Submitted:
06 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
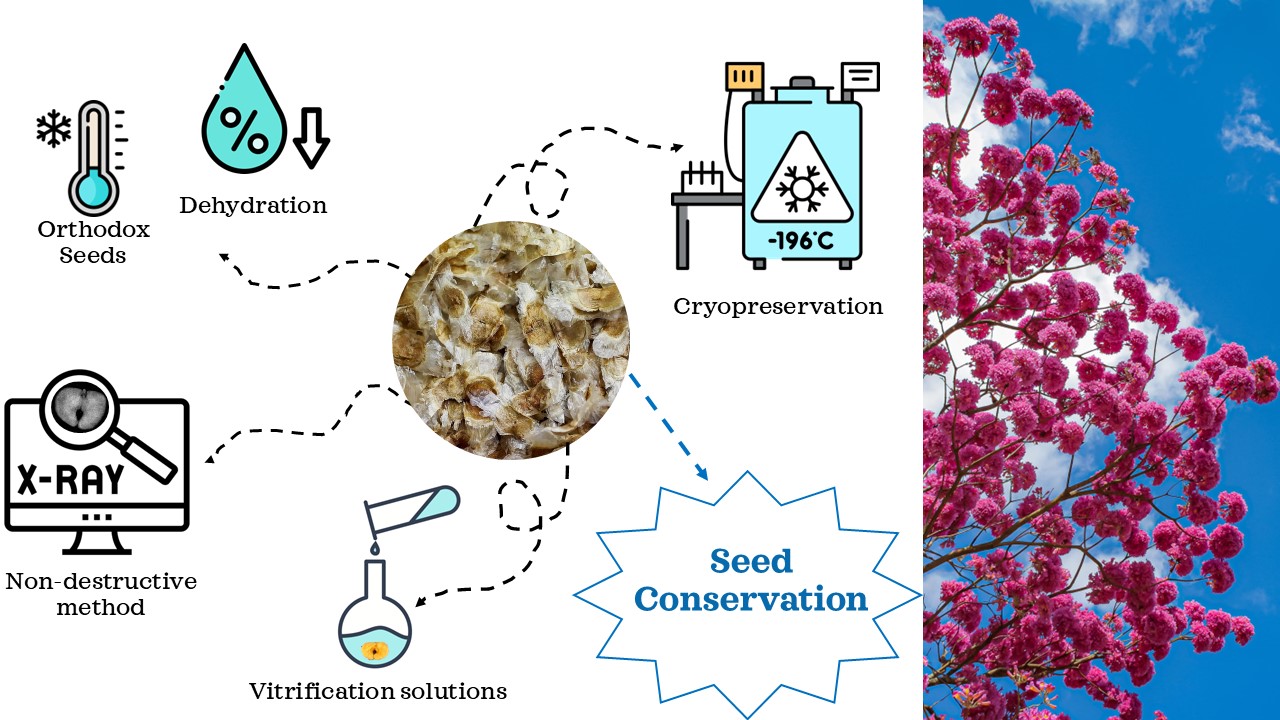
1. Introduction
2. Materials and Methods
2.1. Plant Material
- M1 = Weight of the weighing container with cover in grams.
- M2 = Weight of the weighing container with cover and seeds before drying.
- M3 = Weight of the weighing container with cover and seeds after drying.
- S1= is the moisture loss in the first stage.
- S2= is the moisture loss in the second stage.
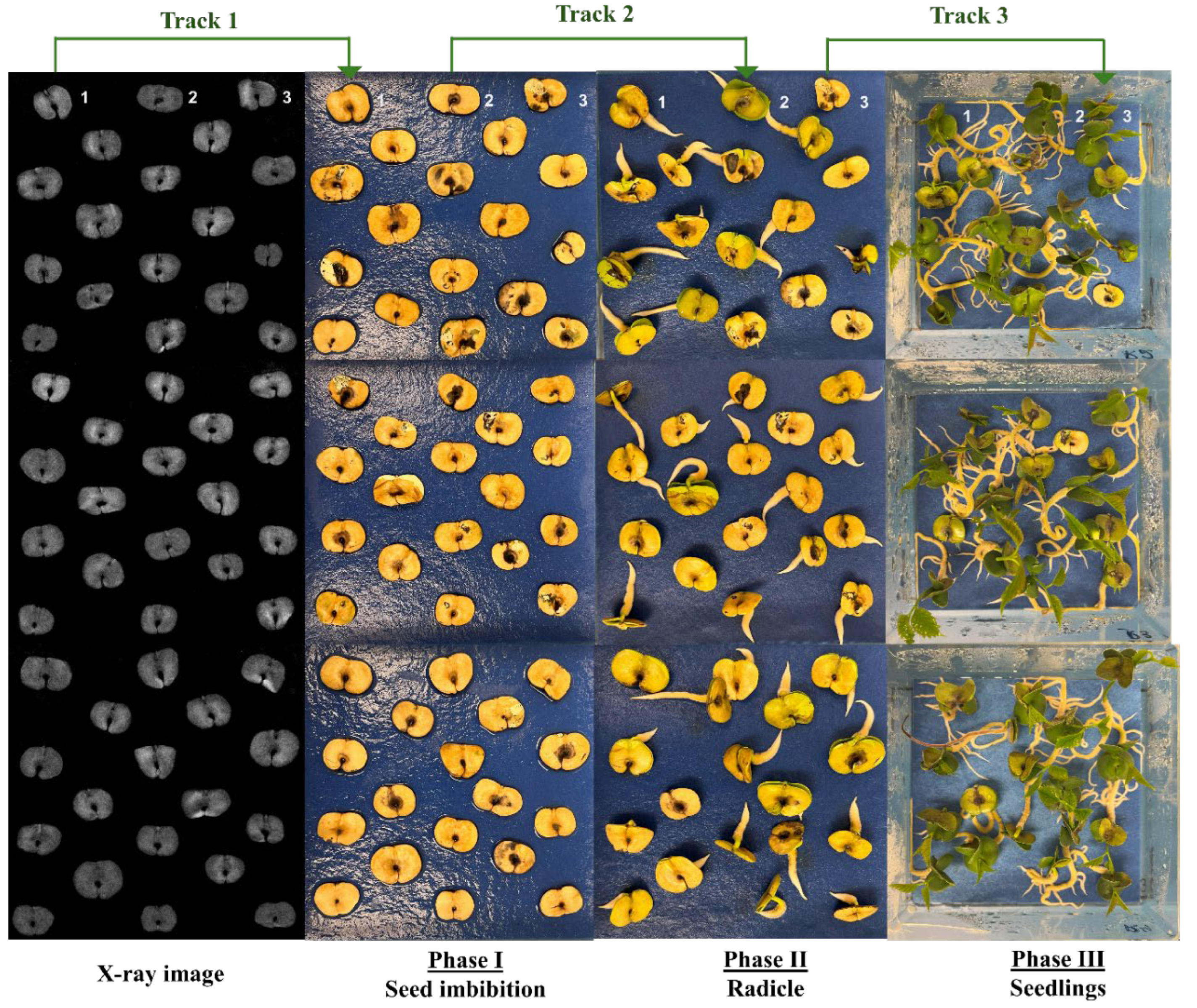
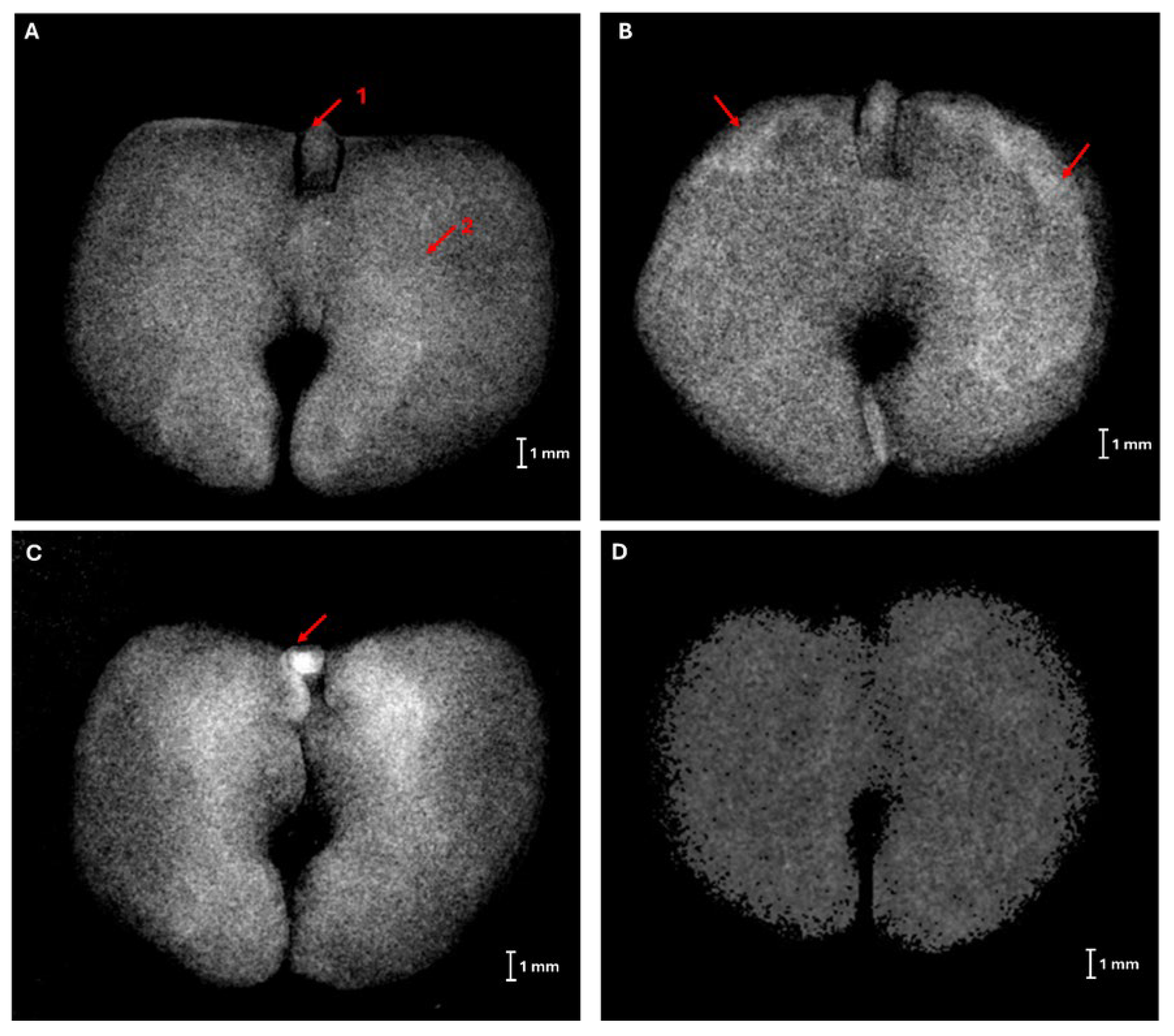
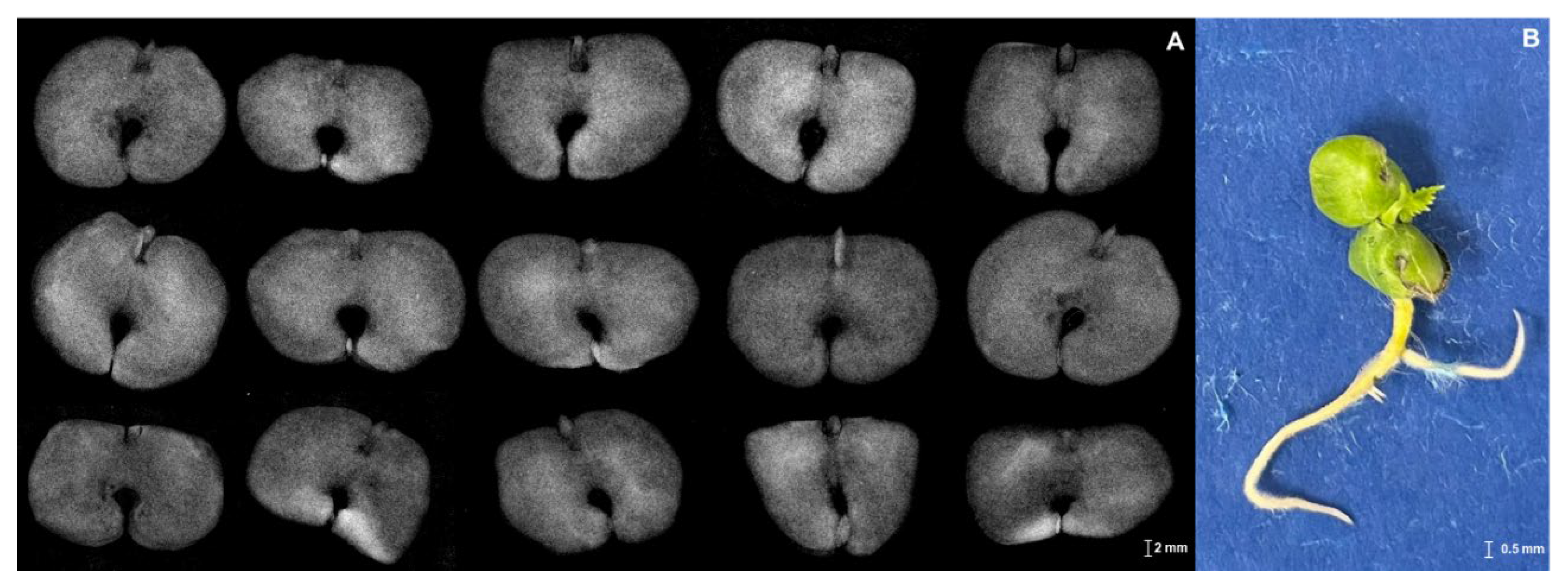
2.2. X-ray Imaging in Seed Quality Assessment
2.3. Seeds Cryopreservation
- T0) Control (seeds were not immersed in liquid nitrogen (LN) or vitrification solution; they were either placed in an ultra-freezer);
- T1) seeds stored in an ultra-freezer at -80 ºC without vitrification solution;
- T2) seeds immersed in LN without vitrification solution;
- T3) seeds immersed in PVS2 followed by LN;
- T4) seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN;
- T5) seeds immersed in PVS3 followed by LN;
- T6) seeds immersed in PVS3 with 1.0% PG followed by LN.
