Submitted:
05 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
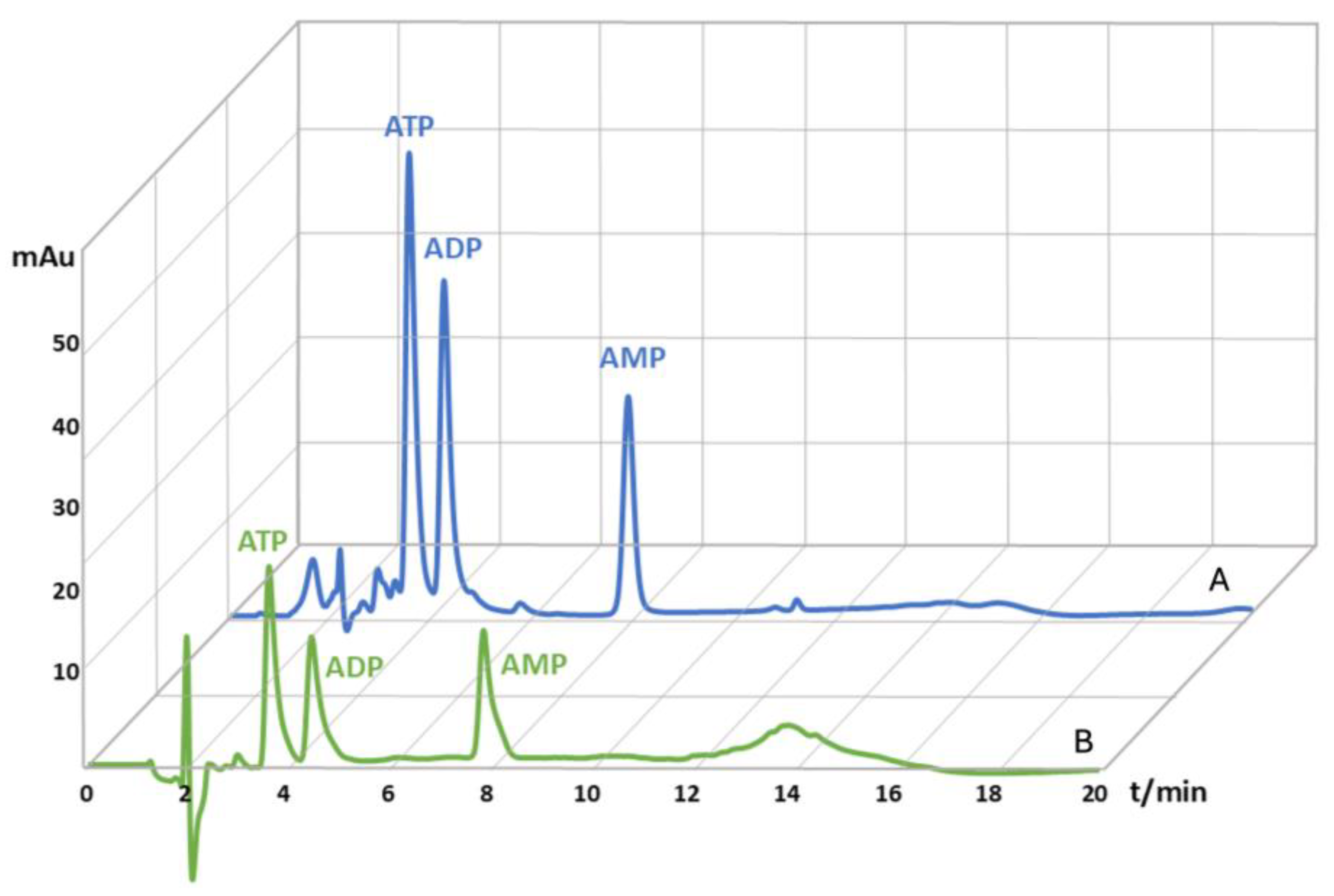
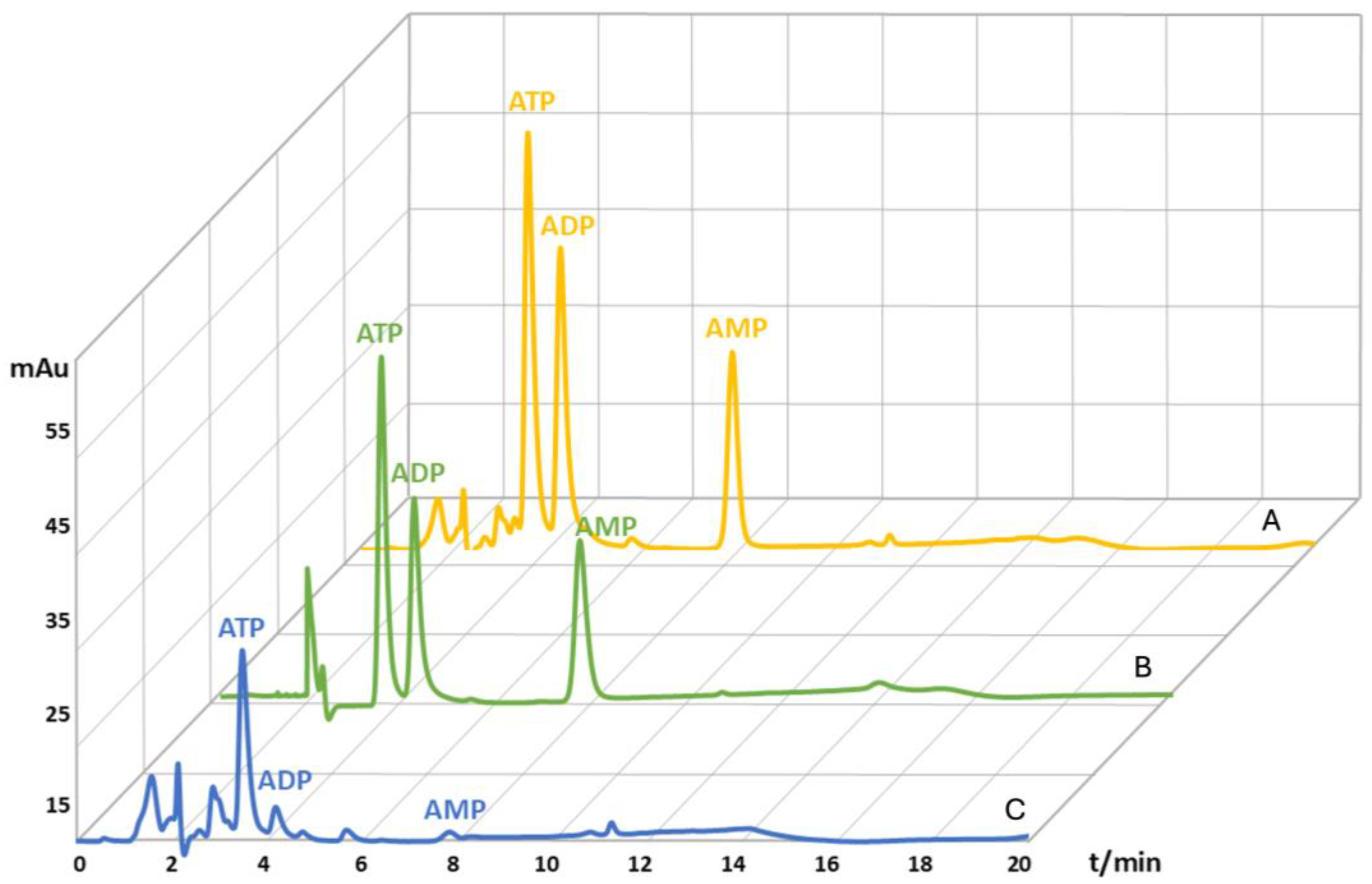
2.1. Protein Precipitation and Membrane Filtration for Adenine Nucleotides Extraction
2.2. µ-SPE Purification of Adenine Nucleotides
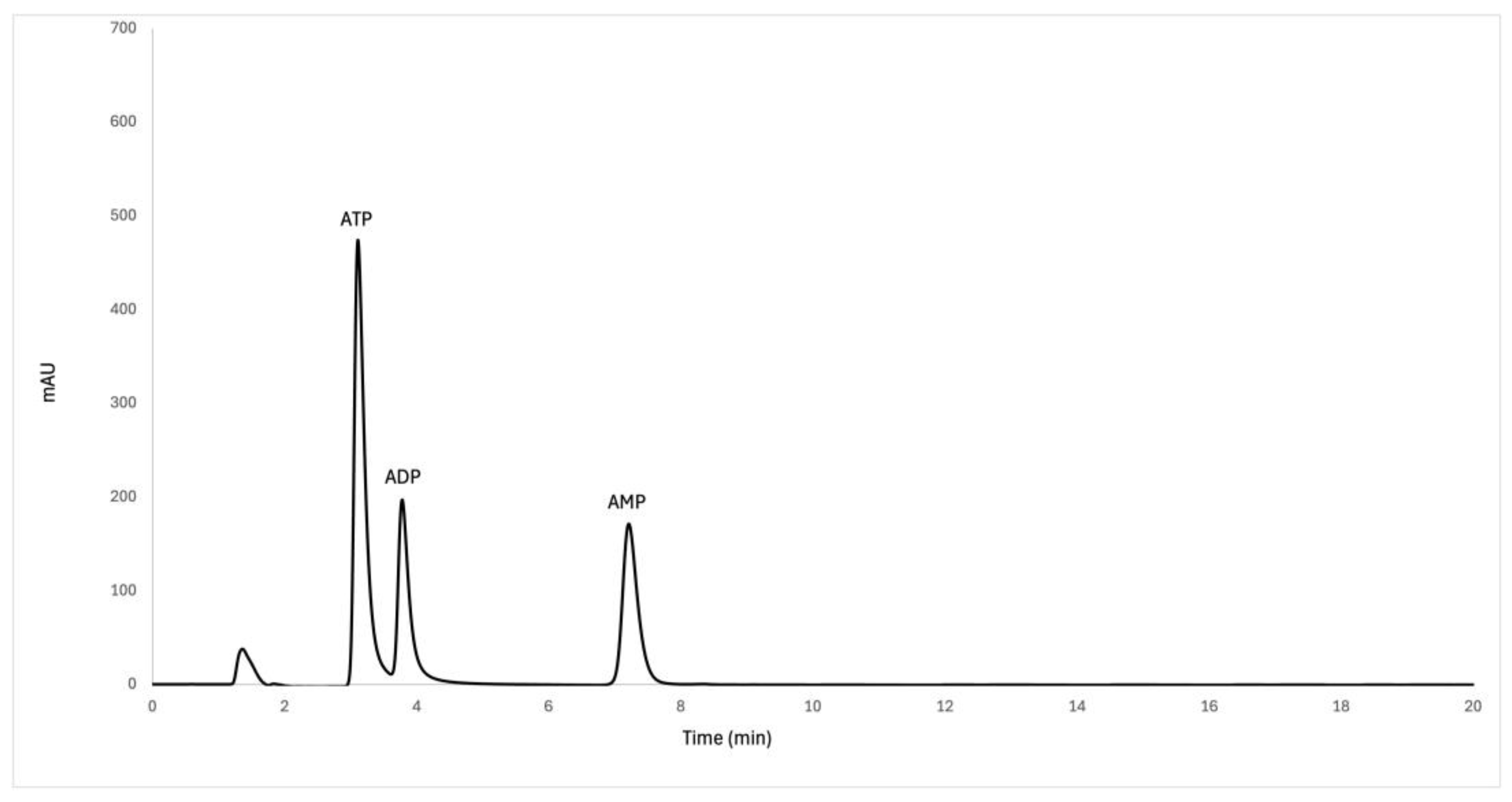
2.3. ATP, ADP and AMP Chromatographic Separation and Quantification
2.4. HPLC Method Validation
3. Materials and Methods
3.1. Chemicals
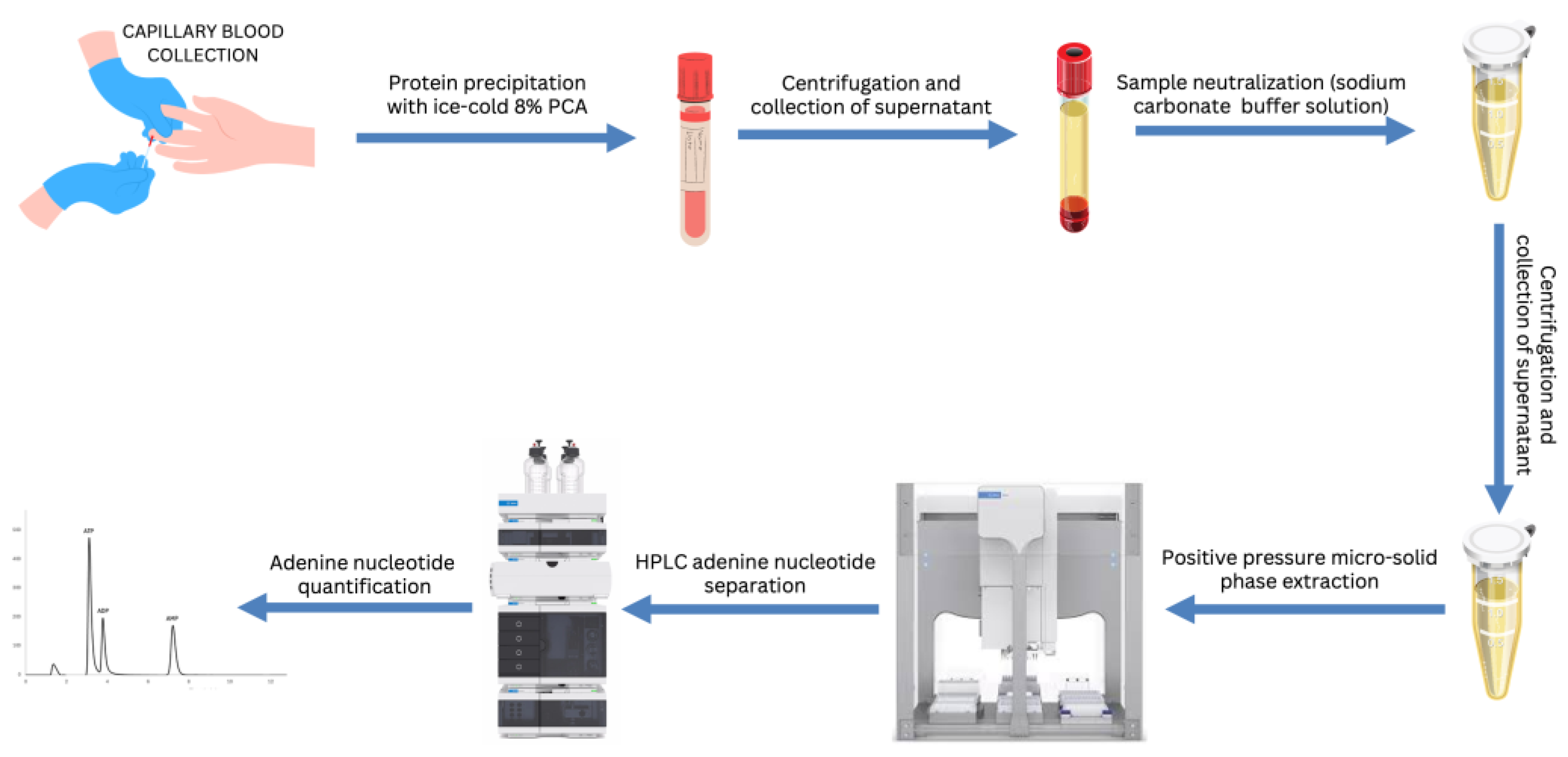
3.2. Blood Sampling and Adenine Nucleotides Liquid-Liquid Extraction
3.3. Positive-Pressure µ-SPE Method
3.4. Standard Solution Preparation
3.5. HPLC Analysis
3.6. HPLC Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- G. Burnstock. Pathophysiology and Therapeutic Potential of Purinergic Signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Martinez, O. Galicia, M.A. Isiordia-Espinoza, F. Martinez-Morales, A novel method for measuring the ATP-related compounds in human erythrocytes. Tohoku J. Exp. Med. 2014, 233, 205–214. [Google Scholar] [CrossRef] [PubMed]
- D. E. Atkinson. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef] [PubMed]
