1. Introduction
Hot air drying is a popular unit operation in commercial production of a wide range ready-to-eat food products, including dehydrated fruits, vegetables, herbs, and protein sources such as meat, eggs, plant-based proteins, and dairy products. While hot air drying has often been assumed effective in reducing microbial contamination, certain bacterial pathogens, particularly
Salmonella, demonstrate high thermal resistance in low-moisture environments. They may survive these processes, rendering products unsafe for direct consumption.
Salmonella has been implicated in nationwide outbreaks and recalls across a wide range of fresh and dehydrated products, including onions, basil, and dried fruits and spices [
1,
2,
3,
4,
5]. Research suggests that
Salmonella’s ability to attach to or internalize in fruits and vegetables significantly contributes to the contamination of susceptible products [
5]. The average cost of a food recall is estimated at
$10 million, with additional losses stemming from brand damage and decreased sales [
6]. The recalls are more costly for Because of long-shelf-life low-moisture foods, leading to an average capitalization loss of over
$400 million per event [
7]. The persistence of heat-resistant pathogens in dehydrated food products represents a growing concern with serious public health and economic implications for the U.S. food system.
Traditional drying processes, designed primarily to maximize efficiency and preserve product quality, frequently fall short in achieving the necessary inactivation levels for bacterial pathogens. For example, spray drying has been shown to achieve less than a 3-log reduction of
Salmonella even with air temperatures of 180°C [
8]. The desiccation of vegetative bacterial cells enhances their thermal resistance, making them more difficult to inactivate. Studies indicate that
Salmonella can survive certain drying conditions due to this increased resistance in low-moisture, desiccated environments [
1,
9,
10].
To comply with the Food Safety Modernization Act (FSMA) and minimize contamination risks, the food industry has adopted two main strategies: 1) adding a validated pasteurization step, such as steam treatment and Propylene Oxide (PPO) fumigation [
11]; and 2) validating the existing (legacy) processes to demonstrate microbial lethality. Although adding pasteurization steps improves safety, it increases production costs and resource demands. Additionally, for fresh ingredients like fruits and vegetables, there have been very few established and effective pasteurization methods. Validating individual food drying operations are costly. Currently, food processors often use surrogate inoculation challenge studies to validate lethality under specific conditions [
12,
13]. However, these studies cannot provide real-time predictions of microbial inactivation or indicate conditions under which the process may fail, especially when accounting for product variability, raw material inconsistencies, process fluctuations, and an increasing prevalence of pathogens linked to climate change [
14,
15,
16]. Thus, a reliable mathematical model that can predict microbial inactivation based on process parameters is critical for ensuring the antimicrobial efficacy of drying processes.
Recent studies have shown that microbial inactivation in low-moisture environments is closely influenced by product temperature and the humidity immediate around the products [
17,
18,
19,
20,
21]. This study aims to develop a mathematical model to predict the microbial reduction in drying and preheating processes (with humidity conservation), using key physical parameters such as air temperature, air humidity, and product surface temperature as inputs [
22].
2. Materials and Methods
2.1. Apple Preparation
Fresh Gala apples were purchased from local stores in Pullman, WA, USA, stored in a refrigerator, and used within two weeks of purchase. Apples with consistent quality and texture were peeled, cored, and diced into 6×6×6 mm cubes using a commercial vegetable dicer (Dynamic CL003, Dynamic International Ltd., Canada).
2.2. Sample Inoculation
A cocktail of three
Salmonella enterica strains—
S. Enteritidis PT30 (ATCC BAA-1045),
S. Montevideo (488275), and
S. Agona (447967)—was prepared using a lawn-based method [
23]. Each strain was cultured in tryptic soy broth (TSB, Bacto™, Sparks, MD, USA) with 0.6% yeast extract (TSBYE) through two consecutive incubations (24 h each at 37°C). Cultures were then plated on tryptic soy agar (TSA, Difco™, Sparks, MD, USA) with yeast extract (TSAYE) for 24 h at 37°C. The bacterial lawn was scraped into buffered peptone water (BPW), centrifuged (3000×g, 15 min), and resuspended in BPW to form a three-strain cocktail. The cocktail was mist-sprayed onto 150 g of apple cubes inside a sterile glass mason jar, with thorough shaking after each spray to ensure even distribution.