2.4. Statistical Procedures
3. Results
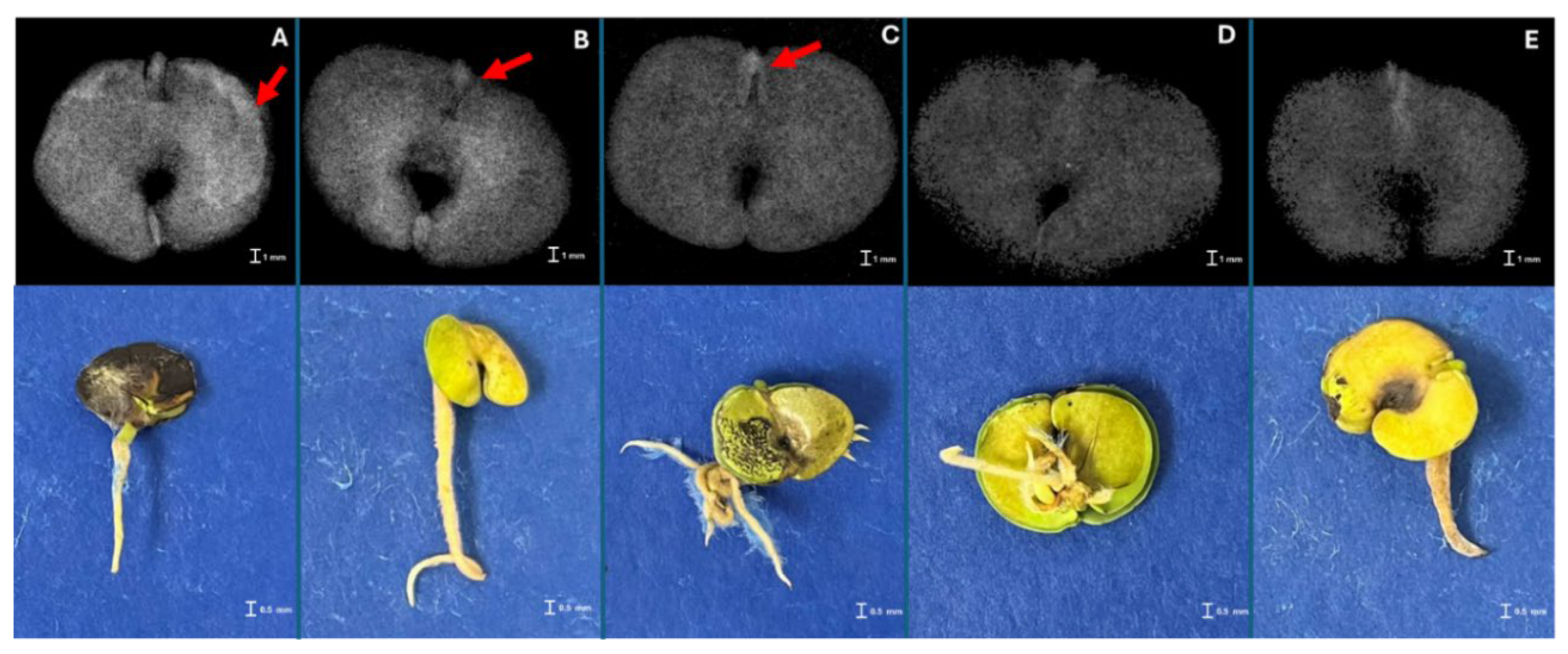
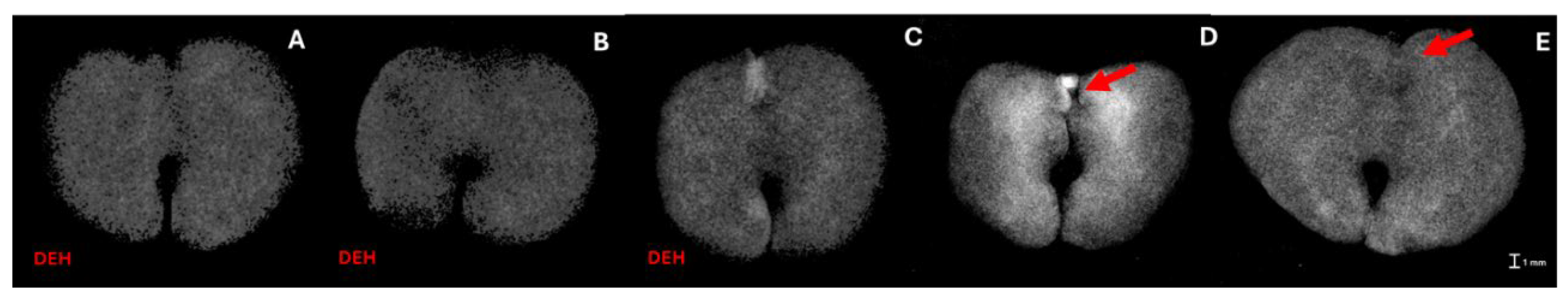
3.1. X-ray Imaging as Seed Quality Assessment
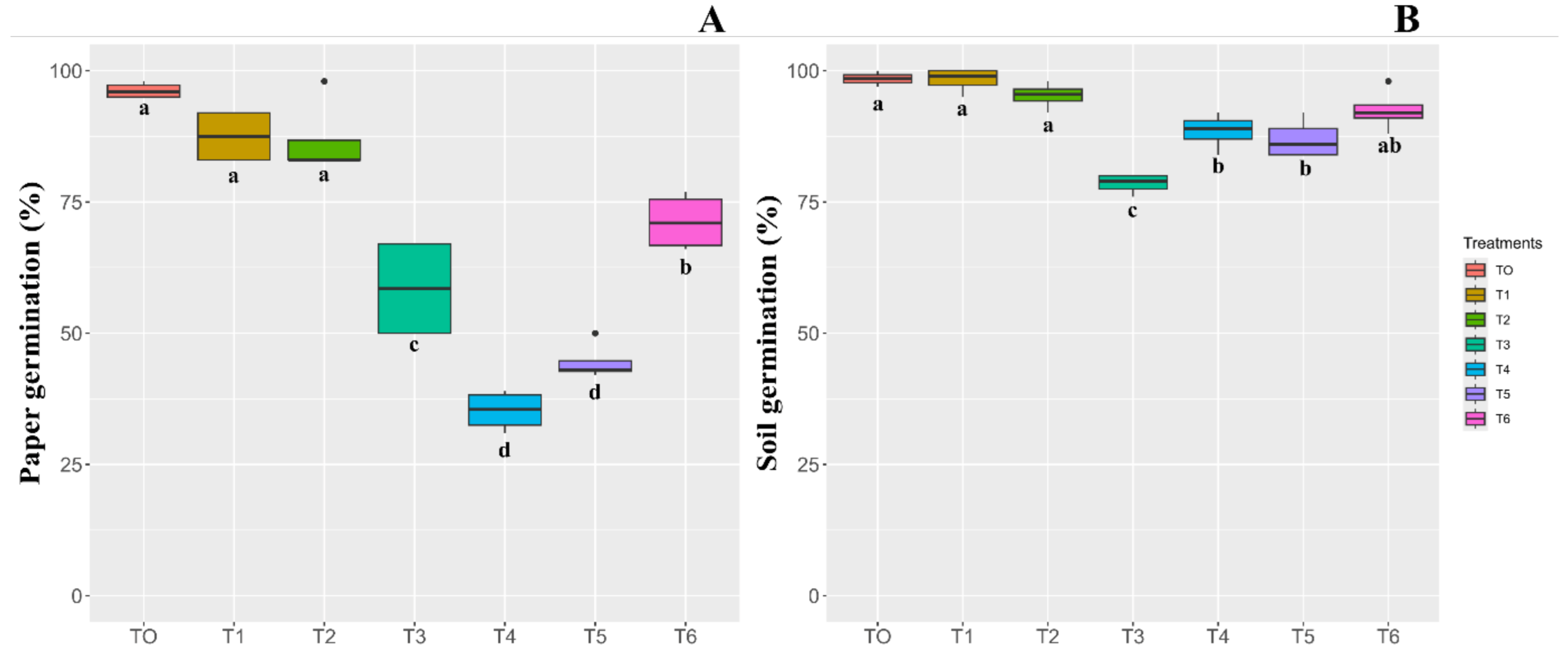
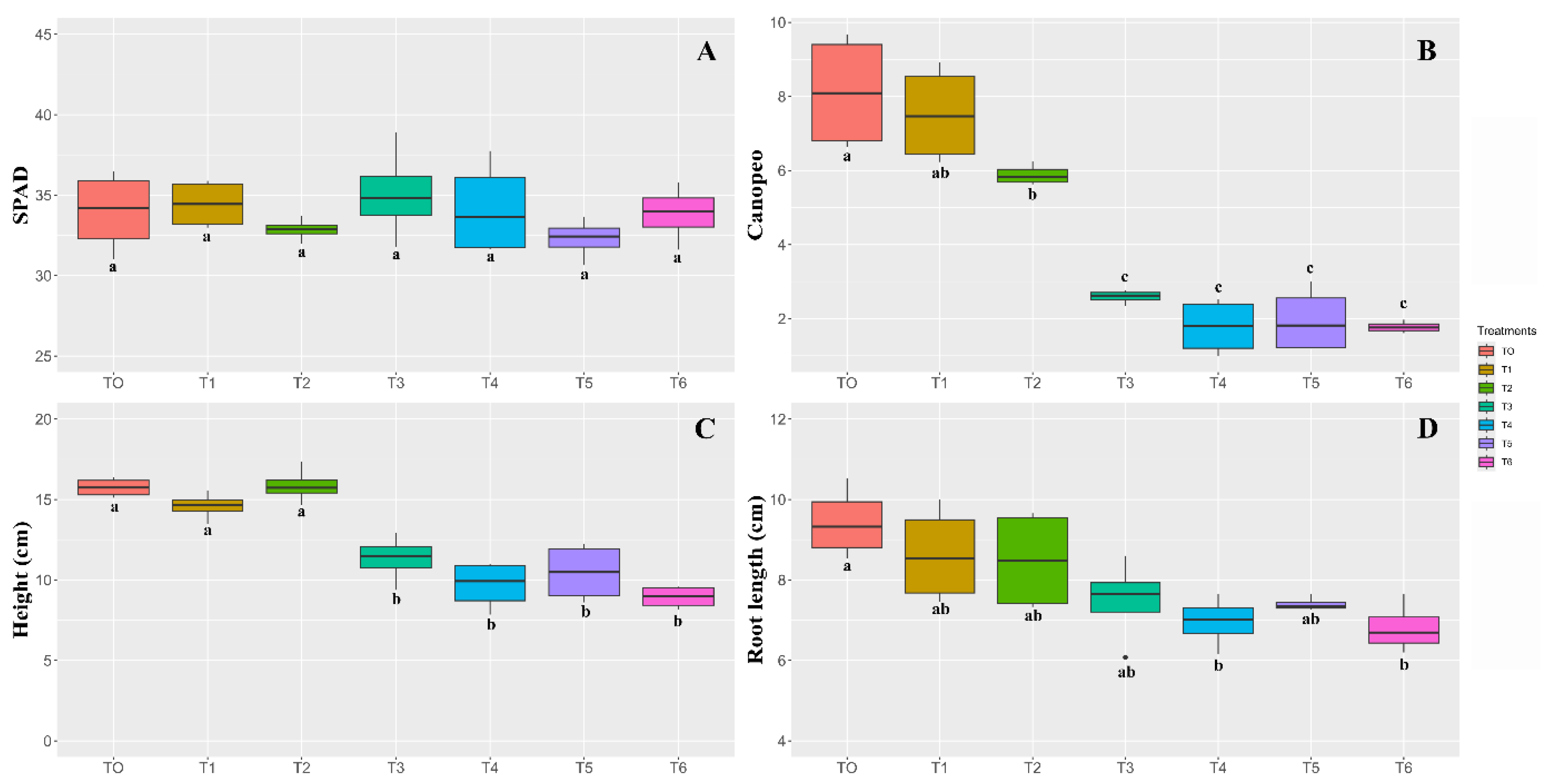
