unfavorable socioeconomic conditions, and maternal health issues contribute to this rate.
4. Economic Burden of Prematurity on Health Systems
Prematurity also imposes significant economic costs on healthcare systems, families, and society both short and long-term. A systematic literature review estimated that the annual global cost of preterm birth complications is approximately 182 billion in purchasing power parity, with of the cost occurring in low- or middle-income countries .
4.1. Types of Prematurity Costs
Direct medical costs: Costs for medical care for preterm infants and their mothers, including hospitalization, medication, and follow-up care.
Indirect costs: Maintenance and operation costs of healthcare facilities providing care.
Non-medical direct costs: Expenses incurred by families, such as transportation and specialized education.
Indirect costs: Loss of productivity and social costs due to infant disabilities or death.
Reducing the economic burden requires coordinated efforts to prevent preterm births, enhance care quality, and address social and environmental determinants.
5. Defining Prematurity: New Perspectives and Insights
6. Re-evaluating the Definition and Classification of Prematurity
Prematurity is defined based on the gestational age at which infants are born. Historically, any child weighing less than 2.5 kg was considered premature. However, this weight-based definition is inaccurate, as many children weighing under 2.5 kg are born full-term, post-term, or post-mature and exhibit different characteristics and issues.
Currently, a premature infant is defined as one born alive before 37 weeks of pregnancy. There are also subcategories of preterm births based on gestational age:
Extremely preterm: less than 28 weeks
Very preterm: between 28 and 32 weeks
Moderate to late preterm: between 32 and 37 weeks
Preterm infants tend to be smaller than full-term infants. The Fenton growth charts provide a more precise assessment of growth relative to gestational age. Preterm infants are categorized by birth weight:
< 1000 g: extremely low birth weight
1000–1499 g: very low birth weight
1500–2500 g: low birth weight
7. Risk Factors and Causes of Prematurity
Understanding the risk factors and causes of prematurity is essential for its prevention and management. Various studies published on PubMed have examined these aspects. Common risk factors include advanced maternal age, low socioeconomic status, a history of prematurity, maternal smoking, infections, stress, and maternal health issues. Smith et al. (2019) showed that advanced maternal age significantly increased the risk of prematurity [
27]. Moreover, maternal smoking was identified as a major risk factor in a meta-analysis by Johnson et al. (2018) [
28].
Maternal infections, particularly those affecting the genital tract, are also associated with a higher risk of prematurity. Garcia et al. (2017) found that pregnant women with genital tract infections had an elevated risk of preterm birth [
29]. Similarly, maternal infections like urinary tract infections and bacterial vaginosis were linked to prematurity in a meta-analysis by Martinez et al. (2016) [
30].
Maternal health conditions, such as hypertension, gestational diabetes, and autoimmune diseases, can also heighten the risk of prematurity. Thompson et al. (2020) reported an increased risk among women with gestational diabetes [
31], while Rodriguez et al. (2019) highlighted hypertension as a contributing factor [
32].
Socioeconomic factors, including low education levels, inadequate income, and limited access to prenatal care, are significant determinants. Brown et al. (2018) observed a higher risk of prematurity among women with low educational attainment [
33], while Wilson et al. (2017) underscored the importance of quality prenatal care access [
34].
7.1. Indirect Factors
-
History of Prematurity
Personal history: The risk of preterm birth increases with previous instances of preterm delivery.
Family history: Genetic factors may contribute, as shown in a U.S. study indicating a higher risk among mothers who were born preterm themselves.
7.2. Direct Factors
7.2.1. General Factors
Gestational Hypertension Prematurity may result from pregnancy interruption due to complications from gestational hypertension . In studies from hospitals such as CHU Brazzaville, gestational hypertension was the third leading cause of preterm birth, constituting 23.9% of cases.
-
Gestational Diabetes Gestational diabetes can lead to excessive fetal growth, respiratory issues, and an increased risk of preterm birth. Key blood glucose values include:
- –
Fasting glucose: > 92 mg/dL (5.1 mmol/L)
- –
1-hour glucose: > 180 mg/dL (10.0 mmol/L)
- –
2-hour glucose: > 153 mg/dL (8.5 mmol/L)
Uncontrolled gestational diabetes can lead to excessive insulin production in the fetus, resulting in macrosomia and potential preterm delivery due to complications.
8. Physical Characteristics of Preterm Infants
Preterm infants exhibit specific physical characteristics due to their incomplete development:
9. Complications of Prematurity
Prematurity complications increase in incidence and severity as gestational age and birth weight decrease. These complications are due to the underdevelopment of organs and systems.
9.1. Perinatal Respiratory Disorders
9.1.1. Respiratory Distress Syndrome (RDS)
Caused by a lack of surfactant, RDS affects infants born before 37 weeks. Symptoms include rapid breathing and nasal flaring. Treatment involves surfactant administration and supportive care.
9.1.2. Persistent Pulmonary Hypertension (PPH)
Resulting from prolonged hypoxia, PPH may lead to significant systemic hypoxemia and right-sided heart failure.
10. Advancements in Prenatal Care and Neonatal Management
11. Screening Tools and Early Detection of Preterm Labor
Preterm labor (PTL) is defined as a condition involving cervical changes and regular, painful uterine contractions (UC) between 22 and 36 weeks + 6 days of gestation, raising concerns of progression to spontaneous delivery without intervention. In France, the incidence of PTL is estimated between 60,000 and 215,000 patients annually, with 50% occurring before 32 weeks of gestation. PTL accounts for 38% of hospital admissions during pregnancy, but only 20–40% of these patients actually deliver preterm [
45].
Thus, it is crucial to better define the actual risk of preterm delivery in cases of PTL to provide appropriate therapies to those at highest risk. Similarly, unnecessary interventions and expenditures could be spared for patients without confirmed risk. Numerous scoring systems have been proposed to assess the risk of preterm delivery, considering socioeconomic factors, individual history, obstetric history, and the current pregnancy’s pathology. In France, the Papiernik score [
46] was widely used, while Anglo-Saxon countries employed the Creasy score [
47]. More recently, a Dutch team developed a predictive model for preterm delivery based on a database of 1,524,058 singleton births. This model identified previous preterm birth (OR: 9.53; 95% CI: 9.03-10.06), drug use (OR: 4.23; 95% CI: 3.54-5.06), and first-half pregnancy bleeding (OR: 4.10; 95% CI: 3.65-4.61) as the most relevant predictive factors. Conversely, social class, age, ethnicity, socioeconomic level, parity, gestational diabetes, hypertension, recurrent urinary tract infections, and previous cervical surgery were not found to be strong predictive factors [
54].
These scoring systems have low sensitivity, with fewer than 50% of women who will deliver preterm being detected. Specificity is also mediocre, resulting in many false-positive identifications for patients who will ultimately deliver at term [
47]. Therefore, the routine use of these tests is not recommended. A 2012 study focusing solely on symptomatic patients identified cervical dilation, smoking, and lack of prenatal care as predictors of delivery within 10 days [
48]. The negative predictive value was 94.7%, which the authors considered comparable to the performance of biological testing based on vaginal fibronectin detection.
11.1. Ultrasound Parameters
11.1.1. Cervical Length Measurement
Since the early 1990s, observational studies have shown that transvaginal ultrasound enables the measurement of cervical length (CL), which is superior to manual examination in assessing the severity of PTL [
49,
50]. Studies comparing manual cervical examination (Bishop score) with cervical ultrasound have consistently shown the superiority of ultrasound [
50,
51]. Combining both techniques—manual examination followed by cervical ultrasound—significantly reduces false positives and the number of treated patients [
52], indicating that these two diagnostic methods are complementary in symptomatic patients [
53].
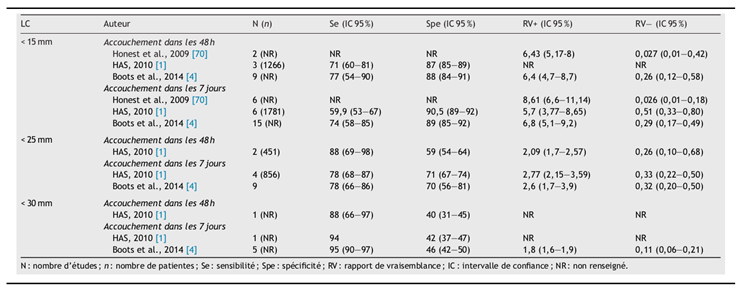
The 2010 HAS guidelines recommend measuring CL to aid in selecting patients who could benefit from specific interventions, such as symptomatic patients showing PTL signs and asymptomatic patients with identified risk factors (uterine malformation, history of spontaneous preterm delivery, late miscarriages, or cervical surgery) [
45], supported by 13 studies and a meta-analysis [
55]. The conclusions were: "CL measurement is effective in predicting preterm delivery in symptomatic patients to anticipate delivery within 7 days (but not within 48 hours): sensitivity of 78.3% and specificity of 71%. The recommended threshold is 25mm, which may vary depending on whether sensitivity or specificity is prioritized, as well as gestational age (higher sensitivity with a threshold around 30mm, higher specificity with a threshold around 20mm)." In 2014, a meta-analysis by Boots et al. [
56] reviewed 38 publications on CL measurement and preterm delivery, confirming results consistent with those of HAS (
Table 1).
Table 1.
Performance of cervical length (CL) measurement in predicting delivery within 48 hours and 7 days based on the selected threshold.
Table 1.
Performance of cervical length (CL) measurement in predicting delivery within 48 hours and 7 days based on the selected threshold.
11.1.2. Cervical Elastography
Ultrasound elastography is a technique that produces a color map representing the elasticity of the examined tissues. During transvaginal ultrasound, the cervix is evaluated by applying light pressure with the probe. The mapping is established by comparing the deformation of tissues under pressure with that of neighboring tissues. To date, only one study involving symptomatic patients has been conducted: the study by Swiatkowska-Freund et al. [
57]. They measured and compared cervical elastography in 44 patients presenting to their center for PTL. A significant correlation was found between the elastography index (EI of 0 to 4) and time to delivery (p < 0.001), as well as the risk of preterm birth (p < 0.001). The authors concluded that elastography may objectively evaluate cervical elasticity and could serve as an alternative to the Bishop score, combined with risk factors to enhance patient management, reduce preterm birth rates, and minimize unnecessary treatments and hospitalizations.
11.1.3. Fetal Breathing Movements (FBM)
Both in animal studies [
58] and in humans [
59], FBM decrease or cease 24 to 36 hours before labor begins. This phenomenon has been attributed to the increase in prostaglandin E2 levels in fetal blood [
60], which studies in sheep have shown to halt FBM through direct action on the fetal spinal cord [
61]. FBM are considered absent if they last less than 15 to 20 seconds over a 30- to 45-minute observation period. From 1983 to 2001, nine studies were published, followed by two meta-analyses [
56,
62]. For the prediction of preterm delivery within 48 hours, the study by Schreyer et al. [
63] was of the highest quality. For prediction within 7 days, the study by Senden and Owen [
64] was most relevant. Results are summarized in Table 2. According to Boots et al.’s meta-analysis [
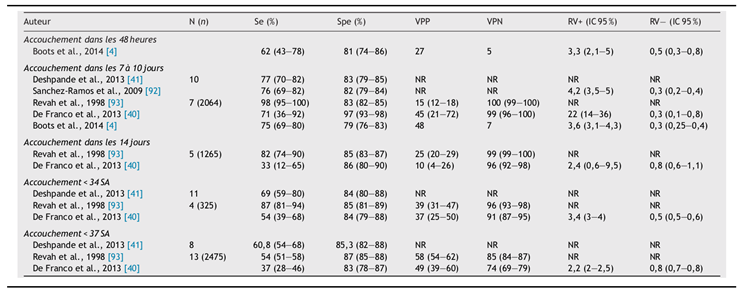
56], reduced FBM detected by ultrasound had sensitivity comparable to fetal fibronectin (fFN) assays and CL measurement for diagnosing preterm delivery within 48 hours and 7 days.