2.3. Apple Drying and Preheating Treatment
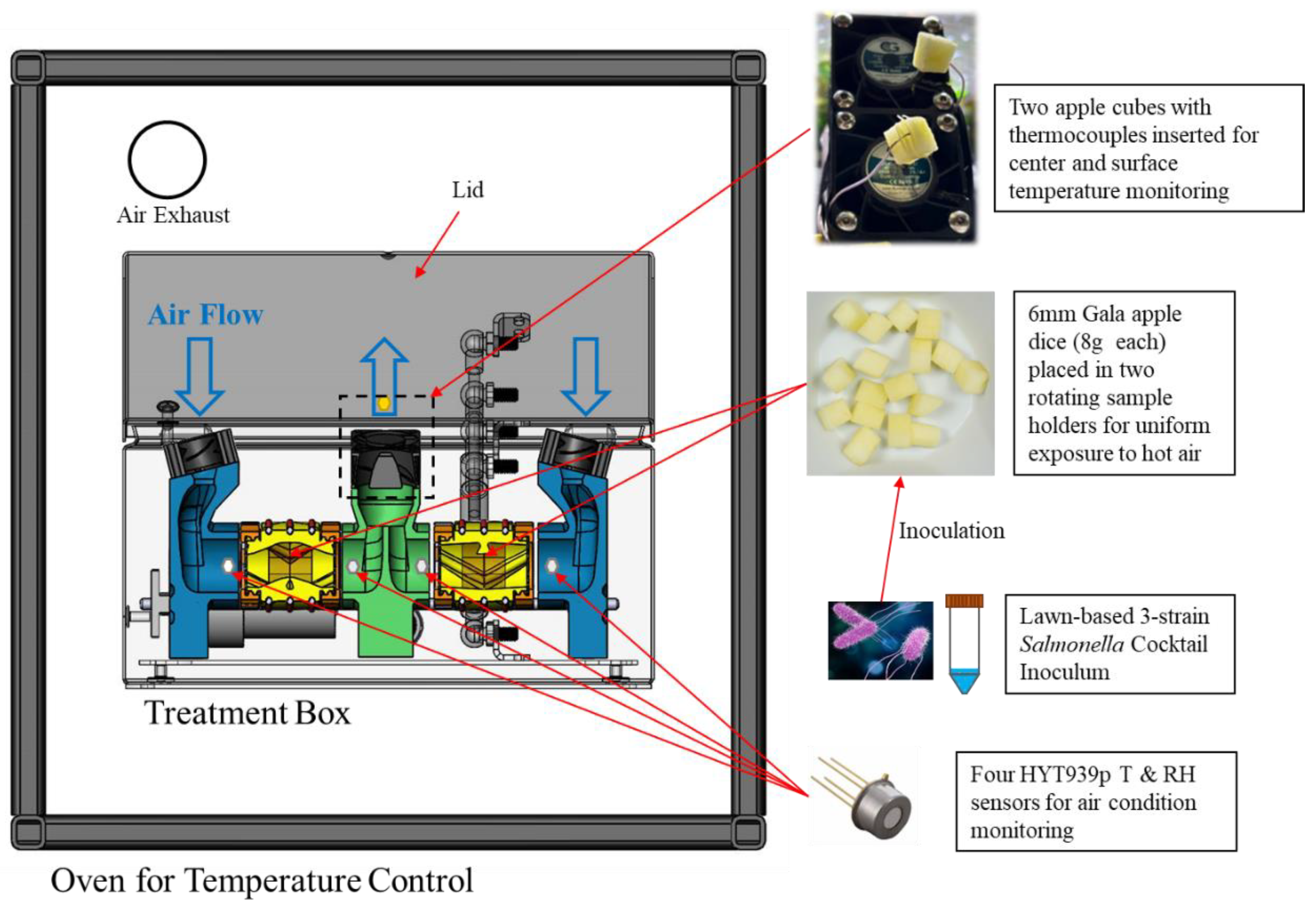
A sample heating box, designed in a previous study [
18] to enhance sample heating uniformity and air condition monitoring, was used in this research (
Figure 1). Inoculated apple cubes (8 g each) were loaded into two drum-shaped sample holders with mesh screens on both sides. These holders were placed inside the preheated treatment box within a convection oven (HCP50, Memmert, Schwabach, Germany) pre-set at 70°C or 90°C. The oven door was sealed, while the box lid was open (for hot air drying) or closed (for high humidity heating).
The tests were conducted under one of three different conditions: 1) oven air at 90°C, box lid was open for open-air drying, (Drying-90); 2) 90°C, box lid was closed (Preheating-90); and 3) 70°C, box lid was closed (Preheating-70). The open-box setup allowed air circulation between the oven and box, with an exhaust vent open to release humid air. The closed-box setup retained moisture inside, creating a high-humidity environment while still allowing heat conduction. A motor rotated the sample holders (37 rpm), and four internal fans (11,000 RPM, 12 Volt, 0.16 Amps) forced hot air through the holders, enhancing heat and moisture transfer as well as heating uniformity.