- J. S. Oakhill, R. Steel, Z.P. Chen, J.W. Scott, N. Ling, S. Tam. AMPK is a direct adenylate charge-regulated protein kinase. Science 2011, 332, 1433–1435. [Google Scholar] [CrossRef]
- L. Zhang and A. Vertes. Energy Charge, Redox State, and Metabolite Turnover in Single Human Hepatocytes Revealed by Capillary Microsampling Mass Spectrometry. Anal. Chem. 2015, 87, 10397–10405. [Google Scholar] [CrossRef]
- M. D. la Fuente, J.M. Cortes, E. Valero, M. Desroches, S. Rodrigues, L. Martinez. On the Dynamics of the Adenylate Energy System: Homeorhesis vs Homeostasis. PLOS ONE 2014, 9, e108676. [Google Scholar] [CrossRef]
- E. J. C. M. Coolen, I. C. W. Arts, E. L. R. Swennen, A. Bast, M. A. C. Stuart, and P. C. Dagnelie. Simultaneous determination of adenosine triphosphate and its metabolites in human whole blood by RP-HPLC and UV-detection. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2008, 864, 43–51. [Google Scholar] [CrossRef]
- P. Yeung, L. Ding, and W. L. Casley. HPLC assay with UV detection for determination of RBC purine nucleotide concentrations and application for biomarker study in vivo. J. Pharm. Biomed. Anal. 2008, 47, 377–382. [Google Scholar] [CrossRef]