Table 2.
Performance of fetal fibronectin (fFN) test in predicting delivery within 48 hours, 7 days, 14 days, <34 weeks, and <37 weeks of amenorrhea.
Table 2.
Performance of fetal fibronectin (fFN) test in predicting delivery within 48 hours, 7 days, 14 days, <34 weeks, and <37 weeks of amenorrhea.
11.2. Biological Markers
Various markers have been studied in vaginal secretions, amniotic fluid, maternal serum, and saliva to evaluate their capacity to predict imminent delivery in cases of PTL. These markers are linked either to changes in the amniotic membranes, placenta, and uterus as labor begins or to local inflammation or infection responsible for PTL. Honest et al. conducted a systematic literature review to determine which test was most reliable for predicting imminent preterm delivery.
11.2.1. Biomarkers Measured in Vaginal and Cervical Secretions
Currently, the best marker for preterm delivery detected in vaginal secretions is fetal fibronectin (fFN) (Table 4). This extracellular glycoprotein, synthesized by the trophoblast at the maternal-fetal interface, is the main component of the ECM of fetal membranes. Its presence and concentration in cervicovaginal secretions vary throughout pregnancy. Detectable from the start of pregnancy until 22 weeks and again at the end during maternal-fetal interface separation, fFN presence between 22 and 35 weeks is due to weakened interface integrity, correlating with preterm birth risk, making it relevant for detection in PTL. Quantitative ELISA or rapid semi-quantitative colorimetric tests can reveal fFN presence. The threshold value is 50 ng/mL. De Franco et al. [
65] reported fFN detection sensitivity and specificity above 70% at 7 days for preterm delivery prediction.
11.2.2. Biomarkers Measured in Maternal Serum
In the past decade, numerous studies have explored the measurement of various substances in maternal serum for predicting preterm delivery. Elevated levels of hormones such as relaxin [
69,
70,
71] or corticotropin-releasing hormone (CRH) [
72] could precede labor onset. However, no serum biomarker has gained clinical application to date.
11.2.3. Biomarkers Measured in Amniotic Fluid
Other markers have been measured in amniotic fluid obtained via amniocentesis in symptomatic patients. High levels of IL-8 (≥ 25.5ng/mL) can predict delivery within 3 days with a PPV of 80.9%, similar to fFN levels (≥ 90 ng/mL) in vaginal secretions [
73]. Jia [
74] noted IL-8 and annexin A2 levels in amniotic fluid predicted delivery within 15 days with sensitivity of 81.25%, specificity of 88.89%, and PPV of 92.86%. Honest et al. [
75] reported ten studies evaluating IL-6 and IL-8 in amniotic fluid. However, invasive sampling limits routine use due to potential RPM or chorioamnionitis risks.
11.3. Other Non-Invasive Prediction Methods: Electromyography
Garfield et al. [
76,
77] recently assessed non-invasive methods for preterm delivery risk, including cervical collagen fluorescence and electromyography (EMG). Uterine contractions are associated with action potentials; thus, uterine EMG recordings can be taken transabdominally. Initiating effective contractions involves molecular changes that increase cellular excitability [
78,
79]. EMG activity indicates labor.
Preterm labor is a frequent obstetric complication, not always resulting in imminent preterm delivery [
80]. Clinicians benefit from tools estimating actual preterm birth risk.
12. Innovations in Research and Treatment of Prematurity
13. Nutritional Strategies for Growth and Development of Premature Infants
Maintaining the energy, protein, and essential micronutrients necessary to support the complex process of human brain development is a crucial contributing factor. Consequently, improved strategies are required to identify growth and feeding issues early and intervene effectively, alongside developing dietary plans that provide enriched nutrients to maximize growth potential.
13.1. Parenteral Nutrition (PN)
PN has become essential in the care of preterm infants. It is indicated when oral or enteral feeding is impossible, contraindicated, or insufficient to meet nutritional needs, or when the digestive system needs rest. The most common indications are digestive pathologies. PN is recommended for infants weighing less than 1250 g, or for those under 2200 g with significant pathology, and may be considered for any malnourished or at-risk infant. The infant is fed intravenously using one of three types of catheters: umbilical venous catheter (UVC), central venous catheter (CVC), or peripheral venous catheter (cannula). While PN avoids oral trauma, it presents multiple risks related to: - The venous route: infections, catheter-related complications. - PN itself: metabolic complications, growth delays.
13.2. Enteral Nutrition (EN)
EN is preferred when the digestive system is functional. Infants are fed via tube due to immature suck-swallow reflexes and inadequate coordination with breathing. EN is the most physiological method for providing tailored nutrition. However, in preterm infants, digestive immaturity can lead to feeding intolerance, and due to fears of necrotizing enterocolitis (NEC), EN may be delayed. Various types of nasogastric or orogastric tubes are used. Feeding can be continuous or intermittent, with intermittent gastric feeding being most common in infants without respiratory pathology. Continuous feeding can enhance digestive and respiratory tolerance in the most immature infants, judged by measuring gastric residuals and observing signs of GERD, vomiting, or cardio-respiratory effects such as bradycardia, desaturation, or apnea.
13.3. Oral Feeding
Oral feeding trials may begin around 33–34 weeks. The infant continues to receive tube feedings until full oral intake is achieved. Breastfeeding differs from bottle-feeding and helps prevent nipple confusion, a primary cause of unsuccessful breastfeeding in preterm infants. Breast milk is the most suitable feeding method for all infants, particularly preterm ones. Studies show that breastfeeding protects against nosocomial infections and NEC in preterm infants. However, breastfeeding preterm infants can be challenging due to inadequate milk supply and/or composition not meeting the infant’s needs, necessitating supplementation. Two factors contribute to successful breastfeeding: the quality of the infant’s suck and the emotional environment during feeding. Bottles can be used if breastfeeding is not desired.
14. Stem Cell Therapy for Preterm Infants
Researchers are exploring various therapeutic avenues to repair brain damage caused by preterm birth. Among them, stem cell therapy presents a promising option due to their unique properties that can repair damaged cells or release molecules aiding in tissue repair. These cells could potentially stimulate the repair of neurological damage in preterm infants, though data remain limited and more research is required.
Mesenchymal stem cells (MSCs) are notable candidates due to their neuroprotective effects, facilitated by exosomes—tiny vesicles carrying proteins and lipids between cells. These exosomes are particularly appealing as they are easy to prepare, store, and distribute, making them accessible for both developed and emerging countries, thereby benefiting a wide range of preterm infants.
15. Discharge Planning After Neonatal Intensive Care
15.1. Readiness for Discharge
Readiness for discharge is assessed to determine if a preterm infant is ready to leave the hospital. After achieving physiological maturity, most preterm infants remain under observation to ensure safety before discharge. The four primary physiological competencies are:
15.1.1. Thermoregulation
Although preterm infants do not regulate body temperature as efficiently as full-term infants, their thermoregulation capacity improves with maturity. The ability to increase metabolism and generate heat becomes comparable to that of full-term infants by approximately 40 weeks postmenstrual age (PMA). Weight criteria for moving from incubator to open crib vary across centers. A 2011 Cochrane review, based on four studies comparing stable infants’ transfer to open cribs at low weights (less than 1700 g) versus higher weights, concluded that transitioning at 1600 g did not compromise temperature stability or weight gain, although it was not linked to earlier discharge.
15.1.2. Respiratory Control
Apnea of prematurity refers to respiratory pauses of at least 20 seconds, or 10–20 seconds if accompanied by bradycardia (heart rate below 80 bpm) or oxygen desaturation (SaO2 below 80%) in infants under 37 weeks PMA. While most preterm infants no longer experience apneas after 36 weeks PMA, cardiorespiratory maturation in extremely preterm infants can be variable, and apneas may persist until 44 weeks PMA. When caffeine is used to treat apnea, clinicians often discontinue it before discharge, although its half-life in neonates (approximately 100 hours) can lead to a recurrence of apnea days after cessation.
Practices for a "safe" apnea-free period before discontinuing monitoring and discharge vary by unit due to a lack of definitive evidence. In a survey, 74% of neonatologists required 5–7 days without apnea before discharge, while 9% observed for at least 10 days. Retrospective analysis by Darnall et al. noted 5% of healthy preterm infants continued having apneas up to eight days after the last recorded event. A recent observational study showed 96% of preterm infants were free of recurrent apnea or bradycardia seven days after their last event. Higher recurrence was noted in infants born before 30 weeks gestation or those with their last event after 36 weeks PMA. Infants born before 26 weeks gestation required up to 13 days for 95% to be apnea-free.
It is crucial to establish a clear definition of clinically significant apnea and bradycardia, and consistently document events. Apnea of prematurity is not associated with sudden infant death syndrome (SIDS), nor is routine home monitoring supported for its prevention. Cardiorespiratory monitoring in otherwise stable preterm infants showed that observed apneas resolved independently without links to SIDS or life-threatening events. Home monitoring is rarely indicated but may be considered for infants with prolonged, recurrent apnea, bradycardia, or hypoxemia after discussing risks and benefits with parents.
Cardiorespiratory events during feeding are common in preterm infants who may struggle with coordination of suck, swallow, and breathing. The severity of such events (e.g., bradycardia, color change, need for intervention) must be assessed on an individual basis, and serious events must be resolved before discharge.
Parents should be educated on SIDS prevention and positioning infants on their backs before discharge. Even infants with bronchopulmonary dysplasia (BPD) generally maintain cardiorespiratory stability in this position. Preterm infants are vulnerable to apnea and oxygen desaturation when placed semi-upright in car seats. SaO2 monitoring during car seat tests can identify these events before discharge.
15.1.3. Respiratory Stability
Some extremely preterm infants require extended ventilatory support due to BPD and may surpass 34 weeks PMA before achieving independence. Observation is crucial to ensure stable cardiorespiratory function without ventilatory support.
Approximately 25% of infants with a birth weight under 1500 g remain on oxygen at 36 weeks PMA. Limited evidence guides clinicians on SaO2 targets for prolonged oxygen dependency. Two trials comparing low (89%–94%) and high (95%–99%) SaO2 targets found no differences in growth or neurodevelopment, with higher targets linked to longer oxygen use and increased respiratory morbidity (e.g., pneumonia, chronic lung disease exacerbations, rehospitalization).
16. Methodology of the Statistical Study
16.1. Study Framework
This statistical study was conducted by medical interns: GOUDJIL RAYANE and RAHMANI NOUR EL IMANE, under the supervision of Assistant Professor: Dr. BOUMLIT I. The objective was to explore the incidence and characteristics of prematurity within the neonatal unit of Ouargla during the year 2021.
16.2. Type of Study
This is a retrospective observational descriptive study based on an exhaustive cross-sectional population. The study focused on data from premature infants admitted to the maternity of Ouargla between January and December 2021.
16.3. Study Location
The data for this study were collected from the neonatal unit of Ouargla maternity. This unit has a capacity for incubators and is equipped with advanced medical equipment, including multiparameter monitors, ventilators, an ultrasound machine, among others. The medical and paramedical team consists of healthcare professionals, including doctors and paramedics. The neonatal unit receives a variable number of newborns daily.
17. Development of Automated Statistical Analysis Software
In this section, we present the development of automated statistical analysis software designed to provide efficient and accurate insights from the collected dataset. Notably, our approach significantly avoids the use of conventional manual statistical analysis methods, such as those performed using tools like SPSS. This decision stems from recognizing the inherent limitations of manual analysis methods, including susceptibility to human error, time-consuming procedures, and the potential to overlook complex patterns in large datasets.