Samples were taken out of the treatment box after 5-7 different time periods, appropriate time intervals and overall test durations were selected for each treatment condition (i.e., Drying-90: 0, 3, 6, 12, 18, 24, 36 min; Preheating-90: 0, 2, 3, 4, 6, 8, 9, 10 min; Preheating-70: 0, 4, 8, 12, 16 min) based on preliminary tests to allow observations of more than 5-log Salmonella reductions.
2.4. Bacteria Survival Enumeration
At the end of each treatment, the oven and the box (if closed) were opened to allow ambient air to flush into the sample holders to cool the samples. Then each sample (8 g) was transferred into a 50-ml centrifugal tube with 32 ml BPW and vortexed for 5 min to release Salmonella. Serial dilutions were prepared using BPW solutions and then plated on TSAYE plates supplemented with 0.05% (w/v) ferric ammonium citrate (Sigma-Aldrich, St. Louis, MO, USA), and 0.03% (w/v) sodium thiosulfate (Mallinckrodt Baker, Phillipsburg, NJ, USA) for colony counting after 24 hours incubation at 37 °C. Colonies with dark spots were counted, and the colony forming unit (CFU) per gram of apple cubes was calculated for each sample. Each condition was tested at least three times.
2.5. Dring Condition Monitoring
Air temperature and relative humidity (RH) were monitored using four humidity and temperature sensor modules (HYT939P, Innovative Sensor Technology, Ebnat-Kappel, Switzerland), positioned near the inlet and outlet of each sample holder (
Figure 1). Sample temperatures were monitored by high-accuracy K-type thermocouples (0.08 mm diameter): one located at the geometric center of an apple cube (uninoculated) and the other tied to the surface of a second apple cube with a cotton thread about 5 mm away from the tip. The sensor modules and thermocouples were connected to a customized data logger (configured with Arduino Mega 2560 Rev3) for communication, calibration, and data recording (with an SD card). Calibration was performed in a high-temperature environmental chamber (HCP50, Memmert) at temperatures of 70, 80, and 90°C, and RH levels of 10, 30, 50, and 70%. The temperature and relative humidity were recorded every second over the duration of each treatment (20 min for Preheating-70, 10 min for Preheating-90, and 45 min for Drying-90).
2.6. Modeling and Statistical Analysis
We used first-order kinetics for
Salmonella inactivation to describe thermal inactivation of
Salmonella under isothermal and iso-relative humidity conditions [
18,
19,
21,
24,
25,
26]. For drying operations in which product temperature and relative humidity change with time, the following relationship was used to calculate accumulated lethality [
22]:
where
N0 and
N are the initial and final microbial population (CFU/g),
T and
RH are current temperature and relative humidity,
Tref and
RHref are reference temperature and humidity,
Dref is the
D-value at the reference condition,
ZT and
ZRH represent changes required for a one-log reduction in
D-value, and
t is the thermal treatment time.
Considering that the drying process is dynamic and nonequilibrium, sample surface RH was used in Equation (1), it was calculated as:
where AH, or absolute humidity (the mass of water vapor per unit volume in g/m3), is calculated based on air temperature and RH:
and Surface Saturation AH corresponds to the absolute humidity at sample surface temperature. Saturated AH at a given temperature T was calculated using the Ideal Gas Law:
where
M is the molar mass of water (18.01528 g/mol),
R is the gas constant (8.3145 m
3PaK
-1mol
-1), and
Ps.T is the saturated water vapor pressure at temperature
T (°C), calculated using Buck’s equation: [
27]:
Model fitting for Equation (1) was performed using the Solver function of Microsoft Excel (Version 2406) to minimize the sum of squared residuals. Model fit quality was evaluated using root mean squared error (RMSE) and the coefficient of determination (R²). Salmonella log reductions at different time points were compared within each treatment group using one-way ANOVA (MATLAB R2024b), with significance set at p < 0.05.