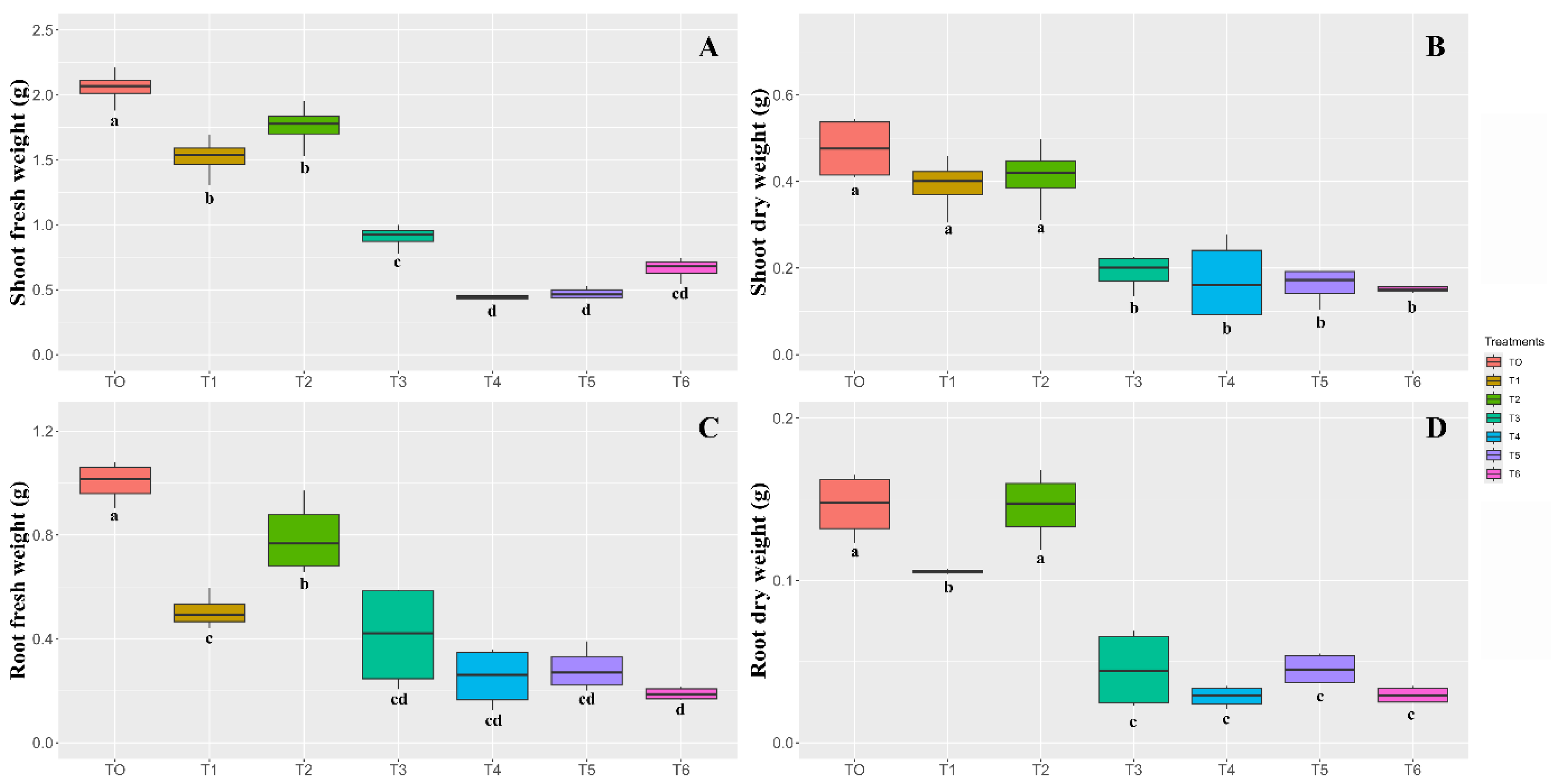
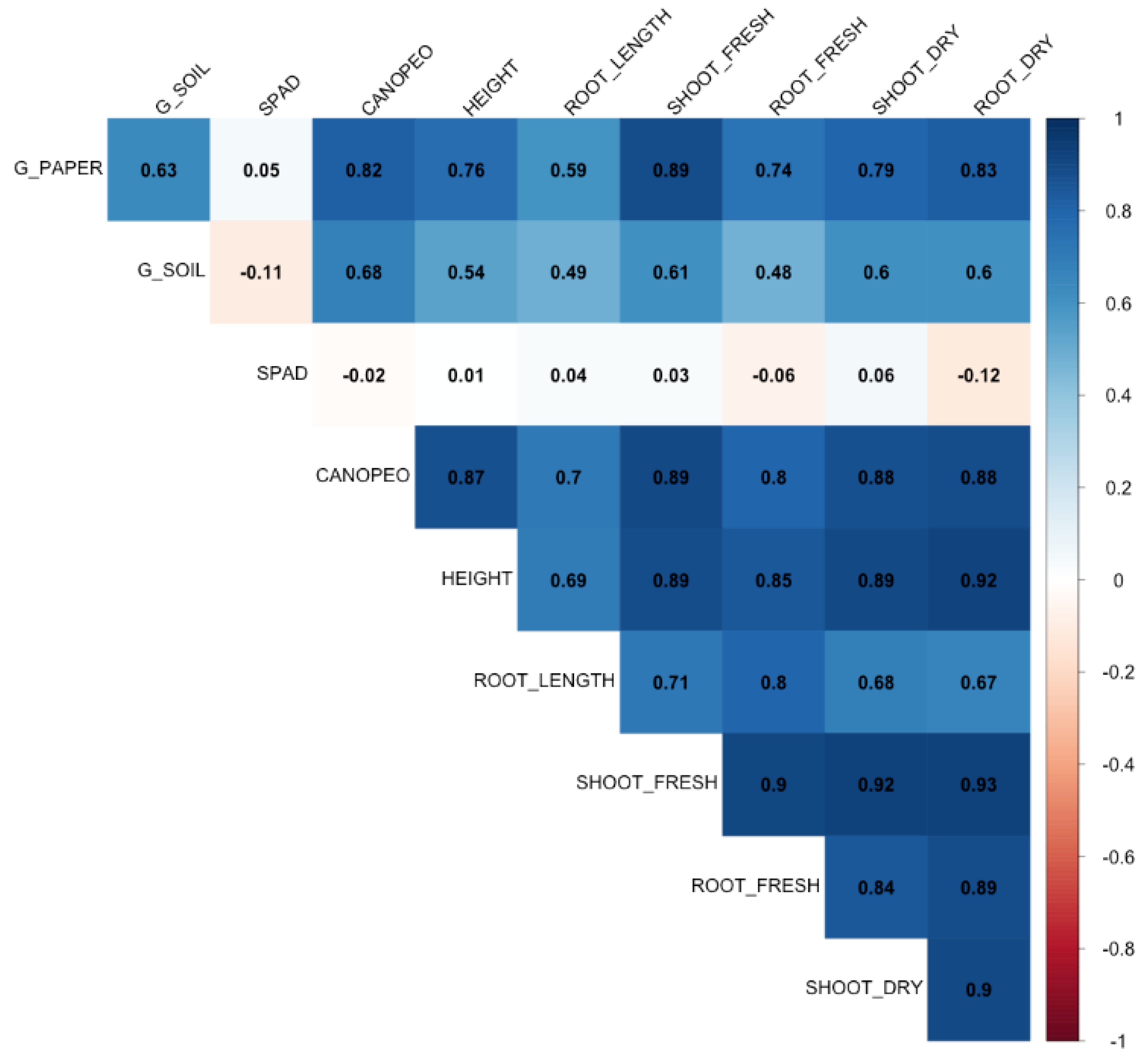
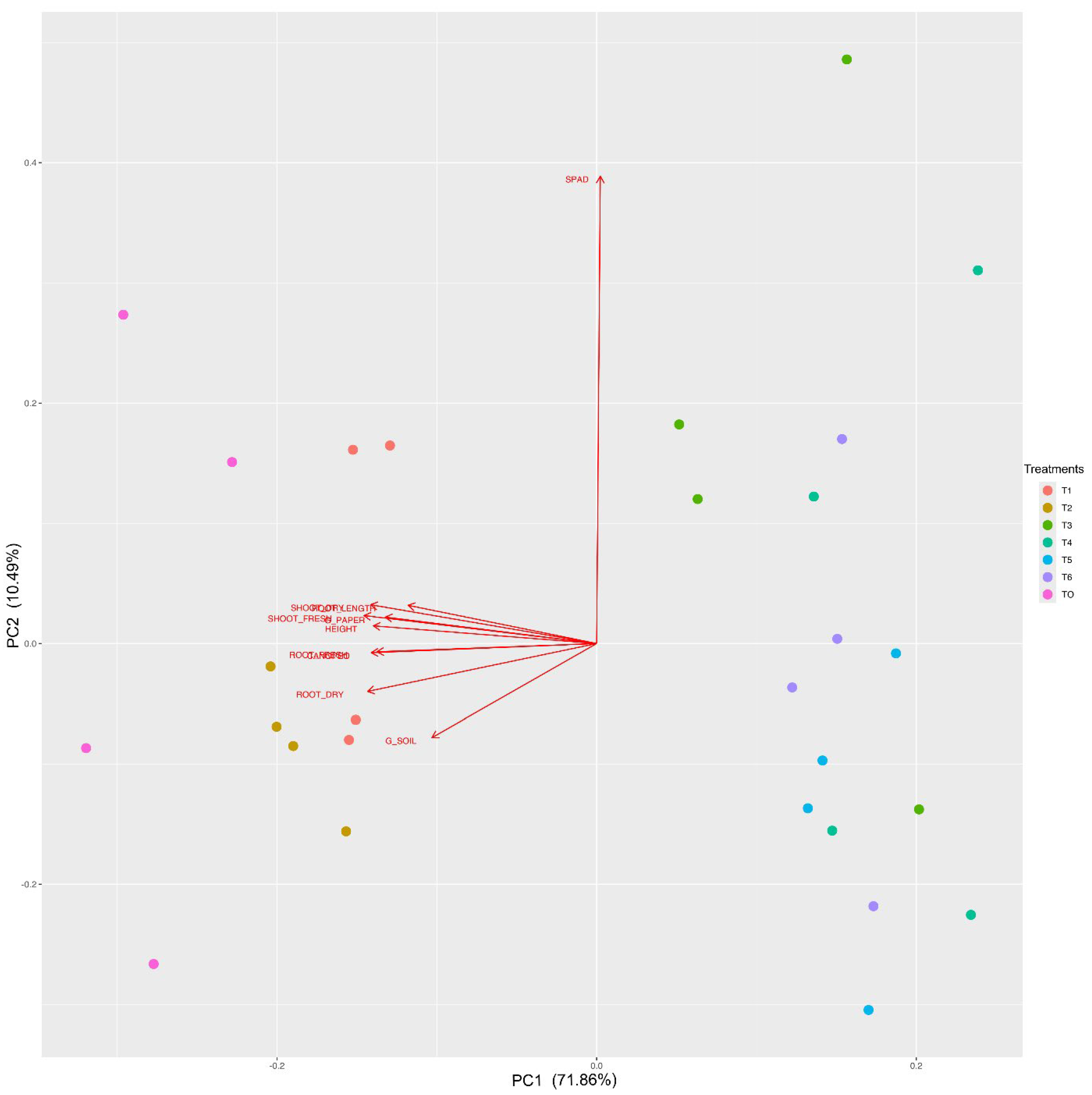
3.2. Seed Cryopreservation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil, 8th ed.; Instituto Plantarum: Nova Odessa, Brazil, 2020; pp. 44–56. [Google Scholar]
- Silva-Junior, O.B.; Grattapaglia, D.; Novaes, E.; Collevatti, R.G. Genome Assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a Highly Valued, Ecologically Keystone Neotropical Timber Forest Tree. Gigascience 2018, 7, gix125. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The Red List of Threatened Species: BGCI, Tree Specialist Group Handroanthus impetiginosus. 2020. Available online: https://www.iucnredlist.org/species/144297143/173394208 (accessed on 20 january 2024).