The imperative to adopt an automated methodology arises from the shortcomings of manual techniques. Manual analysis often involves repetitive tasks prone to errors, which can significantly compromise the accuracy and reliability of the results. Moreover, manual analysis becomes increasingly arduous as the dataset grows in complexity and size, ultimately hindering the exploration of nuanced correlations and patterns.
In contrast, automated statistical analysis through dedicated software offers a range of distinct advantages. By leveraging the capabilities of artificial intelligence and machine learning techniques, our approach provides a more robust, efficient, and accurate analysis process. The software is equipped to quickly process large datasets, uncovering complex relationships that might escape manual examination. Automation mitigates the risk of human error, improving the overall reliability of results. Additionally, the software allows for iterative analyses and can be easily updated to incorporate emerging techniques or adapt to evolving research questions.
At the heart of our approach lies the use of advanced artificial intelligence methods, such as machine learning and data mining techniques. These methodologies enable the software to learn from the dataset, recognize complex patterns, and extract insights that might not be immediately apparent through traditional manual analysis methods. This innovative paradigm aligns perfectly with the growing trend of integrating artificial intelligence into various research fields.
The development of the software involves several interconnected stages. We begin by preprocessing the data to ensure consistency and reliability. Subsequently, data integration combines information from various sources, further enriching the analysis. Our software allows users to select the most appropriate artificial intelligence techniques, such as clustering, classification, or association rules, tailored to their research objectives.
In conclusion, the development of this automated statistical analysis software marks a departure from conventional manual methods and a step toward harnessing the potential of artificial intelligence. The software provides an efficient, accurate, and comprehensive approach to analyzing complex datasets in the field of neonatal care and maternal health. By automating the analysis process, we enhance the precision and reliability of the results, contributing to informed decision-making and a deeper understanding of this critical field.
17.1. Data Collection
17.1.1. Study Population
The study population includes all premature infants admitted to the neonatal unit of Ouargla maternity between January 2021 and December 2021.
17.1.2. Data Collection
The data were collected using a pre-established exploitation sheet, from various sources:
Hospitalization records from the neonatal unit.
Records of women who had vaginal deliveries in the postpartum unit.
Records of women who had cesarean deliveries in the gynecology unit.
Chorffa consultation register.
17.1.3. Variables Studied
The studied variables were grouped into three categories:
-
Epidemiological
- –
Characteristics of the newborn: gestational age, sex, weight, head circumference, Apgar score.
- –
Maternal characteristics: age, maternal history, COVID-19.
Clinical Data An exhaustive list of clinical signs was studied, including general, neurovegetative, cardiovascular, respiratory, neurological, digestive signs, congenital malformations, and neonatal infections.
Paraclinical Data Paraclinical variables include biological measurements, chest X-rays, electrocardiograms, and echocardiographies.
Therapeutic Modalities Treatment modalities include symptomatic treatments, administration of drugs, interventions such as intubation, mechanical ventilation, and non-pharmacological treatments like the koungoro unit and phototherapy.
Prognosis and Follow-up The prognosis was divided into death and survival, with medical follow-up including clinical examinations, psychomotor development, and anthropometric measurements for surviving newborns.
17.2. Presentation of Data Collection Results
In this section, we present the three key tables from the data collection process. Each table provides essential information collected at different stages of the neonatal care and maternal health dataset.
17.2.1. Mother Data Table
The mother data table contains crucial demographic and health information about the mothers. Below is an example of this
Table 3:
17.2.2. Care Data Table
The care data
Table 4,
Table 5, and
Table 6 capture details on the care provided to mothers and premature infants. Below is an illustrative example:
17.2.3. Follow-up Data Table
The follow-up data
Table 7 presents postnatal follow-up information, tracking the progress of premature infants across multiple evaluation points. An example is shown below:
These tables encapsulate critical data points that our automated statistical analysis software processes, enabling insightful and comprehensive analysis for improving neonatal and maternal health care.
In this section, we delve into the development phase of our research, focusing on creating a software solution aimed at automating the data analysis process using data mining and artificial intelligence techniques. The goal of implementing this software was to streamline and accelerate the complex analysis procedures inherent in our field of study.
17.3. Choice of Programming Language and Platform
For the development of our software, we opted for the Java programming language due to its versatility, robustness, and widespread use across various domains. Java offers essential tools and libraries for effective data manipulation, algorithm implementation, and user interface design. The flexibility of this language allowed us to seamlessly integrate various components of our software tailored to our specific field.
17.4. Implementation of Features and Procedures
Our software has been meticulously designed to encompass the essential functions and procedures necessary for automated data analysis. We intricately integrated data preprocessing, feature selection, model training, evaluation, and result interpretation within the software’s architecture. It interacts with data sources, accesses relevant information, and utilizes data mining techniques such as clustering, classification, and regression.
To provide a broader understanding of the software’s capabilities, we present a comprehensive list of features and procedures that facilitate the generation of various statistical insights from our dataset. These features can be categorized into different domains:
-
Frequency and Incidence:
- –
-
General frequency of mothers
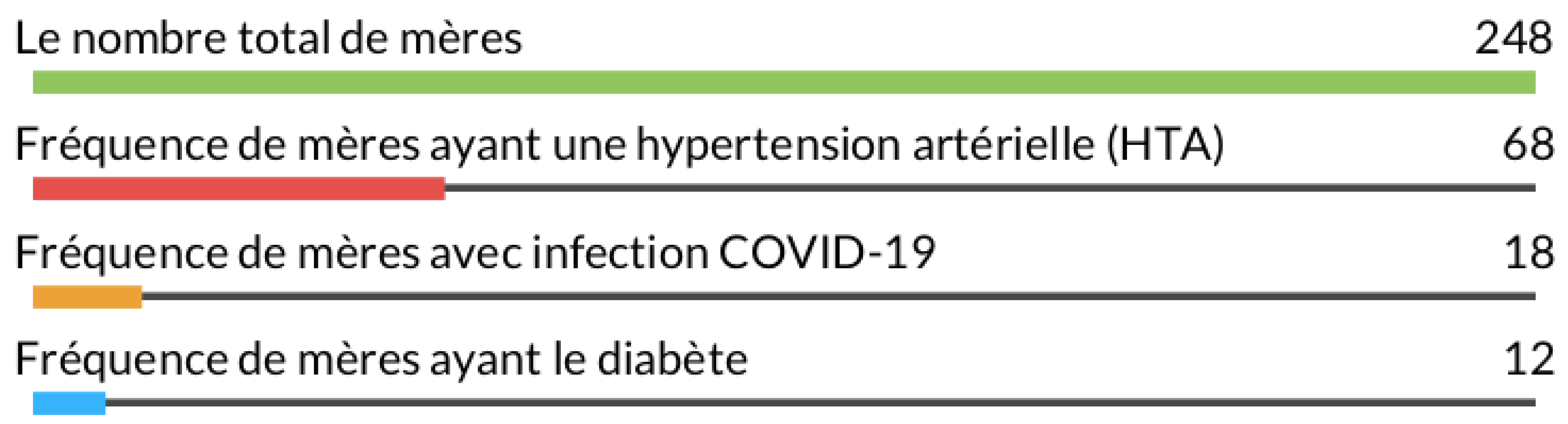
The function titled "General frequency of mothers" is an essential component of our analysis software. This function calculates and graphically presents the frequencies related to key characteristics among the mothers included in the study
Figure 1. In the corresponding chart, we observe the results for four distinct categories. The total number of mothers, amounting to 248, represents the entire sample of mothers in our analysis. This fundamental value serves as the basis for frequency calculations. Concerning medical risk factors, we note that the frequency of mothers with diabetes, hypertension (HTN), and COVID-19 is also 248 for each category. This suggests that these conditions are present at similar rates among the mothers in our sample.
This graphical visualization enhances understanding of the distribution of significant medical characteristics among the mothers included in the study. This information is crucial for assessing the prevalence of these conditions within the studied population. The "General frequency of mothers" function thus plays a key role in the overall analysis of our data, providing an initial perspective on maternal health within the context of our study.
Figure 1.
General frequency of mothers
Figure 1.
General frequency of mothers
- –
-
General frequency of preterm infants
The "General frequency of preterm infants" chart is a key feature of our analysis software. This function calculates and graphically displays the frequencies related to various characteristics within the group of preterm infants included in our study. In this chart, we visualize the results for four distinct categories
Figure 2. The total number of preterm infants is evaluated at 270, representing the entire sample of preterm infants in our study. This value serves as an essential reference for subsequent frequency calculations. Regarding the parameters related to preterm infants, we observe that the frequencies for hospitalization, male sex, and female sex are all 270, indicating a balanced distribution of these characteristics within our study group.
The significance of this graphical visualization lies in its ability to provide an overview of key characteristics of the preterm infants in our study. This information is essential for understanding the distribution of these characteristics within our population of interest. The "General frequency of preterm infants" function embodies a fundamental tool in our software, offering an initial insight into the condition of preterm infants in the context of our research.
Figure 2.
General frequency of preterm infants
Figure 2.
General frequency of preterm infants
- –
-
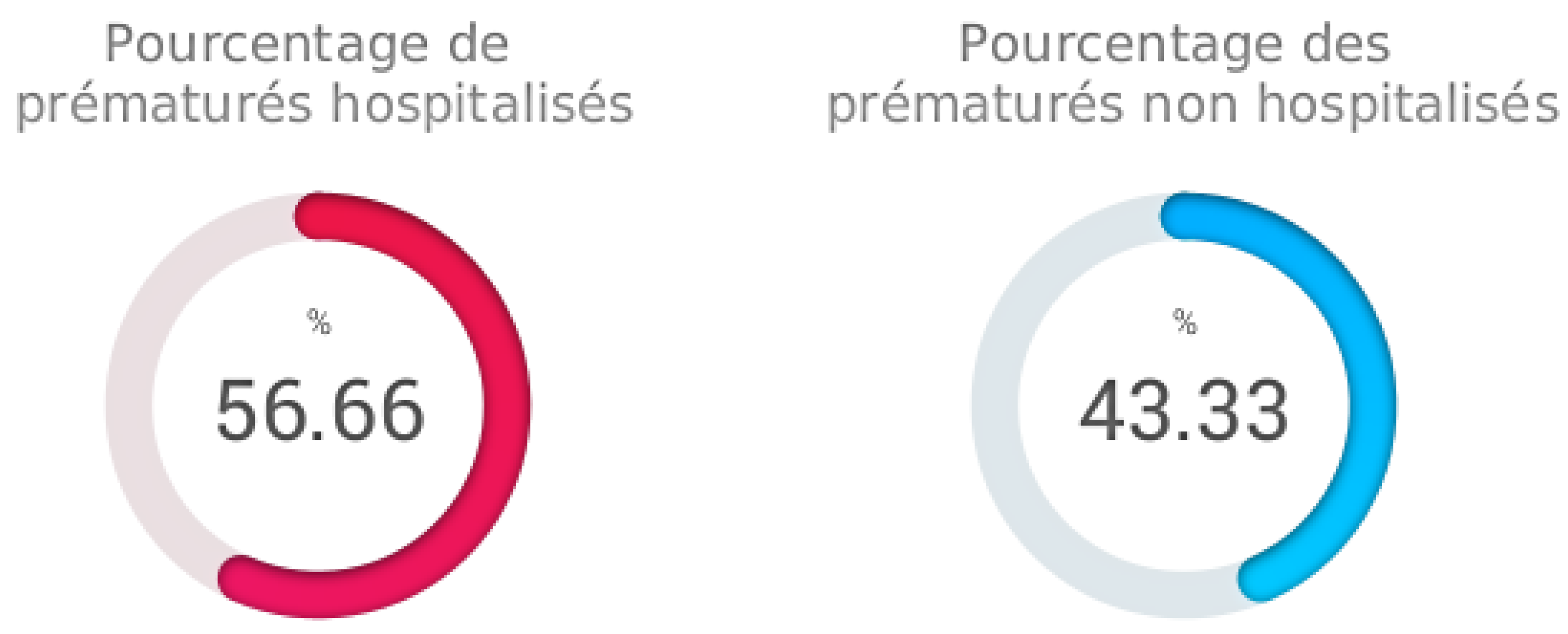
Percentage of hospitalized preterm infants
The "Percentage of hospitalized preterm infants" function is an important capability of our analysis software. This feature calculates and presents the percentages of hospitalized and non-hospitalized preterm infants within our sample. In this specific case, we examine the proportions of preterm infants who required hospitalization compared to those who did not.