3. Results and Discussion
3.1. Humidity and Temperature Profiles
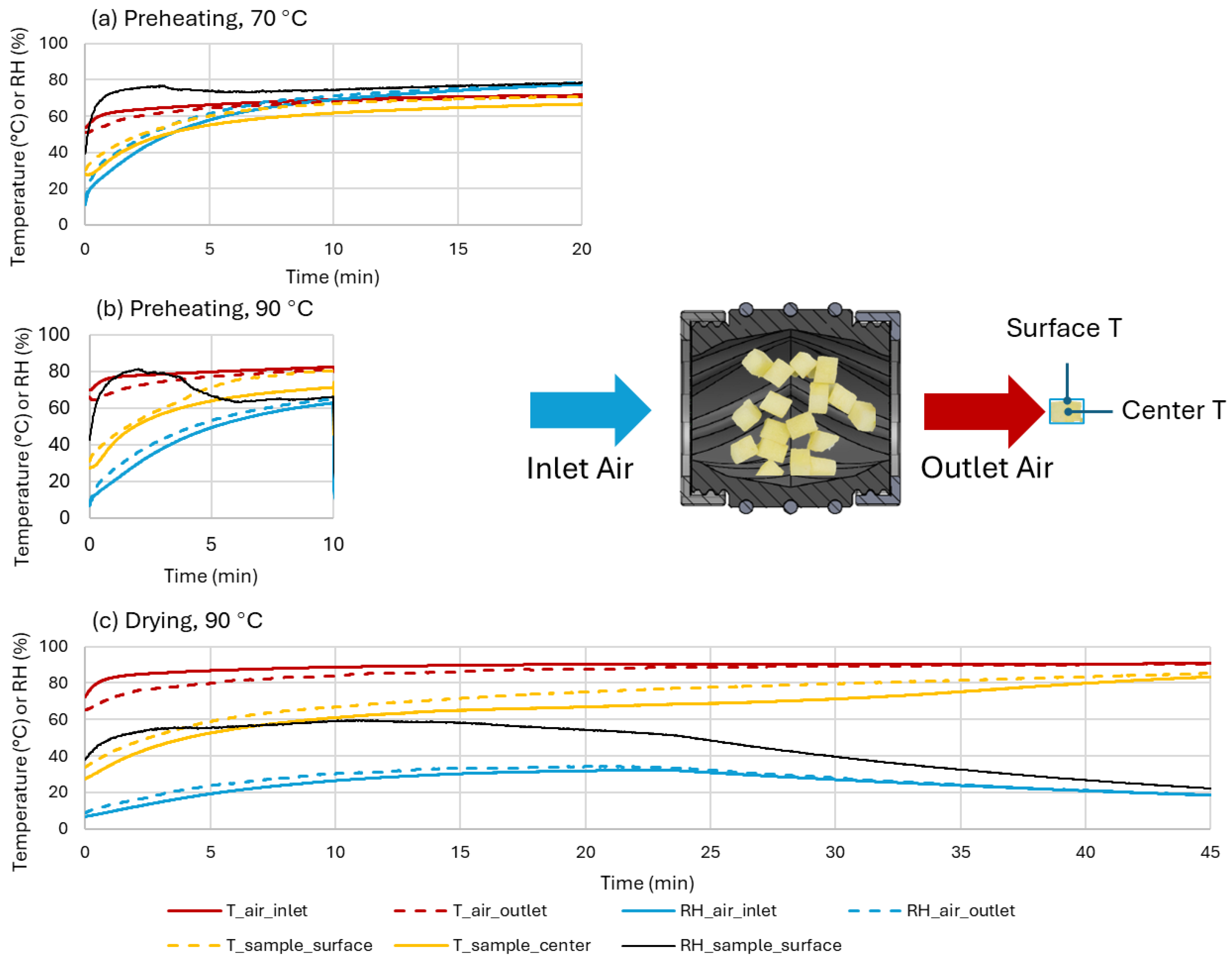
Figure 2 shows the measured sample surface and internal temperatures, as well as air temperature and relative humidity (RH) at the inlet and outlet of the sample holder over the course of a test under each of the three studied conditions. In the conventional drying operation (Drying-90) (
Figure 2c), sample surface and center temperatures increased rapidly in the beginning with the heating slowing down until 10 min, where sample temperatures started to increase at a constant rate. The sample center and surface temperatures reached 61.0 and 66.9 °C, respectively. Then they gradually reached 68.8 and 77.6 °C by 25 min, and ended at 83.4 and 85.3 °C. The hot air temperature at the inlet started at 72.6 °C upon sample addition, then stabilized at 88.7 °C within 10 min. The air inlet and outlet RH reached a plateau at 30.2 and 33.1% by 15 min and gradually declined to 18.4 and 18.6% by 45 min. The calculated sample surface RH rose from 38% to 55% within the first 4 min and remained between 55–60% until 18.5 min.
In the Preheating-90 treatment (
Figure 2b), the sample center temperature reached 71.4 °C within 10 min, approximately 9.6 °C higher than in Drying-90. The surface temperature reached 80.5 °C, 13.6 °C higher than in the Drying-90 condition, with near-equilibrium achieved between the sample surface and outlet air temperatures by 10 min (∆T < 0.1 °C). However, the closed box reduced heat transfer, with the hot air temperature reaching only 80.5 °C, 8.2 °C lower than in Drying-90. RH in the closed box rose above 60% within 10 min, significantly higher than that (<30%) observed in Drying-90. Sample surface RH spiked to 80% within 0.8 min and gradually decreased to 64 ± 2% by 6 min, remaining stable until the end of monitoring (10 min). The increased humidity within the closed box limited moisture evaporation from the sample, accelerating temperature equilibration between the sample and hot air.