- M. Marlewski, R. T. Smolenski, M. Szolkiewicz, Z. Aleksandrowicz, B. Rutkowski, and J. Swierczynski. Accelerated degradation of adenine nucleotide in erythrocytes of patients with chronic renal failure. Mol. Cell. Biochem. 2000, 213, 93–97. [Google Scholar] [CrossRef]
- M. S. D’almeida, J. Jagger, M. Duggan, M. White, C. Ellis, and I. H. Chin-Yee. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus. Med. 2000, 10, 291–303. [Google Scholar] [CrossRef]
- L. Domański, K. Safranow, M. Ostrowski, A. Pawlik, M. Olszewska, G. Dutkiewicz, K. Ciechanowski. Oxypurine and purine nucleoside concentrations in renal vein of allograft are potential markers of energy status of renal tissue. Arch. Med. Res. 2007, 38, 240–6. [Google Scholar] [CrossRef] [PubMed]
- M. A. Lichtman and D. R. Miller. Erythrocyte glycolysis, 2,3-diphosphoglycerate and adenosine triphosphate concentration in uremic subjects: relationship to extracellular phosphate concentration. J. Lab. Clin. Med. 1970, 76, 267–279. [Google Scholar]
- C. H. Wallas. Metabolic studies on red cells from patients with chronic renal disease on haemodialysis. Br. J. Haematol. 1974, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- C. Zhang et al.. Targeted metabolic analysis of nucleotides and identification of biomarkers associated with cancer in cultured cell models. Acta Pharm. Sin. B 2013, 3, 254–262. [Google Scholar] [CrossRef]
- C. Ledderose, E.A. Valsami, M. Newhams, T. Novak, A.G. Randolph, W.G. Junger. ATP breakdown in plasma of children limits the antimicrobial effectiveness of their neutrophils. Purinergic Signal. 2023, 19, 651. [Google Scholar] [CrossRef]
- Z. Redžović, M. Erk, E. Svetličić, L. Dončević, S. Gottstein, A. Hozić, M. Cindrić M. Determination of adenylate nucleotides in amphipod gammarus fossarum by ion-pair reverse phase liquid chromatography: possibilities of positive pressure micro-solid phase extraction. Separations 2021, 8, 1–20. [Google Scholar] [CrossRef]
- Telesiński, The effects of 2, 4-D and dicamba on isoproturon metabolism and selected biochemical parameters in clay soil, Electronic Journal of Polish Agricultural Universities 2010, 13, art.13 ref. 40. http://www.ejpau.media.pl/volume13/issue1/art-13.