- Pimenta, J.M.A.; Souza, W.M.A.T.D.; Ferrari, C.D.S.; Vieira, F.D.A.; Fajardo, C.G.; Pacheco, M.V. Selection of Handroanthus impetiginosus Mother Trees to Support Seed Collection Areas. Revista Árvore 2023, 47, e4706. [Google Scholar] [CrossRef]
- Martins, J.R.; Edvaldo, A.A.S.; Alvarenga, A.A.; Rodrigues, A.C.; Ribeiro, D.E.; Toorop, P.E. Seedling Survival of Handroanthus impetiginosus (Mart. ex DC) Mattos in a Semi-Arid Environment Through Modified Germination Speed and Post-Germination Desiccation Tolerance. Braz. J. Biol. 2015, 75, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, J.M.A.; Felix, F.C.; Araújo, J.S.O.D.; Fajardo, C.G.; Pacheco, M.V. Selection of ISSR Molecular Primers for Studies of Genetic Diversity in Handroanthus impetiginosus (Mart. ex DC) Mattos. Rev. Caatinga 2022, 35, 231–238. [Google Scholar] [CrossRef]
- Piovesan, A.; Vancauwenberghe, V.; Van De Looverbosch, T.; Verboven, P.; Nicolaï, B. X-ray Computed Tomography for 3D Plant Imaging. Trends Plant Sci. 2021, 26, 1171–1185. [Google Scholar] [CrossRef]
- Pessoa, H.P.; Copati, M.G.F.; Azevedo, A.M.; Dariva, F.D.; Almeida, G.Q.D.; Gomes, C.N. Combining Deep Learning and X-ray Imaging Technology to Assess Tomato Seed Quality. Sci. Agricola 2023, 80, e20220121. [Google Scholar] [CrossRef]
- Silva, P.P.; Freitas, R.A.; Cícero, S.M.; Marcos Filho, J.; Nascimento, W.M. Image Analysis to Associate Morphological and Physiological Characteristics in Pumpkin Seeds. Hortic. Bras. 2014, 32, 210–214. [Google Scholar] [CrossRef]
- Medeiros, A.D.D.; Araújo, J.D.O.; León, M.J.Z.; Silva, L.J.D.; Dias, D.C.F.D.S. Parameters Based on X-Ray Images to Assess the Physical and Physiological Quality of Leucaena leucocephala Seeds. Ciência Agrotecnologia 2018, 42, 643–652. [Google Scholar] [CrossRef]
- Gomes Junior, F.G.; Duijn, B.V. Three-Dimensional (3-D) X-Ray Imaging for Seed Analysis. Seed Testing International 2017, 154, 48–52. [Google Scholar]
- Gantait, S.; Kundu, S.; Wani, S.H.; Das, P.K. Cryopreservation of Forest Tree Seeds: A Mini-Review. Journal of Forest and Environmental Science 2016, 32, 311–322. [Google Scholar] [CrossRef]
- Engelmann, F.; Dussert, S. Conservation of Tropical Plant Species; Springer: New York, NY, USA, 2012; pp. 107–119. [Google Scholar] [CrossRef]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. In Vitro Cellular & Developmental Biology - Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Bozkurt, Y. Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: London, United Kidgom, 2018; pp. 129–154. [Google Scholar] [CrossRef]
- Elliott, G. D.; Wang, S.; Fuller, B. J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Roque-Borda, C. A.; Kulus, D.; Souza, A. V.; Kaviani, B.; Vicente, E. F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. International Journal of Molecular Sciences 2021, 22, 6157. [Google Scholar] [CrossRef] [PubMed]
- El Merzougui, S.; Benelli, C.; El Boullani, R.; Serghini, M. A. The cryopreservation of medicinal and ornamental geophytes: application and challenges. Plants 2023, 12, 2143. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y. H.; Kim, U.; Lee, S. G.; Ryu, B.; Kim, J.; Igor, A.; Kim, J. S.; Jung, C. R.; Park, J. H.; Kim, C. Y. Vitrification for cryopreservation of 2D and 3D stem cells culture using high concentration of cryoprotective agents. BMC Biotechnology 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association. Determination of moisture content. International Rules for Seed Testing 2023, 9, 1–22. [CrossRef]
- Brasil. Instruções para a Análise de Sementes de Espécies Florestais; Ministério da Agricultura, Pecuária e Abastecimento (MAPA), Ed.; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2013; pp. 54–79. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/sementes-e-mudas/publicacoes-sementes-e-mudas/instrucoes-para-analise-de-sementes-de-especies-florestais/view (accessed on 20 January 2024).
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of Asparagus (Asparagus officinalis L.) Embryogenic Suspension Cells and Subsequent Plant Regeneration by Vitrification. Plant Science 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification. In Cryopreservation of Plant Germplasm I: Theory and Practice; Bajaj, M.A., Ed.; Springer, 2007; pp. 73–100. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 January 2024).