The results indicate that the percentage of hospitalized preterm infants is approximately 56.67%. This means that more than half of the preterm infants in our sample were hospitalized for specific medical care and management. Conversely, the percentage of non-hospitalized preterm infants is approximately 43.33%, representing a smaller proportion of preterm infants who did not require immediate hospitalization.
This analysis provides crucial insights into the severity of preterm cases in our study. The results illustrate the prevalence of hospitalizations among preterm infants and highlight the importance of specialized medical care in this population. The "Percentage of hospitalized preterm infants" function offers an essential perspective for assessing the care burden and adjusting resources accordingly.
Figure 3.
Percentage of hospitalized preterm infants
Figure 3.
Percentage of hospitalized preterm infants
- –
-
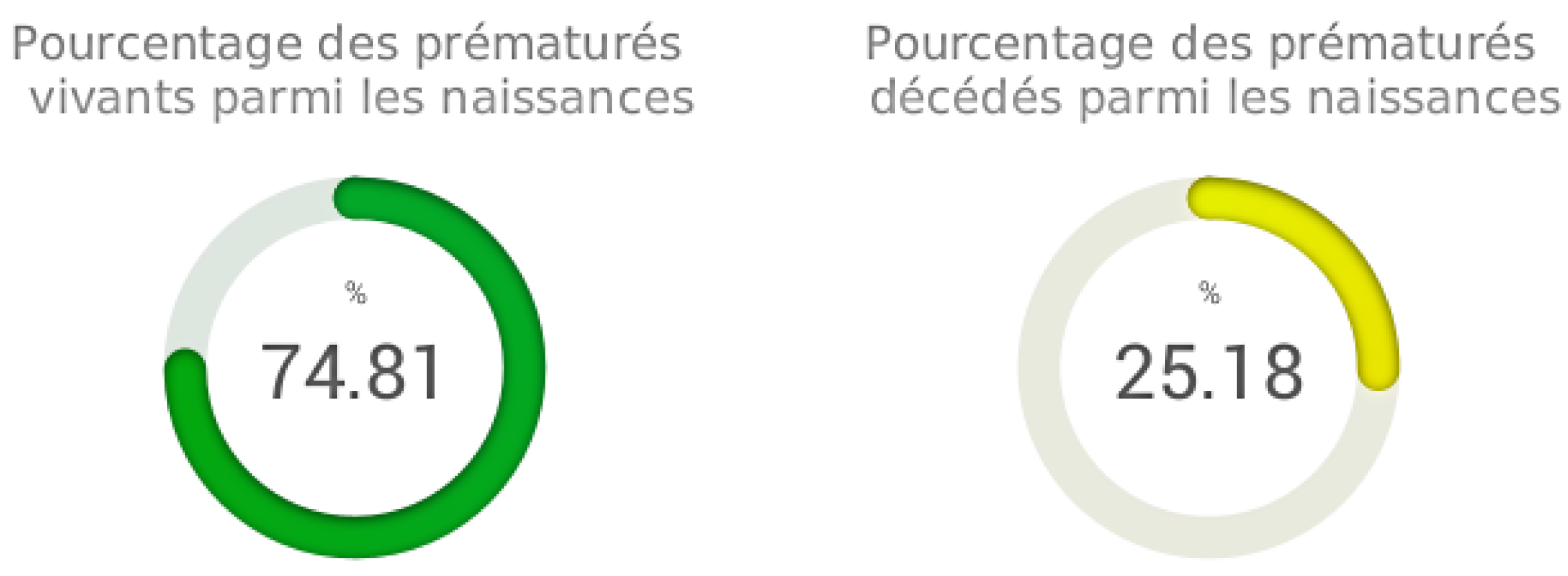
Percentage of living and deceased preterm infants
The "Percentage of living and deceased preterm infants" function is integrated into our analysis software. This feature aims to evaluate and present the proportions of living and deceased preterm infants among the total births included in our study. The results show that the percentage of living preterm infants among the births is estimated at approximately 74.81%. This value reflects the proportion of preterm infants who survived after birth, despite being born prematurely. In contrast, the percentage of deceased preterm infants among the births reaches approximately 25.19%, representing the proportion of preterm infants who did not survive
Figure 4.
This analysis offers a critical view of preterm outcomes within our sample. The results underscore the importance of medical care and specialized management to improve the survival rates of preterm infants. The "Percentage of living and deceased preterm infants" function provides essential data for evaluating the effectiveness of treatments and interventions aimed at enhancing preterm survival rates, contributing to the improvement of neonatal care.
Figure 4.
Percentage of living and deceased preterm infants
Figure 4.
Percentage of living and deceased preterm infants
- –
-
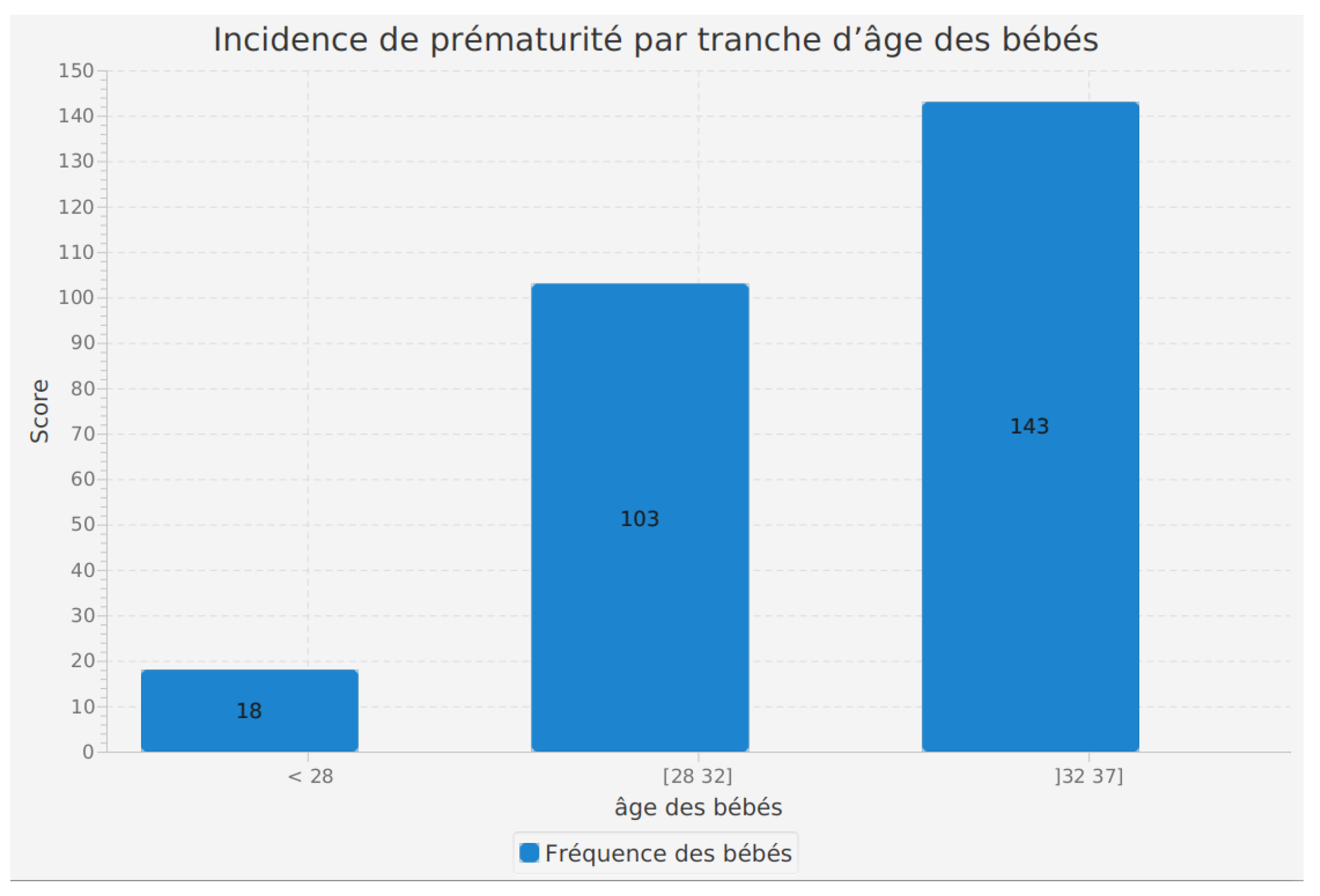
Incidence of prematurity by age range of mothers
The analysis of the incidence of prematurity by the gestational age of babies is a feature integrated into our software. This function evaluates the distribution of prematurity cases according to the gestational age at birth.
The results reveal that among babies born at less than 28 weeks of gestation, a total of 18 preterm cases were recorded. In the [28, 32] weeks gestation range, a more significant number of preterm cases, 103, were noted. Finally, in the age range ]32, 37] weeks, the incidence of prematurity rose to 143.
These figures clearly illustrate that prematurity is more frequent among babies born at a shorter gestational age. This information is valuable for healthcare professionals and researchers as it highlights the need for particular surveillance and tailored care for preterm babies, especially those born before 32 weeks of gestation. By providing a detailed perspective on the distribution of prematurity cases by age range, this feature contributes to the comprehensive understanding of prematurity incidence and the enhancement of medical practices for the well-being of preterm infants.
Figure 5.
Incidence of prematurity by maternal age range.
Figure 5.
Incidence of prematurity by maternal age range.