- Popova and R., B. Kemp. The adenylate energy charge in marine microplankton under different levels of pollution by oil products and the stages of seasonal succession. Int. J. Algae 2002, 4, 234–241. [Google Scholar]
- J. H. E. Koop, C. Winkelmann, J. Becker, C. Hellmann, and C. Ortmann. Physiological indicators of fitness in benthic invertebrates: a useful measure for ecological health assessment and experimental ecology. Aquat. Ecol. 2011, 45, 547–559. [Google Scholar] [CrossRef]
- B. Matoo, G. Lannig, C. Bock, and I. M. Sokolova. Temperature but not ocean acidification affects energy metabolism and enzyme activities in the blue mussel, Mytilus edulis. Ecol. Evol. 2021, 11, 3366–3379. [Google Scholar] [CrossRef]
- F. Wu, E. Y. Zhang, J. Zhang, R. J. Bache, and D. A. Beard. Phosphate metabolite concentrations and ATP hydrolysis potential in normal and ischaemic hearts. J. Physiol. 2008, 586, 4193–4208. [Google Scholar] [CrossRef]
- Baranowska-Bosiacka and A., J. Hlynczak. Effect of lead ions on rat erythrocyte purine content. Biol. Trace Elem. Res. 2004, 100, 259–273. [Google Scholar] [CrossRef] [PubMed]
- W. Dudzinska and A. J. Hlynczak. Purine nucleotides and their metabolites in erythrocytes of streptozotocin diabetic rats. Diabetes Metab. 2004, 30, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Z. Smoleńska, Z. Kaznowska, D. Zarówny, H. A. Simmonds, R. T. Smoleński. Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology 1999, 38, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- W. Dudzinska, A. Lubkowska, B. Dolegowska, K. Safranow, and K. Jakubowska. Adenine, guanine and pyridine nucleotides in blood during physical exercise and restitution in healthy subjects. Eur. J. Appl. Physiol. 2010, 110, 1155–1162. [Google Scholar] [CrossRef]
- M. Zamaraeva, R. Sabirov, E. Maeno, Y. Ando-Akatsuka, S. Bessonova, and Y. Okada. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- S. Patergnani, F. S. Patergnani, F. Baldassari, E. De Marchi, A. Karkucinska-Wieckowska, M. R. Wieckowski, and P. Pinton. Chapter Sixteen - Methods to Monitor and Compare Mitochondrial and Glycolytic ATP Production. in Methods in Enzymology, vol. 542, L. Galluzzi and G. Kroemer, Eds., in Conceptual Background and Bioenergetic/Mitochondrial Aspects of Oncometabolism, vol. 542., Academic Press, 2014, pp. 313–332. [CrossRef]
- Contreras-Sanz, T.S. Scott-Ward, H.S. Gill, J.C. Jacoby, R.E. Birch et al. Simultaneous quantification of 12 different nucleotides and nucleosides released from renal epithelium and in human urine samples using ion-pair reversed-phase HPLC. Purinergic Signal. 2012, 8, 741–751. [Google Scholar] [CrossRef]
- H. Straube, C.-P. Witte, and M. Herde. Analysis of Nucleosides and Nucleotides in Plants: An Update on Sample Preparation and LC-MS Techniques. Cells 2021, 10, 689. [Google Scholar] [CrossRef]
- J. Ullrich and M. Calvin. Alcohol-resistant phosphatase activity in chloroplasts. Biochim. Biophys. Acta 1962, 63, 1–10. [Google Scholar] [CrossRef]
- J. Ullrich. Phosphatase action on phosphoglycolic, 3-phosphoglyceric, and phosphoenol pyruvic acids in spinach chloroplast fragments in the presence and absence of high concentrations of methanol. Biochim. Biophys. Acta 1963, 71, 589–594. [Google Scholar] [CrossRef]
- H. Ikuma, R. M. Tetley. Possible Interference by an Acid-stable Enzyme during the Extraction of Nucleoside Di- and Triphosphates from Higher Plant Tissues. Plant Physiology 1976, 58, 320–323. [Google Scholar] [CrossRef]
- H. Straube, M. Niehaus, S. Zwittian, C.-P. Witte, and M. Herde. Enhanced nucleotide analysis enables the quantification of deoxynucleotides in plants and algae revealing connections between nucleoside and deoxynucleoside metabolism. Plant Cell 2021, 33, 270–289. [Google Scholar] [CrossRef]
- R. L. Bieleski. The problem of halting enzyme action when extracting plant tissues. Anal. Biochem. 1964, 9, 431–442. [Google Scholar] [CrossRef]
- J. X. Khym. An Analytical System for Rapid Separation of Tissue Nucleotides at Low Pressures on Conventional Anion Exchangers. Clinical Chemistry 1975, 21, 1245–1252. [Google Scholar] [CrossRef]
- J. O. Svensson and B. Jonzon. Determination of adenosine and cyclic adenosine monophosphate in urine using solid-phase extraction and high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B. Biomed. Sci. App. 1990, 529, 437–441. [Google Scholar] [CrossRef]