In the Preheating-70 treatment (
Figure 2a), sample center and surface temperatures reached 61.6 °C and 66.9 °C within 10 min, respectively. The air inlet temperature reached 69 °C by 10 min, only 11.5 °C lower than in Preheating-90 despite the oven setting being 20 °C lower. Air RH rose to 67%, higher than in Preheating-90, likely due to the reduced moisture capacity of the cooler air. Sample surface RH reached 75% within 3 min and maintained a narrow range between 73–78.5% throughout the 20 min. The higher sample surface RH in Preheating-70 contributed to a reduced surface temperature, which in turn lowered the surface absolute humidity upon saturation.
Overall, closing the box significantly increased air humidity and sample temperature by keeping the evaporated vapor in the sample box. This raised sample surface RH, particularly during the initial four minutes of the Preheating-90 treatment and throughout Preheating-70.
3.2. Salmonella Survival During Treatment
Table 1 presents the survival populations of inoculated
Salmonella (three-strain cocktail) and the corresponding log reductions (mean ± standard deviation). The initial inoculation levels were consistent across samples, averaging 9.5–9.6 log CFU/g. In the Preheating-70 treatment, a microbial reduction of 3.3 ± 0.8 log CFU/g was achieved within 12 min. Similarly, Preheating-90 resulted in a 3.5 ± 1.3 log CFU/g reduction in just 6 min—half the time required in Preheating-70. In contrast, Drying-90 showed only a 1.0 ± 0.3 log CFU/g reduction at the initial 12 min of drying. 24 min was needed to cause a comparable 3.2 log CFU/g reduction, which was twice the time of Preheating-70 and four times that of Preheating-90. At the first significant log reduction point for each treatment, sample temperatures were above 63 °C. Despite similar product temperature profiles in the first 12 min of Drying-90 and Preheating-70, the significantly lower sample surface RH in Drying-90 likely contributed to reduced microbial inactivation.
3.3. Microbial Reduction Modeling
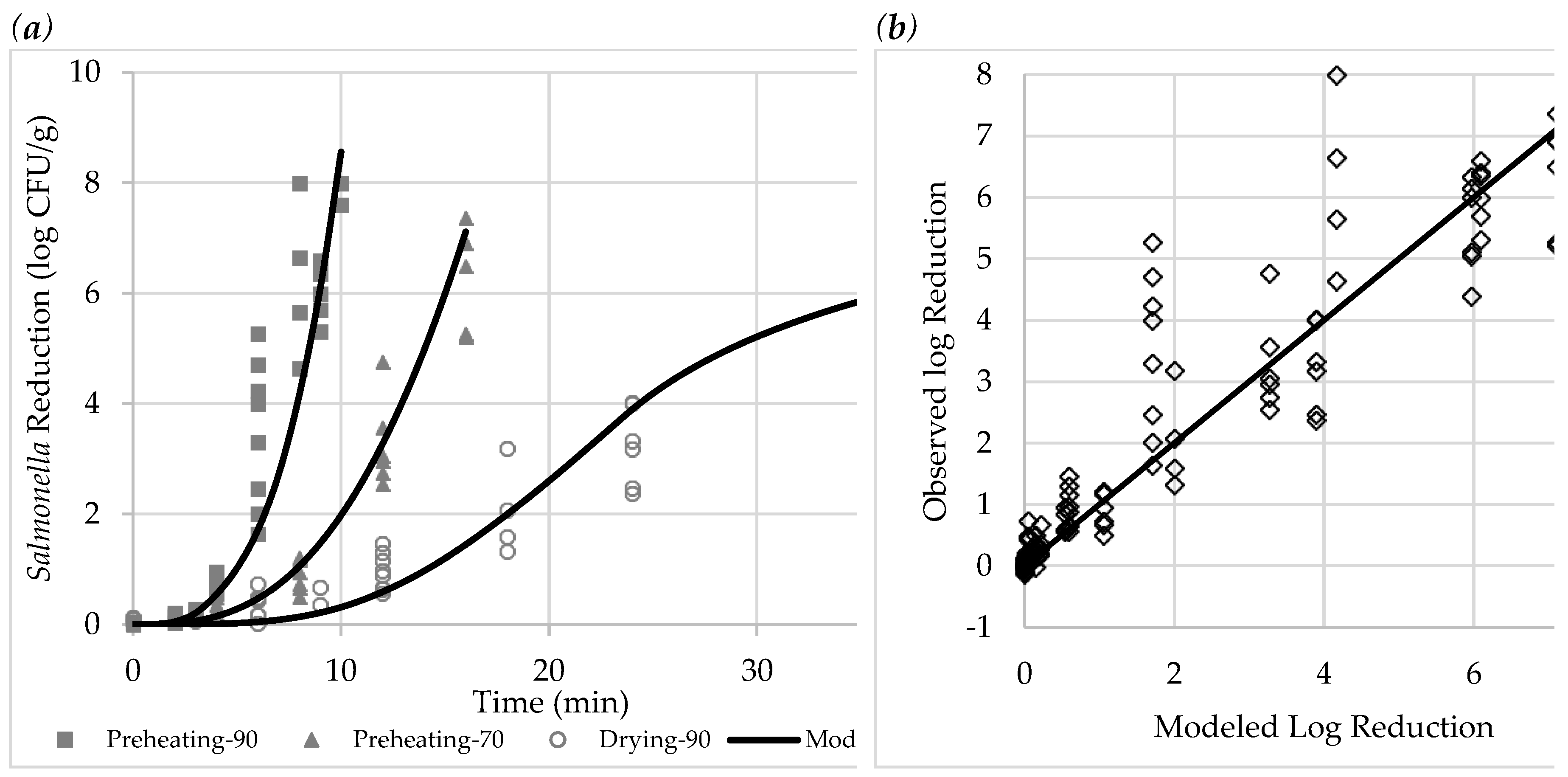
The observed reductions of
Salmonella for the three treatments are shown in
Figure 3, alongside modeled reductions for visual comparison. Modeled parameters for Equation (1), RMSE, and R² are provided in
Table 2. With an RMSE of 0.92 and R² of 0.86, the model accurately predicted
Salmonella reduction for all the treatments. Although some observed reductions deviated by up to 4 logs from the model, all instances of overestimation were within 1.9 logs of the prediction line, suggesting that the model tends to be conservative. Increased uncertainty in observed reductions over time may be attributed to variations in physical conditions across batches. Monitoring sample and air properties for each microbial test could further improve model accuracy in future studies.
The modeled curves in
Figure 3a indicate an accelerated microbial inactivation in the preheating treatments. In Drying-90, microbial reduction followed an S-shaped curve, with the highest inactivation rate at 23.7 min—coinciding with a rapid drop in sample surface RH from 51% to 22%. This suggests that microbial inactivation is most effective when sample temperatures exceeded 60 °C and surface RH remained above 50%. In the later stage of drying, reduced surface RH increases bacterial thermal resistance, slowing inactivation [