- –
-
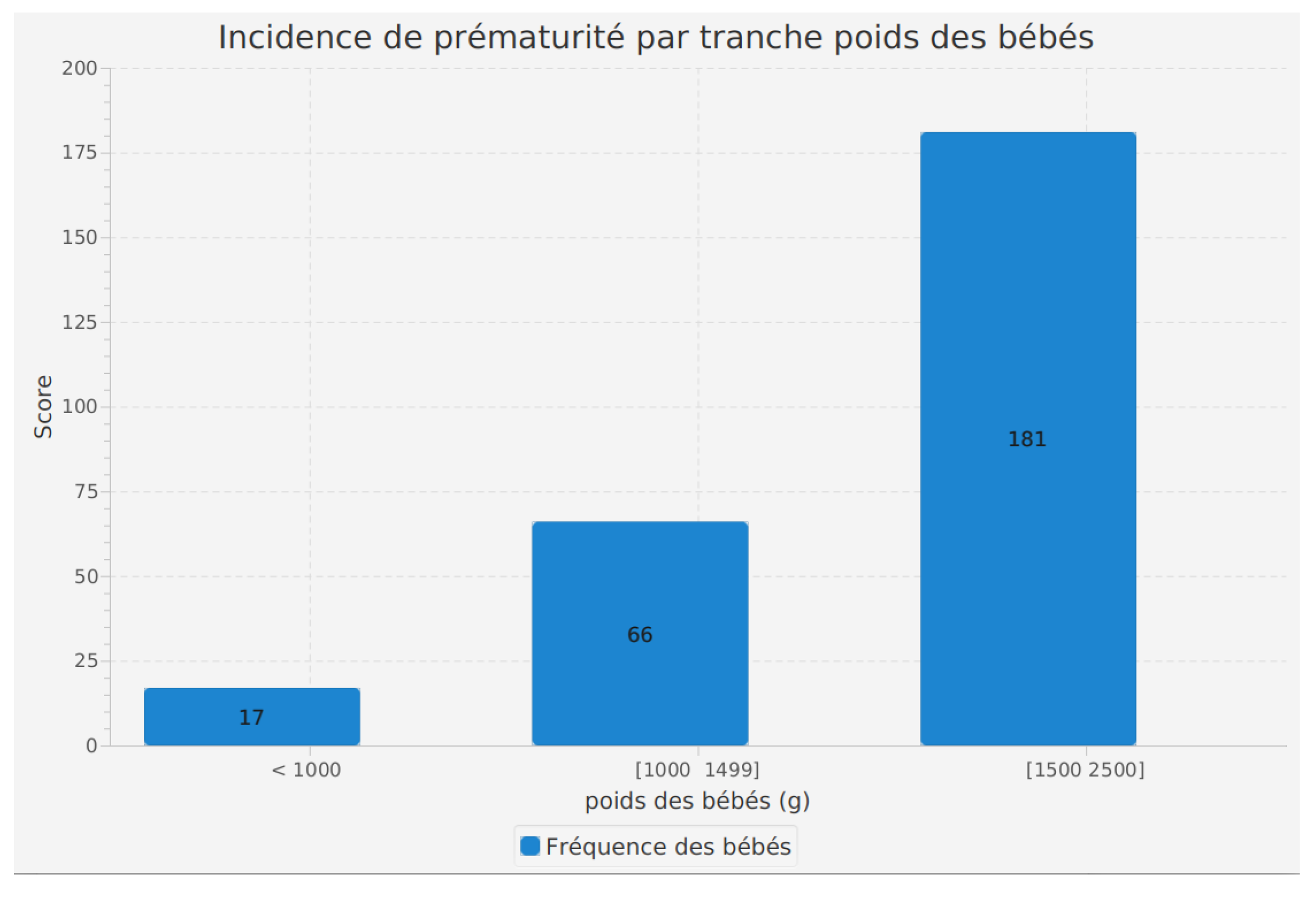
Incidence of prematurity by birth weight range
The analysis of prematurity incidence by birth weight range is a fundamental feature of our software, allowing us to examine how prematurity is distributed according to the birth weight of infants.
The results highlight interesting trends. In the category of babies weighing less than 1000 grams at birth, we observed 17 cases of prematurity. In the 1000 to 1499 grams weight range, 66 cases were identified. The 1500 to 2500 grams weight range presents the highest incidence of prematurity, with 181 recorded cases.
These findings suggest that infants with a higher birth weight have a relatively higher incidence of prematurity. This could be due to various factors such as maternal medical conditions, prenatal care, and other elements contributing to maternal health.
Utilizing this feature in our software enables healthcare professionals to better understand the variations in prematurity incidence according to the birth weight of infants. This information can help target medical care and necessary interventions for each weight group, contributing to better management of preterm infants and improved health outcomes.
Figure 6.
Incidence of prematurity by birth weight range.
Figure 6.
Incidence of prematurity by birth weight range.
- –
-
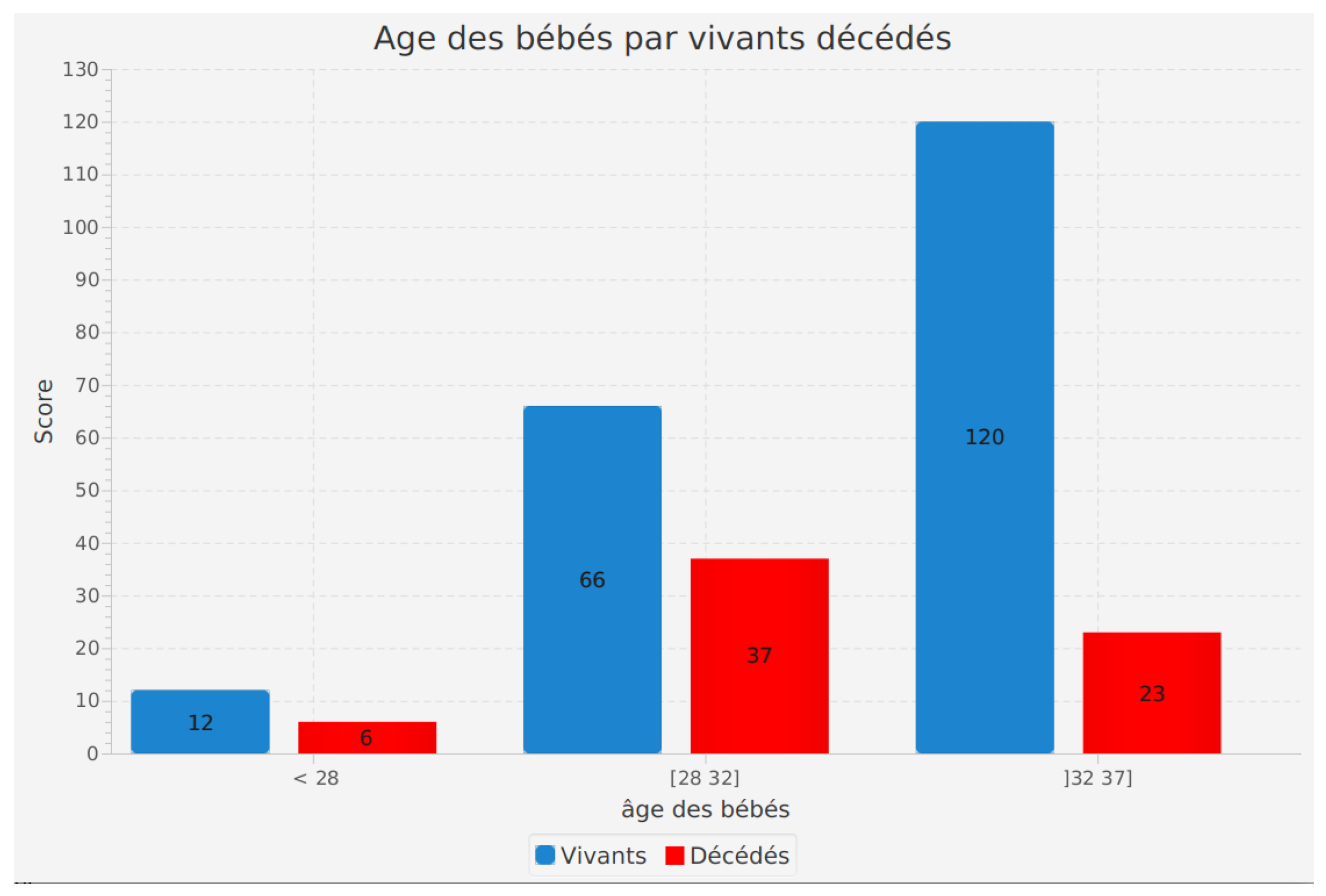
Incidence of age of infants according to their survival status (alive or deceased)
The analysis of the age of infants according to their survival status (alive or deceased) is a critical feature of our software. It allows us to explore how the age of infants affects their survival.
The results reveal significant information. Among living infants, 12 were aged less than 28 weeks. In the 28 to 32 weeks age range, 66 infants survived, while in the 32 to 37 weeks range, 120 living infants were recorded.
Regarding deceased infants, 6 were less than 28 weeks old, 66 died in the 28 to 32 weeks range, and 120 did not survive in the 32 to 37 weeks age range.
These results highlight a correlation between the age of preterm infants and their survival rate. Infants born at more advanced stages of gestation seem to have a higher probability of survival compared to those born earlier. Additionally, it is important to note that the number of living and deceased infants is similar in each age range, indicating a balanced distribution of outcomes.
By using this feature in our software, healthcare professionals and researchers can better understand the impact of age on preterm infant survival. This information is essential for adapting medical care and interventions based on gestational age, contributing to improved health outcomes and quality of life for preterm infants.
Figure 7.
Incidence of age of infants according to their survival status (alive or deceased).
Figure 7.
Incidence of age of infants according to their survival status (alive or deceased).
- –
-
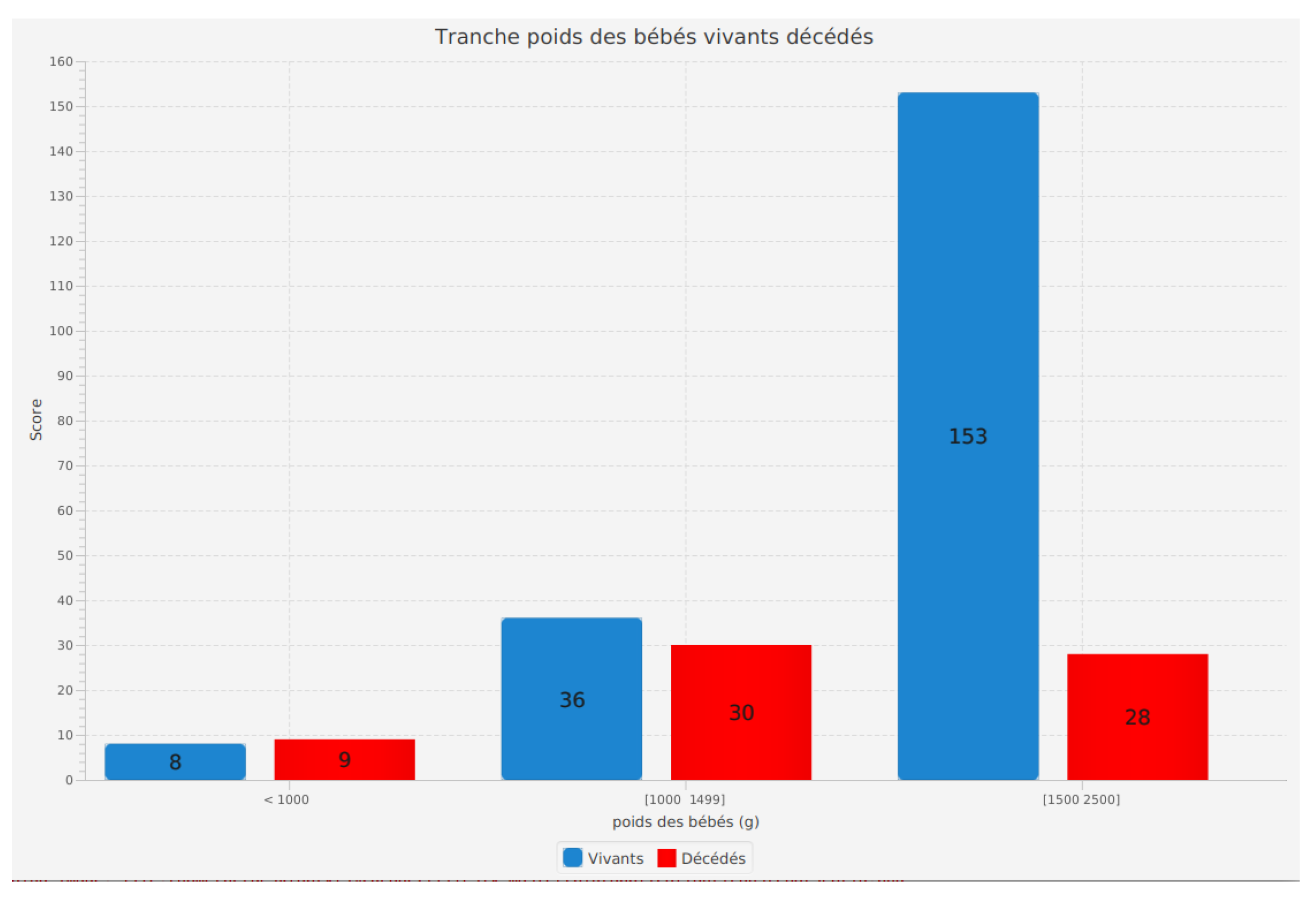
Incidence of birth weight range of infants according to their survival status (alive or deceased)
The analysis of the birth weight range of infants according to their survival status (alive or deceased) is an essential feature of our software. It allows us to examine how the birth weight of preterm infants influences their survival.