- J. Czarnecka, M. Cieślak, and K. Michał. Application of solid phase extraction and high-performance liquid chromatography to qualitative and quantitative analysis of nucleotides and nucleosides in human cerebrospinal fluid. J. Chromatogr. B 2005, 822, 85–90. [Google Scholar] [CrossRef] [PubMed]
- M. Guo, D. Yin, J. Han, L. Zhang, X. Li, D. Tang. Phenylboronic acid modified solid-phase extraction column: Preparation, characterization, and application to the analysis of amino acids in sepia capsule by removing the maltose. J. Sep. Sci. 2016, 39, 3428–3435. [Google Scholar] [CrossRef]
- F. Guérard, P. Pétriacq, B. Gakière, and G. Tcherkez. Liquid chromatography/time-of-flight mass spectrometry for the analysis of plant samples: A method for simultaneous screening of common cofactors or nucleotides and application to an engineered plant line. Plant Physiol. Biochem. 2011, 49, 1117–1125. [Google Scholar] [CrossRef]
- S. R. Strezsak, P. J. Beuning, and N. J. Skizim. Versatile separation of nucleotides from bacterial cell lysates using strong anion exchange chromatography. J. Chromatogr. B 2022, 1188, 123044. [Google Scholar] [CrossRef]
- E. Janas, M. Hofacker, M. Chen, S. Gompf, C. van der Does, and R. Tampé. The ATP Hydrolysis Cycle of the Nucleotide-binding Domain of the Mitochondrial ATP-binding Cassette Transporter Mdl1p*. J. Biol. Chem. 2003, 278, 26862–26869. [Google Scholar] [CrossRef]
- L. C. Bates-Fraser et al. . A practical approach for complete blood count analysis following acute exercise: Capillary vs. venous blood sampling. Adv. Exerc. Health Sci. 2024, 1, 43–50. [Google Scholar] [CrossRef]
- S. Souverain, S. Rudaz, and J.-L. Veuthey. Protein precipitation for the analysis of a drug cocktail in plasma by LC–ESI–MS. J. Pharm. Biomed. Anal. 2004, 35, 913–920. [Google Scholar] [CrossRef] [PubMed]
- J. Stone. Chapter 3 - Sample preparation techniques for mass spectrometry in the clinical laboratory. In Mass Spectrometry for the Clinical Laboratory; H. Nair and W. Clarke, Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 37–62. [Google Scholar] [CrossRef]
- K. Lim. Sample preparation for high-performance liquid chromatography in the clinical laboratory. TrAC Trends Anal. Chem. 1988, 7, 340–345. [CrossRef]
- J. M. High-throughput quantitative bioanalysis by LC/MS/MS. Biomed. Chromatogr. BMC 2000, 14. [Google Scholar] [CrossRef]
- R. D. McDowall. Sample preparation for biomedical analysis. J. Chromatogr. 1989, 492, 3–58. [Google Scholar] [CrossRef]
- L. Makszin, P. Kustan, B. Szirmay, C. Pager, E. Mezo, K. Kalacs, V. Paszthy, E. Gyorgy, F. Kilar, A. Ludany, T. Koszegi. Microchip gel electrophoretic analysis of perchloric acid-soluble serum proteins in systemic inflammatory disorders. Electrophoresis 2018, 40, 447. [Google Scholar] [CrossRef]
- PCA Deproteinization protocol | abcam. Available: https://www.abcam.com/en-us/technical-resources/protocols/deproteinization. (accessed on 28 October 2024).
- Williams and, T. Forrester. Loss of ATP in micromolar amounts after perchloric acid treatment. Pflugers Arch. 1976, 366, 281–283. [Google Scholar] [CrossRef]
- L. B. Thorfinnsdottir, L. García-Calvo, G. H. Bø, P. Bruheim, and L. M. Røst. Optimized Fast Filtration-Based Sampling and Extraction Enables Precise and Absolute Quantification of the Escherichia coli Central Carbon Metabolome. Metabolites 2023, 13, 150. [Google Scholar] [CrossRef]
- M. R. Bladergroen and Y. E. M. van der Burgt. Solid-Phase Extraction Strategies to Surmount Body Fluid Sample Complexity in High-Throughput Mass Spectrometry-Based Proteomics. J. Anal. Methods Chem. 2015, 2015, 250131. [Google Scholar] [CrossRef]
- M. Pabst, J. Grass, R. Fischl, R. Léonard, C. Jin, G. Hinterkörner. Nucleotide and nucleotide sugar analysis by liquid chromatography - electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal. Chem. 2010, 82, 9782–9788. [Google Scholar] [CrossRef]
- Z. Kong, S. Jia, A. L. Chabes, P. Appelblad, R. Lundmark, T. Moritz, A. Chabes, Simultaneous determination of ribonucleoside and deoxyribonucleoside triphosphates in biological samples by hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry, Nucleic Acids Research 2018, 46, e66. [CrossRef]