17].
According to the model, the time required to achieve a 5-log reduction in Salmonella was 8.5 min in Preheating-90, 14.0 min in Preheating-70, and 28.7 min in Drying-90. The moisture retention in the closed-box preheating treatments significantly accelerated microbial reduction, likely due to (1) elevated air RH, which increased sample surface RH, and (2) reduced moisture evaporation, which allowed for faster sample temperature increases.
3.4. Comparing Modeled Thermal Death Parameters with Literature Data
Table 3,
Table 4 and
Table 5 compare the modeled
Dref (70 °C, 80% RH),
ZT, and
ZRH values with values from literature. The
Dref value for
Salmonella (three-strain cocktail) in this study was 0.60 min, aligning with values for
Salmonella in grape juice concentrate and apple/orange juice, and closely matching a recent hot ascorbic acid treatment study on apples (
Dref = 0.63 min) [
28]. This suggests that
Dref values derived from nonlinear treatments and isothermal studies are comparable [
29].
Dref values for low-moisture foods were significantly higher (7.5 and 11.5 folds greater), likely due to the lower acidity in fruit juices, which reduces the thermal resistance of
Salmonella. Similar effects have been observed in studies showing that blanching or acid immersion increases microbial inactivation during drying [
1,
30,
31,
32].
Table 4 presents
ZT values, which range from 6.7–20.6 °C, the value decreased with increasing RH. The fitted
ZT value from this study (12.6 °C) aligns with values observed at RH levels between 25–53%, which is reasonable given the broad sample surface RH ranges in the treatments (
Figure 2). Model accuracy for future applications could be improved by accounting for
ZT temperature dependence.
Table 5 shows
ZRH values ranging from 21–44% across food matrices and processing conditions, with the value from this study closely matching data from a
Salmonella inactivation study on humidity-controlled thermal treatment of black peppercorns [
18].
Overall, the nonlinear model provided reliable predictions for the thermal death parameters of Salmonella. Given the substantial workload required to obtain ZT and ZRH values under isothermal and constant humidity conditions, nonlinear modeling offers a viable alternative.
5. Conclusions
This study provides valuable insights into the role of product surface humidity and temperature in Salmonella inactivation during apple drying and preheating under controlled environments. By comparing traditional drying (Drying-90) with two high-humidity preheating treatments (Preheating-90 and Preheating-70), we demonstrate that keeping water vapor in a closed environment significantly enhances microbial inactivation rates. The closed-box preheating treatments achieved faster reductions in Salmonella populations, with a 5-log reduction obtained in only 8.5 minutes at 90°C and 14.0 minutes at 70°C, compared to 28.7 minutes in conventional drying at 90°C. These results highlight the critical impact of elevated humidity in reducing bacterial thermal resistance, particularly during the initial heating stages.