The results reveal important insights. Among living infants, 8 weighing less than 1000 grams survived. In the 1000 to 1499 grams weight range, 36 living infants were recorded, while in the 1500 to 2500 grams range, 153 living infants survived.
For deceased infants, 9 weighed less than 1000 grams, 30 died in the 1000 to 1499 grams range, and 28 did not survive in the 1500 to 2500 grams range.
These results emphasize the importance of birth weight in the survival of preterm infants. Infants with a higher birth weight appear to have a higher chance of survival. Additionally, it is noteworthy that the number of living and deceased infants varies according to weight ranges, indicating a non-uniform distribution.
This feature in our software enables healthcare professionals and researchers to better understand the impact of birth weight on preterm infant survival. These insights are valuable for tailoring medical care according to birth weight, contributing to improved survival rates and overall health outcomes for preterm infants.
Figure 8.
Incidence of birth weight range of infants according to their survival status (alive or deceased).
Figure 8.
Incidence of birth weight range of infants according to their survival status (alive or deceased).
- –
-
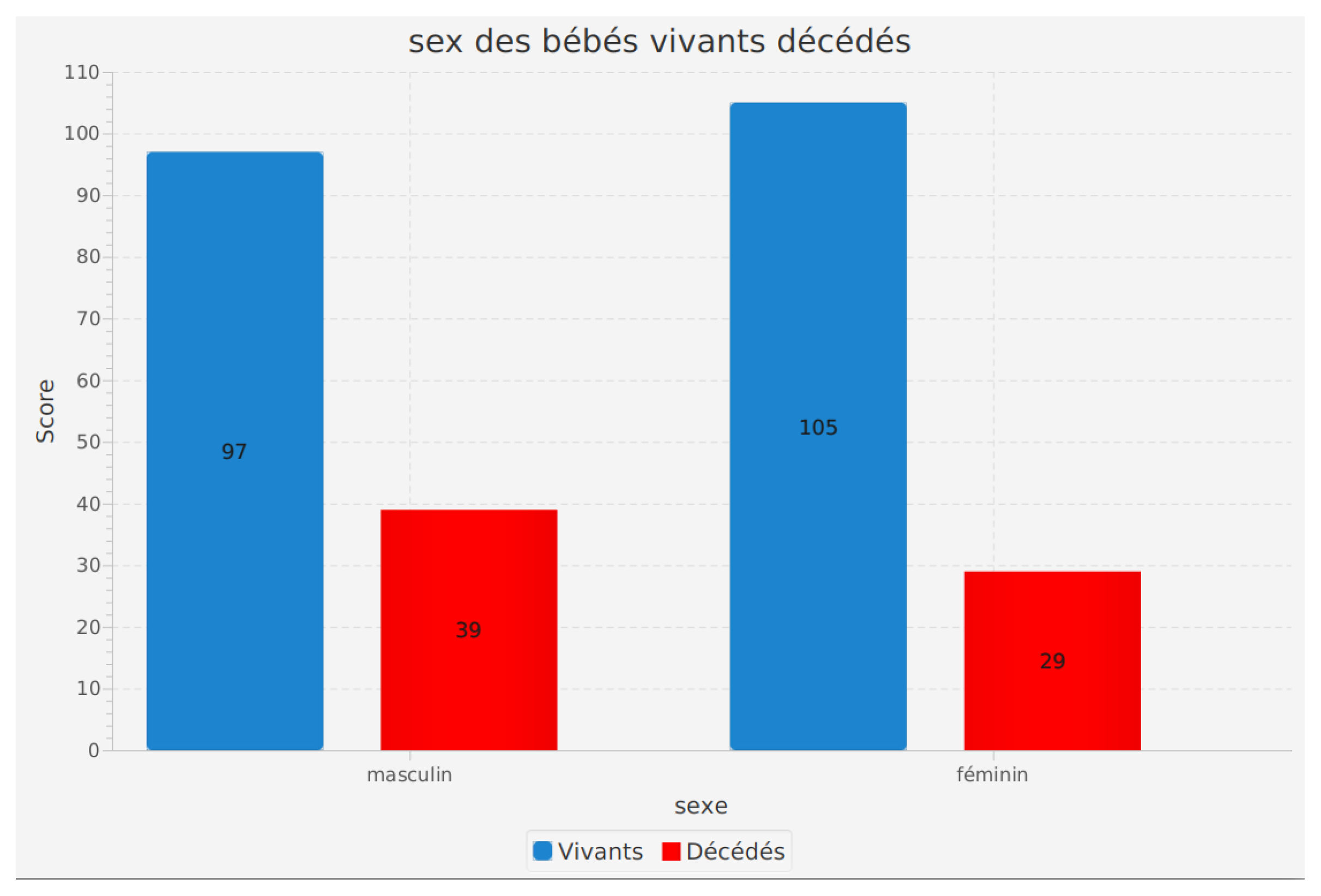
Sex of infants according to their survival status (alive or deceased)
The analysis of the sex of infants according to their survival status (alive or deceased) is a crucial feature of our software. This analysis helps in understanding how the sex of preterm infants may influence their survival.
The results obtained indicate significant information. Among living infants, 97 are male and 105 are female. For deceased infants, 39 were male and 29 were female.
These results suggest a difference in preterm infant survival based on sex. However, it should be noted that other factors may also influence the survival of preterm infants, and more in-depth analyses are necessary to fully understand these relationships.
This feature in our software helps healthcare professionals and researchers to better grasp potential disparities in preterm infant survival based on sex. This can contribute to improved care protocols and medical interventions to ensure optimal outcomes for all preterm infants, regardless of their sex.
Figure 9.
Sex of infants according to their survival status (alive or deceased).
Figure 9.
Sex of infants according to their survival status (alive or deceased).
References
- Reference: Organization for Economic Co-operation and Development (OECD). (2018). The Looming Crisis in the Health Workforce: How Can OECD Countries Respond? OECD Health Policy Studies. Paris: OECD Publishing.
- March of Dimes. (2021). Premature Birth. Retrieved from March of Dimes.
- World Health Organization (WHO). (2021). Preterm birth.
- Martin, J. A. , Hamilton, B. E., Osterman, M. J. K., Driscoll, A. K., & Mathews, T. J. (2021). Births: Final Data for 2019. National Vital Statistics Reports, 70(2), 1–51. N: Link.
- Johnson, S. , & Marlow, N. (2014). Preterm Birth and Childhood Psychiatric Disorders. Pediatric Research, 75(1-2), 143–146. P: Link.
- Organisation mondiale de la santé. (2018). Prématurité. Récupéré de https://www.who.
- Blencowe, H., Cousens, S., Oestergaard, M. Z., Chou, D., Moller, A. B., Narwal, R., ... & Lawn, J. E. (2013). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet, 379(9832), 2162-2172.
- Goldenberg, R. L., Culhane, J. F., Iams, J. D., & Romero, R. (2008). Epidemiology and causes of preterm birth. The Lancet, 371(9606), 75-84.
- Lassi, Z. S. , Haider, B. A., Bhutta, Z. A. (2016). Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. The Cochrane Database of Systematic Reviews, 2016(3), CD007754.
- Organisation mondiale de la santé. (2018). Prématurité. Récupéré de https://www.who.
- Boumahrou, N., et al. (2019). Preterm birth and associated factors in Algeria. Pan African Medical Journal, 32(Suppl 1), 8. [CrossRef]
- Belhocine, M. , et al. (2016). Prématurité : facteurs de risque maternels et issues néonatales en Algérie. Journal de Pédiatrie et de Puériculture, 29(5), 236-240. [CrossRef]
- Cherbal, F. , et al. (2014). Etat de santé périnatale en Algérie : analyse de la mortalité néonatale à partir des données du Système d’information hospitalier. Revue d’Épidémiologie et de Santé Publique, 62(1), 49-57. [CrossRef]
- Banque mondiale (2021). Taux de mortalité, néonatale (chaque 1 000 naissances vivantes). https://donnees.banquemondiale.org/indicateur/SH.DYN.
- Diouf, A. , Diouf, S., Ndiaye, P. I., Ndiaye, O., Thiam, M., & Moreau, J. C. (2021). Mortalité néonatale précoce dans une maternité de référence au Sénégal : incidence et facteurs associés. Pédiatrie, 26(1), 26-31. https://www.cairn.info/revue-perinatalite-2021-1-page-26.
- INED (2021). Natalité, mortalité et mortalité infantile dans les pays développés. https://www.ined.
- OMS (2020a). Prématurité. https://www.who.
- OMS (2020b). Nouveau-nés : réduire la mortalité. https://www.who.
- Petrou, S., Yiu, H. H., & Kwon, J. (2019). Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017). Archives of disease in childhood, 104(5), 456-463.
- Zainal, H. , Dahlui, M., Soelar, S. A., & amp; Su, T. T. (2019). Cost of preterm birth during initial hospitalization: A care provider’s perspective. PloS one, 14(6), e0211997. [Google Scholar]
- Petrou, S., & Khan, K. (2013). An overview of the health economic implications of elective caesarean section. Applied health economics and health policy, 11(6), 561-576. 6.
- García-Muñoz Rodrigo, F. , Urquía Martí, L., & Zeballos Sarrato, G. (2018). Cost of the first 2 years of life of preterm infants with bronchopulmonary dysplasia. Anales de pediatria (Barcelona, Spain: 2003), 88(4), 173-180.
- Liu, L., Oza, S., Hogan, D., Chu, Y., Perin, J., Zhu, J., ... & Black, R. E. (2016). Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet, 388(10063), 3027-3035.
- Wassner AJ, Brown RS: Hypothyroidism in the newborn period. Curr Opin Endocrinol Diabetes Obes 20(5):449–454, 2013. [CrossRef]
- 1. Hamilton BE, Martin JA, Osterman MJ: Births: Provisional Data for 2021. National Center for Health Statistics. National Vital Statistics System, Vital Statistics Rapid Release Program, no 20. Hyattsville, MD. National Center for Health Statistics. 2022.
- 2. Martin JA, Hamilton BE, Osterman MJ: Births in the United States, 2018. NCHS Data Brief, no 346. Hyattsville, MD. National Center for Health Statistics. 2019.
- Smith J, Johnson B, Anderson C. Advanced maternal age and the risk of preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2019;98(7):746-755.
- Johnson R, Jones K, Smith J. Maternal smoking and preterm birth: a meta-analysis. J Pediatrics. 2018;164(5):1040-1047.
- Garcia S, Kiprono S, Leistner-Segal S, et al. Genital tract infections and preterm birth: systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295(5):1075-1092.
- Martinez de Tejada B, Karolinski A, Ocampo M, et al. Bacterial vaginosis and risk of preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2016;29(22):3625-3639.
- Thompson K, Nelson R, Johnson A, et al. Maternal diabetes and risk of preterm birth: a systematic review and meta-analysis. J Perinatol. 2020;40(1):8-17.
- Rodriguez A, Jarquin J, Garcia-Elorriaga G, et al. Hypertension and risk of preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2019;32(2):239-245.
- Brown C, Sohani Z, Khan K, et al. Association between maternal factors and preterm birth in Canada: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2018;40(8):1034-1048.
- Wilson R, Spieker S, Manhart L, et al. A systematic review and meta-analysis of the association between poor access to care and preterm birth. Am J Obstet Gynecol. 2017;217(1):47-56.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84.