- Brancalion, P. H.; De Almeida, D. R.; Vidal, E.; Molin, P. G.; Sontag, V. E.; Souza, S. E.; Schulze, M. D. Fake legal logging in the Brazilian Amazon. Science Advances 2018, 4, eaat1192. [Google Scholar] [CrossRef]
- Franca, C. S.; Persson, U. M.; Carvalho, T.; Lentini, M. Quantifying timber illegality risk in the Brazilian forest frontier. Nature Sustainability 2023, 6, 1485–1495. [Google Scholar] [CrossRef]
- Hay, F. R.; Probert, R. J. Advances in seed conservation of wild plant species: a review of recent research. Conservation Physiology 2013, 1, cot030. [Google Scholar] [CrossRef]
- Arkhipov, M. V.; Priyatkin, N. S.; Gusakova, L. P.; Karamysheva, A. V.; Trofimuk, L. P.; Potrakhov, N. N.; Shchukina, P. A. Microfocus X-ray method for detecting hidden defects in seeds of woody forest species and other types of vascular plants. Technical Physics 2020, 65, 324–332. [Google Scholar] [CrossRef]
- Kunishima, N.; Takeda, Y.; Hirose, R.; Kalasová, D.; Šalplachta, J.; Omote, K. Visualization of internal 3D structure of small live seed on germination by laboratory-based X-ray microscopy with phase contrast computed tomography. Plant Methods 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Felix, F. C.; Medeiros, J. A.; Pacheco, M. V. Morfologia de sementes e plântulas de Handroanthus impetiginosus (Mart ex DC) Mattos. Revista de Ciências Agrárias 2018, 41, 1028–1035. [Google Scholar] [CrossRef]
- Carvalho, L. R. D.; Silva, E. A. A. D.; Davide, A. C. Classificação de sementes florestais quanto ao comportamento no armazenamento. Revista Brasileira de Sementes 2006, 28, 15–25. [Google Scholar] [CrossRef]
- Guimarães, C. C.; Faria, J. M. R.; Oliveira, J. M.; Silva, E. A. A. D. Avaliação da perda da tolerância à dessecação e da quantidade de DNA nuclear em sementes de Peltophorum dubium (Spreng.) Taubert durante e após a germinação. Revista Brasileira de Sementes 2011, 33, 207–215. [Google Scholar] [CrossRef]
- Pereira, W. V. S.; Faria, J. M. R.; Tonetti, O. A. O.; Silva, E. A. A. Perda da tolerância à dessecação em sementes de Copaifera langsdorffii durante a germinação. Brazilian Journal of Biology 2014, 74, 501–508. [Google Scholar] [CrossRef]
- Gomes, K. B. P.; Matos, J. M. M.; Martins, I. S.; Martins, R. C. C. X-ray test to evaluate the physiological potential of Platypodium elegans Vog seeds (Fabaceae). Scientia Agropecuaria 2016, 7, 305–311. [Google Scholar] [CrossRef]
- Pinheiro, D. T.; Medeiros, A. D.; Soares, T. F. N. S.; Capobiango, N. P.; Dias, D. C. T. F. Image analysis using X-ray to evaluate seed quality of Anadenanthera peregrina (L.) Speg. Ciência Florestal 2022, 32. [Google Scholar] [CrossRef]
- Araújo, J. D. O. , Pinheiro, D. T., Queiroz, G. B., Soares, J. M., Hoshide, A. K., Morais Junior, V. T. M. D., Soares da Rocha, S. J. S., & Dias, D. C. F. D. Selection of superior Senna macranthera seeds, carbon stock, and seedling survival, and costs for habitat restoration. Sustainability 2023, 15, 9875. [Google Scholar] [CrossRef]
- Salomão, A. N.; Santos, I. R. I.; José, S. C. B. R. Cryopreservation of Pyrostegia venusta (Ker Gawl) Miers seeds. Hoehnea 2020, 47, e1042019. [Google Scholar] [CrossRef]
- Castro, M. G. B.; Medeiros, A. D.; Silva, J. M.; Silva, L. J. Physical integrity of endive and chicory seeds determined by automated analysis of radiographic images. Revista Brasileira de Ciências Agrárias 2021, 16, e9072. [Google Scholar] [CrossRef]
- Vendrame, W.; Takane, R.; Cardoso, L.; Alvarez, L.; Tadeu, R. Cryopreservation of seeds of Melocactus zehntneri Braun ex Ritter f. and Cereus gounellei Luetzelb ex Schum. by the vitrification method. Agronomy Science and Biotechnology 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Silva, J. D. J.; Alencar, S. D. S.; Gomes, R. A.; Matias, J. R.; Pelacani, C. R.; Dantas, B. F. Conservation and physiological quality of Handroanthus spongiosus (Rizzini) S. Grose (Bignoniaceae) seeds. Journal of Seed Science 2022, 44, e202244007. [Google Scholar] [CrossRef]
- Velasco-García, M. V.; Hernández-Arroyo, D. G.; Muñoz-Gutiérrez, L.; Castillo-Martínez, C. R.; Vallejo-Reyna, M. Á.; García-Campusano, F. Seed cryopreservation of Cedrela odorata L: Germination and early nursery establishment. Revista Mexicana de Ciencias Forestales 2022, 13, 31–55. [Google Scholar] [CrossRef]
- Stegani, V.; Alves, G. A. C.; Bertoncelli, D. J.; Faria, R. T. V. Criopreservação de sementes de rainha do abismo (Sinningia leucotricha). Ornamental Horticulture 2017, 23, 15–21. [Google Scholar] [CrossRef]
- Paula, J. C. B.; Guariz, H. R.; Ribeiro Júnior, W. A.; Shimizu, G. D.; Faria, R. T.; Oliveira, H. C. Cryopreservation of seeds of the Brazilian native species Aroeira-do-sertão (Astronium urundeuva M. Allemão Engl.). Revista Caatinga 2022, 35, 915–924. [Google Scholar] [CrossRef]
- Souza, R. R. D.; Paiva, P. D. D. O.; Freitas, R. T. D.; Silva, D. P. C. D.; Reis, M. V. D.; Nery, F. C.; Paiva, R. Cryopreservation of Genipa americana seeds. Revista Ciência Agronômica 2023, 54, e20228531. [Google Scholar] [CrossRef]
- Zhang, M. J.; Wang, Y. Z.; Xian, Y.; Cui, C. C.; Xie, X. M.; Tong, B. Q.; Han, B. Desiccation sensitivity characteristics and low-temperature storage of recalcitrant Quercus variabilis. Seed Forests 2023, 14, 1837. [Google Scholar] [CrossRef]
- Paula, J. C. B.; Ribeiro Júnior, W. A. R.; Shimizu, G. D.; Men, G. B.; Faria, R. T. Cryopreservation in liquid nitrogen of Brazilian orchid seeds Miltonia flavescens Lindl. Revista Agro@mbiente On-line 2020, 14, e20200016. [Google Scholar] [CrossRef]
- Santana, B. Í. D.; Paiva, R.; Reis, M. V. D.; Vilas-Boas, L. V.; Matos, E. M.; Campos, J. M. S. D. Seed cryopreservation without vitrification (PVS2) induces oxidative stimuli to promote endoreplication in red pitaya seedlings. Plant Cell, Tissue and Organ Culture 2024, 156, 11. [Google Scholar] [CrossRef]
- Best, B. P. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Research 2015, 18, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef] [PubMed]
- Fahy, G.M.; Wowk, B. Principles of Cryopreservation by Vitrification. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 21–82. [Google Scholar] [CrossRef]
- Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitro Cellular & Developmental Biology-Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