A nonlinear mathematical model was successfully developed to predict Salmonella reduction using surface temperature and relative humidity profiles. By calculating sample surface humidity, this model provided accurate predictions across all the treatments, with thermal death parameters consistent with values reported in the literature on isothermal studies. The findings suggest that humidity-controlled preheating can act as a safety harbor, ensuring enhanced microbial inactivation prior to conventional drying and potentially improving overall food safety in commercial drying operations.
Looking forward, these findings underscore the potential of real-time lethality prediction systems for improving the safety and control of commercial drying processes. Expanding this approach across different food matrices and conditions could further validate its application and establish humidity-controlled preheating as a practical intervention in the food industry’s microbial control toolbox.
Author Contributions
Ren Yang: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft. Shuang Zhang: Investigation, Writing – review & editing. Juming Tang: Writing – review & editing, Supervision, Project administration, Conceptualization.
Funding
This research was funded by USDA-NIFA AFRI SAS grant 2020-68012-31822.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors acknowledge assistance from Huimin Lin and Dan Liu during microbial testing. Bacterial strains used in this study were contributed by Dr. Elizabeth Grasso-Kelley of Division of Food Processing Science and Technology of FDA.
Conflicts of Interest
To the best of our knowledge, the named authors have no conflict of interest, financial or otherwise.
References
- Bourdoux, S. , et al., Performance of drying technologies to ensure microbial safety of dried fruits and vegetables. Comprehensive Reviews in Food Science and Food Safety, 2016. 15(6): p. 1056-1066. [CrossRef]
- News, F.S. Norwegian Salmonella outbreak traced to dried fruit from multiple countries. 2021 [cited 2024 07/30]; Available from: https://www.foodsafetynews.com/2021/04/norwegian-salmonella-outbreak-traced-to-dried-fruit-from-multiple-countries/.
- CDC, U.S. 2021 Salmonella Outbreak Linked to Onions. 2022 [cited 2024 07/30]; Available from: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/salmonella/oranienburg-09-21/index.html.
- Yun, J. , et al., Fate of E. coli O157: H7, Salmonella spp. and potential surrogate bacteria on apricot fruit, following exposure to UV-C light. International journal of food microbiology, 2013. 166(3): p. 356-363. [CrossRef]
- Hanning, I.B., J. D. Nutt, and S.C. Ricke, Salmonellosis Outbreaks in the United States Due to Fresh Produce: Sources and Potential Intervention Measures. Foodborne Pathogens and Disease, 2009. 6(6): p. 635-648. [CrossRef]
- FDA, U.S. Recalls, market withdrawals, & safety alerts. Retrieved 12 [cited 19; 2012]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts. 20 March.
- Gomez, C.B. and B.P. Marks, Monetizing the impact of food safety recalls on the low-moisture food industry. Journal of Food Protection, 2020. 83(5): p. 829-835. [CrossRef]
- Steinbrunner, P. , et al., Fate of Salmonella and Enterococcus faecium during pilot-scale spray drying of soy protein isolate. Journal of Food Protection, 2021. 84(4): p. 674-679. [CrossRef]
- Grasso-Kelley, E.M. , et al., Evaluation of hot-air drying to inactivate Salmonella and Enterococcus faecium on apple pieces. Journal of food protection, 2021. 84(2): p. 240-248. [CrossRef]
- Enache, E. , et al., Persistence of Salmonella and other bacterial pathogens in low-moisture foods. Control of Salmonella and other bacterial pathogens in low moisture foods, 2017: p. 67-86. [CrossRef]
- Luo, K.K. , et al., Effect of pasteurization on raw almond (Prunus dulcis) oxidation during storage. ACS Food Science & Technology, 2022. 2(2): p. 260-271. [CrossRef]
- Ahmad, N.H. , et al., Interlaboratory evaluation of Enterococcus faecium NRRL B-2354 as a Salmonella surrogate for validating thermal treatment of multiple low-moisture foods. Journal of Food Protection, 2022. 85(11): p. 1538-1552. [CrossRef]
- California, A.B.o. Guidelines for Using Enterococcus faecium NRRL B-2354 as a Surrogate Microorganism in Almond Process Validation. 2014 [cited 2024 10/25]; Available from: https://www.almonds.com/sites/default/files/2020-05/guidelines_for_using_enterococcus_faecium_nrrl_b-2354_as_a_surrogate_microorganism_in_almond_process_validation.pdf.