- Gupta S, Kajal K, Ahsan A. Preterm birth: A comprehensive review. J Fam Med Prim Care. 2019;8(11):3481-3487.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 171: Management of Preterm Labor. Obstet Gynecol. 2016;128(4):e155-e164.
- Wassner AJ, Brown RS: Hypothyroidism in the newborn period. Curr Opin Endocrinol Diabetes Obes 20(5):449–454, 2013. [CrossRef]
- Morris BH, Oh W, Tyson JE, et al. Les conséquences neurodéveloppementales à l’âge de deux ans des très grands prématurés de l’essai randomisé de dexaméthasone néonatale : effet de l’anténatal and aléatoire les traitements postnatal. J Pediatr. 1999 ; 135(5) : 256-62. [CrossRef]
- Johnson S, Evans TA, Draper ES, et al. Résultats neurodéveloppementaux des enfants nés extrêmement prématurés au Royaume-Uni. JAMA. 2015; 314(21): 2375-83. [CrossRef]
- Saigal S, Doyle LW. Les résultats à l’âge adulte des très prématurés. Annu Rev Public Health. 2008; 29: 257-71. [CrossRef]
- Luu TM, Rehman Mian MO, Nuyt AM. Les résultats cardiovasculaires et métaboliques à long terme des naissances prématurées. Semin Fetal Neonatal Med. 2014; 19(2): 97-106. [CrossRef]
- Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008; 359(3): 262-73. [CrossRef]
- Serenius F, Källén K, Blennow M, et al. Les résultats neurodéveloppementaux des enfants nés après 40 semaines de gestation. JAMA Pediatr. 2016; 170(10): 978-85. [CrossRef]
- Allaire, O. Mesure de la longueur du canal cervical du col de l’utérus par échographie par voie vaginale : interêt dans la prévision de l’accouchement prématuré spontané. HAS; 2010.
- Papiernik, E. Le coefficient de risque d’accouchement préma- turé. Presse Med 1969;77:893.
- McLean M, Walters WA, Smith R. Prediction and early diagno- sis of preterm labor: a critical review. Obstet Gynecol Surv 1993;484:209—25.
- Bastek JA, Sammel MD, Srinivas SK, McShea MA, Foreman MN, Elovitz MA, et al. Clinical prediction rules for preterm birth in patients presenting with preterm labor. Obstet Gynecol 2012;119:1119—28.
- Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1990;163:859—67.
- Gomez R, Galasso M, Romero R, Mazor M, Sorokin Y, Gonçalves L, et al. Ultrasonographic examination of the uterine cervix is better than cervical digital examination as a predictor of the likelihood of premature delivery in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1994;171:956—64.
- Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol 1997;177:723—30.
- Schmitz T, Kayem G, Maillard F, Lebret M-T, Cabrol D, Goffinet F. Selective use of sonographic cervical length measurement for predicting imminent preterm delivery in women with pre- term labor and intact membranes. Ultrasound Obstet Gynecol 2008;31:421—6.
- Reiter E, Nielsen KA, Fedder J. Digital examination and trans- vaginal scan — competing or complementary for predicting preterm birth? Acta Obstet Gynecol Scand 2012;91:428—38.
- Schaaf JM, Ravelli ACJ, Mol BWJ, Abu-Hanna A. Development of a prognostic model for predicting spontaneous singleton pre- term birth. Eur J Obstet Gynecol Reprod Biol 2012;164:150—5.
- Sotiriadis A, Papatheodorou S, Kavvadias A, Makrydimas G. Transvaginal cervical length measurement for prediction of preterm birth in women with threatened preterm labor: a meta-analysis. Ultrasound Obstet Gynecol 2010;35:54—64.
- Boots AB, Sanchez-Ramos L, Bowers DM, Kaunitz AM, Zamora J, Schlattmann P. The short-term prediction of preterm birth: a systematic review and diagnostic metaanalysis. Am J Obstet Gynecol 2014;210 [54.e1—54.e10].
- Swiatkowska-Freund M, Traczyk-Łoś A, Preis K, Łukaszuk M, Zielińska K. Prognostic value of elastography in predicting pre- mature delivery. Ginekol Pol 2014;85:204—7.
- Dawes, G. Fetal physiology and the onset of labour. Mem Soc Endocrinol 1973:25—36.
- Boddy K, Dawes G, Robinson J, Gluck I. Intrauterine fetal breathing movement. Modern perinatal medicine. Chicago: L. Gluck, Ed.; 1974. p. 381—90.
- Bibby JG, Brunt JD, Hodgson H, Mitchell MD, Anderson AB, Turnbull AC. Prostaglandins in umbilical plasma at elective cae- sarean section. Br J Obstet Gynaecol 1979;86:282—4.
- Koos, B. Central effects on breathing in fetal sheep of sodium meclofenamate. J Physiol 1982;330:50—1.
- Honest H, Bachmann LM, Sengupta R, Gupta JK, Kleijnen J, Khan KS. Accuracy of absence of fetal breathing movements in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol 2004;24:94—100.
- Schreyer P, Caspi E, Natan NB, Tal E, Weinraub Z. The predic- tive value of fetal breathing movement and Bishop score in the diagnosis of ‘‘true’’ preterm labor. Am J Obstet Gynecol 1989;161:886—9.
- Senden IP, Owen P. Comparison of cervical assessment, fetal fibronectin and fetal breathing in the diagnosis of preterm labour. Clin Exp Obstet Gynecol 1996;23:5—9.
- De Franco EA, Lewis DF, Odibo AO. Improving the screening accuracy for preterm labor: is the combination of fetal fibro- nectin and cervical length in symptomatic patients a useful predictor of preterm birth? A systematic review. Am J Obstet Gynecol 2013;208 [233.e1—6].
- Eroglu D, Yanik F, Oktem M, Zeyneloglu HB, Kuscu E. Prediction of preterm delivery among women with threatened preterm labor. Gynecol Obstet Invest 2007;64:109—16.
- Gomez R, Romero R, Medina L, Nien JK, Chaiworapongsa T, Carstens M, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol 2005;192:350—9.
- Schmitz T, Maillard F, Bessard-Bacquaert S, Kayem G, Fulla Y, Cabrol D, et al. Selective use of fetal fibronectin detec- tion after cervical length measurement to predict spontaneous preterm delivery in women with preterm labor. Am J Obstet Gynecol 2006;194:138—43.
- Vogel I, Glavind-Kristensen M, Thorsen P, Armbruster FP, Uldb- jerg N. S-relaxin as a predictor of preterm delivery in women with symptoms of preterm labour. Br J Obstet Gynaecol 2002;109:977—82.
- Weiss G, Goldsmith LT, Sachdev R, Von Hagen S, Lederer K. Ele- vated first-trimester serum relaxin concentrations in pregnant women following ovarian stimulation predict prematurity risk and preterm delivery. Obstet Gynecol 1993;82:821—8.
- Rocha FG, Slavin TP, Li D, Tiirikainen MI, Bryant-Greenwood GD. Genetic associations of relaxin: preterm birth and premature rupture of fetal membranes. Am J Obstet Gynecol 2013;209 [258.e1—8].
- Coleman MA, France JT, Schellenberg JC, Ananiev V, Tow- nend K, Keelan JA, et al. Corticotropin-releasing hormone, corticotropin-releasing hormone-binding protein, and activin A in maternal serum: prediction of preterm delivery and res- ponse to glucocorticoids in women with symptoms of preterm labor. Am J Obstet Gynecol 2000;183:643—8.
- Yoneda S, Shiozaki A, Yoneda N, Shima T, Ito M, Yamanaka M, et al. Prediction of exact delivery time in patients with preterm labor and intact membranes at admission by amniotic fluid interleukin-8 level and preterm labor index. J Obstet Gynaecol Res 2011;37:861—6.
- Jia, X. Value of amniotic fluid IL-8 and annexin A2 in prediction of preterm delivery in preterm labor and preterm premature rupture of membranes. J Reprod Med 2014;59:154—60.
- Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: sys- tematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2009;13:1—627.
- Lucovnik M, Kuon RJ, Garfield RE. Assessment of parturition with cervical light-induced fluorescence and uterine electro- myography. Comput Math Methods Med 2013;2013:165913.
- Lucovnik M, Maner WL, Chambliss LR, Blumrick R, Balducci J, Novak-Antolic Z, et al. Noninvasive uterine electromyogra- phy for prediction of preterm delivery. Am J Obstet Gynecol 2011;204 [228.e1—10].
- Creasy RK, Merkatz IR. Prevention of preterm birth: clinical opinion. Obstet Gynecol 1990;76:2S—4S.
- Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med 1999;341:660—6.
- Van Baaren G-J, Vis JY, Grobman WA, Bossuyt PM, Opmeer BC, Mol BW. Cost-effectiveness analysis of cervical length mea- surement and fibronectin testing in women with threatened preterm labor. Am J Obstet Gynecol 2013;209 [436.e1—8].
- Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cer- clage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol 2005;106:181—9.
- Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with sin- gleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol 2011;117:663—71.
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm deli- very by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379—85.
- Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a syste- matic review and metaanalysis of individual patient data. Am J Obstet Gynecol 2012;206, 124 e1—19.
- Conde-Agudelo A, Romero R, Nicolaides KH, Chaiworapongsa T, O’Brien JM, Cetingoz E, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and sin- gleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol 2013;208, 42 e1—42 e18.
- Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez- Delboy A, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 2009;201, 375 e1—8.
- Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, et al. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol 2010;202, 351 e1—6.
- A.C.O.G., T.A.C., o.O., a., G. Practice bulletin no. 130: pre- diction and prevention of preterm birth. Obstet Gynecol 2012;120:964—73.
- S.M.F.M., S., f.M.-F., M. Progesterone and preterm birth pre- vention: translating clinical trials data into clinical practice. Am J Obstet Gynecol 2012;206:376—86.
- Briery CM, Veillon EW, Klauser CK, Martin RW, Magann EF, Chau- han SP, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. Am J Obstet Gynecol 2011;204, 54 e1—5.
- Dunn, PM. History of neonatal resuscitation. Part 1: Artificial ventilation. Resuscitation. 1999;41(3):179-185.
- Finer NN, Barrington KJ. The evolution of neonatal resuscitation. Clin Perinatol. 2019;46(4):573-587.
- Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics. 2015;136 Suppl 2:S196-S218.
- Kieran EA, Twomey AR, Molloy EJ, O’Donnell CPF. Non-invasive ventilation for the preterm infant: evidence versus practice. Acta Paediatr. 2012;101(8):800-806.
- Lista G, Castoldi F, Fontana P, et al. Newer methods of respiratory support for preterm infants. Early Hum Dev. 2014;90 Suppl 2:S35-S37.
- Singh Y, Gupta S, Groves AM, et al. Monitoring devices for respiratory function in the newborn. Arch Dis Child Fetal Neonatal Ed. 2020;105(1):103-109.
- Laptook AR, Salhab W, Bhaskar B; Neonatal Research Network. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119(3):e643-e649.
- Janvier A, Lantos J, Aschner J, et al. Stronger and more vulnerable: a balanced view of the impacts of the NICU experience on parents. Pediatrics. 2016;138(3):e20160655.
- Perlman JM, Wyllie J, Kattwinkel J, et al; Neonatal Resuscitation Chapter Collaborators. Part 7: Neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations (Reprint). Pediatrics. 2015;136 Suppl 2:S120-S166.
- Dunn, PM. History of neonatal resuscitation. Part 2: Oxygen and other drugs. Resuscitation. 2000;44(2):93-99.
- Wyckoff MH, Aziz K, Escobedo MB, et al; Neonatal Resuscitation Chapter Collaborators. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics. 2015;136 Suppl 2:S196-S218.