- L. R. Bushman, J.J. Kiser, J.E. Rower, B. Klein, J.H. Zheng, M.L. Ray, P.L. Anderson. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J. Pharm. Biomed. Anal. 2011, 56, 390–401. [Google Scholar] [CrossRef] [PubMed]
- B. L. Robbins, P. A. Poston, E. F. Neal, C. Slaughter, and J. H. Rodman. Simultaneous measurement of intracellular triphosphate metabolites of zidovudine, lamivudine and abacavir (carbovir) in human peripheral blood mononuclear cells by combined anion exchange solid phase extraction and LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2007, 850, 310–317. [Google Scholar] [CrossRef]
- Emotte, F. Deglave, O. Heudi, F. Picard, and O. Kretz. Fast simultaneous quantitative analysis of FTY720 and its metabolite FTY720-P in human blood by on-line solid phase extraction coupled with liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 58, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Magdenoska, J. Martinussen, J. Thykaer, and K. F. Nielsen. Dispersive solid phase extraction combined with ion-pair ultra high-performance liquid chromatography tandem mass spectrometry for quantification of nucleotides in Lactococcus lactis. Anal. Biochem. 2013, 440, 166–177. [Google Scholar] [CrossRef]
- T. Uesugi, K. Sano, Y. Uesawa, Y. Ikegami, and K. Mohri. Ion-pair reversed-phase high-performance liquid chromatography of adenine nucleotides and nucleoside using triethylamine as a counterion. J. Chromatogr. B. Biomed. Sci. App. 1997, 703, 63–74. [Google Scholar] [CrossRef]
- G. Zhang, A.D. Walker, Z. Lin, X. Han, M. Blatnik, R.C. Steenwyk, E.A. Groeber. Strategies for quantitation of endogenous adenine nucleotides in human plasma using novel ion-pair hydrophilic interaction chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2014, 1325, 129–136. [Google Scholar] [CrossRef]
- J. Kang, L. Lim, and J. Song. ATP binds and inhibits the neurodegeneration-associated fibrillization of the FUS RRM domain. Commun. Biol. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- P. Bhatt, X. P. Bhatt, X. Chen, J. D. Geiger, and T. A. Rosenberger. A sensitive HPLC-based method to quantify adenine nucleotides in primary astrocyte cell cultures. J. Chromatogr. B. [CrossRef]
- L. Mora, A. S. Hernández-Cázares, M.-C. Aristoy, and F. Toldrá. Hydrophilic interaction chromatographic determination of adenosine triphosphate and its metabolites. Food Chem. 2010, 123, 1282–1288. [Google Scholar] [CrossRef]
- D. -S. Hsu and S. S. Chen. Simple anion-exchange chromatography for the determination of adenine nucleotides by using AG MP-1 resin. J. Chromatogr. A 1980, 192, 193–198. [Google Scholar] [CrossRef]
- M. Bartolini, I. W. Wainer, C. Bertucci, and V. Andrisano. The rapid and direct determination of ATPase activity by ion exchange chromatography and the application to the activity of heat shock protein-90. J. Pharm. Biomed. Anal. 2013, 73, 77–81. [Google Scholar] [CrossRef]
- S. Studzińska and B. Buszewski. Effect of mobile phase pH on the retention of nucleotides on different stationary phases for high-performance liquid chromatography. Anal. Bioanal. Chem. 2013, 405, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- T. Qian, Z. Cai, and M. S. Yang. Determination of adenosine nucleotides in cultured cells by ion-pairing liquid chromatography–electrospray ionization mass spectrometry. Anal. Biochem. 2004, 325, 77–84. [Google Scholar] [CrossRef] [PubMed]
- M. Cichna, M. Raab, H. Daxecker, A. Griesmacher, M. M. Müller, and P. Markl. Determination of fifteen nucleotides in cultured human mononuclear blood and umbilical vein endothelial cells by solvent generated ion-pair chromatography. J. Chromatogr. B 2003, 787, 381–391. [Google Scholar] [CrossRef] [PubMed]
- M. Ganzera, P. Vrabl, E. Wörle, W. Burgstaller, and H. Stuppner. Determination of adenine and pyridine nucleotides in glucose-limited chemostat cultures of Penicillium simplicissimum by one-step ethanol extraction and ion-pairing liquid chromatography. Anal. Biochem. 2006, 359, 132–140. [Google Scholar] [CrossRef]
- C. Bolin and F. Cardozo-Pelaez. Assessing biomarkers of oxidative stress: Analysis of guanosine and oxidized guanosine nucleotide triphosphates by high performance liquid chromatography with electrochemical detection. J. Chromatogr. B 2007, 856, 121–130. [Google Scholar] [CrossRef]