- Taiwo, O.R. , et al., Advancements in Predictive Microbiology: Integrating New Technologies for Efficient Food Safety Models. International journal of microbiology, 2024. 2024(1): p. 6612162. [CrossRef]
- Tay, A. , Process Validation and Challenges For Nuts. 2014, Almond Board of California: https://www.almonds.com/sites/default/files/content/attachments/process_validation.pdf.
- Vestergaard, M.S.C.E.M. Do You Need Microbial Challenge Testing? 2001 [cited 2024 08/08]; Available from: https://www.food-safety.com/articles/4410-do-you-need-microbial-challenge-testing.
- Xie, Y. , et al., Moisture content of bacterial cells determines thermal resistance of Salmonella enterica serotype Enteritidis PT 30. Applied and Environmental Microbiology, 2021. 87(3): p. e02194-20. [CrossRef]
- Yang, R. , et al., Inactivation of Salmonella Enteritidis PT30 on black peppercorns in thermal treatments with controlled relative humidities. Food Research International, 2022. 162: p. 112101. [CrossRef]
- Liu, S. , et al., Exponentially increased thermal resistance of Salmonella spp. and Enterococcus faecium at reduced water activity. Applied and Environmental Microbiology, 2018. 84(8): p. e02742-17. [CrossRef]
- Yang, R. , et al., The effect of dry headspace on the thermal resistance of bacteria in peanut oil and peanut butter in thermal treatments. Food Control, 2022. 137: p. 108851. [CrossRef]
- Yang, R. , et al., Oil protects bacteria from humid heat in thermal processing. Food control, 2021. 123: p. 107690. [CrossRef]
- Yang, R. and J. Tang. Developing Thermal Control of Salmonella in Low-Moisture Foods Using Predictive Models. Food Safety Magazine 2023 [cited 2024 10/25]; Available from: https://digitaledition.food-safety.com/august-september-2023/feature-category/.
- Hildebrandt, I.M. , et al., Effects of inoculation procedures on variability and repeatability of Salmonella thermal resistance in wheat flour. Journal of Food Protection, 2016. 79(11): p. 1833-1839. [CrossRef]
- Xu, J. , et al., High temperature water activity as a key factor influencing survival of Salmonella Enteritidis PT30 in thermal processing. Food Control, 2019. 98: p. 520-528. [CrossRef]
- Yang, R. , et al., Desiccation in oil protects bacteria in thermal processing. Food Research International, 2020. 137: p. 109519.
- Yang, R. , et al., Thermal death kinetics of Salmonella Enteritidis PT30 in peanut butter as influenced by water activity. Food Research International, 2022. 157: p. 111288. [CrossRef]
- Lide, D.R. , CRC handbook of chemistry and physics. Vol. 85. 2004: CRC press.
- Zhang, S. , et al., Salmonella control for dried apple cubes. Food Control, 2024. 162: p. 110428. [CrossRef]
- Yang, R. , et al., Experimentally Implementing the Linear Nonisothermal Equation for Simultaneously Obtaining D-and z-Values of Salmonella Senftenberg in Skim Milk with a Differential Scanning Calorimeter. Journal of Food Protection, 2022. 85(10): p. 1410-1417. [CrossRef]
- Phungamngoen, C., N. Chiewchan, and S. Devahastin, Effects of various pretreatments and drying methods on Salmonella resistance and physical properties of cabbage. Journal of Food Engineering, 2013. 115(2): p. 237-244. [CrossRef]
- De W Blackburn, C. , et al., Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157: H7 with temperature, pH and NaCl as controlling factors. International journal of food microbiology, 1997. 38(1): p. 31-44. [CrossRef]
- DiPersio, P.A. , et al., Influence of modified blanching treatments on inactivation of Salmonella during drying and storage of carrot slices. Food Microbiology, 2007. 24(5): p. 500-507. [CrossRef]
- Enache, E. , et al., Thermal resistance parameters for pathogens in white grape juice concentrate. Journal of food protection, 2006. 69(3): p. 564-569. [CrossRef]
- Topalcengiz, Z. , Assessment of recommended thermal inactivation parameters for fruit juices. LWT, 2019. 115: p. 108475. [CrossRef]
- Sun, S. , et al., Survival and thermal resistance of Salmonella in chocolate products with different water activities. Food Research International, 2023. 172: p. 113209. [CrossRef]
- Sun, S. , et al., The influence of temperature and water activity on thermal resistance of Salmonella in milk chocolate. Food Control, 2023. 143: p. 109292. [CrossRef]
- Jin, Y., J. Tang, and M.-J. Zhu, Water activity influence on the thermal resistance of Salmonella in soy protein powder at elevated temperatures. Food Control, 2020. 113: p. 107160. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).