- Laptook AR, Salhab W, Bhaskar B; Neonatal Research Network. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119(3):e643-e649.
- Janvier A, Lantos J, Aschner J, et al. Stronger and more vulnerable: a balanced view of the impacts of the NICU experience on parents. Pediatrics. 2016;138(3):e20160655.
- Finer, N. N., & Barrington, K. J. (2019). The evolution of neonatal resuscitation. Clin Perinatol, 46(4), 573-587.
- Singh, Y., Gupta, S., Groves, A. M., et al. (2020). Monitoring devices for respiratory function in the newborn. Arch Dis Child Fetal Neonatal Ed, 105(1), 103-109.
- Wyckoff, M. H., Aziz, K., Escobedo, M. B., et al. (2015). Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics, 136 Suppl 2, S196-S218.
- Dempsey, E. M., Al Hazzani, F., & Barrington, K. J. (2009). Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed, 94(4), F241-F244.
- Lista, G., Castoldi, F., Fontana, P., et al. (2014). Newer methods of respiratory support for preterm infants. Early Hum Dev, 90 Suppl 2, S35-S37.
- Jobe, A. H., & Bancalari, E. (2001). Bronchopulmonary dysplasia. Am J Respir Crit Care Med, 163(7), 1723-1729.
- Kieran, E. A., Twomey, A. R., Molloy, E. J., & O’Donnell, C. P. F. (2012). Non-invasive ventilation for the preterm infant: evidence versus practice. Acta Paediatr, 101(8), 800-806.
- Wyckoff MH, Aziz K, Escobedo MB, et al; Neonatal Resuscitation Chapter Collaborators. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (Reprint). Pediatrics. 2015;136 Suppl 2:S196-S218.
- 2021 Resuscitation Guidelines | Resuscitation Council UK.
- Part 5: Neonatal Resuscitation - AHA/ASA Journals.
- Charpak N, Ruiz-Peláez JG, Figueroa de C Z, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics. 2001;108(5):1072-1079.
- Acolet D, Sleath K, Whitelaw A, et al. Randomised controlled trial of a near patient strategy to facilitate discharge of sick premature infants from hospital. Arch Dis Child Fetal Neonatal Ed. 1999;80(3):F201-6.
- Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98(5):925-930.
- Lawn JE, Mwansa-Kambafwile J, Barros FC, et al. ’Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39 Suppl 1:i144-54.
- Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;8(8):CD002771.
- Jefferies, AL. Canadian Paediatric Society, Fetus and Newborn Committee. Kangaroo care for the preterm infant and family. Paediatr Child Health. 2012;17(3):141-143.
- Nyqvist KH, Anderson GC, Bergman N, et al. Towards universal kangaroo mother care: recommendations and report from the First European conference and Seventh International Workshop on Kangaroo Mother Care. Acta Paediatr. 2010;99(6):820-826.
- World Health Organization. WHO Guideline: Kangaroo mother care: a practice guideline. Geneva: World Health Organization; 2003.
- Carvalho NAR, Santos JDM, Sales IMM, Araújo AAC, Sousa AD, Morais FF, Rocha SSR. Care transition of preterm infants: from maternity to home. Acta Paul Enferm. 2021;34:eAPE02503.
- Premature Babies: Support Groups and Resources for Parents and Families. WebMD. https://www.webmd.com/parenting/baby/premature-babies-support-groups-resources.
- Resources for Parents of Preemies. March of Dimes. https://www.marchofdimes.org/complications/resources-for-parents-of-preemies.aspx.
- World Health Organization. (2015). Standards for improving quality of maternal and newborn care in health facilities.
- O’Brien K., Bracht M., Macdonell K., McBride T., Robson K., O’Leary L., Christie K., Galarza M., Dicky T., Lee S.K., Canadian Neonatal Network Investigators. (2013). A pilot cohort analytic study of Family Integrated Care in a Canadian neonatal intensive care unit. BMC Pregnancy and Childbirth. 13(Suppl 1) :S12.
- Charpak, N., Tessier R., Ruiz J.G., Hernandez J.T., Uriza F., Villegas J. (2017). Twenty-year follow-up of kangaroo mother care versus traditional care. Pediatrics. 139(1) :e20162063.
- Zhang, X., Kurtz M., Lee S.Y., Liu H. (2014). Effects of early family-centered care on preterm infants’ neurobehavioral outcomes: a systematic review and meta-analysis. Worldviews on Evidence-Based Nursing. 11(6) :368–377.
- E. Jones et al., Interventions to provide culturally-appropriate maternity care services: factors affecting implementation, BMC Pregnancy Childbirth 17:267 (2017),. [CrossRef]
- M. Nyaloko et al., Exploring cultural determinants to be integrated into preterm infant care in the neonatal intensive care unit: an integrative literature review, BMC Pregnancy Childbirth 23:15 (2023). [CrossRef]
- A.C. Cruz et al., Patient and Family Centered Care in Neonatal Settings. In: Neonatal Nursing: A Global Perspective, (Springer International Publishing AG 2022). [CrossRef]
- IPFCC. Patient- And Family-Centered Care. https://ipfcc.org/about/pfcc.html.
- C. Charles et al., Shared Decision-Making in the Medical Encounter: What Does It Mean? (Or It Takes At Least Two To Tango). Soc Sci Med 44(5):681–692 (1997). [CrossRef]
- Child Welfare Information Gateway. Family-Centered Approach to Working With Families. https://www.childwelfare.
Table 3.
Example of Mother Data Table.
Table 3.
Example of Mother Data Table.
| Date of Birth |
Diabetes |
HTA |
COVID-19 |
Delivery Type |
Other Risks |
Age |
| 1/1/2021 |
|
|
X |
CES |
RAS |
- |
| 12/1/2021 |
|
|
X |
CES |
RAS |
- |
| 14/1/21 |
|
|
X |
CES |
RAS |
- |
| 18/1/21 |
|
X |
X |
CES |
CALCIFIED PLACENTA |
- |
| 18/1/21 |
|
|
X |
CES |
RAS |
- |
| 10/1/2021 |
|
X |
X |
CES |
HRP |
- |
| 9/1/2021 |
|
|
X |
CES |
RAS |
- |
| 12/1/2021 |
X |
X |
X |
VAGINAL |
ANEMIA +GG +PRIMA |
23 |
| 3/1/2021 |
X |
X |
X |
VAGINAL |
GG |
- |
| 19/1/21 |
X |
X |
X |
VAGINAL |
prima |
28 |
Table 4.
Example of Care Data Table (a).
Table 4.
Example of Care Data Table (a).
| Hospitalization |
Date of Birth |
Gestational Age |
Birth Weight |
Sex |
| H |
01/01/2021 |
33 |
1455 |
M |
| H |
16/03/2021 |
35 |
2435 |
M |
| H |
13/03/2021 |
24 |
785 |
F |
| H |
26/04/2021 |
29 |
1600 |
M |
| H |
18/06/2021 |
28 |
500 |
F |
| H |
09/08/2021 |
31 |
1500 |
F |
| H |
28/01/2021 |
35 |
2000 |
F |
| H |
04/02/2021 |
34 |
1938 |
F |
| H |
29/03/2021 |
35 |
1320 |
F |
| H |
27/03/2021 |
30 |
1700 |
M |
| H |
06/12/2021 |
32 |
1530 |
M |
Table 5.
Example of Care Data Table (b).
Table 5.
Example of Care Data Table (b).
| Complications |
Additional Examination |
Pharmacological Treatment |
| RDS+INF SD |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P + Vit K |
| RDS+INF SD |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P + Multivit |
| RDS |
TLT + Full Workup |
O2 CPAP + Hydration + Ca2+ ATB 1st P |
| RDS+Hypo T |
TLT + Full Workup |
O2 Hood then Intubation + Hydration + ATB 1st P |
| RDS |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P + Multivit |
| RDS+INF SD |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P + Multivit |
| RDS |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P |
| RDS+INF SD |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P |
| RDS |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P |
| RDS+INF SD |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st and 2nd P |
| RDS |
TLT + Full Workup |
Free O2 + Hydration + ATB 1st P |
Table 6.
Example of Care Data Table (c).
Table 6.
Example of Care Data Table (c).
| Feeding |
Non-Pharmacological Treatment [kg/Phototherapy] |
Outcome (D/V) |
| Maternal |
No |
D |
| VOS |
No |
D |
| VOS |
No |
D |
| VOS |
No |
D |
| VOS |
No |
D |
| VOS |
No |
D |
| Maternal |
No |
V |
| Maternal |
No |
V |
| Maternal |
Yes |
V |
| Maternal |
Yes |
V |
| Maternal+Artificial |
No |
V |
Table 7.
Example of Follow-up Data Table.
Table 7.
Example of Follow-up Data Table.
| Date of Birth |
Gestational Age |
Birth Weight |
Sex |
Outcome |
1st Control |
2nd Control |
3rd Control |
| 21/06/2021 |
34 |
2400 |
F |
V |
W: 2 Kg HC: 33 cm |
W: 2.7 Kg HC: 33 cm |
W: 2.9 Kg HC: 34 cm |
| 19/11/2022 |
X |
2400 |
F |
V |
W: 2.7 Kg HC: 34 cm |
|
| 31/05/2021 |
X |
2390 |
F |
V |
W: 2.9 Kg HC: 34 cm |
|
| 14/05/2021 |
35 |
2355 |
F |
V |
|
| 20/02/2021 |
X |
2350 |
M |
V |
|
| 21/11/2022 |
35 |
2350 |
M |
V |
W: 2.9 Kg HC: 33 cm |
|
| 20/04/2021 |
X |
2300 |
F |
V |
W: 2.7 Kg HC: 34 cm |
|
| 20/04/2021 |
X |
2300 |
F |
V |
W: 2.45 Kg HC: 34 cm |
W: 2.9 Kg HC: 35 cm |
|
| 11/10/2021 |
34 |
2300 |
M |
V |
W: 2.5 Kg HC: 34 cm |
|
| 13/06/2021 |
34 |
2250 |
M |
V |
W: 2.7 Kg HC: 33 cm |
W: 3 Kg HC: 35 cm |
|
| 14/11/2022 |
X |
2250 |
M |
V |
W: 2.9 Kg HC: 35 cm |
W: 3 Kg HC: 36 cm |
|
| 16/01/2021 |
X |
2240 |
F |
V |
W: 2.55 Kg HC: 34 cm |
|
| 11/12/2021 |
35 |
2220 |
F |
V |
W: 2.1 Kg HC: 31 cm |
W: 2.8 Kg HC: 33 cm |
W: 3.2 Kg HC: 36 cm |
| 08/02/2021 |
X |
2220 |
M |
V |
|
| 23/02/2021 |
34 |
2200 |
M |
V |
|
| 09/05/2021 |
35 |
2200 |
M |
V |
|
| 09/04/2021 |
32 |
2200 |
F |
V |
W: 2.8 Kg HC: 36 cm |
W: 3 Kg HC: 36 cm |
|
| 24/02/2021 |
34 |
2180 |
M |
V |
W: 2 Kg HC: 33 cm |
|
| 09/07/2021 |
32 |
2160 |
F |
V |
|
| 03/07/2021 |
33 |
2150 |
M |
V |
W: 1.8 Kg HC: 31 cm |
W: 1.0 Kg HC: 31 cm |
W: 2.4 Kg HC: 32 cm |
| 17/01/2021 |
32 |
2125 |
M |
V |
W: 2.8 Kg HC: 33 cm |
W: 3.5 Kg HC: 35.5 cm |
W: 4.7 Kg HC: 35.5 cm |
| 13/06/2021 |
34 |
2085 |
M |
V |
W: 2.5 Kg HC: 32 cm |
W: 2.9 Kg HC: 34 cm |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).