- S. Zur Nedden, R. Eason, A. S. Doney, and B. G. Frenguelli. An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze–thaw degradation of ATP. Anal. Biochem. 2009, 388, 108–114. [Google Scholar] [CrossRef]
- Patel, L. Malinovska, S. Saha, J. Wang, S. Alberti, Y. Krishnan, A.A. Hyman. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- M. Cox and D. Nelson, Lehninger Principles of Biochemistry 2000, 5. [CrossRef]
- M. Rice and M. K. Rosen. ATP controls the crowd. Science 2017, 356, 701–702. [Google Scholar] [CrossRef]
- Harmsen, J. W. de Jong, and P. W. Serruys. Hypoxanthine production by ischemic heart demonstrated by high pressure liquid chromatography of blood purine nucleosides and oxypurines. Clin. Chim. Acta Int. J. Clin. Chem. 1981, 115, 73–84. [Google Scholar] [CrossRef]
- Crescentini and, V. Stocchi. Fast reversed-phase high-performance liquid chromatographic determination of nucleotides in red blood cells. J. Chromatogr. A 1984, 290, 393–399. [Google Scholar] [CrossRef]
- V. Stocchi, L. Cucchiarini, F. Canestrari, M. P. Piacentini, and G. Fornaini. A very fast ion-pair reversed-phase HPLC method for the separation of the most significant nucleotides and their degradation products in human red blood cells. Anal. Biochem. 1987, 167, 181–190. [Google Scholar] [CrossRef] [PubMed]
- K. K. Tekkanat and I. H. Fox. Isocratic separation of ATP and its degradation products from biological fluids by automated liquid chromatography. Clin. Chem. 1988, 34, 925–932. [Google Scholar] [CrossRef]
- R. Caruso, J. Campolo, C. Dellanoce, R. Mariele, O. Parodi, and R. Accinni. Critical study of preanalytical and analytical phases of adenine and pyridine nucleotide assay in human whole blood. Anal. Biochem. 2004, 330, 43–51. [Google Scholar] [CrossRef] [PubMed]
- M. F. Wahab, D. C. Patel, and D. W. Armstrong. Total peak shape analysis: detection and quantitation of concurrent fronting, tailing, and their effect on asymmetry measurements. J. Chromatogr. A 2017, 1509, 163–170. [Google Scholar] [CrossRef] [PubMed]
- T. Ivanov. Low pH-induced hemolysis of erythrocytes is related to the entry of the acid into cytosole and oxidative stress on cellular membranes. Biochim. Biophys. Acta 1999, 1415, 349–360. [Google Scholar] [CrossRef]
- J. E. T. Andersen. The standard addition method revisited. TrAC Trends Anal. Chem. 2017, 89, 21–33. [Google Scholar] [CrossRef]
| Adenine nucleotide | Blood (µM) |
Spike (µM) |
Blood + Spike (µM) |
Recovery (%) |
|---|---|---|---|---|
| ATP | 1393.1 | 1983.4 | 2807.9 | 71.6 |
| ADP | 254.8 | 1618.8 | 2228.2 | 121.8 |
| AMP | 76.9 | 1332.2 | 1573.8 | 112.1 |
| Adenine nucleotide | Theoretical concentration (µM) |
Intra-day | Inter-day | ||
|---|---|---|---|---|---|
| Observed concentration (µM) |
RSD (%) | Observed concentration (µM) |
RSD (%) | ||
| ATP | 10 | 9.47 ± 0.75 | 0.24 | 9.16 ± 0.21 | 0.07 |
| 50 | 50.27 ± 3.35 | 0.22 | 49.97 ± 2.80 | 0.19 | |
| 100 | 100.22 ± 4.16 | 0.14 | 98.43 ± 2.25 | 0.08 | |
| 200 | 199.9 ± 2.12 | 0.03 | 201.43 ± 1.40 | 0.02 | |
| 333.3 | 332.21 ± 1.15 | 0.01 | 331.74 ± 3.13 | 0.03 | |
| ADP | 10 | 9.61 ± 2.51 | 0.86 | 9.22 ± 0.92 | 0.32 |
| 50 | 50.91 ± 3.16 | 0.22 | 49.22 ± 1.70 | 0.12 | |
| 100 | 99.14 ± 2.60 | 0.09 | 100.15 ± 0.71 | 0.03 | |
| 200 | 198.24 ± 1.53 | 0.03 | 200.00 ± 5.37 | 0.10 | |
| 333.3 | 332.52 ± 1.10 | 0.01 | 331.87 ± 3.68 | 0.04 | |
| AMP | 10 | 10.23 ± 1.30 | 0.46 | 10.37 ± 3.48 | 1.22 |
| 50 | 50.69 ± 1.82 | 0.13 | 49.47 ± 4.36 | 0.33 | |
| 100 | 99.46 ± 0.91 | 0.03 | 99.87 ± 0.50 | 0.02 | |
| 200 | 198.81 ± 2.76 | 0.05 | 198.14 ± 0.62 | 0.01 | |
| 333.3 | 333.71 ± 1.81 | 0.02 | 331.20 ± 1.25 | 0.01 | |
| µ-SPE step | Solution | Volume (µL) |
Flow rate (µL/min) |
|---|---|---|---|
| Priming | acetonitrile in 3% formic acid, pH 9, 60:40; v/v | 300 | 100 |
| Equilibration | ultra-pure water | 100 | 100 |
| Sample load | standard solution and capillary blood | 100 | 5 |
| Washing | ultra-pure water | 100 | 5 |
| Elution | acetonitrile in 3% formic acid, pH 9, 60:40; v/v | 25 | 5 |
| Time (min) | Flow Rate (mL/min) | Mobile Phase Solution A 50 mM Phosphate Buffer (%) |
Mobile Phase Solution B 100% Methanol (%) |
|---|---|---|---|
| 0.0 | 0.6 | 100.0 | 0.0 |
| 2.0 | 0.6 | 100.0 | 0.0 |
| 10.0 | 0.6 | 87.5 | 12.5 |
| 12.0 | 0.6 | 87.5 | 12.5 |
| 20.0 | 0.6 | 100.0 | 0.0 |
| 30.0 | 0.6 | 100.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





